Abstract
Aims
To compare information on drug–drug interactions (DDIs) reported on two standard drug-related information sources (Summary of Product Characteristics and Drugdex system by Micromedex), by assessing the prevalence and predictors of potential DDI with proton pump inhibitors (PPIs) in general practice.
Methods
From the ‘Caserta-1’ Local Health-Service database, 156 general practitioners (GPs) were recruited. From more than 180 000 individuals registered on their lists, we selected patients receiving co-prescription of PPI and medications at interaction risk, according to the Italian Summary of Product Characteristics (SPC) of PPI and Drugdex information, during the year 2003. Thereafter, we carried out a regression analysis to identify the predictors of co-prescription at interaction risk with PPI, on the basis of the two information sources. A number of analyses were performed to evaluate agreement on DDI information between the SPC of PPI and Drugdex.
Results
According to SPC and Drugdex, 324 (3.0%) and 958 (9.0%) patients, respectively, received co-prescriptions of PPI and potentially interacting medications during the study period. PPI users’ age, type of medication and number of other drug prescriptions per month were independent predictors of receiving co-prescriptions at interaction risk, when considering only Drugdex. With regard to potential DDIs with PPI, a significant disagreement (P < 0.0001) between the two drug-related information sources, was shown through agreement analyses.
Conclusions
Potential DDIs with PPI are a common health issue in general practice. Estimates of prevalence and predictors of potential DDIs with PPI significantly changes according to the drug information source being used.
Keywords: drug information sources, Drugdex, drug–drug interaction, general practice, proton pump inhibitors, Summary of Product Characteristics
Introduction
Symptoms related to gastrointestinal diseases are a common reason for consultation and referrals in general practice. Every year, from 20% to 40% of outpatients visit a general practitioner, at least once, because of dyspepsia or gastroesophageal reflux disease (GERD) [1, 2].
The most effective treatment strategy for these symptoms in primary care is reduction of gastric acid secretion, which can be achieved by using H2-receptor antagonists or proton-pump inhibitors (PPIs) [3].
Indeed, randomized, controlled clinical trials have shown that PPIs are highly effective and relatively safe in treating acid-related upper gastrointestinal diseases, including GERD, gastroduodenal ulcer and gastro-duodenitis [4–6].
All PPI medications are extensively metabolized in the liver via CYP3A4 and CYP2C19. As a consequence, PPIs might interact with drugs sharing the same metabolic pathway [7].
Although no significant differences have been shown in the metabolism of the different PPIs, pantoprazole is claimed to have a lower potential for drug–drug interactions (DDIs) than other proton pump inhibitors [8, 9].
Furthermore, it is well known that all PPIs might alter the degree of absorption of certain medications through modifying intragastric pH [10].
The Summary of Product Characteristics (SPCs) is the primary source of information about DDIs for health care professionals. However, potential drug–drug interactions cannot be listed exhaustively, since the space in the SPC is limited. As a result, information on potential DDIs might be insufficiently described on SPC in comparison with other standard drug-related information sources, based on current evidence from the literature [11], such as the Drugdex System (Thomson Micromedex, Greenwood Village, Colo) [12].
The aim of this study was to compare the estimate of prevalence and predictors of potential drug–drug interactions with PPI, according to the risk reported on two different standard information sources: Italian SPC of PPIs and the Drugdex system.
Methods
Data source
Data were obtained from the Arianna Database, which was set up by the ‘Caserta 1’ Local Health-Service in the year 2000, with the aim to perform studies on drug-utilization. Briefly, the Arianna Database currently contains information about a population of almost 300 000 individuals living in a city (Caserta) in the Campania region and registered on the lists of 225 general practitioners (GPs) and 18 family paediatricians. Such a sample of physicians represents 69.4% (243/350) of total GPs and family paediatricians who practice in the same area, as described in a previous study [13].
The participating GPs record data during their daily clinical practice through dedicated software and send monthly complete, but anonymous, clinical data concerning their patients to the Arianna Database. Information collected include patients’ demographics, drug prescriptions, coded according to the Anatomical Therapeutic Chemical classification system (ATC), and medical diagnoses (linked to any recorded prescription), coded by the ninth edition of International Classification of Diseases (ICD-9).
Data are submitted monthly to a number of checks to verify completeness rate by analyzing several parameters, such as the number of daily filled prescriptions and the proportion correctly linked to medical diagnosis. Any variation within defined ranges is investigated and submitted to each participating GP, in order to receive an immediate feedback about data quality and information completeness. GPs who fail to meet standard quality criteria are not taken into account for pharmacoepidemiological studies [14].
Study population
Among 225 GPs participating in the Arianna database, 156 (69.3%) were recruited in the study, since 69 GPs did not meet standard quality criteria. On the other hand, we decided to exclude all 18 family paediatricians since only four FPs met standard quality criteria and these FPs provided a very low number of PPI prescriptions during the study period. GPs enrolled into the study had a patient population of 188 715 individuals > 15 years old (mean age 45.1 ± 19.1) and 1821 277 drug prescriptions recorded during the whole of 2003. Within the study sample, we first selected patients receiving at least one PPI prescription during 2003. Secondly, we identified PPI users who were simultaneously (i.e. at the same date) prescribed with potentially interacting medications, thus defined as patients at interaction risk.
Outcome definition
Only exposure to PPIs and potentially interacting medications was considered, while no clinical outcomes were evaluated.
PPIs included in the study were omeprazole (ATC: A02BC01), pantoprazole (A02BC02), lansoprazole (A02BC03), rabeprazole (A02BC04) and esomeprazole (A02BC05).
We selected as drugs at interaction risk with each PPI, those listed in the Italian SPC of PPIs or in the Drugdex System (Table 1). The Drugdex System (developed by Micromedex) is a standard information source based on current evidence from the literature that provides unbiased information on drug–drug interactions, their effects, and clinical significance [12, 15]. This information is drug-specific rather than class-specific. Therefore, it helps more accurately to interpret interaction data. This system provides independently reviewed data gathered from the major drug centres and pharmacology services worldwide [15].
Table 1.
Drugs* potentially interacting with proton pump inhibitors (PPIs), according to the Italian Summary of Product Characteristics (SPCs) of PPIs or Drugdex by Micromedex
| Information source | Pantoprazole | Lansoprazole | Omeprazole | Esomeprazole | Rabeprazole |
|---|---|---|---|---|---|
| SPC | Ketoconazole | Ampicillin | Clarithromycin | Citalopram | Digoxin |
| Iron (oral formulations) | Itraconazole | Imipramine | Ketoconazole | ||
| Ketoconazole | Ketoconazole | Itraconazole | |||
| Oral contraceptives | Phenytoin | Ketoconazole | |||
| Phenytoin | Warfarin | Phenytoin | |||
| Warfarin | Warfarin | ||||
| Drugdex | Ampicillin | Aluminium hydroxide and magnesium hydroxide | Ampicillin | Atorvastatin | Ampicillin |
| Ampicillin/sulbactam | Ampicillin/Sulbactam | Digoxin | Ampicillin/sulbactam | ||
| Iron (oral formulations) | Ampicillin | Carbamazepine | Iron (oral formulations) | ||
| Itraconazole | Ampicillin/sulbactam | Cyanocobalamin | Ketoconazole | Digoxin | |
| Ketoconazole | Clarythromicin | Cyclosporine | Iron (oral formulations) | ||
| Digoxin | Digoxin | ||||
| Iron (oral formulations) | Disulfiram | Itraconazole | |||
| Itraconazole | Fluvastatin | Ketoconazole | |||
| Ketoconazole | Iron (oral formulations) | Warfarin | |||
| Sucralfate | Itraconazole | ||||
| Theophylline | Ketoconazole | ||||
| Warfarin | Methotrexate | ||||
| Phenytoin | |||||
| Tacrolimus | |||||
| Warfarin |
Only medications marketed in Italy and reimbursed by the Italian National Health System were included in the study.
Since GPs register into the Caserta database only prescriptions of drugs reimbursed by the Italian Health National System, we took out from our analysis those medications charged to citizens, such as benzodiazepines. Moreover, we ruled out medications listed in Drugdex system, but never marketed in Italy.
Statistical analysis
At first, we used the chi-square test for categorical variables and Student’s t-test for continuous variables, in order to compare demographic and clinical characteristics of different PPI users and the proportion exposed to a risk of interaction, on the basis of both SPC and Drugdex.
A logistic regression analysis was carried out to identify any covariate independently associated with an increased risk to receive co-prescription of medications potentially interacting with PPI, according to the two different standard information sources. We took into account, as potential confounders, patient’s age and sex, PPI type, indication of use, number of PPI and other drug prescriptions per month prior to the index date. Index date was defined as the date of co–prescription at interaction risk, or the date of the last PPI prescription for patients not exposed to interaction risk during the study period.
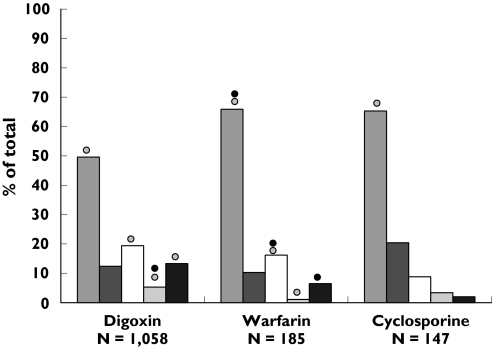
As a consequence of drug–drug interactions, certain medications (i.e. digoxin, warfarin and cyclosporin) are particularly associated with the risk of clinically severe outcomes, whether or not they are co-prescribed with some of the PPIs. Therefore, we explored the distribution of concomitant prescriptions of digoxin, warfarin and cyclosporin to evaluate whether co-prescriptions of these medications were differently distributed among PPI users (Figure 1).
Figure 1.
Distribution of concomitant prescriptions at interaction risk with PPIs*, stratified by PPI and concomitant drugs. *Among medications potentially interacting with PPIs, we took into account those that were mostly prescribed and associated with major clinical relevance, as a consequence of drug-drug interactions. Risk of potential drug-drug interaction with PPI described on SPC (•) or Drugdex ( ). Omeprazole (
). Omeprazole ( ), Pantoprazole (
), Pantoprazole ( ), Lansoprazole (□), Rabeprazole (
), Lansoprazole (□), Rabeprazole ( ), Esomeprazole (▪).
), Esomeprazole (▪).
Thereafter, an agreement analysis was performed to evaluate the concordance on information about drug–drug interactions from the two leading drug-related information sources. Weighted Cohen’s kappa [16] was subsequently computed to correct for the degree of disagreement. According to Landis & Koch [17], the K-value was categorized as fair (0.2–0.4), moderate (0.4–0.6), good (0.6–0.8), and very good (0.8–1).
Furthermore, a z-test was performed to reject the null hypothesis that no significant agreement exists between the two information sources. Marginal homogeneity was evaluated by the Maxwell test for disagreement.
Finally, a logistic regression was performed to describe relationships between the disagreement of the information sources, considered as event, and predictor variables. Age was put into the model categorized by quintiles (<42, 42–54, 54–64, 64–74, >74 years). Pearson chi-square goodness of fit and deviance goodness of fit were performed for model analysis.
A two-sided P value of < 0.05 was considered as statistically significant in all analyses which were carried out.
StatsDirect Statistical Software (ver. 2.5.5 – StatsDirect Ltd.) was used to perform all the statistical analyses.
Results
Overall, 188 715 subjects older than 15 years were included in the study. Among these, 10 648 patients (5.6%) received at least one PPI prescription during 2003. Characteristics of PPI users are described in Table 2. GERD and gastro-duodenal ulcer were the main indications of use for PPIs. Most of patients received from one to five PPI prescriptions during the study period, even though almost 10% of PPI users had more than 10 prescriptions within a 1 year follow-up. Among PPI users, 324 (3.0%) and 958 (9.0%) were concomitantly prescribed with medications potentially interacting with PPIs, according to the risk described in SPC and Drugdex, respectively.
Table 2.
Characteristics of users of PPIs*, stratified by medication
| Total users n = 10 648 (%) | Omeprazole n = 3939 (%) | Esomeprazole n = 2773 (%) | Pantoprazole n = 1888 (%) | Lansoprazole n = 2668 (%) | Rabeprazole n = 966 (%) | |
|---|---|---|---|---|---|---|
| Mean age (years) | 58.1 ± 16.9 | 61.4 ± 15.7** | 53.3 ± 17.0** | 58.2 ± 16.8 | 59.0 ± 16.7 | 54.8 ± 16.6** |
| Age group | ||||||
| 15–29 years | 594 (5.6) | 119 (3.0)** | 243 (8.8)** | 118 (6.3) | 134 (5.0) | 62 (6.4) |
| 30–44 years | 1894 (17.8) | 514 (13.0)** | 697 (25.1)** | 299 (15.8) | 439 (16.5) | 217 (22.5)** |
| 45–59 years | 2862 (26.9) | 1019 (25.9) | 774 (27.9) | 518 (27.4) | 699 (26.2) | 295 (30.5) |
| 60–74 years | 3341 (31.4) | 1409 (35.8)** | 719 (25.9)** | 615 (32.6) | 871 (32.6) | 262 (27.1) |
| >74 years | 1957 (18.4) | 878 (22.3)** | 340 (12.3)** | 338 (17.9) | 525 (19.7) | 130 (13.5)** |
| Gender | ||||||
| Male | 5477 (51.4) | 2136 (54.2) | 1458 (52.5) | 925 (49.0) | 1291 (48.4) | 491 (50.8) |
| Female | 5171 (48.6) | 1803 (45.8) | 1317 (47.5) | 963 (51.0) | 1377 (51.6) | 475 (49.2) |
| Indication of use for PPI | ||||||
| GERD | 4263 (40.0) | 1218 (30.9)** | 1332 (48.0)** | 855 (45.3)** | 1035 (38.8) | 437 (45.2) |
| Gastroduodenal ulcer | 2635 (24.7) | 1138 (28.9)** | 708 (25.5) | 444 (23.5) | 515 (19.3) | 260 (26.9) |
| Gastroduodenitis | 1407 (13.2) | 574 (14.6) | 294 (10.6)** | 219 (11.6) | 356 (13.3) | 100 (10.4) |
| Other GI diseases § | 1107 (10.4) | 473 (12.0) | 344 (12.4) | 225 (11.9) | 282 (10.6) | 114 (11.8) |
| Gastroprotection | 665 (6.2) | 257 (6.5) | 34 (1.2)** | 87 (4.7) | 312 (11.7)** | 16 (1.7) |
| Not reported | 571 (5.4) | 279 (7.1)** | 63 (2.3)** | 58 (3.1)** | 168 (6.3) | 39 (4.0) |
| Interaction risk (SPC)∧ | ||||||
| No | 10 324 (97.0) | 3737 (94.9) | 2688 (96.9) | 1868 (98.9) | 2584 (96.9) | 937 (97.0) |
| Yes | 324 (3.0) | 202 (5.1)** | 85 (3.1) | 20 (1.1)** | 84 (3.1) | 29 (3.0) |
| Interaction risk (Drugdex)∧ | ||||||
| No | 9690 (91.0) | 3461 (87.9) | 2590 (93.4) | 1769 (93.7) | 2309 (86.5) | 911 (94.3) |
| Yes | 958 (9.0) | 478 (12.1)** | 183 (6.6)** | 119 (6.3)** | 359 (13.5)** | 55 (5.7)** |
Values are numbers (percentages) unless stated otherwise. Legend: GI = gastrointestinal, GERD = Gastroesophageal reflux disease.
Patients receiving at least one prescription of PPI during the study period (categories are not mutually exclusive).
Statistically significant difference (P < 0.05) in comparison with other users of PPI.
Cirrhosis, GI bleeding, GI functional disorders, gastric cancer, GI multiple disease.
Patients receiving at least 1 concomitant prescription of PPI and potentially interacting drugs, according to the Italian SPC of PPIs or Drugdex.
As regards to different PPIs, the most prescribed drug was omeprazole, followed by lansoprazole and esomeprazole which has been marketed in Italy since 2002.
Some variations concerning the characteristics of different PPI users are highlighted in Table 2. Users of omeprazole are significantly older than users of other PPIs and they are treated mostly because of gastro-duodenal ulcer. On the contrary, esomeprazole and rabeprazole are significantly prescribed more to younger people who are affected by GERD. Lansoprazole and omeprazole, the only PPIs approved for gastroprotection in Italy, were significantly more prescribed than other medications for this indication.
According to the SPC, the highest proportion of patients at interaction risk is reported for omeprazole users (5.1%) while it is for lansoprazole users (13.5%), when Drugdex information is taken into account. On the other hand, patients treated with pantoprazole seem to be less exposed to co-prescriptions at interaction risk compared with other PPI users, independently of the information source that was used.
Risk distribution in GPs and patients
During the study period, 150 (96.2%) out of 156 GPs filled in total 2024 co-prescriptions for PPIs and medications potentially interacting with PPIs, according to the risk described on Drugdex. On average, 6.4 patients per GP (median value 5, range 1–18), received this co-prescription at interaction risk, once at least. Users of PPIs took a medium number of 2.1 potentially interacting co-prescriptions (median value 1, range 1–15) on the basis of the Drugdex source.
Taking into account the SPC, 122 GPs (78.2%) filled a total of 514 co-prescriptions for PPIs and other medications at interaction risk. On average, 2.7 patients per GP (median value 2, range 1–9) received this potential drug–drug interaction. In particular, 324 PPI users received a medium number of 1.6 co–prescriptions at interaction risk (median value 1, range 1–8) during the whole of 2003.
Predictors of concomitant prescription at interaction risk
Variables associated with an increased probability to receive co–prescriptions at interaction risk, according to both SPC and Drugdex, are shown in Table 3.
Table 3.
Predictors of drug–drug interactions in users of PPI, according to the risk described on SPC and Drugdex
| SPC Patients at interaction risk∧n = 324 (%) | Adjusted* OR (95% CI) | Drugdex Patients at interaction risk∧n = 958 (%) | Adjusted* OR (95% CI) | |
|---|---|---|---|---|
| Age group | ||||
| 15–29 years | 15 (4.6) | 1.00 | 31 (3.2) | 1.00 |
| 30–44 years | 42 (13.0) | 0.89 (0.49, 1.60) | 75 (7.8) | 0.73 (0.48, 1.11) |
| 45–59 years | 85 (26.2) | 1.22 (0.70, 2.12) | 155 (16.2) | 0.94 (0.64, 1.39) |
| 60–74 years | 118 (36.4) | 1.51 (0.87, 2.62) | 351 (36.6) | 1.73 (1.19, 2.51) |
| 74 years | 64 (19.8) | 1.46 (0.81, 2.62) | 346 (36.1) | 2.89 (1.96, 4.16) |
| Sex | ||||
| Male | 164 (50.6) | 1.02 (0.82, 1.27) | 468 (48.9) | 1.03 (0.91, 1.17) |
| Female | 160 (49.4) | 1.00 | 490 (51.1) | 1.00 |
| Indication of use for PPI | ||||
| GERD | 103 (31.8) | 1.00 | 284 (29.6) | 1.00 |
| Gastroduodenal ulcer | 91 (28.1) | 1.40 (1.06, 1.87) | 250 (26.1) | 1.26 (1.06, 1.49) |
| Gastro-duodenitis | 44 (13.6) | 1.26 (0.88, 1.80) | 145 (15.1) | 1.35 (1.10, 1.65) |
| Other GI diseases | 47 (14.5) | 1.78 (1.25, 2.52) | 132 (13.8) | 1.49 (1.21, 1.83) |
| Gastroprotection | 6 (1.9) | 0.35 (0.15, 0.80) | 62 (6.5) | 1.14 (0.86, 1.50) |
| Not reported | 33 (10.2) | 2.33 (1.57, 3.48) | 85 (8.9) | 1.87 (1.46, 2.39) |
| Type of PPI | ||||
| Omeprazole | 149 (46.0) | 1.00 | 356 (37.2) | 1.00 |
| Pantoprazole | 1 (0.3) | 0.02 (0.01, 0.11) | 47 (4.9) | 0.36 (0.26, 0.48) |
| Rabeprazole | 12 (3.7) | 0.40 (0.22, 0.72) | 25 (2.6) | 0.45 (0.30, 0.68) |
| Lansoprazole | 45 (13.9) | 0.48 (0.34, 0.67) | 246 (25.7) | 1.20 (1.01, 1.41) |
| Esomeprazole | 32 (9.9) | 0.37 (0.25, 0.54) | 80 (8.4) | 0.51 (0.40, 0.65) |
| Combinations | 85 (26.2) | 1.40 (1.07, 1.83) | 204 (21.3) | 1.42 (1.19, 1.68) |
| Number of PPI prescriptions per month | ||||
| 0 | 199 (61.4) | 1.00 | 534 (55.7) | 1.00 |
| 1 | 81 (25.0) | 1.67 (1.28, 2.18) | 284 (29.7) | 1.70 (1.46, 1.97) |
| >1 | 44 (13.6) | 1.97 (1.41, 2.76) | 140 (14.6) | 1.85 (1.54, 2.23) |
| Number of other drug prescriptions per month | ||||
| 0 | 64 (19.8) | 1.00 | 113 (11.8) | 1.00 |
| 1–5 | 208 (64.2) | 1.45 (1.25, 1.70) | 641 (66.9) | 1.65 (1.34, 2.05) |
| >5 | 52 (16.0) | 1.36 (1.00, 1.85) | 204 (21.3) | 3.01 (2.33, 3.90) |
Patients receiving at least one concomitant prescription of PPI and potentially interacting drugs, according to the Italian SPC of PPIs or Drugdex.
Adjusted for all variables included in the table.
After adjusting for a number of potential confounders, PPI users older than 60 years seem to be associated with a significantly increased risk to receive simultaneously medications at interaction risk with PPIs compared with younger people, but only considering Drugdex information. According to both SPC and Drugdex, a lower risk of receiving potentially interacting co-prescriptions is reported for patients treated with PPIs because of GERD in comparison with those treated with PPIs for other indications. Considering exclusively SPC information, patients taking PPIs as gastroprotection therapy were less likely to be exposed to potential interaction risk compared with PPI users with GERD.
Omeprazole users appear to be associated with a higher likelihood to concomitantly receive potentially interacting medications, than users of other PPIs. In particular, pantoprazole users have a 64% and 98% risk reduction compared with omeprazole users, when taking into account Drugdex and SPC, respectively. Interestingly, in comparison with omeprazole users, the risk to receive potentially interacting co-prescriptions for lansoprazole users completely changes when information from SPC (OR 0.55, 95% CI 0.39, 0.76) or Drugdex (OR 1.36, 95% CI 1.16, 1.61) are considered.
The higher the monthly number of PPI and other drug prescriptions taken by PPI users prior to the potentially interacting co-prescription date, the higher is the risk to be exposed to potential DDIs.
Distribution of co–prescriptions at interaction risk within users of different PPI
Among different types of PPI, omeprazole (n = 1012) accounts for half of the co-prescriptions at interaction risk, on the basis of Drugdex. Digoxin (525), warfarin (122) and iron (143) are the medications mostly involved in potential drug–drug interactions with omeprazole, and, in general, with all PPIs.
In Figure 1, the distribution of co-prescriptions of certain medications potentially interacting with PPIs and associated with clinically relevant outcomes is shown. Seventy % of co-prescriptions of cyclosporin were given to users of omeprazole, although the interaction risk with cyclosporin is described only for omeprazole in Drugdex.
Concerning co-prescriptions of warfarin and digoxin with PPI, prescriptions of pantoprazole account only for approximately 10% of total PPI co-prescriptions.
Agreement analysis
A number of analyses were carried out to assess the agreement on drug–drug interactions as reported in the Italian SPC of PPIs and the Drugdex system.
We calculated a weighted kappa of 0.23 (95% CI 0.21, 0.25) indicating a significantly poor agreement between the SPC and Drugdex (P < 0.0001). The Maxwell test also confirmed the occurrence of significant disagreement (P < 0.0001) between the two drug-related information sources under consideration.
Use of omeprazole (OR 3.0; 95% CI 2.5, 3.6) and lansoprazole (OR 2.8, 95% CI 2.3, 3.3) and age (categorized by quintiles) of PPI users (OR 1.4, 95% CI 1.3, 1.5) were independent predictors of disagreement in identifying interaction risk with PPIs between the two standard information sources (data not shown).
Discussion
In this cross-sectional study, we found that the 3.0% of PPI users were exposed to potential drug–drug interactions within 1 year of follow-up, according to the risk described in the Italian Summary of Product Characteristics of PPIs. On the other hand, this proportion was three-fold higher (9.0%) when information about drug–drug interaction risk with PPIs, reported on Drugdex, was considered. In particular, the highest proportion of patients at potential interaction risk was reported for omeprazole users (5.1%) on the basis of SPC, while for lansoprazole users (13.5%), according to Drugdex. These data seem to be in contrast with the results achieved from a prior study performed in a US community setting [18], which reported of 9.9% of patients at interaction risk for omeprazole users compared with 0.3% for lansoprazole users. A different time window for exposure to interaction risk and different drugs considered as potentially interacting with PPIs might be plausible explanations for such a divergence between the two studies.
Our results are in line with a recently published review about PPIs [6] where it was reported that omeprazole and lansoprazole had a greater potential for drug–drug interactions than other PPIs. Among all PPI medications, pantoprazole was strikingly the least likely to cause an interaction risk, in comparison with omeprazole (on the basis of Drugdex: OR 0.36, 95% CI 0.26, 0.48; on the basis of SPC: OR 0.02, 95% CI 0.01, 0.11). This finding is strongly supported by scientific evidence suggesting that pantoprazole lacks the cytochrome P450 interaction with concomitantly administered drugs and, as a consequence, it has the lowest potential for interactions with other drugs, among all PPIs [19–21]. This difference might be particularly relevant when medications for the treatment of acid-related diseases in older patients receiving polypharmacy are chosen [22].
Interestingly, in addition to the estimate of prevalence of potential drug–drug interactions, the predictors of co-prescribing medications at interaction risk with PPI change as well in our analysis, when taking into account the interaction risk described in the two considered information sources. Indeed, advanced age and monthly number of other drug prescriptions, taken by PPI users, appear to be factors strongly associated with the risk of receiving co-prescriptions at potential interaction risk with PPIs during 1-year of follow-up, but only according to Drugdex information. In line with our results, a recent study performed in an Asiatic outpatient setting highlighted that the rate of DDIs increased with the number of drugs prescribed and with the patient’s age [23].
Another finding of our analysis was that digoxin, warfarin and iron were the medications mainly involved in the potential DDIs during therapy with PPIs. Apart from pantoprazole, an increased risk of digoxin toxicity (nausea, vomiting, arrhythmias) is well documented, when digoxin is co-prescribed with all PPIs [24–27]. Concerning warfarin–PPI interaction, a UK retrospective study [28] reports omeprazole and lansoprazole as being increasingly used with anticoagulants in general practice, despite the Association of the British Pharmaceutical Industry alerts about the risk of interaction between these PPIs and warfarin. A recently published review suggests prescribing omeprazole in warfarinized patients should be avoided, on the basis of consistent reports of an omeprazole–warfarin interaction [29].
Furthermore, our analysis showed that omeprazole accounted for approximately 70% of concomitant prescriptions of PPIs with cyclosporin, although the risk of potential interaction with this immunosuppressant drug is described on Drugdex only for omeprazole among PPI medications, and it is classified as moderately severe [30]. Conversely, no mention about this potential DDI is reported on the Italian SPC of omeprazole.
Even if most of the co–prescriptions at potential interaction risk might be associated with moderately harmful adverse events, it is essential, to ensure patient safety, to prevent the use of medications associated with clinically relevant DDI risk. Computerized drug interaction alerts and screening of prescriptions by community pharmacists might be two important safety nets to prevent co-prescribing at interaction risk in primary care [31].
At the moment, the current information on DDI with PPIs to general practitioners appears to be inconsistent, incomplete and probably unreliable, in some cases reflecting scientific uncertainty and lack of documentation [32]. As a first step, an attempt should be made to achieve a general agreement among different drug-related information sources on the actual risk of clinically relevant drug–drug interactions, explicitly associated with any drug combinations. Our study highlights this need, as a very low agreement was shown between two standard drug-related information sources, in line with previously published papers [33–34].
The critical issue is how to provide general practitioners with knowledge on drug–drug interactions, with the aim of reducing clinically relevant DDIs. Strategy interventions should be planned by local health authorities to supply general practitioners with such information.
Several previous studies [11, 15, 35] carried out in primary care have confirmed the validity of Drugdex in providing information on potential drug–drug interactions. In particular, the severity of potential DDIs is classified as minor, moderate and major on the basis of how clinically relevant an interaction might be. Interaction of any severity might potentially be adverse, as the effect of an interaction is not predictable in all patients. Nevertheless, Drugdex appears to be an incomplete information source for commonly prescribed drug pairs and therefore additional sources might help to complete and evaluate information on drug interactions [36].
To our knowledge, this is the first population-based study to compare the estimated prevalence and predictors of potential DDIs with any PPI in general practice by using two different information sources.
A number of limitations of the study, however, warrant cautions. We defined as patients at interaction risk, those PPI users receiving medications potentially interacting with PPI at the same date. This methodology did not allow us to evaluate if PPI was added to a chronically used medication at interaction risk or vice versa. On the other hand, this procedure may underestimate the real prevalence since the ideal analysis would consider the overlapping of time windows of different pharmacological therapies. Nevertheless, a similar study [13] on statin–macrolide interaction risk, previously carried out through the same database, highlighted such a procedure as being reliable for estimate of potential DDI prevalence.
The Arianna database contains only prescription data of medications that are reimbursed by the Italian National Health system. As a consequence, we were not able to evaluate the extent of self-medications and drugs charged to citizens on the prevalence of potential DDIs with PPIs in these general practices of Southern Italy.
Moreover, we used outpatient prescription data and we had no information whether the concomitant prescriptions at interaction risk were actually filled and taken.
However, the purpose of this study was to assess GPs’ prescribing appropriateness, in relation to interaction risk with PPIs as described by different standard information sources. We did not evaluate any clinical outcome as a result of potential drug–drug interaction and, consequently, from this point of view, the study results should be interpreted carefully.
Another clinically relevant issue should be underlined. For drugs that undergo polymorphic enzyme metabolism, such as omeprazole, lansoprazole, esomeprazole by CYP2C19, genetic makeup is an important determinant of the degree of the interaction [37]. The normal drug interaction sources such as the SPC and Drugdex make generalizations about interacting drugs that might influence their predictive value of DDIs. Therefore, a critical evaluation of these data from a clinical point of view, also taking into account pharmacogenetic features, might be helpful. Nevertheless, this drug-utilization study provides important insights into the global risk of potential DDIs with PPIs in the general population.
In conclusion, potential DDIs during therapy with PPIs are a common health issue in these general practices of Southern Italy. However, the estimate of prevalence and predictors of such a potential interaction risk significantly changes when different drug information sources are considered. As regards to medications potentially interacting with PPIs, there seems to be a relevant discrepancy between the Italian Summary of Product Characteristics of PPIs and the Drugdex system.
Further investigations should be targeted to evaluate whether significant disagreement in identifying potential DDI risk exists between different standard information sources for other commonly prescribed drug pairs.
Information on drug–drug interactions derived from different standard sources should be made homogeneous and should be constantly updated on the basis of current evidence from the literature. The National Health system should urgently plan a strategy intervention to keep physicians adequately aware of potential drug–drug interactions, with particular regard to widely used medications.
Vincenzo Arcoraci had full access to all the data in the study and had final responsibility for the decision to submit for publication.
This study was not supported by any grant.
References
- 1.Jones R, Lydeard S. Prevalence of symptoms of dyspepsia in the community. BMJ. 1989;298:30–2. doi: 10.1136/bmj.298.6665.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Bommel MJ, Numans ME, de Wit NJ, Stalman WA. Consultations and referrals for dyspepsia in general practice, a one year database survey. Postgrad Med J. 2001;77:514–8. doi: 10.1136/pmj.77.910.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delaney BC, Innes MA, Deeks J, Wilson S, Oakes R, Moayyedi P, Hobbs FD, Forman D. Initial management strategies for dyspepsia. Cochrane Database Syst Rev. p. CD001961. [DOI] [PubMed]
- 4.Blum RA. Lansoprazole and omeprazole in the treatment of acid peptic disorders. Am J Health-Syst Pharm. 1996;53:1401–15. doi: 10.1093/ajhp/53.12.1401. [DOI] [PubMed] [Google Scholar]
- 5.Shin JM, Besancon M, Prinz C, Simon A, Sash G. Continuing development of acid pump inhibitors: site of action of pantoprazole. Aliment Pharmacol Ther. 1994;8:11–23. doi: 10.1111/j.1365-2036.1994.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 6.Horn J. The proton-pump inhibitors: similarities and differences. Clin Ther. 2000;22:266–80. doi: 10.1016/S0149-2918(00)80032-6. [DOI] [PubMed] [Google Scholar]
- 7.Li XQ, Andersson TB, Ahlstrom M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004;32:821–7. doi: 10.1124/dmd.32.8.821. [DOI] [PubMed] [Google Scholar]
- 8.Simon WA, Budingen C, Fahr S, Kinder B, Koske M. The H+, K+-ATPase inhibitor pantoprazole (BY1023/SK and F96022) interacts less with cytochrome P450 than omeprazole and lansoprazole. Biochem Pharmacol. 1991;42:347–55. doi: 10.1016/0006-2952(91)90722-h. [DOI] [PubMed] [Google Scholar]
- 9.Reilly JP. Safety profile of the proton-pump inhibitors. Am J Health Syst Pharm. 1999;56:S11–7. doi: 10.1093/ajhp/56.suppl_4.S11. [DOI] [PubMed] [Google Scholar]
- 10.Robinson M, Horn J. Clinical pharmacology of proton pump inhibitors: what the practising physician needs to know. Drugs. 2003;63:2739–54. doi: 10.2165/00003495-200363240-00004. [DOI] [PubMed] [Google Scholar]
- 11.Bergk V, Haefeli WE, Gasse C, Brenner H, Martin-Facklam M. Information deficits in the summary of product characteristics preclude an optimal management of drug interactions: a comparison with evidence from the literature. Eur J Clin Pharmacol. 2005;61:327–35. doi: 10.1007/s00228-005-0943-4. [DOI] [PubMed] [Google Scholar]
- 12.Klasko RK, editor. DRUGDEX System. 1. Thomson Micromedex: Greenwood Village CO; 2003. [Google Scholar]
- 13.Piacentini N, Trifiro G, Tari M, Moretti S, Arcoraci V UVEC group. Statin–macrolide interaction risk: a population-based study throughout a general practice database. Eur J Clin Pharmacol. 2005;61:615–20. doi: 10.1007/s00228-005-0972-z. [DOI] [PubMed] [Google Scholar]
- 14.Lawrenson R, Williams T, Farmer R. Clinical information for research: the use of General Practice databases. J Public Health Med. 1999;21:299–304. doi: 10.1093/pubmed/21.3.299. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim IA, Kang E, Dansky KH. Polypharmacy and possible drug–drug interactions among diabetic patients receiving home health care services. Home Health Care Serv Q. 2005;24:87–99. doi: 10.1300/J027v24n01_07. [DOI] [PubMed] [Google Scholar]
- 16.Altman DG. Practical statistics for medical research. Chapman & Hall; 1991. [Google Scholar]
- 17.Landis JR, Koch G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 18.Saltiel E, Fask A. Prevalence of potential proton–pump inhibitor drug interactions: a retrospective review of prescriptions in community pharmacies. Clin Ther. 1999;21:1812–9. doi: 10.1016/S0149-2918(99)80059-9. [DOI] [PubMed] [Google Scholar]
- 19.Steinijans VW, Huber R, Hartmann M, Zech K, Bliesath H, Wurst W, Radtke HW. Lack of pantoprazole drug interactions in man. Int J Clin Pharmacol Ther. 1994;32:385–99. [PubMed] [Google Scholar]
- 20.Cheer SM, Prakash A, Faulds D, Lamb HM. Pantoprazole. an update of its pharmacological properties and therapeutic use in the management of acid-related disorders. Drugs. 2003;63:101–33. doi: 10.2165/00003495-200363010-00006. [DOI] [PubMed] [Google Scholar]
- 21.Meyer UA. Interaction of proton pump inhibitors with cytochromes P450: consequences for drug interactions. Yale J Biol Med. 1996;69:203–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Humphries TJ, Merritt GJ. Review article: drug interactions with agents used to treat acid-related diseases. Aliment Pharmacol Ther. 1999;13(Suppl 3):18–26. doi: 10.1046/j.1365-2036.1999.00021.x. [DOI] [PubMed] [Google Scholar]
- 23.Janchawee B, Wongpoowarak W, Owatranporn T, Chongsuvivatwong V. Pharmacoepidemiologic study of potential drug interactions in outpatients of a University hospital in Thailand. J Clin Pharm Ther. 2005;30:13–20. doi: 10.1111/j.1365-2710.2004.00598.x. [DOI] [PubMed] [Google Scholar]
- 24.Oosterhuis B, Jonkman JH, Andersson T, et al. Minor effect of multiple dose omeprazole on the pharmacokinetics of digoxin after a single oral dose. Br J Clin Pharmacol. 1991;32:569–72. doi: 10.1111/j.1365-2125.1991.tb03953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Product Information. Willmington, DE, AstraZeneca: February 2001. Nexium (TM), esomeprazole. [Google Scholar]
- 26.Product Information. Lake Forest, IL: TAP Pharmaceuticals; August 2002. Prevacid (R), lansoprazole. [Google Scholar]
- 27.Humphries TJ. Vienna, Austria: World Congress; 1999. A review of the drug–drug interaction potential of rabeprazole sodium based on CYP-450 interference or absorption effects. [Google Scholar]
- 28.Hungin AP, Rubin GP, O’Flanagan H. Co-prescription of H2 receptor blockers and proton pump inhibitors with warfarin in general practice. Postgrad Med J. 1999;75:721–2. doi: 10.1136/pgmj.75.890.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutfin T, Balmer K, Bostrom H, Eriksson S, Hoglund P, Paulsen O. Stereoselective interaction of omeprazole with warfarin in healthy men. Ther Drug Monit. 1989;11:176–84. doi: 10.1097/00007691-198903000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Arranz R, Yanez E, Franceschi JL, Fernandez-Ranada JM. More about omeprazole–cyclosporine interaction. Am J Gastroenterol. 1993;88:154. [PubMed] [Google Scholar]
- 31.Chen YF, Avery AJ, Neil KE, Johnson C, Dewey ME, Stockley IH. Incidence and possible causes of prescribing potentially hazardous/contraindicated drug combinations in general practice. Drug Saf. 2005;28:67–80. doi: 10.2165/00002018-200528010-00005. [DOI] [PubMed] [Google Scholar]
- 32.Landes BD, Petite JP, Flouvat B. Clinical pharmacokinetics of lansoprazole. Clin Pharmacokinet. 1995;28:458–70. doi: 10.2165/00003088-199528060-00004. [DOI] [PubMed] [Google Scholar]
- 33.Abarca J, Malone DC, Armstrong EP, Grizzle AJ, Hansten PD, Van Bergen RC, Lipton RB. Concordance of severity ratings provided in four drug interaction compendia. J Am Pharm Assoc (Wash DC) 2004;44:136–41. doi: 10.1331/154434504773062582. [DOI] [PubMed] [Google Scholar]
- 34.Chao SD, Maibach HI. Lack of drug interaction conformity in commonly used drug compendia for selected at-risk dermatologic drugs. Am J Clin Dermatol. 2005;6:105–11. doi: 10.2165/00128071-200506020-00005. [DOI] [PubMed] [Google Scholar]
- 35.Eames CH, Klein MS. Increasing access to clinical information on hospital wards. Proceedings of the Annu Symp Comput Appl Medical Care. 1994. p. 981. [PMC free article] [PubMed]
- 36.Bergk V, Gasse C, Rothenbacher D, Loew M, Brenner H, Haefeli WE. Drug interactions in primary care: impact of a new algorithm on risk determination. Clin Pharmacol Ther. 2004;76:85–96. doi: 10.1016/j.clpt.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Lee LS, Nafziger AN, Bertino JS., Jr Evaluation of inhibitory drug interactions during drug development: genetic polymorphisms must be considered. Clin Pharmacol Ther. 2005;78:1–6. doi: 10.1016/j.clpt.2005.04.006. [DOI] [PubMed] [Google Scholar]