Abstract
GABAergic signalling exerts powerful inhibitory control over spinal tactile and nociceptive processing but during development GABA can be depolarizing and the functional consequences of this upon neonatal pain processing is unknown. Here we show a postnatal switch in tonic GABAA receptor (GABAAR) modulation of cutaneous tactile and nociceptive reflexes from excitation to inhibition, but only in the intact spinal cord. Neonatal and 21-day old (P21) rats were intrathecally treated with one of the GABAAR antagonists bicuculline and gabazine with both compounds dose-dependently decreasing hindpaw mechanical and thermal withdrawal thresholds in P21 rats but increasing them in P3 neonates. Intrathecal gabazine also produced an increase in the cutaneous evoked EMG response of the biceps femoris in P21 rates while lowering the response in neonates. Injections of 3H-gabazine in the L4-L5 region at P3 confirmed that gabazine binding was restricted to the lumbar spinal cord. Spinalisation of P3 neonates at the upper thoracic level prior to drug application reversed the behavioural and EMG responses to GABA antagonists so that they resembled those of P21 rats. The effects of spinalisation were consistent with gabazine facilitation of ventral root potentials observed in isolated neonatal spinal cord. These data show a marked postnatal developmental switch in GABAergic control of neonatal nociception that is mediated by supraspinal structures and illustrate the importance of studying developmental circuits in the intact nervous system.
Keywords: Rat, neonate, brain stem, pain
Introduction
Pain is a sensory experience that is not restricted to mature organisms; even the youngest animals exhibit acute responses to nociceptive stimuli. However, it is also clear that nociceptive circuitry undergoes considerable tuning in the postnatal period which affects both infant pain behaviour and responsiveness to analgesic agents(Pattinson and Fitzgerald, 2004;Waldenstrom et al., 2003). Dorsal horn cell receptive fields are large, cutaneous mechanical thresholds are low and nociceptive reflexes have poor spatial organisation in the neonate compared to the adult (Andrews and Fitzgerald, 1994;Andrews et al., 2002;Torsney and Fitzgerald, 2002). A key mechanism for postnatal tuning may be the maturation of spinal cord inhibition (Fitzgerald and Jennings, 1999;Fitzgerald, 2005); substantial changes in dorsal horn synaptic inhibitory signalling occur over the postnatal period (Baccei and Fitzgerald, 2004;Keller et al., 2001;Keller et al., 2004;Schaffner et al., 1993)
The majority of fast inhibitory neurotransmission in the nervous system is mediated by GABAA receptors and GABAergic interneurons in the spinal dorsal horn play an important role in the processing of somatosensory and nociceptive information. In the adult, application of the GABAAR antagonist bicuculline results in dorsal horn neuronal excitation, revealing the presence of strong GABAergic inhibition (Cronin et al., 2004;Reeve et al., 1998;Chiang et al., 1999;Ishikawa et al., 2000). Alterations in the strength of this inhibitory tone are thought to have a role in neuropathic pain (Moore et al., 2002;Woolf and Salter, 2000;Kontinen et al., 2001).
GABA containing interneurons and terminals are concentrated in the superficial laminae of the dorsal horn from before birth (Allain et al., 2004) but neonatal CNS levels of GABA are up to 50% higher than in the adult (Schaffner et al., 1993). GABA receptors are among the first to be expressed in the embryonic spinal cord but their subunit composition is developmentally regulated; expression of α2, α3, β3 and γ2 subunits is high in the neonatal dorsal horn but falls to adult levels within a week of birth (Ma et al., 1992) followed by a four-fold acceleration in the decay rate of GABA mIPSCs observed in laminae I-II neurons between P8 and P23 (Keller et al., 2004). In many areas of the developing CNS, GABAAR activity initially produces neuronal depolarization and even excitation which switches later to hyperpolarization due to a maturational shift in the chloride reversal potential (Ben Ari, 2002). Accumulation of intracellular Cl- in the adult is prevented by the K+-Cl- cotransporter, KCC2 and the developmental onset of KCC2 coincides with GABA hyperpolarizing activity (Stein et al., 2004;Wang et al., 2002). In the rat dorsal horn the depolarizing action of GABA is present until some days after birth (Baccei and Fitzgerald, 2004)
Despite extensive cellular studies on the regulation of GABA signalling in the developing brain and spinal cord, the functional development of spinal GABAAR transmission upon neonatal tactile and nociceptive reflexes is not known. Here we have used behavioural and electrophysiological techniques to test the effects of intrathecal GABAA receptor antagonists upon mechanical and thermal pain thresholds and reflex activity in neonatal intact and spinalized rats.
Methods
All animal use procedures were licensed by the U.K. Home Office and performed in accordance with the Animals (Scientific Procedures) Act 1986. Sprague Dawley rats of either sex were used. Animals were kept in cages with their littermates and mothers in a room with a 12hr light dark cycle.
Behavioural Testing
Mechanical withdrawal thresholds were measured in 3 (P3) and 21 (P21) day old rat pups by application of graded Von Frey Hairs (vFh; 0.01-220g; Stoelting, Wood Dale, Illinois, USA). Animals were habituated to the testing room and apparatus for 45mins prior to behavioural testing. Older (P21) animals were placed on top of a wire mesh grid to allow the application of the vFh to the plantar surface of the foot. Younger (P3) rats were placed on a cotton wool sheet to allow vFh application to the dorsal surface of the foot. Five applications of each filament were made at 1min intervals and the threshold recorded as the vFh that elicited a withdrawal response in 40% (2 out of 5) or more of applications.
Thermal withdrawal thresholds were determined using the technique described by Hargreaves et al. (1988). Briefly animals were habituated to the test apparatus (Plantar Test Apparatus, Stoelting USA) for 45min (P21) and 10min (P3). Baseline withdrawal latencies to an infrared heat stimulus were measured and recorded. An i.t. injection of gabazine or saline were made as described below, and withdrawal latencies were measured every ten minutes for one hour. Each foot was tested three times during each 10minute period; each test was evenly spaced over that period with successive tests being separated by at least three minutes.
Intrathecal Injections
Following measurement of baseline mechanical and thermal thresholds animals were anaesthetized with 4% halothane in O2 in a gas chamber and maintained at 1.5% halothane via a nose cone. Drugs (bicuculline (10, 5, 1ng/g): Sigma, U.K. or gabazine (1ng/g): Sigma U.K; all dissolved in saline) or saline were injected at the L4-L5 level intrathecally (i.t.) using a 10μl Hamilton syringe (26S gauge, model 801 RN, Hamilton Bonaduz AG, Switzerland) with a fixed needle. Injected volumes were 2μl in P3 and 7μl in P21 rats. Following injections animals were returned to the test apparatus (wire grid for P21 and cotton wool for P3 rats) and thresholds were measured every five minutes for the subsequent 40mins after the injection and then again at 50mins and 60mins post-injection.
EMG Recordings
P3 or P21 rat pups were anaesthetized with halothane (2-4%) in oxygen. A tracheotomy was performed and animals were ventilated with a Harvard small animal ventilator (Harvard Apparatus Ltd, Edenbridge, Kent U.K.). Body temperature was monitored and normothermia was maintained through the use of a thermostatically controlled heat source. Pups were placed in a small animal spinal frame and supported in a mould (Impregum F, ESPE) with one hindlimb secured in slight extension and plantar flexion on a fixed platform. Baseline EMG recordings to plantar mechanical stimulation were obtained. Intrathecal injections of saline or gabazine (1ng/g; volumes described above) were performed with a sterile 30-guage needle attached to a glass 5mcl or 25mcl Hamilton syringe. Recordings were repeated at 5-minute intervals following intrathecal injection. At the end of the experiment, animals were terminally anaesthetized with intraperitoneal pentobarbitone (100mg/kg).
Bipolar EMG electrodes (Ainsworks, London, U.K.) were placed through a small skin incision into the belly of the biceps femoris muscle. Raw signals were amplified using conventional amplification and filtering (Neurolog, Digitimer, Welwyn Garden City, U.K.) and analysed with, PowerLab 4S (AD Instruments, Castle Hill, Australia). Raw data was digitized at a frequency of 4kHz. VFh of graded intensity were applied to the hindpaw and the EMG response recorded. The mechanical withdrawal threshold was defined as the lowest number von Frey hair that elicited an EMG response. Up to three vFh above threshold were sequentially applied at one-minute intervals.
Spinalisation
An analogous series of experiments were performed in spinalized animals. In these experiments animals had their baseline mechanical withdrawal thresholds measured prior to spinalization. Pups were anaesthetized with halothane (2-4%) in oxygen and a laminectomy was performed in the upper thoracic region and the cord divided. Animals were allowed to recover for 2-3 hours (P3) and 6 hours (P21) prior to measurement of mechanical thresholds as detailed above. At the end of the experiment the upper thoracic cord was dissected, and data only included if complete transection was confirmed.
Visualization of gabazine binding
P3 rat pups were anaesthetized as described above and a syringe containing 0.5μl of 0.9%saline with 2.2μCi of 3[H]Gabazine ((Specific activity = 55.3Ci/mmol) Perkin Elmer Boston, MA) was inserted intrathecally. The compound was injected and 10min post injection the animals were terminally anaesthetized with sodium pentobarbitone. Tissue was snap frozen on dry ice and mounted on a cryostat chuck. Frozen transverse sections (30μm) were thaw mounted on superfrost plus microscope slides dried overnight and exposed to a phosphorimage screen for 10 days. The phosphorimage screen was then scanned using a Typhoon Phosphoimager (Amersham, Little Chalfont, U.K.).
Ventral Root Potential Electrophysiology
Neonatal Sprague-Dawley pups (P3) were killed by an overdose of halothane (5% in medical oxygen), decapitated and the spinal column quickly removed and placed in an ice-cold dissection solution consisting of (in mM): 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.0 NaH2PO4, 6 MgCl2, 0.5 CaCl2, 25 glucose and continuously bubbled with 95% O2 / 5% CO2. The spinal cord was removed and hemisected leaving the dorsal and ventral roots attached, and placed in a chamber filled with oxygenated dissection solution and allowed to recover for 1.5 - 2 hours at room temperature. Spinal cords were transferred to a submersion-type recording chamber (RC-22; Warner Instruments) and mounted on the stage of an upright microscope (Zeiss Axioskop 2; Welwyn Garden City, UK). The cords were continually perfused at room temperature with oxygenated aCSF solution containing (in mM): 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.0 NaH2PO4, 1.0 MgCl2, 2.0 CaCl2, 25 glucose at a rate of 1-3 mL/min. Dorsal roots were electrically stimulated via a suction electrode connected to a constant-current stimulator (NeuroLog system, Digitimer Ltd., Hertfordshire, UK). Ventral root potentials (VRPs) were recorded with the use of a suction electrode connected to a Multiclamp 700A amplifier (Axon Instruments; Union City, CA, USA). The root-mean-square of the VRP was calculated and the area under the waveform measured for a 1 second period from the stimulus onset. Signals were filtered, displayed and analysed with a data acquisition system (Digidata 1322A with pClamp 8.0 software, Axon Instruments).
Statistics
Behavioral data was analyzed using GraphPad Prism® (GraphPad Software Inc, San Diego, U.S.A.). Statistically significant differences between groups were analyzed using ANOVAs with Bonferonni Post-tests where appropriate. Comparisons were made between age groups and also between drug treated and saline treated animals.
The duration of the EMG response was outlined from the display of the raw data and the integral of the rectified (RMS) signal was calculated (Chart, Powerlab AD Instruments). The integral of the RMS signal (response) was plotted against the von Frey hair number (mechanical stimulus) and the area under the stimulus-response curve (AUC) calculated (Prism, GraphPad) to quantify the reflex response both at baseline and following intrathecal injection of saline or gabazine. Data was also expressed as the percentage change from the baseline value i.e. % change = [(post AUC - pre AUC / pre AUC) × 100]. To determine the effects of gabazine a two-way ANOVA was performed with Bonferroni Post-tests where appropriate. To determine the effects of spinalisation a one way ANOVA was performed with Bonferroni post-tests where appropriate.
Results
The effect of spinal GABA antagonists on mechanical and thermal behavioural thresholds reverses between P3 and P21
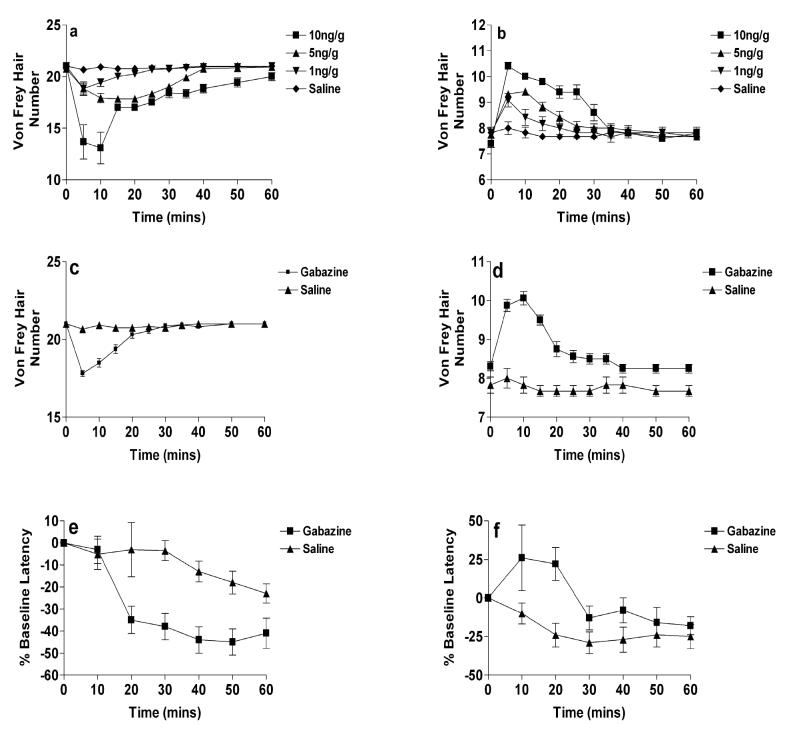
Our results provide evidence of a striking age difference in the effects of intrathecal GABA antagonists upon hindpaw mechanical and thermal nociceptive reflex thresholds in awake animals at P3 and P21. Intrathecal application of bicuculline produced dose-dependent changes in the mechanical withdrawal thresholds in both P21 (Figure 1a) and P3 (Figure 1b) rats. Each dose produced its’ maximal effect within the first ten minutes following injection in P21 rats (P<0.001; Figure 1a) and within five minutes in P3 rats (P<0.001; Figure 1b). However, while in P21 rats the highest dose of bicuculline (10ng/g) decreased the mechanical withdrawal threshold by 7vFh (200g to 10g), the same dose in P3 rats had the opposite effect and increased withdrawal thresholds by 3vFh (0.85g to 5.0g). Saline elicited no effect in any age rat tested (P>0.05). Thresholds returned to pre-injection values within 30mins in P3 and 25mins in P21 rats.
Figure 1.
The effects of intrathecal bicuculline upon hindpaw mechanical and thermal withdrawal thresholds (see supplementary methods). Thresholds are expressed as mean vFh number ± SEM, or as a percentage of baseline in hot plate withdrawal latency compared to controls (n=8-14 rats per point). (a) In P21 rats thresholds were decreased significantly within five minutes of injection (P<0.05; n=12) at all doses when compared to saline treated animals. (b) In P3 rats all doses of bicuculline significantly increased withdrawal thresholds within five minutes of injection when compared to saline (P<0.05; n=12). In both age groups values returned to baseline values within sixty minutes. (c & d) Intrathecal gabazine(1ng/g) had the same effect on mechanical thresholds as bicuculline at (c) P21 and (d) P3. (e & f) The hot plate withdrawal latency was decreased by intrathecal gabazine in (d) P21 rats and increased in (e) P3 rats when compared to saline treated animals. Time zero (0 mins) reflects baseline, drug-free, responses in all cases.
The specific dependence of this switch upon GABAAR activity was confirmed using the more selective GABAAR antagonist, gabazine, which unlike bicuculline, has no effect on other GABA receptors and channels at high doses (Mestdagh and Wulfert, 1999). Gabazine (1ng/g) also decreased the mechanical withdrawal threshold in P21 rats, in this case by 4vFh (200g to28.5g; P<0.001; Figure 1c), whilst increasing the threshold in P3 rats by 2vFh (0.85g to 2.40g; P<0.001; Figure 1d).
To determine if the developmental switch in GABAA activity extended to other sensory modalities, the effect of gabazine upon noxious thermal withdrawal thresholds was also measured. Intrathecal gabazine (1ng/g) decreased thermal thresholds in P21 (Figure 1e) rats but increased them in P3 (Figure 1f) rats (P21 - 45±5%, P<0.05; P3 26±21%, P>0.05). Values returned to baseline values in P3 animals but not in P21 rats.
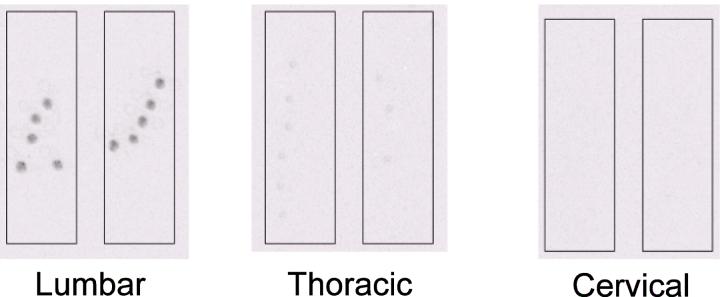
To confirm the spinal site of binding of gabazine, tritiated gabazine (1ng/g) was injected intrathecally and gabazine binding sites examined in spinal cord sections via a phosphoimager. This experiment illustrated that binding was restricted to the lumbar spinal cord with little binding being found at the thoracic level. Binding was completely absent in the cervical spinal cord or in the brain stem (Figure 2).
Fig 2.
Phosphoimages of lumbar, thoracic and cervical spinal cord sections from P3 rats showing 3H-gabazine sites. Binding can be observed in the lumbar and to a lesser extent thoracic sections but none was present in the cervical cord.
These data together provide evidence that tonic spinal GABAergic influences on cutaneous sensory reflex thresholds in awake animals switches from excitation to inhibition over the first 3 postnatal weeks.
The effect of spinal GABA antagonists on nociceptive EMG activity also reverses between P3 and P21
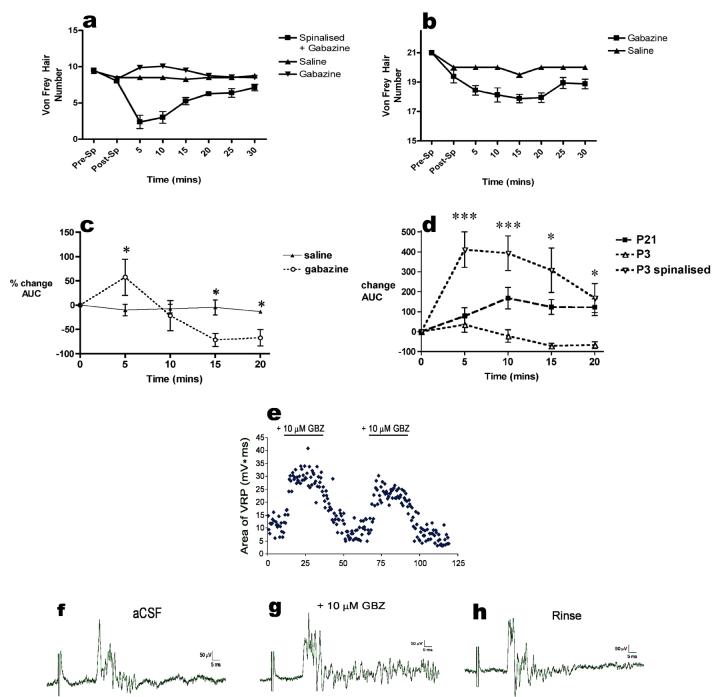
The age dependent effects of intrathecal application of gabazine were also observed upon the amplitude of the nociceptive EMG response recorded from flexor muscles in response to mechanical hindpaw stimulation in halothane anesthetised rats. Fig 3c illustrates the percentage changes in EMG response following intrathecal application of gabazine (1ng/g) in P3 rats. At P21, the drug significantly increased the reflex amplitude within 10mins of application (168±54%; P<0.05) and this increase remained significantly higher than baseline and saline injected animals for 60mins (Fig 3d). In P3 rats, however, a bi-phasic response to intrathecal gabazine was observed. Initially the drug significantly increased EMG activity (57±37%; P<0.05; Fig 3c), this was abolished after a further 5min. Subsequently EMG size was significantly decreased when compared to baseline values. At 15mins EMG size was decreased by -71±13% (P<0.05) and at 20mins the reduction was -67±16% (P<0.05). Values did not recover to baseline levels for the duration of the experiment.
Fig 3.
(a) The inhibitory effects of intrathecal gabazine (1ng/g) on hindpaw mechanical withdrawal thresholds at P3 are abolished following spinalization, so that the response resembles that in intact P21 animals. (b) Spinalization attenuates but does not abolish the ability of intrathecal gabazine (1ng/g) to reduce mechanical thresholds in P21 rats. (c) Intrathecal gabazine initially increases and then decreases flexor EMG responses to hindpaw mechanical stimulation at P3. Asterisks indicate statistical significance (Gabazine vs saline; *P<0.05 n=4 in each group). (d) Gabazine decreases the flexor muscle EMG responses at P3, which is reversed by spinalization. (168± 54% vs. -71±13% at 15mins post-gabazine; P<0.05, n=4 in each group). Asterisks indicate statistical significance (*P<0.05; ***P<0.001) between P3 and P3 spinalized. (e) Block of spinal GABAARs enhances ventral root potentials (VRPs) in the P3 isolated perfused spinal cord. Plot of normalized VRP area (expressed as a percentage of the mean VRP area in aCSF) as a function of time for a population of P3 spinal cords (n = 6). (f,g,h) Example of VRP evoked by dorsal root stimulation at C-fiber intensity (1 mA, 1 ms) before, during and after bath application of gabazine. Time zero (0 mins) reflects baseline, drug-free, responses in all cases.
The tonic GABAergic influence on nociceptive reflexes in lightly anaesthetised animals also therefore switches from excitatory to inhibitory over the first postnatal three weeks.
Tonic GABAergic excitation is not observed ‘in vivo’ in P3 spinalized animals
The effects of intrathecal gabazine (1ng/g) on hindpaw mechanical sensory thresholds in awake P3 rats that had been spinalized 2 hours previously are shown in figure 3a. Spinalisation itself did not significantly affect baseline mechanical withdrawal thresholds. In these animals, the effects of gabazine were similar to those observed at P21. Thresholds fell by 7vFh (0.85g to 0.02g) with the maximal effect of the drug appearing 5mins post-injection compared to an increase of 2vFh (0.85g to 2.40g) observed in intact animals. The effects of saline were unchanged by spinalisation. The effect of spinalisation upon P21 rats is shown in figure 3b. In these animals spinalisation decreased baseline mechanical thresholds, subsequent i.t. gabazine further decreased mechanical thresholds but to a lesser degree than in intact animals, whilst saline had no effect in spinalized animals. This decreased effect of gabazine may reflect the continued presence of spinal shock resulting from the spinalisation.
Figure 3d also shows that spinalisation of P3 rats also abolished the inhibitory effect of gabazine upon nociceptive EMG reflex amplitudes. The magnitude of the response was significantly increased from baseline values and reached its’ maximum 5mins following injection (411±89%; P<0.05). The effect of spinalisation upon the effects of intrathecal gabazine when compared to intact animals were maintained for a further 10mins. Spinalisation therefore changes the effect of tonic GABAAR activation in P3 spinal cord unmasking a mature phenotype.
Since spinalisation abolished the tonic GABAergic excitation of spinal nociceptive reflexes in P3 intact rat pups ‘in vivo’, we predicted that it would also not be observed in ventral root reflex responses recorded from isolated perfused P3 spinal cord ‘in vitro’. The effect of bath application of the GABAAR antagonist gabazine (10 μM) upon both the threshold and amplitude of the ventral root potential (VRP) evoked by dorsal root stimulation at C fibre intensity is illustrated in Figures3e-h. Gabazine did not have a significant effect on VRP threshold (240 ± 40 μA; n = 5; p = 0.24). However, in all spinal cords examined (n = 6), gabazine increased the magnitude (area under the curve) of the VRP evoked by C-fibre stimulation in a reversible manner (Fig. 3f). This resulted from a slight enhancement in the peak amplitude of the VRP along with a striking increase in the VRP duration (Fig. 3e). Across the population of spinal cords tested, gabazine facilitated VRP area by up to 242.0 ± 15.2 % (n = 6; Fig. 3g). The net effect of activating spinal GABAARs at P3 in isolated cord is therefore to inhibit spinal cord activity.
Discussion
In the present study we have shown that the GABAAR, a major target of clinically prescribed analgesics and sedatives, differentially modulates the responses to nociceptive stimuli in neonatal and mature rats. Responses to spinal GABAAR antagonism revealed that in P3 rats GABA activity is having a net excitatory influence upon neonatal spinal cord cutaneous reflexes in vivo whereas in older animals the net effect is inhibitory.
Especially important is the fact that the excitatory influence of GABA activity at P3 is abolished when the spinal cord is isolated from the brainstem, either by spinalisation or by studying the isolated spinal cord in vitro. This means that GABA signaling is not directly excitatory at the level of spinal cord circuitry. At early postnatal stages GABA has been shown to act as an excitatory neurotransmitter in the CNS due to an inverted electrochemical gradient for Cl- in neonatal neurons (Ben Ari, 2002). Although there is evidence for a depolarizing effect of GABA in the newborn dorsal horn, the ECl was found to be never above firing threshold suggesting that GABA does not directly excite dorsal horn neurons at this age as it does in the hippocampus (Baccei and Fitzgerald, 2004). Nevertheless it is possible that the greater spike activity ‘in vivo’ could lead to an accumulation of chloride ions and a further shift in the ECl within the dorsal horn and resulting in direct GABA excitation. Our results here show that this is not the case. Isolated or spinalized spinal cord reflexes are excited by gabazine showing that GABA has a direct and powerful inhibitory effect upon the developing networks in the dorsal and ventral horn.
The major question, then, is how spinal GABA activity can have an excitatory influence upon spinal reflexes only when the spinal cord is connected to the brain. Clearly a supraspinal element is involved in modulating the effects of GABAAR antagonists in the dorsal horn of neonatal rats. One possible explanation is a technical one, namely that in P3 animals the drugs diffused rostrally and had a direct action on the brain. Intrathecal 3H-gabazine injected permeated the entire thickness of the spinal cord at lumbar regions but only in low levels into the superficial dorsal horn in thoracic cord and no 3H-gabazine binding was found in the cervical cord or brainstem. It is reasonable to conclude from this that the GABA antagonism was restricted to the lumbar and possibly thoracic spinal cord.
It is likely then that at P3, as well as at P21, gabazine disinhibits dorsal horn cell responses leading to enhanced transmission of cutaneous evoked activity projecting to higher centres in the brainstem. This may explain the bi-phasic nature of the EMG response in P3 animals (Fig 3c) with perhaps the first phase reflecting dorsal horn disinhibition. However, the effect of this increased activity upon those supraspinal centres and the descending activity that form part of the feedback loop back down to the spinal cord, must be very different at the two ages causing inhibition in neonates but not in older animals.
In the adult, ascending nociceptive pathways from lamina 1 and deeper laminae ascend in the spinothalamic tract (STT) and the spinoparabrachial tract to innervate the thalamus, reticular areas and the parabrachial area which in turn sends projections to the hypothalamus, amygdala, periaqueductal grey and the rostroventral medulla (RVM) (Hunt and Mantyh, 2001). The RVM is a source of powerful descending projections via the dorsolateral funiculus to the spinal dorsal horn, which have both inhibitory and excitatory influences upon dorsal horn nociceptive responses (Millan, 2002;Millan, 2002;Gebhart, 2004;Suzuki et al., 2004). Within the RVM are ‘off-cells’ that inhibit and ‘on-cells’ that facilitate spinal nociceptive transmission and reflexes. The activity of these cells is modified in prolonged pain states and by analgesic agents. Nociceptive stimulation in adults appears to inhibit ‘off cells’ through a GABAergic pathway, thereby relieving tonic descending inhibition and allowing ‘on cells’ to facilitate reflexes through descending excitatory pathwys (Gilbert and Franklin, 2001;Fields and Heinricher, 1985;Fields et al., 1995).
Little is known about the postnatal development of brainstem spinal cord interactions in cutaneous nociceptive processing, Lamina 1 projection neurons are born early in the embryonic period, before local circuit neurons (Bice and Beal, 1997a;Bice and Beal, 1997b) and appear to project to the brainstem soon after birth (Qin et al., 1993). Descending pathways from the RVM to the dorsal horn are also present at birth but functional studies of descending inhibition from the DLF have shown that this is not fully developed until P21 (Fitzgerald and Koltzenburg, 1986;van Praag and Frenk, 1991), most likely due to low levels of neurotransmitter. The current results raise the intriguing possibility that the dominant baseline influence of the brainstem over spinal reflex function in the newborn is an excitatory one, perhaps through ‘on cells’ rather than an inhibitory one through ‘off cells’ as in the adult. Blockade of GABA receptors in the dorsal horn and subsequent increase of ascending activity to the brainstem, could possibly diminish ‘on cell’ rather than ‘off cell’ activity and blunt descending excitation. A further possibility is that the GABAergic pathways in the brainstem that normally inhibit ‘off cells’ may actually be excitatory due to the chloride shift and thereby enhance their activity, although the absence of descending inhibition via the DLF in newborns argues against this (Fitzgerald and Koltzenburg, 1986;van Praag and Frenk, 1991), further experiments need to be performed to determine the developmental processes that occur in the RVM and other brainstem nuclei that may influence nociception in the neonate. For example in a recent study, auditory brain stem nuclei have been shown to undergo a developmentally regulated change whereby future inhibitory GABAergic and glycinergic cells also transiently express glutamate that acts on transient NMDA and AMPA receptors until P9 (Gillespie et al., 2005). Such a process could also be occurring in brainstem nuclei associated with nociception.
In conclusion, by blocking GABA signaling in neonatal spinal cord tactile and nociceptive circuits, we have shown that spinal GABAergic activity in these circuits has profoundly differing effects in neonatal compared to older animals, switching from a net excitatory to a net inhibitory effect between P3 and P21. The early excitatory influence of GABA on spinal reflexes is entirely dependent upon a spinal brainstem loop and is proposed to arise from an altered balance of descending excitation and inhibition over the postnatal period. This data supports the suggestion that supraspinal systems play a critical role in the postnatal tuning of spinal nociceptive systems (Levinsson et al., 1999).
Abbreviations
- EMG
electromyography
- P3
postnatal day three
- P21
Postnatal day 21
- RVM
rostro ventral medulla
- vFh
Von Frey Hair
- VRP
ventral root potential
Reference List
- 1.Allain AE, Bairi A, Meyrand P, Branchereau P. Ontogenic changes of the GABAergic system in the embryonic mouse spinal cord. Brain Res. 2004;1000:134–147. doi: 10.1016/j.brainres.2003.11.071. [DOI] [PubMed] [Google Scholar]
- 2.Andrews K, Fitzgerald M. The cutaneous withdrawal reflex in human neonates: sensitization, receptive fields, and the effects of contralateral stimulation. Pain. 1994;56:95–101. doi: 10.1016/0304-3959(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 3.Andrews KA, Desai D, Dhillon HK, Wilcox DT, Fitzgerald M. Abdominal sensitivity in the first year of life: comparison of infants with and without prenatally diagnosed unilateral hydronephrosis 1. Pain. 2002;100:35–46. doi: 10.1016/s0304-3959(02)00288-9. [DOI] [PubMed] [Google Scholar]
- 4.Baccei ML, Fitzgerald M. Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. J.Neurosci. 2004;24:4749–4757. doi: 10.1523/JNEUROSCI.5211-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat.Rev.Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 6.Bice TN, Beal JA. Quantitative and neurogenic analysis of neurons with supraspinal projections in the superficial dorsal horn of the rat lumbar spinal cord. J.Comp Neurol. 1997a;388:565–574. doi: 10.1002/(sici)1096-9861(19971201)388:4<565::aid-cne5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Bice TN, Beal JA. Quantitative and neurogenic analysis of the total population and subpopulations of neurons defined by axon projection in the superficial dorsal horn of the rat lumbar spinal cord. J.Comp Neurol. 1997b;388:550–564. doi: 10.1002/(sici)1096-9861(19971201)388:4<550::aid-cne4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Chiang CY, Kwan CL, Hu JW, Sessle BJ. Effects of GABA receptor antagonist on trigeminal caudalis nociceptive neurons in normal and neonatally capsaicin-treated rats. J.Neurophysiol. 1999;82:2154–2162. doi: 10.1152/jn.1999.82.5.2154. [DOI] [PubMed] [Google Scholar]
- 9.Cronin JN, Bradbury EJ, Lidierth M. Laminar distribution of G. Pain. 2004;112:156–163. doi: 10.1016/j.pain.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Fields HL, Heinricher MM. Anatomy and physiology of a nociceptive modulatory system. Philos.Trans.R.Soc.Lond B Biol.Sci. 1985;308:361–374. doi: 10.1098/rstb.1985.0037. [DOI] [PubMed] [Google Scholar]
- 11.Fields HL, Malick A, Burstein R. Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J.Neurophysiol. 1995;74:1742–1759. doi: 10.1152/jn.1995.74.4.1742. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald M. The development of nociceptive circuits. Nat.Rev.Neurosci.. 2005 doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- 13.Fitzgerald M, Jennings E. The postnatal development of spinal sensory processing. Proc.Natl.Acad.Sci.U.S.A. 1999;96:7719–7722. doi: 10.1073/pnas.96.14.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzgerald M, Koltzenburg M. The functional development of descending inhibitory pathways in the dorsolateral funiculus of the newborn rat spinal cord. Brain Res. 1986;389:261–270. doi: 10.1016/0165-3806(86)90194-x. [DOI] [PubMed] [Google Scholar]
- 15.Gebhart GF. Descending modulation of pain. Neurosci Biobehav.Rev. 2004;27:729–737. doi: 10.1016/j.neubiorev.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert AK, Franklin KB. GABAergic modulation of descending inhibitory systems from the rostral ventromedial medulla (RVM). Dose-response analysis of nociception and neurological deficits. Pain. 2001;90:25–36. doi: 10.1016/s0304-3959(00)00383-3. [DOI] [PubMed] [Google Scholar]
- 17.Gillespie DC, Kim G, Kandler K. Inhibitory synapses in the developing auditory system are glutamatergic. Nat.Neurosci. 2005;8:332–338. doi: 10.1038/nn1397. [DOI] [PubMed] [Google Scholar]
- 18.Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat.Rev.Neurosci. 2001;2:83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa T, Marsala M, Sakabe T, Yaksh TL. Characterization of spinal amino acid release and touch-evoked allodynia produced by spinal glycine or GABA(A) receptor antagonist. Neuroscience. 2000;95:781–786. doi: 10.1016/s0306-4522(99)00461-3. [DOI] [PubMed] [Google Scholar]
- 20.Keller AF, Breton JD, Schlichter R, Poisbeau P. Production of 5alpha-reduced neurosteroids is developmentally regulated and shapes GABA(A) miniature IPSCs in lamina II of the spinal cord. J.Neurosci. 2004;24:907–915. doi: 10.1523/JNEUROSCI.4642-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keller AF, Coull JA, Chery N, Poisbeau P, De Koninck Y. Region-specific developmental specialization of GABA-glycine cosynapses in laminas I-II of the rat spinal dorsal horn. J.Neurosci. 2001;21:7871–7880. doi: 10.1523/JNEUROSCI.21-20-07871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kontinen VK, Stanfa LC, Basu A, Dickenson AH. Electrophysiologic evidence for increased endogenous gabaergic but not glycinergic inhibitory tone in the rat spinal nerve ligation model of neuropathy. Anesthesiology. 2001;94:333–339. doi: 10.1097/00000542-200102000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Levinsson A, Luo XL, Holmberg H, Schouenborg J. Developmental tuning in a spinal nociceptive system: effects of neonatal spinalization. J.Neurosci. 1999;19:10397–10403. doi: 10.1523/JNEUROSCI.19-23-10397.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma W, Behar T, Barker JL. Transient expression of GABA immunoreactivity in the developing rat spinal cord. J.Comp Neurol. 1992;325:271–290. doi: 10.1002/cne.903250210. [DOI] [PubMed] [Google Scholar]
- 25.Millan MJ. Descending control of pain. Prog.Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 26.Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J.Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pattinson D, Fitzgerald M. The neurobiology of infant pain: development of excitatory and inhibitory neurotransmission in the spinal dorsal horn 1. Reg Anesth.Pain Med. 2004;29:36–44. doi: 10.1016/j.rapm.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Qin YQ, Wang XM, Martin GF. The early development of major projections from caudal levels of the spinal cord to the brainstem and cerebellum in the gray short-tailed Brazilian opossum, Monodelphis domestica. Brain Res.Dev.Brain Res. 1993;75:75–90. doi: 10.1016/0165-3806(93)90067-k. [DOI] [PubMed] [Google Scholar]
- 29.Reeve AJ, Dickenson AH, Kerr NC. Spinal effects of bicuculline: modulation of an allodynia-like state by an A1-receptor agonist, morphine, and an NMDA-receptor antagonist. J.Neurophysiol. 1998;79:1494–1507. doi: 10.1152/jn.1998.79.3.1494. [DOI] [PubMed] [Google Scholar]
- 30.Schaffner AE, Behar T, Nadi S, Smallwood V, Barker JL. Quantitative analysis of transient GABA expression in embryonic and early postnatal rat spinal cord neurons. Brain Res.Dev.Brain Res. 1993;72:265–276. doi: 10.1016/0165-3806(93)90192-d. [DOI] [PubMed] [Google Scholar]
- 31.Stein V, Hermans-Borgmeyer I, Jentsch TJ, Hubner CA. Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. J.Comp Neurol. 2004;468:57–64. doi: 10.1002/cne.10983. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol.Sci. 2004;25:613–617. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Torsney C, Fitzgerald M. Age-dependent effects of peripheral inflammation on the electrophysiological properties of neonatal rat dorsal horn neurons. J.Neurophysiol. 2002;87:1311–1317. doi: 10.1152/jn.00462.2001. [DOI] [PubMed] [Google Scholar]
- 34.van Praag H, Frenk H. The development of stimulation-produced analgesia (SPA) in the rat 732. Brain Res.Dev.Brain Res. 1991;64:71–76. doi: 10.1016/0165-3806(91)90210-a. [DOI] [PubMed] [Google Scholar]
- 35.Waldenstrom A, Thelin J, Thimansson E, Levinsson A, Schouenborg J. Developmental learning in a pain-related system: evidence for a cross-modality mechanism. J.Neurosci. 2003;23:7719–7725. doi: 10.1523/JNEUROSCI.23-20-07719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C, Shimizu-Okabe C, Watanabe K, Okabe A, Matsuzaki H, Ogawa T, Mori N, Fukuda A, Sato K. Developmental changes in KCC1, KCC2, and NKCC1 mRNA expressions in the rat brain. Brain Res.Dev.Brain Res. 2002;139:59–66. doi: 10.1016/s0165-3806(02)00536-9. [DOI] [PubMed] [Google Scholar]
- 37.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]