Abstract
Aims
To describe how changes in legislation to restrict paracetamol sales have affected overdose discharges and death associated with the drug in Scotland.
Methods
A descriptive analysis of routine death and hospital discharge data for the entire Scottish population between 1995 and 2004. Patients in Scotland participated who were discharged from hospital with a diagnosis of poisoning; deaths in Scotland from diagnosis of poisoning 1995–2003 were also analysed. Outcome measures were changes in mortality and overdose due to poisoning involving paracetamol. A comparison was made of in-hospital and out-of-hospital mortality in fatalities involving paracetamol.
Results
The majority of paracetamol-associated deaths were due to co-proxamol. Deaths associated with paracetamol alone or with ethanol occurred principally in hospital and were a minority of deaths overall. The proportion of in-hospital deaths attributed to paracetamol increased (post/pre ratio 1.347; 95% confidence interval 1.076, 1.639; P = 0.013). Overall numbers of cases discharged with poisoning fell. The proportion of these involving paracetamol in any form increased significantly in all groups except young men aged 10 to <20 years.
Conclusions
Legislation has not reduced mortality or proportional use of paracetamol in overdose, both of which appear to have increased in Scotland since pack-size limitations. Other approaches are necessary to reduce the death rate from overdoses involving paracetamol.
Keywords: co-proxamol, paracetamol poisoning, self-harm, suicide
Introduction
In September 1998 paracetamol sales in Scotland were, as in the rest of the UK, affected by legislation [1–3] This restricted the amount of paracetamol and aspirin that could be bought over the counter to 32 tablets in pharmacies and 16 tablets in other retail outlets. Paracetamol had to be supplied in blister packs accompanied by written ‘health warnings’.
In several early studies the legislation has been reported to have led to a reduction in both the number of paracetamol overdoses and the number of tablets consumed [4–9]. While beneficial effects on death rates and trends in paracetamol-related liver damage are reported in England, in Scotland no such change was initially observed [10, 11]. Assessment of this apparent difference may be complicated by the fact that more admissions for paracetamol-induced liver failure in Scotland were from disadvantaged populations [11]. Paracetamol-associated morbidity and mortality in Scotland is deprivation related and initially appeared unchanged [1].
Health statistics relating to paracetamol are, however, complex. In Scotland hospital discharge and mortality datasets include all episodes in which paracetamol is recorded, whatever the formulation or source. The overall rates of self-harm in the community are another factor that can change with time. Furthermore, disadvantaged populations may receive more prescription medicines, or combination products, which could be confounders in any analysis. Thus the influence of paracetamol pack-size change on these prescription drugs, particularly co-proxamol (paracetamol with dextropropoxyphene), needs to be considered and the effect of pack-size restriction on hospital activity or death rate cannot be easily extracted. We now present further work examining overdose and, in particular, death rates, from paracetamol-related poisoning in Scotland’s population from 1995 to 2004 inclusive.
Methods
Overall numbers of deaths by poisoning where paracetamol was recorded as implicated on the death certificate were provided by the General Register Office for Scotland (GROS) for 1996–2003. These data were further analysed by taking individual records and assessing the drugs recorded in each case and location of death. We reasoned that in-hospital deaths were more likely to reflect the effects of paracetamol, which takes at least 48–72 h to be fatal, whereas out-of-hospital deaths were more likely to reflect coingested toxins, particularly opioids. In the case of co-proxamol (paracetamol 325 mg, dextropropoxyphene 32.5 mg) diagnosis is generally reached on a combination of laboratory tests and history. Dextropropoxyphene is virtually never prescribed alone in Scotland and so we regard detection of dextropropoxyphene, or its metabolite norproxyphene, to indicate ingestion of this compound product in overdose.
For deaths, overall in Scotland, we have examined absolute numbers per annum relating to four categories in the years 1995–2003. We have examined absolute numbers of deaths per annum related to four categories: 1, paracetamol alone, or with ethanol; 2, paracetamol in any dosage form other than co-proxamol and other coingested medicines; 3, co-proxamol overall and without other potentially fatal coingestions; and 4, co-codamol (paracetamol and codeine) and co-dydramol (paracetamol and dihydrocodeine) alone. Having examined these data we then chose to concentrate our analysis on in-hospital deaths in which paracetamol was mentioned (excluding co-proxamol), as this was the target population for the legislation. We have specifically examined three periods, one before legislation, one transitional, and one more than 2 years after legislation, in order better to understand change over time.
The Information Services Division (ISD) of NHS National Services Scotland routinely collects information on activity in Scottish hospitals using the Scottish Morbidity Records scheme (SMR). Using the SMRs for acute hospital discharges (SMR01), all episodes were identified where overdose was recorded from 1995 to 2004 inclusive (paracetamol overdose defined as ICD10 code T39.1 in any of the six available diagnostic fields. All poisonings were defined as T36–T51).
The numbers of paracetamol overdoses and all other overdoses were calculated quarterly and the proportions of all overdoses in which paracetamol were recorded calculated.
Prescription data (items) for the common compound analgesics containing paracetamol were also obtained from ISD. As this legislation also affected aspirin availability, we examined the profile of aspirin poisoning in a similar way in that to which we explored paracetamol.
Statistical methods
The overall statistical approach was the use of Generalized Linear Modelling (GLM) [12]. The software used was the R system [13]. Models involving numbers of poisonings were fitted using Poisson family models with the canonical ln link function. Models involving proportions were fitted with the Binomial family with logistic link function.
Models
The obvious periods to consider were before and after legislation, but it was anticipated that a transient disturbance might occur around the time that the legislation became current. The modelling was data-driven to the extent that the duration of the transitional interval was estimated by examination of the data.
A period factor was used to index the three periods studied: prelegislation, transitional and post-legislation. It was decided on the basis of observation of graphical data to take the transitional period as 1998 Q2 to 2000 Q2 inclusive. Observations by Hawton et al.[9] regarding commercially available pack sizes and information related to supermarket procedures in the UK (F.R.H., personal communication from management source) suggest that, for obvious reasons, availability of large quantities of analgesics on single purchase changed in anticipation of the legislation. In addition, we reasoned household stocks would reduce over time.
It was expected that sex would be an important factor in behaviour, and this was therefore modelled.
A suggested logical reason for the legislation was that even though adults could easily collect supplies from several shops, infants and the elderly would be less at risk of accidental ingestion if only small, difficult to open packs are available. Age was therefore included in the model as a factor. Patients were grouped as follows: 0 to < 10 years (child); 10 to < 20 years (youth); 20 to < 70 years (adult); ≥ 70 years (elderly).
For reasons related to numerical stability and to make comparisons of means easier to describe, 1996 Q1 and 2002 Q3 were taken as pre- and post-legislation reference points for comparison of means and a time variate was constructed by subtracting 1996 Q1 from all prelegislation times and 2002 Q3 from post-legislation times. As this study was exploratory in approach, all P-values and confidence intervals are reported without any adjustment for multiplicity.
Modelling was conducted starting with a full model, expressed in R notation as:
Nonsignificant effects at the customary (arbitrary) level of P = 0.05 were progressively stripped out starting with highest-order interactions. The order of removal proved not to be critical. Since some models showed slight residual over-dispersion a quasi-likelihood approach was adopted throughout. Nonsignificant terms were replaced in some models to enable confidence intervals for selected parameters to be given where they seemed likely to be of interest.
The reference times are, respectively, 2.25 years before and after the limits of the adopted transitional period. Comparisons between fitted values at these times (period main effects) need to be interpreted with care as the times are arbitrary – different choices of reference times would be expected to lead to different fitted values if a time trend is present. Much of the interest in this study lies in trends and interactions; a period:time interaction, for example, indicates different time trends between periods. Results are expressed as proportions or percentage trends with 95% confidence intervals.
Results
The main purpose of the study was to examine trends in paracetamol overdose and effects on mortality. First, examining paracetamol mortality overall for the period 1995–2003 (Table 1), it is clear that the majority of overdose deaths in which paracetamol was mentioned occurred outside hospital. Only 327 of the 929 deaths occurred in hospital. It is the in-hospital deaths (including 2004) which we concentrated on in our analysis. The mode of action of toxicity of paracetamol is extremely unlikely to cause out-of-hospital deaths, but of course it is possible that some of the in-hospital deaths coded as paracetamol related were due to other factors. Since our interest was in the effect of licence change on deaths relating to paracetamol (as opposed to co-proxamol), we concentrated on this cohort of in-hospital deaths.
Table 1.
Deaths in which reference to paracetamol is made on death certificates, 1995–2003
| Paracetamol ± ethanol | Paracetamol + other drugs | Co-proxamol | Paracetamol in combination products excluding co-proxamol | Total | |
|---|---|---|---|---|---|
| Out-of-hospital deaths | |||||
| 1995 | 5 | 13 | 34 | 0 | 52 |
| 1996 | 9 | 11 | 45 | 0 | 65 |
| 1997 | 7 | 7 | 49 | 1 | 64 |
| 1998 | 6 | 15 | 51 | 3 | 75 |
| 1999 | 5 | 9 | 37 | 1 | 52 |
| 2000 | 4 | 18 | 54 | 3 | 79 |
| 2001 | 7 | 13 | 53 | 3 | 76 |
| 2002 | 9 | 18 | 50 | 4 | 81 |
| 2003 | 7 | 9 | 39 | 3 | 58 |
| Total | 58 | 113 | 412 | 19 | 602 |
| In-hospital deaths | |||||
| 1995 | 22 | 4 | 5 | 0 | 31 |
| 1996 | 19 | 2 | 6 | 2 | 29 |
| 1997 | 24 | 5 | 17 | 0 | 46 |
| 1998 | 14 | 2 | 12 | 1 | 29 |
| 1999 | 17 | 2 | 7 | 1 | 27 |
| 2000 | 28 | 6 | 9 | 0 | 43 |
| 2001 | 25 | 6 | 17 | 5 | 53 |
| 2002 | 13 | 4 | 20 | 3 | 40 |
| 2003 | 21 | 2 | 6 | 0 | 29 |
| Total | 183 | 33 | 99 | 12 | 327 |
| Overall | 241 | 146 | 511 | 31 | 929 |
Deaths are shown as ‘out-of-hospital’ and ‘in-hospital’ for four categories: paracetamol ± ethanol alone; paracetamol + other drugs; co-proxamol (alone or in combination); and paracetamol in combination products excluding co-proxamol.
Over the full 10-year period covered by this study, when all overdose-related deaths are considered, no explanatory variables were statistically significant. A model comprising just period and sex was fitted to provide a summary and confidence intervals for the data in Table 2. This shows that total numbers of deaths fell slightly after the legislation came into force and the sex difference was small (ratio M:F 0.98; 0.85, 1.13). When paracetamol-related deaths were examined, only the main effect of sex was statistically significant (M:F 0.73; 0.57, 0.94). Thus deaths related to paracetamol were more common in females. Binomial modelling of the proportion of paracetamol-related deaths (Table 3) showed significant period and sex main effects but no interaction. The proportion of deaths related to paracetamol was significantly higher after legislation and was greater in females at all times.
Table 2.
Numbers of all poisoning-related deaths, per quarter (upper panel) and paracetamol deaths per quarter (lower panel) expressed as an average over the respective periods (see text)
| Sex | Pre-legislation, deaths per quarter (95% CI) | Transitional, deaths per quarter (95% CI) | Post-legislation, deaths per quarter (95% CI) | Ratio (95% CI) P post relative to pre |
|---|---|---|---|---|
| All poisonings | ||||
| F | 12 (10, 13) | 9.8 (8.3, 12) | 10 (9.0, 12) | 0.88 (0.75, 1.04) P = 0.13 |
| M | 11 (9.9, 13) | 9.6 (8.1, 11) | 10 (8.8, 11) | |
| Paracetamol poisonings | ||||
| F | 3.4 (2.6, 4.3) | 3.5 (2.6, 4.6) | 4.1 (3.3, 5.0) | 1.22 (0.91, 1.63) P = 0.19 |
| M | 2.5 (1.9, 3.2) | 2.5 (1.9, 3.4) | 3.0 (2.4, 3.8) | |
In addition, ratios pre and post regulation are shown.
Table 3.
Proportion of deaths related to paracetamol in periods before, during and after licence change
| Sex | Pre-legislation (95% CI) | Transitional (95% CI) | Post-legislation (95% CI) | Post/pre ratio (95% CI) P | M/F ratio (95% CI) P |
|---|---|---|---|---|---|
| F | 0.294 (0.235, 0.362) | 0.353 (0.276, 0.440) | 0.397 (0.336, 0.461) | 1.347 (1.076, 1.639) P = 0.013 | 0.734 (0.573, 0.925) P = 0.0095 |
| M | 0.216 (0.167, 0.275) | 0.265 (0.200, 0.343) | 0.303 (0.247, 0.365) |
Although examined, only 16 aspirin-related deaths occurred over the 10-year period of study and no time effect was detected.
Overdoses
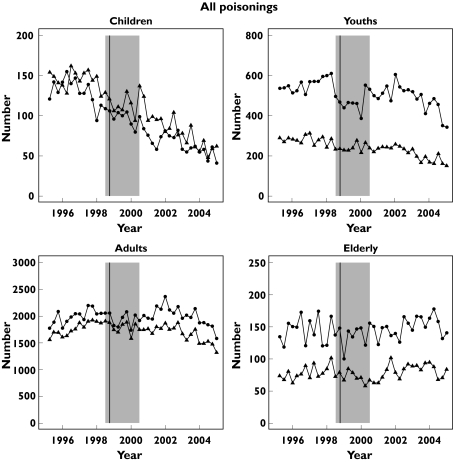
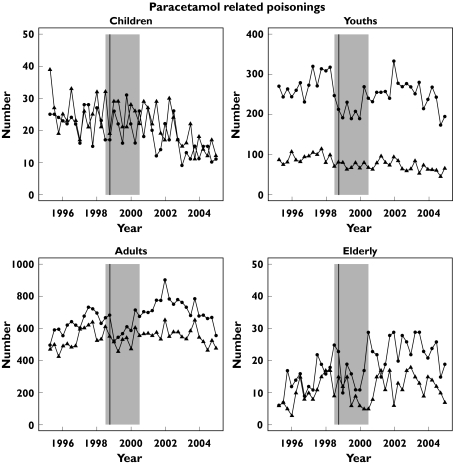
Figure 1 shows the quarterly trends in all poisonings in four age groups and Figure 2 similar data for paracetamol.
Figure 1.
Number of discharges per quarter in Scotland with a diagnosis of poisoning 1996–2004 by age group (children < 10 years, youth 10 to < 20 years, adult 20 to < 70 years, elderly ≥ 70 years). The vertical line identifies the quarter of the licence change and the shaded area indicates the period of transition
Figure 2.
Number of paracetamol-related discharges per quarter in Scotland with a diagnosis of poisoning 1996–2004 by age group (children < 10 years, youth 10 to < 20 years, adult 20 to < 70 years, elderly ≥70 years). The vertical line identifies the quarter of the licence change and the shaded area indicates the period of transition
For all poisonings clear time trends within periods were apparent. When the data overall were fitted using the model Sex × Age + Period × Sex + Period × Age + Age × Time + Period × Time a number of significant interactions were identified (Table 4), with reduction in patients < 20 years. The trend in reduction increased after legislation (difference − 9.3%; − 7.8; − 11; P < 0.001).
Table 4.
All overdoses
| Age group | Sex | Fitted value at 1996 Q1 | Fitted value at 2002 Q3 | Ratio at reference times (95% CI) P |
|---|---|---|---|---|
| Child | F | 128 | 68 | 0.53 (0.48, 0.59) P < 2 × 10−16 |
| M | 153 | 77 | 0.50 (0.45, 0.55) P < 2 × 10−16 | |
| Youth | F | 560 | 482 | 0.86 (0.82, 0.90) P < 2.2 × 10−9 |
| M | 269 | 217 | 0.81 (0.76, 0.85) P = 2.2 × 10−13 | |
| Adult | F | 1929 | 2003 | 1.04 (1.01, 1.07) P = 0.010 |
| M | 1708 | 1662 | 0.97 (0.94, 1.003) P = 0.075 | |
| Elderly | F | 135 | 149 | 1.12 (1.02, 1.22) P = 0.015 |
| M | 76 | 11 | 1.05 (0.96, 1.15) P = 0.32 |
Fitted values and their ratios from the statistical model are shown at the reference times stratified by Age and Sex.
A similar model to that used for all poisonings was used for paracetamol-related overdoses. Table 5 illustrates the key findings, with significant reductions in total numbers of paracetamol overdoses in those aged < 20 years and increases in the older cohorts. As with all poisonings, rates of change were greater after pack-size limitation (difference in trend −12%; −9.6, −14; P < 0.001).
Table 5.
Number of paracetamol-related overdoses
| Age group | Sex | Fitted value at 1966 Q1 | Fitted value at 2002 Q3 | Ratio at reference times (95% CI) P |
|---|---|---|---|---|
| Child | F | 22 | 17 | 0.75 (0.63, 0.89) P = 0.0011 |
| M | 27 | 18 | 0.67 (0.57, 0.80) P = 8.7 × 10−6 | |
| Youth | F | 269 | 250 | 0.93 (0.88, 0.99) P = 0.021 |
| M | 86 | 71 | 0.83 (0.78, 0.90) P = 2.0 × 10−6 | |
| Adult | F | 601 | 736 | 1.22 (1.17, 1.27) P < 2 × 10−16 |
| M | 510 | 560 | 1.10 (1.05, 1.15) P = 5.5 × 10−5 | |
| Elderly | F | 13 | 23 | 1.69 (1.38, 2.08) P = 9.8 × 10−7 |
| M | 9 | 13 | 1.52 (1.24, 1. 87) P = 9.8 × 10−5 |
Fitted values and their ratios from the statistical model are shown at the reference times stratified by Age and Sex.
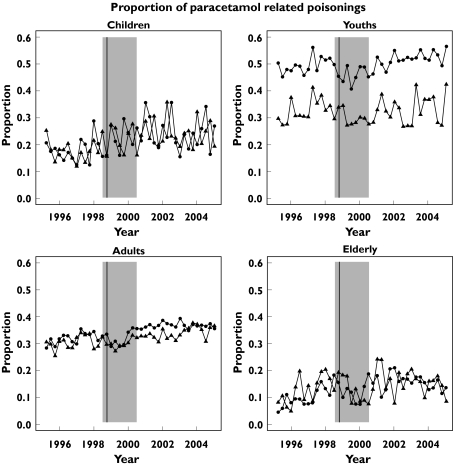
As there were trends in both poisoning overall and in paracetamol, we sought to examine the proportion of paracetamol-related overdoses using a model of Sex × Age + Sex × Period + Period × Time × Age(Figure 3). The data from the reference times, for illustration, are shown in Table 6. The proportion of overdoses in Scotland in which paracetamol is involved increased in all cohorts examined, apart from males aged 10 –19 years, in whom there was no significant change.
Figure 3.
Proportion of paracetamol-related discharges of all poisoning discharges per quarter in Scotland 1996–2004 by age group (children < 10 years, youth 10 to < 20 years, adult 20 to < 70 years, elderly ≥ 70 years). The vertical line identifies the quarter of the licence change and the shaded area indicates the period of transition
Table 6.
Proportion of paracetamol-related overdoses
| Age Group | Sex | Fitted value at 1966 Q1 | Fitted value at 2002 Q3 | Ratio at reference times (95%CI) P |
|---|---|---|---|---|
| Child | F | 0.175 | 0.247 | 1.410 (1.231, 1.606) P = 2.7 × 10−6 |
| M | 0.178 | 0.239 | 1.343 (1.171, 1.531) P = 4.9 × 10−5 | |
| Youth | F | 0.486 | 0.517 | 1.064 (1.024, 1.103) P = 0.0018 |
| M | 0.317 | 0.330 | 1.042 (0.983, 1.103) P = 0.16 | |
| Adult | F | 0.313 | 0.367 | 1.171 (1.138, 1.205) P < 2 × 10−16 |
| M | 0.299 | 0.337 | 1.128 (1.092, 1.165) P = 1.5 × 10−11 | |
| Elderly | F | 0.0867 | 0.153 | 1.760 (1.444, 2.130) P = 1.2 × 10−7 |
| M | 0.100 | 0.166 | 1.652 (1.358, 1.996) P = 1.9 × 10−6 |
Fitted values and their ratios from the statistical model are shown at the reference times stratified by Age and Sex.
The number of aspirin-related overdoses was small, and general trends were similar to those for paracetamol.
Prescriptions
Prescriptions for co-proxamol in Scotland remained relatively stable throughout this period: 1.42 million in 1995, peaking at 1.49 million in 1997 and slowly falling to 1.26 million in 2003. Prescriptions for co-codamol and co-dydramol have shown proportional increases which are not reflected in the mortality statistics.
Discussion
Assessing the impact of any intervention in poisoning in Scotland is problematical in view of the changing baselines we have identified. Thus, rates of poisoning were increasing during the late 1990s, and subsequently declined. Any measurement of the effect of a change in licensing of paracetamol therefore needs to take this into account. Obviously, an overall decline in poisoning could merely reflect a decline in the use of paracetamol in overdose; we have shown in this study that trends in paracetamol poisoning and in poisoning overall are different. This further emphasizes the points made by Morgan and Majeed [14] in their review of previous studies of the effects of this legislation, in which they make a plea for planned evaluation of interventions of this type in future.
One problem with hospital discharge statistics is that the coding of agents involved is not specific. Thus it is difficult to be certain across the country what proportion of overdoses relate to over-the-counter sales. Our own unit in Edinburgh treats approximately 10% of the Scottish population, and in this population over-the-counter supply accounts for approximately 70% of paracetamol ingestions.
Case fatality with paracetamol is low, and an important finding in this study is the impact of co-proxamol on overall mortality in cases in which paracetamol is a component of the overdose. Recent legislation removing co-proxamol from prescription lists in the UK is therefore likely to have a significant impact on mortality rates [15]. By concentrating on hospital mortality relating to paracetamol we have attempted to focus on the main target group of the legislation. While we cannot be certain that all the deaths would be directly due to paracetamol-related toxicity, the effect of the legislation was anticipated to be a reduction in paracetamol mortality. By excluding co-proxamol we have attempted to avoid a major confounder in the analysis. The analysis has been made more difficult by underlying trends in poisoning rates, and to address this we have specifically considered the time periods before, during and after legislation, and expressed our results as a proportion of all overdoses, with respect both to total numbers of discharges and to numbers of deaths. The findings of this study are concerning. It is relevant that Newsome [11] and colleagues noted no reduction in serious paracetamol-related liver damage in the admissions to the Scottish Liver Transplant Unit up to March 2001. Our present study suggests that the legislation intended to reduce paracetamol-related mortality and admission rates by reducing availability of the drug for general sale has been unsuccessful in Scotland. This contrasts with the report by Hawton and colleagues [10], who reported that in England there appeared to be an immediate reduction of about 20% in mortality from paracetamol. They also calculated that the numbers of tablets taken in overdose had reduced by around 1 g paracetamol, which is toxicologically insignificant. Hawton’s work concentrated on the time immediately after the licence change, in the period we have designated as a transitional time, whereas this present study extends well beyond any temporary impact of the publicity associated with this change, and reflects the longer-term impact.
The detailed analysis of overdose patterns is subject to a variety of potential biases in determining and recording which particular drug or preparation causes death or was the ‘main component’ implicated in an overdose. At present, Procurators Fiscal in Scotland and Coroners in England, together with their respective pathologists, play a large role in the collation of national statistics. In practice, the level of detail of information collected about any overdose is dependent upon its seriousness, recording systems and local toxicology protocols. With deaths, the presence and detail of toxicology reports and death certificate terminology may also vary. We have based our assessments on the material recorded in the Scottish system.
Studies in other countries have not been on similar interventions. In Australia manufacturer product recall due to extortion threat had a mixed effect, with some reduction in admissions in Western Australia [16] although no obvious change in calls to poisons centres about paracetamol nationwide [17]. In addition, there was an apparent increase in overdose from other analgesics [17]. In contrast, increasing availability of paracetamol for sale in Canada did not result in an increase in hospitalization for poisoning [18].
In the UK reduction of availability of a prescription antipsychotic, thioridazine, resulted in profound alterations in patterns of self-harm with this drug [19]. Licence change for co-proxamol [15] is also anticipated to produce similar dramatic change. Changing availability of over-the-counter medicines by pack-size limitation has not had a similar impact in Scotland. Paracetamol remains the most common drug taken in overdose and although the case fatality rate is low, these data do not suggest that the primary aim of pack-size limitation, namely reduction in mortality, has been achieved. While it may be argued by some that the reasons for increasing hospital discharge rates is a result of increased caution in medical practitioners, UK protocols for managing paracetamol are based on plasma concentration measurement, and have not changed over the period of this study.
In conclusion, the major public health problem with paracetamol poisoning appears to remain that of getting patients to hospital rapidly to receive appropriate antidote medication within the time window in which this works.
Competing interests
None declared.
Acknowledgments
We thank Mr Graham Jackson of the General Register Office for Scotland for the death registration data.
References
- 1.Inglis JHC. Restricting sales of paracetamol tablets: effect on deaths and emergency admissions for poisoning in Scotland 1991–2002. Scot Med J. 2004;49:142–3. doi: 10.1177/003693300404900408. [DOI] [PubMed] [Google Scholar]
- 2.Sheen CL, Dillon JF, Bateman DN, Simpson KJ, MacDonald TM. Poisoning with paracetamol, ibuprofen and aspirin. How much is due to prescribed medication. J R Coll Physicians Edin. 2003;33:258–61. [Google Scholar]
- 3.Bateman DN, Bain M, Gorman D, Murphy D. Changes in paracetamol, antidepressants and opioid poisoning in Scotland during the 1990s. Q J Med. 2003;96:125–32. doi: 10.1093/qjmed/hcg015. [DOI] [PubMed] [Google Scholar]
- 4.Hawton K. United Kingdom legislation on pack size of analgesics: background, rationale, and effects on suicide and deliberate self-harm. Suicide Life Threat Behav. 2002;32:223–9. doi: 10.1521/suli.32.3.223.22169. [DOI] [PubMed] [Google Scholar]
- 5.Hawton K, Townsend E, Deeks J, Appleby L, Gunnell D, Bennewith O, Cooper J. Effects of legislation restricting pack sizes of paracetamol and salicylate on self poisoning in the United Kingdom: before and after study. BMJ. 2001;322:1203–7. doi: 10.1136/bmj.322.7296.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turvill JL, Burroughs AK, Moore KP. Change in occurrence of paracetamol overdose in UK after introduction of blister packs. Lancet. 2000;355:2048–9. [PubMed] [Google Scholar]
- 7.Robinson D, Smith AMJ, Johnson GD. Severity of overdose after restriction of paracetamol availability: retrospective study. BMJ. 2000;321:926–7. doi: 10.1136/bmj.321.7266.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prince MI, Thomas SHL, James ONW, Hudson M. Reduction in incidence of severe paracetamol poisoning. Lancet. 2000;355:2047–8. doi: 10.1016/S0140-6736(00)02354-0. [DOI] [PubMed] [Google Scholar]
- 9.Hawton K, Simkin S, Deeks J, Cooper J, Johnston A, Waters K, Arundel M, Bernal W, Gunson B, Hudson M, Suri D, Simpson K. UK legislation on analgesic packs: before and after study of long term effect on poisonings. BMJ. 2004;329:1076–81. doi: 10.1136/bmj.38253.572581.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheen CL, Dillon JF, Bateman DN, Simpson KJ, Macdonald TM. Paracetamol related deaths in Scotland, 1994–2000. Br J Clin Pharmacol. 2002;54:430–2. doi: 10.1046/j.1365-2125.2002.t01-1-01671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newsome PN, Bathgate AJ, Henderson NL, MacGilchrist AJ, Plevris JN, Masterton G, Garden OJ, Lee A, Hayes PL, Simpson KJ. Referral patterns and social deprivation in paracetamol-induced liver injury in Scotland. Lancet. 2001;358:1612–3. doi: 10.1016/S0140-6736(01)06663-6. [DOI] [PubMed] [Google Scholar]
- 12.McCullagh P, Nelder J. Generalized Linear Models. 2. London: Chapman & Hall; 1989. [Google Scholar]
- 13.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; Available at http://www.R-project.org2004 (last accessed: March 2006). [Google Scholar]
- 14.Morgan O, Majeed A. Restricting paracetamol in the United Kingdom to reduce poisoning: a systematic review. J Public Health. 2005;27:12–8. doi: 10.1093/pubmed/fdh200. [DOI] [PubMed] [Google Scholar]
- 15.Committee on the Safety of Medicines. Available at http://www.mhra.gov.uk2005 (last accessed: March 2006).
- 16.Kisely SR, Lawrence D, Preston NJ. The effect of recalling paracetamol on hospital admissions for poisoning in Western Australia. Med J Aust. 2003;178:1612–3. doi: 10.5694/j.1326-5377.2003.tb05067.x. [DOI] [PubMed] [Google Scholar]
- 17.Balit CR, Isbister GK, Peat J, Dawson AH, Whyte IM. Paracetamol recall: a natural event influencing paracetamol poisoning. Med J Aust. 2002;176:162–5. doi: 10.5694/j.1326-5377.2002.tb04346.x. [DOI] [PubMed] [Google Scholar]
- 18.Prior MJ, Cooper K, Cummins P, Bowen D. Acetaminophen availability increases in Canada with no increase in the incidence of reports of inpatient hospitalizations with acetaminophen overdose and acute liver toxicity. Am J Ther. 2004;11:443–52. doi: 10.1097/01.mjt.0000140217.48324.e3. [DOI] [PubMed] [Google Scholar]
- 19.Bateman DN, Good AM, Afshari R, Kelly CA. Effects of licence change on prescribing and poisons enquiries for antipsychotic agents in England and Scotland. Br J Clin Pharmacol. 2003;55:596–603. doi: 10.1046/j.1365-2125.2003.01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]