Abstract
Aim
To assess residual psychomotor and cognitive effects of a modified-release formulation of zolpidem (zolpidem-MR), developed to provide sustained hypnotic efficacy during the whole night, compared with placebo and flurazepam.
Methods
Twenty-four healthy elderly volunteers received four study treatments (zolpidem-MR 6.25 mg and 12.5 mg, placebo and flurazepam 30 mg) using a randomized, cross-over, double-blind design. Residual psychomotor and cognitive effects were assessed with a psychometric test battery. Quality of sleep and residual effects were evaluated subjectively with the Leeds Sleep Evaluation Questionnaire.
Results
Psychometric performance was significantly impaired with flurazepam but not with zolpidem-MR at either dose. Ease of falling asleep and sleep quality were significantly improved with both doses of zolpidem-MR and with flurazepam. Neither active drug modified perception of well-being on awakening.
Conclusion
In elderly subjects, zolpidem-MR showed no residual functional impairment in psychometric or cognitive tests sensitive to flurazepam,
Keywords: elderly subjects, formulation, Leeds Sleep Evaluation Questionnaire, psychomotor performance, zolpidem
Introduction
The pharmacological treatment of sleep disorders in elderly subjects presents particular challenges [1]. The prevalence of sleep disturbances secondary to other physical and psychiatric disorders as well as of idiopathic insomnia appears to be particularly high among older people [2, 3]. Changes in hepatic function with aeging lead to a reduction in the efficiency of drug metabolism and subsequent increase in exposure to hypnotic drugs [4]. In particular, the continued presence of pharmacologically active amounts of hypnotic drugs or their active metabolites many hours after drug administration may be responsible for residual sedative effects the next day [5]. These can increase the risk of accidents, notably falls and subsequent hip fracture [6, 7]. In addition, there is some evidence that the elderly subject may be more sensitive to the sedative effects of hypnotic drugs independently of pharmacokinetic considerations, perhaps due to changes in the sensitivity of target receptors in the brain or to a generalized decrease in neuronal excitability [8]. For these reasons, dose reduction and choice of short-acting hypnotics are appropriate treatment strategies for the management of insomnia in elderly patients [9].
Zolpidem (Ambien®, Stilnox®, Myslee®; Sanofi-Aventis, Great Valley, PA, USA) is a short-acting nonbenzodiazepine hypnotic drug which has demonstrated efficacy in the treatment of insomnia in elderly patients as well as in the general population [10]. The mean elimination half-life of zolpidem is 2.4 h in the healthy adult and 2.9 h in the elderly subject [11]. Consistent with its short duration of action, several studies have demonstrated that this hypnotic possesses little if any residual sedative effects, either in young [12] or in elderly subjects [13–15].
Pharmacokinetic studies with zolpidem have shown older people to present a lower clearance and volume of distribution together with higher maximum plasma concentration and AUC compared with young adults [16]. For this reason, it was thought that a lower dose of zolpidem would be appropriate for the elderly subject. This hypothesis was confirmed in dose-ranging efficacy studies of zolpidem in older subjects which showed that a 5-mg dose of zolpidem had clinically valuable efficacy combined with a lower incidence of adverse events [13]. In consequence, this dose (i.e. half that used for younger subjects) is recommended for subjects >65 years old.
In order to improve the duration of hypnotic action of zolpidem over the course of the night, a modified-release formulation of this drug was developed (zolpidem-MR), including a layer of a standard-release formulation of zolpidem and another of a slow-release formulation. Exploratory studies in healthy young volunteers [17] have demonstrated the prolonged absorption of the modified formulation and that a 12.5-mg dose of zolpidem-MR provided a longer protection against nocturnal awakenings without the emergence of significant residual effects the next day. Other exploratory studies in elderly subjects have shown that, similar to standard zolpidem, half the standard dose of zolpidem-MR (i.e. 6.25 mg) should be used in this population. This dose was chosen for this study together with a full dose (12.5 mg zolpidem-MR) to reflect clinical usage, where a possible increase in dose regimen could occur in instances when an elderly patient does not respond optimally to 6.25 mg.
The objective of the present study was to assess the potential residual psychomotor and cognitive effects of two doses of zolpidem-MR 6.25 and 12.5 mg 8 h after a single oral dose in healthy elderly subjects. The study was placebo controlled and flurazepam 30 mg was used as a positive internal control at a dose double that usually administered in the elderly patient, to validate the sensitivity of the test battery to the residual effects of the nocturnal administration of the test substances.
Methods
This study used a double-blind, randomized, four-way, cross-over design and was performed in a single centre in the UK (Human Psychopharmacology Research Unit, University of Surrey) between January and June 2003. The study was conducted according to the Declaration of Helsinki (Hong Kong Amendment), Good Clinical Practices (European Guidelines) and pertinent national legal and regulatory requirements. Written informed consent was obtained from each subject. Subjects were free to withdraw from the study at any time for any reason. The protocol was submitted to and approved by the Quorn Ethics Review Committee.
Study participants
The study included 24 healthy elderly volunteers of either sex aged at least 65 years. Subjects underwent a comprehensive medical examination to ensure that they were healthy and within normal range values for blood pressure, heart rate, ECG and standard haematological and biochemical laboratory tests, including plasma alanine aminotransferase, aspartate aminotransferase, creatinine and creatinine clearance. Body weight was required to be between 45 and 90 kg, body mass index between 16 and 32 kg m−2 and subjects were required to have normal vision (with glasses if needed).
No subject was seropositive for hepatitis B, hepatitis C or human immunodeficiency virus. Any subject with an ongoing clinically relevant condition, currently receiving medication likely to interfere with the conduct of the study, or with a history of drug allergy (particularly a known hypersensitivity to flurazepam or zolpidem) or with a past or present history of drug and substance abuse (including smokers over 5 cigarettes per day and excessive consumers of alcohol and xanthine-containing beverages) and those with special dietary habits, were excluded from the study.
Study procedures
The study consisted of several phases. After a screening visit in which entry criteria were validated, each subject took part in a familiarization session on the battery of psychometric tests to be used during the study, to preclude learning effects [17]. The subjects were then randomized to a treatment sequence and received zolpidem-MR 6.25 mg, zolpidem-MR 12.5 mg, flurazepam 30 mg and placebo, given as single oral doses in identical treatments using a double-dummy procedure. Treatment visits lasted 2 days and were separated by at least 28 days and no more than 42 days wash-out. Each treatment visit commenced with a re-familiarization session on the psychometric test battery in the afternoon preceding drug administration. The subject received one of the four study treatments before bedtime. The test session was held the next morning, 8 h after treatment. Subjects were monitored clinically for a further 18 h. An end-of-study medical examination was performed within 7–14 days after the last treatment.
Psychometrics
The test battery consisted of five psychometrics: Critical Flicker Fusion (CFF) [18, 19], the Choice Reaction Time (CRT) [20], word recall (WR) [21], the Continuous Tracking Test (CTT) [22] and the Digit Symbol Substitution Test (DSST) [23].
Critical Flicker Fusion
The CFF task assesses the integrative capacity of the central nervous system and, more specifically, the ability to discriminate discrete ‘bits’ of sensory information [24]. Subjects were required to discriminate flicker from fusion, and vice versa, in four light-emitting diodes arranged in a 1-cm square on a black background. The diodes were held in foveal fixation at a distance of 1 m. Individual thresholds were determined by the psychophysical method of limits on four ascending (flicker to fusion) and four descending (fusion to flicker) scales [25]. The mean of these four ascending and four descending presentations gave the threshold frequency in hertz (Hz). CFF has been shown to be sensitive to a variety of psychoactive compounds [26–28]. A decrease in the CFF threshold was indicative of a reduction in the overall integrative activity of the central nervous system [29].
Choice Reaction Time
The CRT task is used as an indicator of sensorimotor performance, assessing the ability to attend and respond to a critical stimulus. Subjects were required to extinguish one of six equidistant red lights, illuminated at random, by pressing the associated response button as quickly as possible. The mean reaction time of 50 trials was recorded in ms for three components: recognition, motor and total reaction time. Recognition reaction time (RRT) was the time it takes for the subject to notice the light, being the time between stimulus onset and the subject lifting their finger from the start button. Motor reaction time (MRT) indexed the movement component of this task and was the time between the subject lifting their finger from the start button and touching the response button. Total reaction time (TRT) was the sum of RRT and MRT. CRT is sensitive to the effects of psychotropic drugs [26, 30].
Memory recall
Immediate recall is thought to involve the central executive component of working memory [21, 31], while delayed recall is assumed to be a measure of explicit memory. Each word list consists of 20 nouns, based on norms for concreteness, imagery and meaningfulness [32]. They are additionally matched for number of syllables. Subjects were given 2 min exposure and learning time to examine each list, immediately followed by a 2-min period in which to write down as many of the words as they could recall (WRi). After approximately 30 min had elapsed (from the learning period), subjects were given a further 2 min to write down as many words as they could recall (WRd). WRi and WRd score was calculated as the number of words recalled correctly.
Continuous Tracking Task
The CTT is an interactive task of psychomotor function which entails using a computer mouse to keep a cursor in alignment with a moving target on a visual display unit (VDU) screen. The movement of the target is a function of an irregular sine wave. The response measure is the mean difference between the centres of the target and cursor in pixels, sampled five times per second. Lower scores are indicative of more accurate tracking. A peripheral awareness task was included, in which the subject responded to a stimulus presented in the periphery of vision by clicking the left mouse button, while simultaneously attending to the tracking test. The mean reaction time in ms to these stimuli over the trial period was taken as the response measure for this component of the divided attention task [33]. CTT performance is sensitive to psychoactive compounds [34, 35].
Digit Symbol Substitution Test
The DSST [23] is a pen and paper test which consists of rows of blank squares paired with randomly assigned digits (between 0 and 9). Subjects were required to substitute each digit with a different nonsense symbol, according to a key printed at the top of the sheet which indicated the nonsense symbol that corresponded to each digit. Alternate forms of the test are available, whereby the symbols which correspond to the digits are scrambled or reversed. The test involves the recognition and recoding of sensory information and has been described as a measure of simple information processing [36] and of psychomotor performance [37]. It also assesses visuomotor coordination, sustained attention and concentration skills.
Subjective evaluation of sleep
The Leeds Sleep Evaluation Questionnaire (LSEQ) is an instrument which measures subjective evaluation of sleep through 10 items rated with visual analogue scales [26, 38, 39]. It evaluated four factors: the perceived ease of getting to sleep, the quality of sleep, the ease of awakening and the integrity of behaviour following waking.
Pharmacokinetic sampling
Zolpidem plasma samples were collected from each subject before administration of study drug and approximately 8.5 h later, just after completion of the psychometric test battery. Samples were analysed for the presence of zolpidem using a liquid chromatography–tandem mass spectroscopy detection system with a detection limit of 0.5 ng ml−1. Plasma concentrations of flurazepam were not determined.
Safety assessment
Adverse events were monitored throughout the study via spontaneous reporting and classified as Treatment-Emergent Adverse Events (TEAEs), repetition of TEAE and Non-Treatment Emergent Adverse Events (NTEAEs) according to chronological criteria. They were coded according to the Medical Dictionary for Regulatory Authorities.
Heart rate and blood pressure were measured before each treatment administration and three times during the following day.
An electrocardiogram (ECG) was recorded at the screening visit and at the final study follow-up visit.
Standard biochemical and haematological tests were performed at the screening visit and at the final study follow-up visit. An alcohol breath test and a urine drug screen were performed before each treatment administration.
Statistical analysis
A priori power calculations based on the known effect of zolpidem on the CFF test were used to define the sample size. The objective was to detect a minimal clinically relevant difference in this test of 1.6 Hz between zolpidem and placebo with a power of at least 90% and an α error of 0.05. Assuming a potential drop-out rate of 20%, a sample size of 24 was calculated to be sufficient.
Statistical differences between treatment groups were assessed using a linear mixed effects model. For each test variable, pairwise comparison for each of the verum treatments and the placebo group were performed using linear contrasts and 95% confidence intervals for the difference calculated. A P-value <0.05 was taken to be statistically significant.
Results
Study subjects
The study included 10 male and 14 female subjects aged between 65 and 78 years. The mean (± SD) age of the male subjects was 71.2 ± 3.8 years and of the female subjects 70.7 ± 4.2 years. One female subject was prematurely withdrawn from the study after the first treatment period (zolpidem-MR 12.5 mg) due to poor compliance with the protocol and her data have been excluded from the efficacy analysis but kept in the safety analysis.
Psychometric tests
There were no significant differences in performance between either of the two doses of zolpidem-MR and placebo. In contrast, statistically significant differences in performance were observed between flurazepam and placebo for all the tests with the exception of the DSST (Table 1), although even for this test a trend toward impairment was observed with flurazepam (26.6 ± 1.5) vs. placebo (28.5 ± 1.9) at a probability level of 0.0526). For the CRT, the impairment was attributable to motor reaction time rather than recognition time. For the CTT, the impairment was in reaction time rather than with tracking accuracy.
Table 1.
Performance in psychomotor and cognitive tests
| Placebo | Zolpidem-MR 6.25 mg | Zolpidem-MR 12.5 mg | Flurazepam 30 mg | Pairwise comparison P-value | Mean difference estimate (95% CI) | |
|---|---|---|---|---|---|---|
| CFF threshold (Hz) | 28.0 ± 0.6 | 28.2 ± 0.5 | 27.6 ± 0.6 | 27.3 ± 0.5 | 6.25 vs. pbo 0.4970 12.5 vs. pbo 0.1312 flu vs. pbo 0.0011 | 0.20 [−0.38, 0.77) −0.44 (−1.02, 0.14)−1.01 (−1.59, –0.42) |
| CRT recognition time (ms) | 524 ± 16 | 514 ± 18 | 536 ± 19 | 548 ± 19 | 6.25 vs. pbo 0.4212 12.5 vs. pbo 0.3962 flu vs. pbo 0.0709 | −10.60 (−36.75, 15.56) 11.18 (−14.97, 37.33) 24.05 (−2.10, 50.20) |
| CRT motor reaction time (ms) | 318 ± 15 | 327 ± 16 | 315 ± 17 | 343 ± 19 | 6.25 vs. pbo 0.4015 12.5 vs. pbo 0.8383 flu vs. pbo 0.0243 | 9.16 (−12.51, 30.82) 2.22 (−23.89, 19.44) 25.03 (3.36, 46.69) |
| CRT total reaction time | 842 ± 23 | 841 ± 25 | 851 ± 25 | 890 ± 28 | 6.25 vs. pbo 0.9322 12.5 vs. pbo 0.5972 flu vs. pbo 0.0050 | −1.44 (− 35.13, 32.26) 8.96 (− 24.74, 42.65) 49.07 (15.38, 82.77) |
| Immediate word recall | 9.2 ± 0.5 | 8.8 ± 0.6 | 8.5 ± 0.7 | 6.4 ± 0.4 | 6.25 vs. pbo 0.5424 12.5 vs. pbo 0.2328 flu vs. pbo 0.0001 | −0.34 (−1.47, 0.78) –0.68 (−1.80, 0.45) −2.76 (−3.88, −1.63) |
| Delayed word recall | 6.0 ± 0.7 | 5.9 ± 0.6 | 4.9 ± 0.8 | 3.0 ± 0.5 | 6.25 vs. pbo 0.8935 12.5 vs. pbo 0.0876 flu vs pbo 0.0001 | −0.08 (−1.29, 1.13) −1.05 (−2.26, 0.16) −2.94 (−4.15, −1.73) |
| CTT mean deviation (pixels) | 31.1 ± 4.1 | 28.4 ± 3.1 | 35.2 ± 5.3 | 38.6 ± 6.1 | 6.25 vs. pbo 0.5260 12.5 vs. pbo 0.2974 flu vs pbo 0.0732 | −2.55 (−10.53, 5.43) 4.20 (−3.78, 12.18) 7.28 (−0.70, 15.26) |
| CTT mean response time (ms) | 655 ± 31 | 635 ± 27 | 697 ± 53 | 768 ± 60 | 6.25 vs. pbo 0.6785 12.5 vs. pbo 0.3552 flu vs pbo 0.0198 | 19.59 (−113.56, 74.39) 43.80 (−50.17, 137.78) 112.46 (18.49, 206.43) |
| DSST | 28.5 ± 1.9 | 29.2 ± 1.6 | 29.3 ± 1.7 | 26.6 ± 1.5 | 6.25 vs. pbo 0.4817 12.5 vs. pbo 0.4519 flu vs. pbo 0.0526 | 0.68 (−1.24, 2.61) 0.73 (−1.20, 2.65) −1.90 (−3.83, 0.020) |
Data are expressed as mean± SEM of data obtained in 23 subjects. CFF, Critical Flicker Fusion; CRT, Choice Reaction Time; CTT, Continuous Tracking Test; DSST, Digit Symbol Substitution Test.
Subjective assessments of sleep
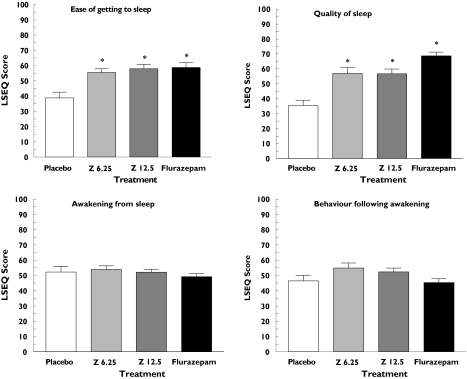
Subjective assessments of sleep and awakening were derived from the LSEQ. Statistically significant improvements in ease of getting to sleep and in sleep quality compared with placebo were observed for flurazepam and both doses of zolpidem-MR using LSEQ rating scales (Figure 1). In contrast, none of the active treatment groups varied significantly from placebo on visual analogue scale ratings of awakening from sleep or behaviour after awakening (Figure 1).
Figure 1.
Mean scores (mm) on each of four sleep factors in healthy elderly volunteers treated the night before with placebo (□), zolpidem-MR 6.25 mg (Z 6.25,  ), zolpidem-MR 12.5 mg (Z 12.5;
), zolpidem-MR 12.5 mg (Z 12.5;  ) or flurazepam 30 mg (▪). Data represent mean ± SEM from 23 subjects. *A significant difference with placebo (P < 0.05)
) or flurazepam 30 mg (▪). Data represent mean ± SEM from 23 subjects. *A significant difference with placebo (P < 0.05)
Safety assessment
All adverse events consisted of TEAEs. They were reported in 10 subjects with zolpidem-MR 6.25 mg, 14 subjects with zolpidem-MR 12.5 mg, 14 subjects with flurazepam and 15 subjects with placebo in the 18 h following administration of the different study treatments (Table 2). The frequency of adverse events was similar in all four treatment conditions. The most frequently reported adverse event was somnolence, although its frequency was nearly twice as high following flurazepam administration as after placebo or zolpidem-MR. Insomnia was reported more frequently after placebo than after zolpidem-MR or flurazepam. Dizziness was reported only after treatment with zolpidem-MR. None of the adverse events was serious or led to withdrawal from the study. No clinically relevant abnormalities were observed in biochemical or haematological parameters, vital signs or ECG parameters.
Table 2.
Number of Treatment-Emergent Adverse Events (TEAE) presented by system organ class and preferred term
| Placebo (N = 23) | Flurazepam 30 mg (N = 23) | Zolpidem 6.25 mg (N = 23) | Zolpidem 12.5 mg (N = 24) | ||
|---|---|---|---|---|---|
| Any class | Any TEAE | 15 (65.2%) | 14 (60.9) | 10 (43.5) | 14 (58.3) |
| Gastrointestinal disorders | Any TEAE | 3 (13.0%) | 0 (0) | 1 (4.3) | 0 (0) |
| Eructation | 1 (4.3%) | 0 (0) | 0 (0) | 0 (0) | |
| Nausea | 2 (8.7%) | 0 (0) | 1 (4.3) | 0 (0) | |
| Vomiting NOS | 1 (4.3%) | 0 (0) | 0 (0) | 0 (0) | |
| General disorders and administration site conditions | Any TEAE | 3 (13.0%) | 5 (21.7) | 2 (8.7) | 4 (16.7) |
| Fatigue | 2 (8.7%) | 1 (4.3) | 1 (4.3) | 2 (8.3) | |
| Feeling of relaxation | 1 (4.3%) | 0 (0) | 0 (0) | 0 (0) | |
| Gait abnormal | 0 (0) | 2 (8.7) | 0 (0) | 1 (4.2) | |
| Lethargy | 0 (0) | 3 (13.0) | 1 (4.3) | 1 (4.2) | |
| Infections and infestations | Any TEAE | 0 (0) | 0 (0) | 0 (0) | 1 (4.2) |
| Nasopharyngitis | 0 (0) | 0 (0) | 0 (0) | 1 (4.2) | |
| Musculoskeletal disorders | Any TEAE | 0 (0) | 0 (0) | 0 (0) | 1 (4.2) |
| Muscle cramp | 0 (0) | 0 (0) | 0 (0) | 1 (4.2) | |
| Nervous system disorders | Any TEAE | 9 (39.1%) | 10 (43.5) | 8 (34.8) | 7 (29.2) |
| Attention disturbance | 1 (4.3%) | 0 (0) | 0 (0) | 0 (0) | |
| Dizziness | 0 (0) | 0 (0) | 2 (8.7) | 1 (4.2) | |
| Dysgeusia | 1 (4.3%) | 1 (4.3) | 0 (0) | 0 (0) | |
| Headache | 3 (13.0%) | 0 (0) | 3 (13.0) | 1 (4.2) | |
| Somnolence | 6 (26.1%) | 10 (43.5) | 5 (21.7) | 6 (25.0) | |
| Psychiatric disorders | Any TEAE | 7 (30.4%) | 0 (0) | 4 (17.4) | 1 (4.2) |
| Disorientation | 0 (0) | 0 (0) | 1 (4.3) | 0 (0) | |
| Insomnia | 7 (30.4%) | 0 (0) | 3 (13.0) | 1 (4.2) |
Data are presented as the number and percentage of subjects in whom at least one TEAE was reported. NOS, Not otherwise specified.
Pharmacokinetic measurements
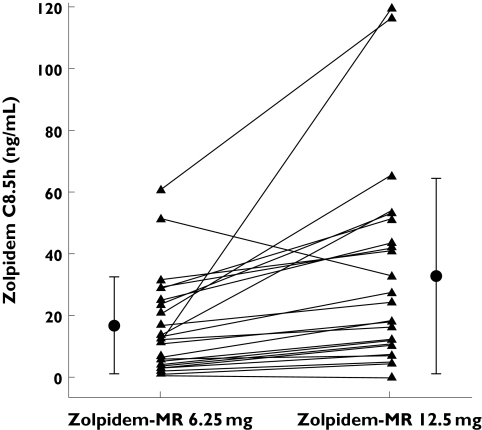
Plasma concentrations of zolpidem were determined 8.5 h after administration. The mean plasma zolpidem concentrations after the 6.25-mg and 12.5-mg doses of zolpidem-MR were 17.0 ± 15.9 ng ml−1 and 33.9 ± 32.3 ng ml−1 respectively, although intersubject variability was high. This corresponds to a concentration ratio of 1.96 between the two doses, which is consistent with dose linearity for mean plasma concentrations. The individual values are presented in Figure 2.
Figure 2.
Plasma concentrations of zolpidem-MR in healthy elderly volunteers treated the night before with zolpidem-MR 6.25 mg or zolpidem-MR 12.5 mg. Individual values (▴), mean (SD) values (•)
Discussion
This placebo- and positive- (flurazepam) controlled study demonstrated the lack of residual cognitive or psychomotor effects the morning after administration of zolpidem-MR at doses of 6.25 or 12.5 mg. The validity of the results is shown by the observation of impairment on most tests with flurazepam 30 mg, a sleep-promoting agent with well-established residual effects [40], used as a positive control. Since this study did not include a comparison with immediate-release zolpidem – in order not to add a fifth treatment period – we cannot conclude anything about the relative merits of the two formulations. However, such a comparison has been carried out in a previous study with young healthy volunteers [41]. When assessed 8 h post dose in a model of insomnia using the same battery of psychomotor tests, no residual effect was observed for either formulation.
Subjective assessment of sleep with the LSEQ revealed an improvement in scores for the factors relating to ease of falling asleep and quality of sleep after treatment with either dose of zolpidem-MR as well as with flurazepam 30 mg. Although sleep disorders were an exclusion criterion for the subjects evaluated in the study, the perceived improvement in sleep with zolpidem-MR may be indicative of a hypnotic effect that could be evaluated further in patients with insomnia. There was no difference in subjective assessment of next day awakening and well-being compared with placebo with any of the active study treatments.
One of the hypotheses of the study was that zolpidem-MR may not be completely eliminated from the patient due to reduced clearance, as has been demonstrated with the standard formulation [16]. Even though a complete pharmacokinetic profile was not obtained in the study, the mean plasma levels observed 8.5 h after administration of zolpidem-MR 12.5 mg (33.9 ng ml−1) were very similar to those reported (36.6 ng ml−1) in an identical paradigm in younger subjects (mean age 26 years) [17]. Although some residual plasma concentrations of zolpidem were found the day following treatment, these were not associated with any pharmacodynamic effects, as none of the individual psychometric assessments was significantly impaired or modified to any noticeable extent.
These data demonstrate that the zolpidem-MR formulation can be used safely in elderly subjects without the occurrence of clinically meaningful residual cognitive and psychomotor effects at either of the doses evaluated. The results highlight the freedom from next-day residual effects in clinical use for even those patients requiring a dose of zolpidem-MR of 12.5 mg.
This study was funded by Sanofi-Synthélabo Groupe (now Sanofi Aventis).
References
- 1.Morgan K. Hypnotics in the elderly. What cause for concern? Drugs. 1990;40:688–96. doi: 10.2165/00003495-199040050-00004. [DOI] [PubMed] [Google Scholar]
- 2.Hohagen F, Kappler C, Schramm E, Rink K, Weyerer S, Riemann D, Berger M. Prevalence of insomnia in elderly general practice attenders and the current treatment modalities. Acta Psychiatr Scand. 1994;90:102–8. doi: 10.1111/j.1600-0447.1994.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 3.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 4.Greenblatt DJ, Harmatz JS, Shader RI. Clinical pharmacokinetics of anxiolytics and hypnotics in the elderly. Therapeutic considerations (Part I) Clin Pharmacokinet. 1991;21:165–77. doi: 10.2165/00003088-199121030-00002. [DOI] [PubMed] [Google Scholar]
- 5.Hicks R, Dysken MW, Davis JM, Lesser J, Ripzckyj A, Lazarus L. The pharmacokinetics of psychotropic medication in the elderly; a review. J Clin Psychiatry. 1981;42:374–85. [PubMed] [Google Scholar]
- 6.Ray WA, Griffin MR, Downey W. Benzodiazepines of long and short elimination half-life and the risk of hip fracture. JAMA. 1989;262:3303–7. [PubMed] [Google Scholar]
- 7.Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: I. Psychotropic drugs. J Am Geriatr Soc. 1999;47:30–9. doi: 10.1111/j.1532-5415.1999.tb01898.x. [DOI] [PubMed] [Google Scholar]
- 8.Greenblatt DJ, Harmatz JS, Shader RI. Clinical pharmacokinetics of anxiolytics and hypnotics in the elderly. Therapeutic considerations (Part II) Clin Pharmacokinet. 1991;21:262–73. doi: 10.2165/00003088-199121040-00003. [DOI] [PubMed] [Google Scholar]
- 9.Wortelboer U, Cohrs S, Rodenbeck A, Ruther E. Tolerability of hypnosedatives in older patients. Drugs Aging. 2002;19:529–39. doi: 10.2165/00002512-200219070-00006. [DOI] [PubMed] [Google Scholar]
- 10.Holm KJ, Goa KL. Zolpidem: an update of its pharmacology, therapeutic efficacy and tolerability in the treatment of insomnia. Drugs. 2000;59:865–89. doi: 10.2165/00003495-200059040-00014. [DOI] [PubMed] [Google Scholar]
- 11.Drover DR. Comparative pharmacokinetics and pharmacodynamics of short-acting hypnosedatives zaleplon, zolpidem and zopiclone. Clin Pharmacokinetics. 2004;43:227–38. doi: 10.2165/00003088-200443040-00002. [DOI] [PubMed] [Google Scholar]
- 12.Morselli PL, Larribaud J, Guillet P, Thiercelin JF, Bartolini G. 1988 Daytime residual effects of zolpidem: a review of available data. In: Sauvanet JP, Langer SZ, Morselli PL, editors. Imidazopyridines in Sleep Disorders. New York: Raven Press; 1988. pp. 183–91. [Google Scholar]
- 13.Scharf MB, Mayleben DW, Kaffeman M, Krall R, Ochs R. Dose–response effects of zolpidem in normal geriatric subjects. J Clin Psychiatry. 1991;52:77–83. [PubMed] [Google Scholar]
- 14.Fairweather DB, Kerr JS, Hindmarch I. The effects of acute and repeated doses of zolpidem on subjective sleep, psychomotor performance and cognitive function in elderly volunteers. Eur J Clin Pharmacol. 1992;43:597–601. doi: 10.1007/BF02284957. [DOI] [PubMed] [Google Scholar]
- 15.Allain H, Bentue-Ferrer D, Tarral A, Gandon JM. Effects on postural oscillation and memory functions of a single dose of zolpidem 5 mg, zopiclone 3.75 mg and lormetazepam 1 mg in elderly healthy subjects. A randomized, cross-over, double-blind study versus placebo. Eur J Clin Pharmacol. 2003;9:179–88. doi: 10.1007/s00228-003-0591-5. [DOI] [PubMed] [Google Scholar]
- 16.Salvà P, Costa J. Clinical pharmacokinetics and pharmacodynamics of zolpidem. Therapeutic implications. Clin Pharmacokinet. 1995;29:142–53. doi: 10.2165/00003088-199529030-00002. [DOI] [PubMed] [Google Scholar]
- 17.Hindmarch I, Legangneux E, Stanley N. A randomised double-blind placebo-controlled 10-way cross-over study shows that a new Zolpidem modified release formulation improves sleep maintenance compared to standard Zolpidem (abstract) Sleep. 2004;27:A55. (Suppl.) [Google Scholar]
- 18.Parkin CE, Kerr JS, Hindmarch I. The effects of practice on choice reaction time and critical flicker fusion threshold. Hum Psychopharmacol. 1997;12:65–70. [Google Scholar]
- 19.Curran S. Critical Flicker Fusion techniques in psychopharmacology. In: Hindmarch I, Stonier PD, editors. Human Psychopharmacology: Measures and Methods. Vol. 3. Chichester: John Wiley and Sons; 1990. [Google Scholar]
- 20.Sherwood N, Kerr JS. The reliability, validity and pharmacosensitivity of 4 psychomotor tests. In: Hindmarch I, Stonier PD, editors. Human Psychopharmacology: Measures and Methods. Vol. 4. Chichester: John Wiley and Sons; 1993. [Google Scholar]
- 21.Baddeley AD. Working Memory. Oxford: Oxford University Press; 1986. [Google Scholar]
- 22.Parkin C, Fairweather DB, Shamsi Z, Stanley N, Hindmarch I. The effects of cigarette smoking on overnight performance. Psychopharmacology. 1998;136:172–8. doi: 10.1007/s002130050553. [DOI] [PubMed] [Google Scholar]
- 23.Wechsler D. Wechsler Adult Intelligence Scale. New York: The Psychological Corporation; 1981. (Revised 1981) [Google Scholar]
- 24.Hindmarch I. Relevant psychometric tests for antidepressants and anxiolytics. Int Clin Psychopharmacol. 1994;9(Suppl. 1):27–33. doi: 10.1097/00004850-199403001-00005. [DOI] [PubMed] [Google Scholar]
- 25.Woodworth R, Schlosberg H. Experimental Psychology. London: Methuen; 1958. [Google Scholar]
- 26.Hindmarch I. A 1,4 benzodiazepine, temazepam (K 3917), its effect on some psychological parameters of sleep and behaviour. Arz Forsch. 1975;25:1836–9. [PubMed] [Google Scholar]
- 27.Smith JM, Misiak H. Critical Flicker Fusion Frequency (CFF) and psychotropic drugs in normal human subjects: a review. Psychopharmacology. 1976;47:175–82. doi: 10.1007/BF00735818. [DOI] [PubMed] [Google Scholar]
- 28.Hindmarch I. Critical Flicker Fusion Frequency (CFF): the effects of psychotropic compounds. Pharmacopsychiatria. 1982;15(Suppl. 1):44–8. [Google Scholar]
- 29.Fairweather DB, Dal Pozzo C, Kerr JS, Lafferty SV, Hindmarch I. Citalopram compared to dothiepin and placebo: effects on cognitive function and psychomotor performance. Hum Psychopharmacol. 1997;12:119–26. [Google Scholar]
- 30.Baselt RC. Drug Effects on Psychomotor Performance. Foster City: Biomedical Publications; p. 2001. [Google Scholar]
- 31.Baddeley AD, Hitch G. Working memory. In: Bower GA, editor. Recent Advances in Learning and Motivation. Vol. 8. New York: Academic Press; [Google Scholar]
- 32.Paivio A, Yuille JC, Madigan S. Concreteness, imagery and meaningfulness values for 925 nouns. J Exper Psychol Monogr Suppl. 1968;76:1–25. doi: 10.1037/h0025327. Suppl. [DOI] [PubMed] [Google Scholar]
- 33.Parkin C, Fairweather DB, Shamsi Z, Stanley N, Hindmarch I. The effects of cigarettes smoking on overnight performance. Hum Psychopharmacol. 1991;6:197–207. doi: 10.1007/s002130050553. [DOI] [PubMed] [Google Scholar]
- 34.Hindmarch I, Subhan Z, Stoker MJ. Comparison of the effects of zimelidine and amitriptylline on car driving performance. Acta Psychiatr Scandinavica. 1983;308:141–6. doi: 10.1111/j.1600-0447.1983.tb11115.x. [DOI] [PubMed] [Google Scholar]
- 35.Hindmarch I. Three antidepressants (amitriptylline, dothiepin and fluoxetine) with and without alcohol, compared with placebo on tests of psychomotor ability related to car driving. Hum Psychopharmacol. 1987;2:177–83. [Google Scholar]
- 36.Parrott AC. Performance tests in human psychopharmacology (3): construct validity and test interpretation. Hum Psychopharmacol. 1991;6:197–207. [Google Scholar]
- 37.Lezak MD. Neuropsychological Assessment. 3. New York: Oxford University Press; 1995. [Google Scholar]
- 38.Parrott AC, Hindmarch I. The Leeds Sleep Evaluation Questionnaire in psychopharmacological investigations – a review. Psychopharmacology. 1980;71:173–9. doi: 10.1007/BF00434408. [DOI] [PubMed] [Google Scholar]
- 39.Zisapel N, Laudon M. Subjective assessment of the effects of CNS active drugs on sleep by the Leeds Sleep Evaluation Questionnaire: a review. Hum Psychopharmacol. 2003;18:1–27. doi: 10.1002/hup.455. [DOI] [PubMed] [Google Scholar]
- 40.Salkind MR, Silverstone T. A clinical and psychometric evaluation of flurazepam. Br J Clin Pharmacol. 1975;2:223–6. doi: 10.1111/j.1365-2125.1975.tb01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hindmarch I, Stanley N, Legangneux E, Emegbo S. Zolpidem modified-release significantly reduces latency to persistent sleep 4 and 5 hours post dose compared with standard Zolpidem in a model assessing the return to sleep following nocturnal awakening (abstract) Sleep. 2005;28:A246. (Suppl.) [Google Scholar]