Abstract
Aims
To evaluate the pharmacokinetics of nevirapine and any possible influencing factors in pregnant women (n = 16), nonpregnant women (n = 13) and men (n = 14), who received nevirapine 200 mg twice daily together with nucleoside reverse transcriptase inhibitors.
Methods
Blood samples were taken for 12 h at steady state. Nevirapine concentrations were measured by liquid chromatography-tandem mass spectrometry. The influence of gender, age, body weight and comedication on minimum and maximum concentrations (Cmin, Cmax), area under the concentration-time curve (AUC), total clearance (CLtot), half-life (t1/2) and volume of distribution (Vd) was analysed by multivariate techniques.
Results
Mean [95% confidence interval (CI)] Cmax, AUCss and clearance were 5221 ng ml−1 (4267, 6175), 50 789 ng −1h ml−1 (43 453, 58 125) and 69.9 ml min−1 for men, 5871 ng ml−1 (4848, 6895), 57 045 ng h−1 ml−1 (45 997, 68 093) and 65.6 ml min−1 for nonpregnant women and 4505 ng ml−1 (3644, 5366), 44 579 ng h−1 ml−1 (36 564, 52 594) and 82.1 ml min−1 for pregnant women. The differences between pregnant and nonpregnant women (% difference, 95% CI) in Cmax (−30.3; −28.5, −33.0), AUCss (−28.0; − 25.8, − 29.5) and clearance (20.2; 26.6, 15.6) reached statistical significance (P = 0.010, P = 0.028 and P = 0.028, respectively). The multivariate analysis underscored the influence of bodyweight on the plasma exposure to nevirapine.
Conclusions
Pregnant women exhibited an increased nevirapine clearance and comparably low plasma concentrations, whereas women with a low bodyweight achieved high plasma nevirapine concentrations. The large variability in nevirapine concentrations in women may lead to loss of efficacy and viral resistance, or drug toxicity, and therefore these patients should be monitored frequently.
Keywords: nevirapine, pharmacokinetics, pregnancy
Introduction
Nevirapine is a non-nucleoside reverse transcriptase inhibitor (NNRTI) which is frequently used as part of a combination therapy in the treatment of HIV-1 infection. International guidelines for the prevention of mother to child transmission (PMTCT) of HIV recommend nevirapine in combination with nucleoside reverse transcriptase inhibitors (NRTI) as one treatment option [1–3]. Nevirapine is widely used in resource poor areas and it is available as part of generic (fixed) drug combinations [4]. Nevirapine is well absorbed with a bioavailability > 90%, has a high volume of distribution of 1.2 l kg−1 in adults and is about 60% bound to plasma proteins. Nevirapine crosses the placenta, resulting in a drug concentration ratio of 0.8 in cord blood compared with maternal blood. Nevirapine dose has to be increased after 2 weeks of therapy from 200 mg day−1 to 400 mg day−1 due to autoinduction of its metabolism. Elimination is prolongued in women during labour and in newborn children. Nevirapine is metabolized primarily by CYP3A4 and CYP2B6 and to a lesser extent by CYP2D6 and CYP2C9. Induction of the CYP3A4 by nevirapine is the reason for its potential interaction with other drugs [4–8]. The in vitro 50% inhibitory concentration (IC50) of nevirapine is 100 nm against clinical isolates of HIV. The drug has been proven to be effective and its side-effects profile is well characterized [7–10]. When administered in suboptimal regimens, drug-resistant viruses emerge rapidly, limiting future options for treatment with NNRTI. Viral mutations causing resistance against nevirapine include K103N, V106A, M, Y181C,I, Y188C,H and G190A,S and 230L. K103N, V106A,M and Y181C lead to cross-resistance against the other NNRTIs [6–10]. A recent study has characterized the relationship between plasma nevirapine concentrations and the durability of viral suppression and selection of primary mutations [11]. A trough nevirapine concentration of > 4300 ng ml−1 was found to predict longer viral suppression. The mean (± SD) trough concentration (n = 242) documented in the product information given by the manufacturer was 4500 ng ml−1 (±1900) [7]. Skin rash and exanthema are the most common adverse reactions to nevirapine, with Stevens-Johnson Syndrome occurring on rare occasions. Recently published data suggest a higher risk of severe hepatotoxicity in women with a body mass index < 18.5 and/or a CD4 cell count of > 250 µl−1[8–10]. In addition, other patient characteristics may influence the pharmacokinetics of nevirapine, leading to either very high or suboptimal plasma exposure [12–14]. Recent work has shown a high risk of the development of NNRTI resistance in women who received nevirapine as part of MTCT prophylaxis in late pregnancy [15]. One reason for this may be the comparatively short treatment period with NRTI of 5 days following the cessation of nevirapine after birth. It has been shown that variability in nevirapine half-life is high and, as a result, the median (range) predicted time for nevirapine plasma concentration to fall below the inhibitory concentration of wild-type virus (IC50) is 168 h (108–246) [16]. Therefore, plasma nevirapine concentrations which predispose to the selection of NNRTI-resistant viruses may persist in patients for up to 21 days [17].
In the present study we have compared the pharmacokinetics and pharmacodynamics of nevirapine in men, women and pregnant women in the late stage of pregnancy, all receiving 200 mg of nevirapine twice daily combined with nucleoside/nucleotide reverse transcriptase inhibitors.
Methods
Study protocol
After at least 2 weeks’ treatment with a nevirapine 200 mg twice daily plus NRTI regimen, the pharmacokinetics of the drug was assessed using a standardized protocol. The schedule of HIV drug intake was documented by the patients for 3 days prior to the study. In addition, all other drugs taken were recorded by the patient and physician, including daily intake of herbal agents or nutrition supplements. On the day of the study, fasting trough concentrations were recorded immediately before drug intake, followed by a standardized continental breakfast. Plasma samples were then collected at 1, 2, 4, 6, 9 and 12 h after drug administration. We included all patients studied between September 2001 and September 2004 and excluded all subjects without complete documentation or who had been taking comedication expected to influence the cytochrome P450-mediated metabolism of nevirapine. All data were obtained as part of the therapeutic drug monitoring (TDM) programme, which is routinely performed in the medical HIV treatment and research unit. Verbal consent for TDM was obtained from all patients and documented in their records. This study design was observational, with no additional intervention being performed. Ethics approval was not obtained based on the National Medical Act and the advice of the responsible ethics committee.
Drug analysis
Nevirapine concentrations were measured at the HIV-Laboratory, Berlin, Germany as described before [18]. Nevirapine was provided by Boehringer Ingelheim, Germany, and deuterium-labelled methadone (6-Di(trideuteromethyl)amino-4,4-diphenyl-1-trideuteromenthyl-3-heptanone), which was used as the internal standard, was obtained from Promochem® (LGC Promochem GmbH, Wesel, Germany). One hundred-microlitre aliquots of serum were added to polypropylene vials. After protein precipitation and extraction with 500 µl of acetonitrile containing the internal standard (1000 ng), the samples were spun at 13 000 g for 6 min. The supernatants were transferred into clean tubes, centrifuged and injected onto a Eurospher C18 (5 µm; 4.6 × 30 mm) column (Knauer, Berlin, Germany). The mobile Phase A was H2O: acetonitrile 95: 5 v:v and 0.0025 m ammonium acetate. Mobile Phase B was acetonitrile containing 0.1% formic acid. The high-performance liquid chromatography (HPLC) system consisted of the following components: a Perkin Elmer® Series 200 mobile phase delivery pump (Perkin Elmer, Wellesley, MA, USA) and a Gilson Abimed® 233 XL autosampler (ABIMED Analysen-Technik GmbH, Langenfeld, Germany). HPLC separation was achieved with mobile phase gradient elution (flow 1.5 ml min−1) using the following sequence: 0 min, 100% A; − 0.3 min, 100% A; − 0.4 min, 25% A; − 3.3 min, 25% A; − 3.5 min, 100% A. The injection volume was 50 µl. During the first 90 s of each run, the effluent was directed to waste. The flow to the MS was maintained with a second pump (Merck, Hitachi® L-7100) delivering methanol:H2O, 50: 50, v:v at a rate of 1.0 ml min−1.
An API 365 mass spectrometer (Applied Biosystems, Ontario, Canada) equipped with an electrospray ionization interface (ESI) and run with Analyst 1.2 software was used for detection. The majority (80%) of the effluent was diverted before entering the interface.
Analytes were monitored in the positive multiple reaction monitoring mode with the following transitions of precursor to product ions: m/z 267.0-226.3 (nevirapine) and 319.2-268.2 (deuterium-labelled methadone).
Interday and intraday coefficients of variation were < 5% at a concentration of 1000 ng ml−1 and < 8% at a concentration of 250 ng ml−1. Mean deviations from nominal concentrations were < 8% for all concentrations throughout all runs [18].
Pharmacokinetic analysis
Pharmacokinetic parameters were calculated according to a noncompartmental approach from the 0–12-h data. Cmin and Cmax values were read directly from the plasma concentration-time data over the standard dosing interval (12 h) The following pharmacokinetic parameters were obtained using a noncompartmental model: AUC(τ) = AUCss (0,12) the area under the concentration-time curve at steady-state conditions from time zero (trough) over the time span of the dosing interval τ = 12 h, obtained using the logarithmic trapezoidal rule. The oral clearance (CLtot) of nevirapine was determined from the expression D/AUC(τ) assuming complete bioavailability. The steady-state half-life (t1/2) was calculated from the elimination constant λz using the equation t1/2 = ln2/λz = 0.69315/λz (time). The last four plasma concentration points were weighted equally. The volume of distribution (Vz) following oral administration of the drugs was calculated from the equation Vz = CL/λz. All pharmacokinetic analyses were performed using TOPFIT2.0® software [19].
Statistical analysis
Pharmacokinetic parameters were subject to exploratory statistics (mean, SD). Primary target variables were AUCss (τ), Cmax, Cmin, CLtot, Vd and t1/2. The statistical analysis was based on the comparison of the primary target variables of group 2 (women) vs. group 1 (men) or group 3 (pregnant women). The T-test ws used to assess the absolute difference between the groups, and including the Levene variance ratio test for the equality of the group means. Normal distribution of values was determined for each time point and group, according to the Kolmogoroff-Smirnoff-Liljefors test, prior to the statistical analyses of parameters.
A multiple linear regression analysis described the effect on each pharmacokinetic parameter by weighted correlation of changes in the following variables: baseline age, CD4 cell count, HIV-RNA-PCR, gender and/or pregnancy, weight and comedication at the time of the study. Ethnic background was not considered to be an influencing factor [20]. The multiple linear regression test was performed by the stepwise deletion of variables and included the analysis of variance of all parameters in each group. As we tested for six different variables, the statistical significance level was set as P < 0.001 according to the Bonferoni correction. All statistical analyses used SPSS 11.5 for Windows [21] (SPSS Inc., Chicago, IL, USA) except the Pearson linear regression analysis of the body weight-dependent exposure to nevirapine, which was performed with Graphpad Prism 4.01 (Graphpad, San Diego, CA, USA) [22].
Results
The demographics and characteristis of patients at baseline were not comparable for all groups (Table 1). Mean age differed between the groups of male (47.2 years) and female patients (nonpregnant women 33.4 and pregnant women 29.4 years). The majority of males were caucasian and the majority of females belonged to various ethnic backgrounds, such as black African (n = 12), Asian (n = 3) and Hispanic (n = 2). At baseline of therapy a higher number of male patients had an AIDS diagnosis in their medical history (n = 9; 64.3%) compared to nonpregnant (n = 2; 15.4%) or pregnant women (n = 3; 18.8%). Viral load was higher in pregnant women (4.09 log10) than in nonpregnant women (3.1 log10) and men (2.57 log10), whereas pregnant women had the highest absolute CD4 cell count (333 µl−1) compared with nonpregnant women (209 µl−1) and men (280 µl−1). Both groups of women had approximately three previous treatments, whereas men underwent five previous treatments. Mean body weight also differed between all groups (men 76.3 kg; nonpregnant women 58.8 kg and pregnant women 70.2 kg).
Table 1.
Patients’ baseline characteristics
| Groups Parameter | Men (1) n = 14 | Women (2) n = 13 | Pregnant women (3) n = 16 | |||
|---|---|---|---|---|---|---|
| Mean | % (range) | Mean | % (range) | Mean | % (range) | |
| Cauc4/other | 11/3 | 78.5/21.5 | 5/8 | 38.5/61.5 | 7/9 | 43.8/56.2 |
| Age (years) | 47.2 | (33–61) | 33.4 | (27–58) | 29.4 | (22–41) |
| Bodyweight (kg) | 76.3 | (65–87) | 58.8 | (44–84) | 70.2 | (54–87) |
| CDC status B/C3 | 3/9 | 21.4/64.3 | 7/2 | 53.8/15.4 | 5/3 | 31.3/18.8 |
| HCV-PCR positive | 1 | 7.1 | 1 | 7.7 | 3 | 18.8 |
| CD4 cell count | 280 | (15–720) | 209 | (17–610) | 333 | (116–696) |
| Viral load (log10) | 2.57 | (1.28–5.27) | 3.10 | (1.28–5.55) | 4.09 | (1.28–5.62) |
| Previous treatments | 5.0 | (0–11) | 2.9 | (0–8) | 2.6 | (0–4) |
| NNRTI-naïve | 9 | 64.3 | 7 | 53.8 | 5 | 31.3 |
| Treatment-naive | 2 | 14.3 | 4 | 30.1 | 5 | 31.3 |
| Concomitant nucleoside or nucleotide reverse transcriptase inhibitors | ||||||
| Zidovudine | 5 | 35.7 | 5 | 38.5 | 16 | 100 |
| Lamivudine | 11 | 78.6 | 12 | 92.3 | 16 | 100 |
| Tenofovir | 5 | 35.7 | 4 | 30.8 | – | – |
| Stavudine | 3 | 21.4 | 4 | 30.8 | – | – |
| Abacavir | 4 | 28.6 | 1 | 7.7 | – | – |
| Didanosine | 2 | 14.3 | 1 | 7.7 | – | – |
The majority of pregnant women (n = 14, 87.5%) were started on nevirapine therapy in late pregnancy with a mean [95% confidence interval (CI)] of 9 weeks (6.6, 11.2) from first dose to birth. The mean (95% CI) baseline viral load declined from 4.09 log10 (3.58, 4.6) to 1.65 log10 (1.44, 1.86) at birth with a mean decrease in viral load of 2.46 log10 (1.98, 2.92). At the time of birth four women had an undetectable viral load, seven women had concentrations between 20 and 50 copies ml−1 and five women between 51 and 350 copies ml−1. The mean (95% CI) CD4 cell count of 333 µl−1 (255, 411) at baseline showed an increase of 116 µl−1 (52, 180) to a mean of 457 µl−1 (370, 544) at time of birth. Viral load decreased in all pregnant women, whereas the CD4 cell count decreased in two cases between the start of treatment and birth (−122 µl−1 and − 56 µl−1). Two women were already on combination treatment with nevirapine plus NRTIs, when β-human chorionic gonadotropin was tested positive. They remained on treatment throughout pregnancy (time from first dose until birth was 104 and 160 weeks, respectively). Both had a viral load < 500 copies ml−1 when they started treatment and an undetectable viral load when they gave birth. Two women underwent an emergency caesarean operation at the 31st and 34th week of pregnancy due to complications (preliminary uterine contractions and amniorrhexis). The remaining 14 women had an elective caesarean section at the 36th and 37th weeks. None of the children (n = 17) was HIV-RNA-PCR positive at birth.
Baseline genotypic resistance testing was performed in 12 pregnant women (75%), who showed no resistance to NNRTI prior to therapy baseline. The pharmacokinetics of nevirapine was studied in pregnant women in either the third (n = 12; mean pregnancy week 32 + 0 (days)) or the second trimester (n = 4; mean pregnancy week 22 + 5 (days)).
Mean values plus 95% CIs for all pharmacokinetic parameters are shown in Table 2. Men exhibited mean (95% CI) values of AUCss, Cmin and Cmax of 50 789 ng ml−1 (43 453, 58 125), 3343 ng ml−1 (2907, 3779) and 5221 ng ml−1 (4267, 6175), respectively, plasma nevirapine concentrations were highest in nonpregnant female patients with mean (95% CI) values of AUCss, Cmin and Cmax of 57 045 ng h−1 ml−1 (45 997, 68 093), 3462 ng ml−1 (2536, 4387) and 5871 ng ml−1 (4848, 6895), respectively, compared with pregnant women with corresponding mean (95% CI) values of 44 579 ng h−1 ml−1 (36 564, 52 594), 2925 ng ml−1 (2312, 3538) and 4505 ng ml−1 (3644, 5366). The differences in the AUCss values for nevirapine (P = 0.028) and Cmax (P = 0.010) reached statistical significance. The oral clearance also differed markedly between nonpregnant and pregnant patients (66 ml min−1 vs. 82 ml min−1; P = 0.028). The body weight-adjusted AUCss mg−1 kg−1 differed significantly between male and nonpregnant female patients (19 324 vs. 15 678 ng h−1 ml−1; P = 0.036), as did the dose adjusted by weight (2.62 vs. 3.62 mg kg−1; P < 0.001). The mean volume of distribution was higher in pregnant women (106 l) compared with men (87 l) and nonpregnant females (73 l). The elimination half-life of nevirapine was comparable for all groups. Nonpregnant women showed the greatest variablity in nevirapine plasma concentrations and nevirapine dose related to body weight (mg/kg).
Table 2.
Pharmacokinetic parameters for nevirapine in parients given 200 mg twice daily
| Parameter | Men, group 1, mean (95% CI) | Women, group 2 mean (95% CI) | Pregnant women, group 3 mean (95% CI) | Difference group 1 vs. 2 % (95% CI) | T-test, P-valuea | Difference group 3 vs. 2 % (95% CI) | T-test, P-valuea |
|---|---|---|---|---|---|---|---|
| Cmin (ng ml−1) | 3343 (2907, 3779) | 3462 (2536, 4387) | 2925 (2312, 3538) | −3.4 (−13.9, 14.6) | NS | −18.4 (−9.4, −24.0) | NS |
| Cmax (ng ml−1) | 5221 (4267, 6175) | 5871 (4848, 6895) | 4505(3644, 5366) | −11.1(−12.0, − 10.5) | NS | −30.3 (−28.5, − 33.0) | 0.010 |
| AUCss (ng h−1 ml−1) | 50 789 (43 453, 58 125) | 57 045 (45 997, 68 093) | 44 579 (36 564, 52 594) | −11.0 (−14.6, − 5.5) | NS | −28.0 (−25.8, − 29.5) | 0.028 |
| t1/2 (h) | 15.02 (10.34, 19.70) | 14.09 (10.13, 18.05) | 15.16 (11.95, 18.37) | 6.6 (2.1, 9.1) | NS | 7.1 (15.5, 1.7) | NS |
| Cltot (ml min−1) | 69.9 (58.9, 80.9) | 65.5 (49.9, 81.2) | 82.1 (68.0, 96.2) | 6.7 (0.4, 18.0) | NS | 20.2 (26.6, 15.6) | 0.028 |
| Vd (l) | 86.6 (65.5, 107.8) | 72.9 (54.3, 91.5) | 106.3 (80.5, 132.0) | 18.8 (17.8, 20.6) | NS | 31.4 (32.5, 30.7) | 0.062 |
| AUCss mg−1 kg−1 (ng h−1 ml−1) | 15 679 (13 332, 18 025) | 19 325 (16 656, 21 994) | 15 722 (12 400, 19 045) | −18.9 (−20.0, − 18.0) | 0.036 | −23 (−15.5, −34.3) | NS |
| Dose/weight (mg kg−1) | 2.62 (2.50, 2.74) | 3.62 (3.19, 4.06) | 2.90 (2.70, 3.10) | −27.6 (−32.5, − 21.6) | <0.001−24.8 (−18.1, −140.0) | 0.002 |
Difference between the groups according the T-Test; CI = Confidence Interval; AUCss = area under the concentration-time curve at steady state; Cmin = minimum concentration; Cmax = maximum concentration; Cl tot = total clearance; t1/2 = elimination half-life; V d = volume of distribution.
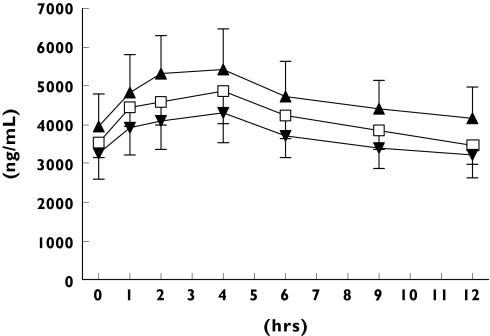
Plasma exposure to nevirapine was significantly influenced by weight-related parameters. The independent predictor for a low Cmin and AUCss is the dose/weight (mg kg−1; P < 0.001), whereas nevirapine Cmax is related to body weight (P < 0.001) and volume of distribution (Vd; P < 0.001) A further correlation between plasma exposure and bodyweight was detected by the Pearson regression test (r = −0.378; 95% CI − 0.609, − 0.087, P = 0.013) (Figure 1). The r2-values for the linear regression test differed between the groups. Thus, a good correlation was found for nonpregnant women (r2 = 0.48), whereas pregnant women (r2 = 0.02) and men (r2 = 0.14) showed no or a weak correlation between weight and nevirapine AUCss.
Figure 1.
Steady-state nevirapin concentration-time curves in men, nonpregnant and pregnant women over the 12-h dosing interval. NVP men (□), NVP women (▴), NVP pregnant (▾)
Gender, age, nucleotide/nucleoside reverse transcriptase comedication, baseline CD4 cell count and viral load appeared to have no significant influence on the plasma concentrations of nevirapine.
Discussion
Although nevirapine exhibited lower plasma concentrations in pregnant women, it appeared to be effective in PMTCT and HIV treatment during late pregnancy. The viral load decrease in pregnant women was significant over a mean time of 9 weeks of treatment before birth. Immunological and virological response to therapy with nevirapine (200 mg twice daily) plus NRTI in our study was comparable to previously published data [6, 7].
Since 25% of the pregnant women studied exhibited a minimum steady-state nevirapine concentration between 2345 and 1250 ng ml−1 and nevirapine concentrations may be also subject to an intraindividual variability of 26%[23], there exists considerable potential for subinhibitory plasma exposure and development of viral NNRTI resistance in pregnancy. A minimum nevirapine concentration of < 2300 ng ml−1 was associated with a trend towards a higher risk of developing virological failure, although no precise value associated with a significant increase in the hazard ratio has been defined [24]. The product labelling for nevirapine recommends minimum plasma concentrations of 3500 ng ml−1 to maintain long-term efficacy [7] and minimum concentrations > 4300 ng ml−1 were found to be predictive of sustained virological response [11].
Twenty-five percent of the nonpregnant/pregnant women achieved high maximum plasma concentrations between 7234/5198 ng ml−1 and 8760/9150 ng ml−1, which may lead to adverse reactions. A low body weight was independently associated with high plasma nevirapine concentrations. Pregnancy is known to influence body weight, volume of distribution, plasma protein binding of drugs, hepatic blood flow and the activity of metabolic enzymes. A new compartment of considerable volume in late pregnancy (the fetal compartment) is opened and weight is gained due to the increase in body fat, extracellular body water and the growing fetus. Nevirapine is distributed into these compartments, but this does not explain the lowered steady-state plasma nevirapine concentrations in pregnant women. The latter finding is probably the result of the higher clearance of the drug during pregnancy. The activities of several forms of cytochrome P450 contributing to drug metabolism are increased during pregnancy, namely CYP2C9 [25–27], CYP2D6 [28] and CYP3A4 [29, 30]. Together with decreased albumin concentrations and an increased fraction of unbound drug, increased CYP activity is likely to be the reason for the significantly lowered plasma concentrations in pregnant women. However, even comparatively low plasma nevirapine concentrations produce sufficient antiviral activity in pregnant women, which may be explained by the absence of changes or even an increase in unbound drug [31].
Nevirapine concentrations should be monitored and if necessary adjusted in women with a low body weight and/or during pregnancy, and particularly in those with high plasma nevirapine concentrations. Furthermore female gender, abnormally high alanine aminotransferase level and CD4 cell count > 250 µl−1 at baseline, time on previous antiretroviral teatment and hepatitis B or C coinfection were reported to be independent risk factors for the development of hepatotoxicity during nevirapine-containing treatments [10, 32–35]. Although these data are somewhat contradictory and serious adverse events such as liver failure and hepatic related mortality occurred mainly after long-term treatment with nevirapine plus protease inhibitors in patients who were infected with hepatitis C [28], these risk factors should be considered at the start of therapy and carefully monitored throughout treatment. In addition, women who are not hepatitis C positive or who have a history of previous exposure to nevirapine have developed liver failure, which in some cases has been fatal, during pregnancy [36, 37].
Bearing in mind the limitations of an observational study with a comparably small number of participants, it is clear that nevirapine plus zidovudine/lamivudine can be an effective and safe therapy in the late second and third trimesters of pregnancy, if attention is paid to the potential risk factors for toxicity and viral resistance. Following the recent US guidelines for the treatment of HIV during pregnancy and the present mother to child transmission (PMTCT) services, nevirapine therapy should not be started in women with a baseline CD4 cell count of > 250 µl−1 blood, unless ‘the benefit clearly outweighs the risk’[1]. The variability in plasma exposure to nevirapine underlines the use of therapeutic drug monitoring, especially in pregnant women. Further data on the influence of genetic variation in drug transporters, metabolizing enzymes [38] and of nevirapine pharmacokinetics on the clinical outcome are warranted. A link between plasma nevirapine concentrations and its main side-effects, rash and hepatotoxicity [39], has not yet been established.
Although studies in outpatients are not common for the investigation of pharmacokinetics or pharmacokinetic-pharmacodynamic relationships, the present sampling strategy followed a standardized protocol, which was designed to assess data from patients in an ambulatory setting. The analysis of mean (plus 95% CI) pharmacokinetic parameter values between normally distributed groups of patients, under steady-state conditions by equal weighting of seven samples over the dosing interval, should minimize the influence of intraindividual variability. The interindividual range of plasma drug concentrations in this study was seen as representative and not rejected as a bias. Interindividual variations point to the necessity of therapeutic drug monitoring and to its potential benefit in certain populations of patients [40, 41]. Therapeutic drug monitoring should follow a standardized protocol and be repeated frequently. As it is difficult for women in the very late stage of pregnancy to remain in the study for 12 h for blood sampling, easier ways of generating valid data are desirable and should lead to the use of sparse data pharmacokinetic models [42] for monitoring in pregnant women.
References
- 1.Centers for Disease Control. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1 Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV-1 Transmission in the United States. 24 February 2005. Available at http://AIDSinfo.nih.gov Last accessed 15 March 2005. [PubMed]
- 2.Centers for Disease Control. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1 Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV-1 Transmission in the United States. Supplement: Safety and Toxicity of Individual Antiretroviral Agents in Pregnancy. 24 February 2005. Available at http://AIDSinfo.nih.gov Last accessed15 March 2005.
- 3.Deutsche AIDS-Gesellschaft (DAIG), Österreichische AIDSGesellschaft (ÖAG), Robert Koch Institut et al. DeutschÖsterreichische Empfehlungen zur HIV-Therapie in der Schwangerschaft. May 2003. Available at http://rki.de Last accessed 15 March 2005.
- 4.Penzak SR, Acosta EP, Turner M, Tavel JA, Masur H. Analysis of generic nevirapine products in developing countries. JAMA. 2003;289:2648–9. doi: 10.1001/jama.289.20.2648-c. [DOI] [PubMed] [Google Scholar]
- 5.Mirochnick M, Clarke DF, Dorenbaum A. Nevirapine: pharmacokinetic considerations in children and pregnant women. Clin Pharmacokinetics. 2000;39:281–93. doi: 10.2165/00003088-200039040-00004. [DOI] [PubMed] [Google Scholar]
- 6.Mirochnick M. Antiretroviral pharmacology in pregnant women and their newborns. Ann NY Acad Sci. 2000;918:287–97. doi: 10.1111/j.1749-6632.2000.tb05498.x. [DOI] [PubMed] [Google Scholar]
- 7.Nevirapine Product Information. Ridgefield, USA: Boehringer Ingelheim Pharmaceuticals, Inc.; 27 March 2002. Version revised. [Google Scholar]
- 8.Boehringer-Ingelheim. Viramune. Summary of Product Characteristics. Available at http://www.viramune.com (last accessed: February 2005).
- 9.Change of the Product Information for Nevirapine 200mg Tablets. Ingelheim: Boehringer Ingelheim Pharma; 12 February 2004. [Google Scholar]
- 10.Milinkovic A, Martinez E. Nevirapine in the treatment of HIV. Expert Rev Anti-Infect Ther. 2004;2:367–73. doi: 10.1586/14787210.2.3.367. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez de Requena D, Bobora S, Garazzino S, Sciandra M, d’Avolio A, Raiteri R, Marrone R, Boffito M, De Rosa FG, Sinicco A, Di Perri G. Nevirapine plasma exposure affects both durability of viral suppression and selection of nevirapine primary resistance mutations in a clinical setting. Antimicrob Agents Chemother. 2005;49:3966–9. doi: 10.1128/AAC.49.9.3966-3969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanne I, Mommeja-Marin H, Hinkle J, Bartlett JA, Lederman MM, Maartens G, Wakeford C, Shaw A, Quinn J, Gish RG, Rousseau F. Severe hepatotoxicity associated with nevirapine use in HIV-1 infected subjects. J infect Dis. 2005;191:825–9. doi: 10.1086/428093. [DOI] [PubMed] [Google Scholar]
- 13.Kappelhoff BS, van Leth F, MacGregor TR, Lange J, Beijnen JH, Huitema AD NN Study Group. Nevirapine and efavirenz pharmacokinetics and covariate analysis in the 2NN study. Antiviral Ther. 2005;10:145–55. [PubMed] [Google Scholar]
- 14.Haberl A, von Hentig N, Carlebach A, Kurowski M, Harder S, Staszewski S. Nevirpaine plasma exposure is decreased in pregnant women. In 15th International AIDS Conference, July 2004, Bangkok, Thailand. 2004;1 Abstract published in eJIAS Tu PeB4644. [Google Scholar]
- 15.Lyons FE, Coughlan S, Byrne CM, Hopkins SM, Hall WW, Mulcahy FM. Emergence of antiretroviral resistance in HIV-positive women receiving combination antiretroviral therapy in pregnancy. AIDS. 2005;19:63–7. doi: 10.1097/00002030-200501030-00007. [DOI] [PubMed] [Google Scholar]
- 16.Muro E, Droste J, ter Hofstede H, Bosch M, Dolmans W, Burger D. San Francisco, USA: 8–11 February 2004. Nevirapine plasma concentrations are still detectable after more than 2 weeks in the majority of women receiving single-dose NVP. Implications for intervention studies 11th Conference on Retroviruses and Opportunistic Infections. Abstract 891. [DOI] [PubMed] [Google Scholar]
- 17.Mackie NE, Fidler S, Tamm N, Clarke JR, Back D, Weber JN, Taylor GP. Clinical implications of stopping nevirapine-based antiretroviral therapy: relative pharmacokinetics and avoidance of drug resistance. HIV Med. 2004;5:180–4. doi: 10.1111/j.1468-1293.2004.00208.x. [DOI] [PubMed] [Google Scholar]
- 18.Stocker H, Kruse G, Kreckel P, Herzmann C, Arasteh K, Claus J, Jessen H, Cordes C, Hintsche B, Schlote F, Schneider L, Kurowski M. Nevirapine significantly reduces the levels of racemic methadone and (R)-methadone in human immunodefficiency virus-infected patients. Antimicrob Agents Chemother. 2004;48:4148–53. doi: 10.1128/AAC.48.11.4148-4153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinzel G, Woloszczak R, Thomann P. TOPFIT 20 Pharmacokinetic and Pharmacodynamic Data Analysis System for the PC. Stuttgart: Gustav Fischer Verlag; 1993. [Google Scholar]
- 20.De Maat MM, Nellen JF, Huitema AD, Wit FW, Mulder JW, Prins JM, Beijnen JH. Race is not associated with nevirapine pharmacokinetics. Therapeut Drug Monit. 2004;26:456–8. doi: 10.1097/00007691-200408000-00018. [DOI] [PubMed] [Google Scholar]
- 21.SPSS for Windows®. Version 11.5 (German).
- 22.Graphpad Prism®. Version 4.01.
- 23.Nettles R, Kieffer T, Parsons T, Johnson J, Quinn T, Jackson B, Cofranesco J, Gallant J, Carson K, Siliciano R, Flexner C. Frequent sampling in virologically suppressed patients taking HIV protease inhibitors or non-nucleoside reverse transcriptase inhibitors defines intra-individual pharmacokinetic variability. 12th Conference on Retroviruses and Opportunistic Infections; 22–25 February 2005; Boston, USA. Abstract 642. [Google Scholar]
- 24.van Leth F, Kappelhoff B, Johnson D, Losso M, Boron-Kaczmarska A, Saag M, Livrozet JM, Hall D, Huitema A, Wit F, Beijnen J, Lange J, Anderson GD the 2NN Study Group. Minimum plasma coincentrations of nevirapine and efavirenz in relation to virologic failure in antiretroviral-naïve patients. In: 12th Conference on Retroviruses and Oprtunistic Infections 2005 February 22–25, Boston, USA. Abstract 8025. Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989–1008. [Google Scholar]
- 25.Wadelius M, Darj E, Frenne G, Rane A. Induction of CYP2D6 in pregnancy. Clin Pharmacol Ther. 1997;62:400–7. doi: 10.1016/S0009-9236(97)90118-1. [DOI] [PubMed] [Google Scholar]
- 26.Hogstedt S, Lindberg B, Peng DR, Regardh CG, Rane A. Pregnancy-induced increase in metoprolol metabolism. Clin Pharmacol Ther. 1985;37:688–92. doi: 10.1038/clpt.1985.114. [DOI] [PubMed] [Google Scholar]
- 27.Heikkinen T, Ekblad U, Palo P, Laine K. Pharmacokinetics of fluoxetine and norfluoxetine in pregnancy and lactation. Clin Pharmacol Ther. 2003;73:330–7. doi: 10.1016/s0009-9236(02)17634-x. [DOI] [PubMed] [Google Scholar]
- 28.Tomson T, Lindbom U, Ekqvist B, Sundqvist A. Disposition of carbamazepine and phenytoin in pregnancy. Pilepsia. 1994;35:131–5. doi: 10.1111/j.1528-1157.1994.tb02922.x. [DOI] [PubMed] [Google Scholar]
- 29.Ohkita C, Goto M. Increased 6-hydrocortisol excretion in pregnant women: implication of drug-metabolizing enzyme induction. DICP. 1990;24:814–6. doi: 10.1177/106002809002400902. [DOI] [PubMed] [Google Scholar]
- 30.Kosel BW, Beckermann KP, Hayashi S, Homma M, Aweeka FT. Pharmacokinetics of nelfinavir and indinavir in HIV-1 infected pregnant women. AIDS. 2003;17:1195–9. doi: 10.1097/00002030-200305230-00011. [DOI] [PubMed] [Google Scholar]
- 31.Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 32.Martinez E, Blanco JL, Arnaiz JA, Perez-Cuevas JB, Mocroft A, Cruceta A, Marcos MA, Milinkovic A, Garcia-Viejo MA, Mallolas J, Carne X, Phillips A, Gatell JM. Hepatotoxicity in HIV-1 infected patients receiving nevirapine-containing antiretroviral therapy. AIDS. 2001;15:1261–8. doi: 10.1097/00002030-200107060-00007. [DOI] [PubMed] [Google Scholar]
- 33.Sulkowski MS, Thomas DL, Mahta SH, Chisson RE, Moore RD. Hepatotoxicity associated with nevirapine- or efavirenz-containing antiretroviral therapy: role of hepatitis C and B infections. Hepatology. 2002;35:182–9. doi: 10.1053/jhep.2002.30319. [DOI] [PubMed] [Google Scholar]
- 34.De Maat MM, Mathot RA, Veldkamp AI, Huitma AD, Mulder JW, Meenhorst PL, Van Gorp EC, Carlier H, Beijnen JH. Hepatotoxicity following nevirapine-containing regimens in HIV-1 infected individuals. Pharmacol Res. 2002;46:295–300. doi: 10.1016/s1043-6618(02)00146-9. [DOI] [PubMed] [Google Scholar]
- 35.Stern JO, Robinson PA, Love J, Lanes S, Imperails MS, Mayers DL. A comprehensive hepatic safety analysis of nevirapine in different populations of HIV infected patients. J Acquir Immune Def Syndr. 2003;34(Suppl. 1):S21–33. doi: 10.1097/00126334-200309011-00005. [DOI] [PubMed] [Google Scholar]
- 36.Clendennen N, Barry C, McConkey S. Hepatotoxicity occuring in the second pregnancy of a patient on longterm nevirapine. 10th European AIDS Conference; 17–20 November 2005; Dublin, Ireland. Abstract PS 8/1. [Google Scholar]
- 37.Devitt E, Jackson A, Sheehan G, Powderly WG. Nevirapine associated hepatitis occuring after prior successful nevirapine therapy in a pregnant woman. 10th European AIDS Conference; 17–20 November 2005; Dublin, Ireland. Abstract PS 8/2. [Google Scholar]
- 38.Haas DW, Bartlett J, Andersen J, Sanne I, Wilkinson G, Quinn J, Rousseau F, Ingram C, Shaw A, Lederman M, Kim R. Pharmacogenetics of nevirapine and hepatotoxicity: NWCS220, an ACTG Collaborative Study. 12th Conference on Retroviruses and Opportunistic Infections; 22–25 February 2005; Boston, USA. Abstract 833. [Google Scholar]
- 39.Crommentuyn KM, Huitema AD, Brinkman K, van der Ende ME, de Wolf F, Beijnen JH. Therapeutic drug monitoring of nevirapine reduces pharmacokinetic variability but does not affect toxicity or virologic success in the ATHENA study. J AIDS. 2005;39:249–50. [PubMed] [Google Scholar]
- 40.Back D, Blaschke T, Boucher C, Burger D, Fletcher C, Flexner C, Gerber J, Schapiro J. Optimising TDM in HIV Clinical Care: a Practical Guide to Performing Therapeutic Drug Monitoring (TDM) for Antiretroviral Agents. Version 1.0 Available at: http://www.hivpharmacology.com Last accessed 14 March 2005.
- 41.van Heeswijk RPG, Veldkam A, Mulder JW, Meenhorst PL, Lange JM, Beijnen JH, Hoetelmans RM. Combination of protease inhibitors for the treatment of HIV-1-infected patients: a review of pharmacokinetics and clinical experience. Antiviral Ther. 2001;6:201–29. [PubMed] [Google Scholar]
- 42.Barrett JS, Labbe L, Pfister M. Application and impact of population pharmacokinetics in the assessment of antiretroviral pharmacotherapy. Clin Pharmacokinetics. 2005;44:591–625. doi: 10.2165/00003088-200544060-00003. [DOI] [PubMed] [Google Scholar]