Abstract
An immunization regimen was evaluated in rabbits consisting of the soluble, oligomeric form of envelope glycoprotein of HIV-1, strain R2 (gp140R2), or the surface component of the same envelope (Env), gp120R2, in the adjuvant AS02A. The gp140R2 was selected based on its unusual CD4-independent phenotype and the exceptionally broad neutralizing response in the infected donor. The gp140R2 immunogen induced antibodies that achieved 50% neutralization of 48/48, and 80% neutralization of 43/46 primary strains of diverse HIV-1 subtypes tested. The strains tested included members of standard panels of subtype B and C strains, and other diverse strains known to be neutralization resistant. The gp120R2 induced antibodies that neutralized 9/48 of the same strains. Neutralization was IgG-mediated and HIV-1-specific. These results demonstrate that induction of truly broad spectrum neutralizing antibodies is an achievable goal in HIV-1 vaccine development.
Keywords: adjuvant, envelope, glycoprotein, neutralization
A major goal of efforts to develop a vaccine against HIV type 1 (HIV-1) is the induction of broadly cross-reactive neutralizing antibodies (1). HIV-1-neutralizing antibodies are directed against epitopes on the envelope (Env) of the virus (2). Conformation-independent neutralization epitopes are located on both the surface, gp120, and transmembrane, gp41, components of the Env (3–5). There are also conformational epitopes associated with the heterotrimeric complex, some of which overlap receptor or coreceptor binding sites (6). Among most HIV-1-infected patients, the degree of neutralizing antibody cross-reactivity that develops is limited, but there are occasional patients who develop extensively cross-reactive antibody responses (7).
This study utilizes Env derived from an HIV-1-infected individual whose serum antibodies exhibit extensive neutralizing cross-reactivity against many primary strains of HIV-1 of diverse virus subtypes (8, 9). This Env, designated R2, is highly unusual as a naturally occurring HIV-1 Env that is be capable of mediating CD4-independent infection (9). In immunogenicity studies conducted in small animals and nonhuman primates, we have demonstrated that this Env induces neutralizing antibodies against multiple HIV-1 strains, and in nonhuman primates we have shown induction of protection against i.v. challenge with a heterologous strain of simian-human immunodeficiency virus (SHIV) (8, 10). The neutralizing cross-reactivity we observed in those studies was greater than that previously reported in studies of other envelope immunogens, and the prevention of infection by heterologous SHIV challenge was previously undescribed.
In our previous studies, R2 glycoprotein immunizations were conducted by using either an alphavirus expression vector, soluble, oligomeric gp140R2 in RiBi adjuvant, or both (8, 10). The gp140R2 was produced as a recombinant secreted form in which most of the molecules remain as uncleaved, multimeric species. This soluble, oligomeric gp140 possesses many of the functions of mature Env spikes on the surface of the virus (8). We and others have also studied the immunogenicity of the surface component of HIV-1 Env, gp120. In general, gp120 immunization has been reported to induce neutralizing antibodies that possess a narrow spectrum of cross-reactivity, which may be directed against linear epitopes in variable loops (11). One gp120 preparation induced immunity that protected chimpanzees from challenge with a laboratory adapted strain of HIV-1, but did not protect people from infection in a vaccine efficacy trial (12). Two studies did report that immunization with gp120 in AS02A adjuvant resulted in immunity that contributed to protection of monkeys from challenge with SHIV (13, 14). The adjuvant AS02A is an oil in water emulsion containing 3D-monophosphoryl lipid A and saponin QS21 (14). Li et al. (15) have reported on the use of that adjuvant in conjunction with gp140 and gp120 for immunization of guinea pigs. They found that the AS02A and related adjuvants produced by GlaxoSmithKline Biologicals were associated with more potent responses than RiBi, and that more potent and cross-reactive neutralizing responses were induced by gp140 than gp120. We have also examined the immunogenicity of R2 gp120 and gp140 in AS02A adjuvant in mice (unpublished data). The gp140 induced more potent and cross-reactive neutralizing antibody responses than gp120, and the cross-reactivity of the gp140-induced response was similar to that which we observed previously in monkeys. In addition, the adjuvant AS02A has been used in clinical trials as a component of an HIV vaccine containing gp120 and Nef and Tat protein antigens (18, 19).‖,** Based on these previous studies we proceeded to conduct the present study of immunization of rabbits with the R2 gp120 and gp140 in AS02A adjuvant.
Results
Development of HIV-1 Inhibitory Activity in Sera of Immunized Rabbits.
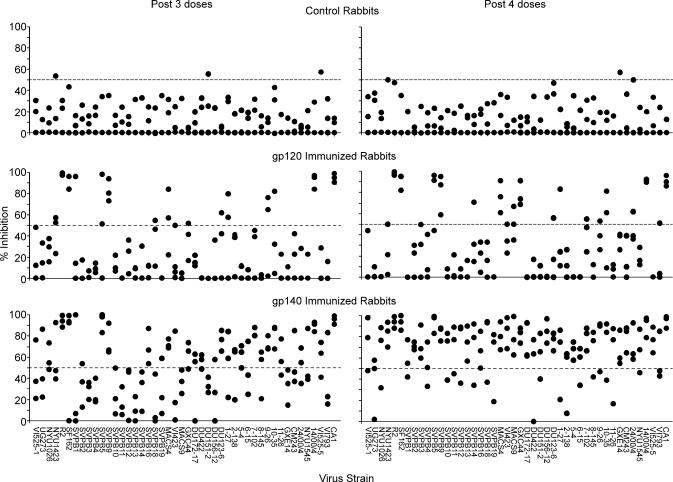
Results of neutralizing antibody testing at a 1:5 dilutions of sera obtained after the third and fourth doses are shown in Fig. 1. Results are shown for testing of four subtype A, 19 subtype B, 15 subtype C, and eight other strains of various subtypes. These strains and their neutralization sensitivity are described in detail in supporting information (SI) Text. The majority of these strains have been demonstrated to be resistant to neutralization. Included were subtype B and C strains recently established as a panel considered to be representative of the current epidemic (20). The variation among the results for control sera at the 1:5 dilution was sufficiently limited that inhibition of luminescence by ≥50% of the control average by individual control sera was observed in only 4 of 276 possible events. In contrast, after four immunizations, the sera from either two or all three of the gp120 immunized rabbits inhibited ≥50% in the case of nine strains (strains R2, SF162, SVPB5, SVPB9, MACS4, GXC44, 10–35, 14/00/4, and CA1). The frequency of neutralization was significantly greater by Chi Square test by sera from gp120 immunized than control rabbits after both the third (P = 1.9 × 10−6) and fourth (P = 1.7 × 10−8) doses. Immunization with gp140 resulted in more broadly cross reactive neutralization than immunization with gp120. After three doses either two or three of the sera from the gp140 immunized rabbits neutralized 23 strains of HIV-1, and after four doses all but one of the strains was neutralized by at least two of the sera. The differences after three (P = 2.98 × 10−6) and four (P = 4.1 × 10−24) doses were statistically significant. Antibodies that neutralized the nine strains that were sensitive to gp120-induced antibodies developed more rapidly than antibodies that neutralized strains that were only sensitive to gp140-induced antibodies, as is further illustrated in SI Fig. 4. Neutralization of strains sensitive to gp120-induced antibodies reached near maximal levels after two doses of either gp120 or gp140, whereas maximal responses against the other strains did not occur until after four doses of gp140. The rabbit sera were tested for neutralization of various SHIV and the HIV-1 strains from which they were derived, as shown in SI Fig. 5. After four doses of gp140 sera from all three rabbits neutralized HIV and corresponding SHIV strains: DH12 and DH12R(Clone 7), SF162 and SF162P3, and 89.6 and 89.6p (10, 21–24). Some of the strains were also neutralized by gp120-induced antibodies.
Fig. 1.
Comparative inhibition of HIV-1 infection by sera from gp120R2 and gp140R2 immunized rabbits, as manifested by levels of luciferase reporter gene expression. The viruses were pseudotyped with Env of the HIV-1 strains and subtypes indicated. See SI Text regarding the sources and characteristics. Viruses were incubated in the presence of 1:5 diluted test or control sera before cell culture inoculation. Mean luminescence after infection in the presence of control sera was calculated. Luminescence obtained in the presence of individual test and control sera was calculated and used to determine percent inhibition in comparison with the control mean. Percent inhibition by individual control sera is shown to illustrate the variance observed.
Endpoint Neutralization Titers in Sera from Immunized Rabbits.
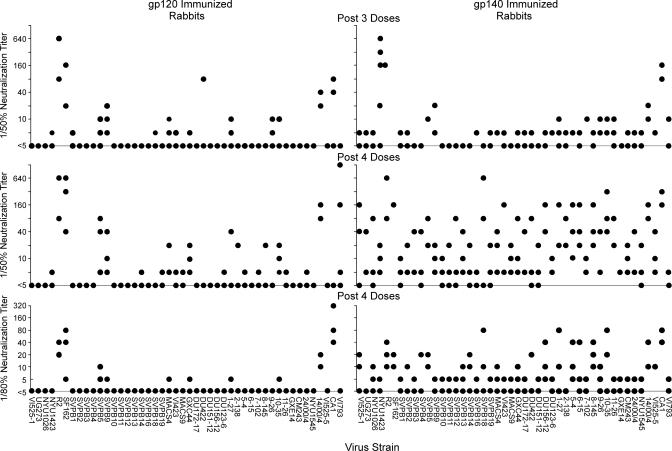
The endpoint neutralization titers obtained for the sera from the gp120 and gp140 immunized rabbits are shown in Fig. 2. At least one of the three sera from rabbits that received four doses of gp140 had a 50% neutralization endpoint titer ≥1:10 for 43/46 strains, and ≥1:20 for 39/46 strains shown in Fig. 1. Similarly, at least one of the three sera from rabbits that received four doses of gp140 had an 80% neutralization endpoint titer ≥1:5 for 44/46 strains, and ≥1:10 for 23/46 strains. One rabbit tended to have the highest titers against many of the strains (rabbit 4). The geometric mean of the titers of the three sera from the rabbits after four doses of gp140 against all of the virus strains was 1:19.1, whereas that of the serum from rabbit 4 was 1:62.
Fig. 2.
Neutralization endpoint titers of sera from gp120R2 and gp140R2 immunized rabbits against various strains of HIV-1. Results are shown for sera obtained after three or four doses of immunogen. Sera that inhibited <50% were assigned titers <1:5. Sera that inhibited ≥50–74% were assigned titers of 1:5. Sera that inhibited ≥75% were tested by serial dilution. Serial dilutions of test sera were compared with serial dilutions of pooled, concurrent control sera. The end point was considered to be the highest dilution that resulted in ≥50% or ≥80% inhibition of luminescence compared with the same dilution of the control serum pool.
HIV-1 Specificity of Neutralizing Antibody Responses.
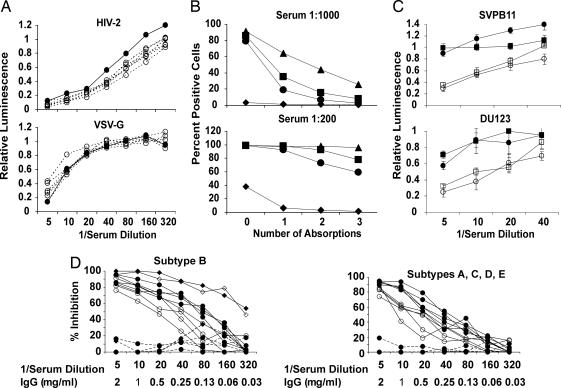
Sera were tested for neutralization of viruses pseudotyped with HIV-2 Env and VSV G protein, both produced by transfection of 293T cells, as shown in Fig. 3A. Compared with control sera, the post-fourth-dose sera from the immunized rabbits did not neutralize either HIV-2 or VSV. Similar results were observed in repeat experiments. In experiments not shown, virus pseudotyped with Nipah virus F and G proteins was prepared and tested for neutralization by the same sera. No significant differences were observed.
Fig. 3.
The HIV-1-neutralizing activity induced by gp140 is virus-specific and IgG-mediated. (A) Sera from gp120R2- and gp140R2-immunized rabbits do not neutralize HIV-2 Env or VSV G pseudotyped viruses. Rabbit sera obtained after four doses of gp120R2 or gp140R2 (both open circles and dashed lines) and pooled concurrent control sera (filled circles) were tested in triplicate at serial dilutions. (B and C) Extensive absorption of gp140-immune rabbit sera with 293T cells does not deplete primary HIV-1-neutralizing activity. (B) FACS analysis of sera from rabbits 4 (▴), 5 (■), and 6 (●) after fourth-dose of gp140 and pooled prebleed sera from the same rabbits (♦) before and after one, two, or three consecutive absorptions with 293T cells. Percent positive cells compared with negative control results obtained by using PBS plus goat serum without rabbit sera are shown. (C) Inhibition of neutralization resistant subtype B (SVPB11) and C (DU123) strains of HIV-1 by post-fourth-dose serum from rabbit 4 (open symbols), in comparison with pooled sera from the control rabbits (filled symbols) at the same time point, before (■, □) and after (●, ○) three consecutive absorptions with 293T cells. Standard deviations are shown in relation to each data point. (D) Neutralizing activity in serum is IgG mediated. IgG (filled symbols) was purified from post-sixth-dose sera from rabbit 4 and controls, and tested in comparison with the same sera (open symbols) for neutralization. Results obtained by using immune sera and IgG are shown as solid lines, whereas results obtained by using control IgG are shown as dashed lines. Neutralization of R2 virus by IgG (▴) and serum (▵) was essentially identical. The five addition subtype B strains tested (Left) were SVPB5, SVPB11, SVPB14, SVPB16, and SVPB19. The remaining strains (subtypes) were DU422 (C), DU165.12 (C), UG273 (A), NYU1545 (D), and CM243 (E) (Right).
The possibility that the virus inhibitory activity in the rabbit sera was due to antibodies directed against cell antigens was investigated. In preliminary experiments using fluorescence-activated cell sorting (FACS) we found significant binding activity against both BSC-1 and 293T cells in the sera from the gp120 and gp140 immune rabbits, although the levels were greater in the gp140-immune sera. We investigated the level of cell binding IgG in sera from rabbits immunized with regimens that induced less cross reactive neutralizing activity. The levels in the gp140R2-immune sera were similar to those in sera from rabbits immunized with HIV-1 gp140CM243 in RiBi adjuvant, which did not induce neutralizing antibodies (data not shown). They were also similar to those in sera from rabbits immunized with a regimen that involved priming with Venezuelan Equine Encephalitis virus replicon particles expressing gp160R2 followed by boosting with gp140R2 in RiBi adjuvant (8). Sera from these latter rabbits have antibodies that neutralize several strains of HIV-1, but not a number of neutralization resistant strains shown in Fig. 1 (8). These results demonstrated that the presence of 293T cell-binding Ig in sera did not correlate with the cross reactivity of the neutralizing response to gp140.
In view of those preliminary FACS data, we conducted the experiment shown in Fig. 3 B and C. Sera from after four doses of gp140R2 and pooled sera collected before immunization from the same rabbits were absorbed with 293T cells and tested for cell binding activity in FACS and for neutralizing activity. Absorptions were conducted at high serum concentrations, so that sera could be used subsequently in neutralization assays. At such high serum concentrations exhaustive removal of cell binding activity could not be accomplished. However, substantial reduction in cell binding activity was achieved by three sequential absorptions, because there was almost no activity remaining when sera was diluted 1:1000 for testing in FACS assay, and significant reduction was reflected in assays that were conducted by using 1:200 dilutions of the rabbit sera (Fig. 3B). Interestingly, preimmunization sera also possessed significant cell binding activity, detected in sera diluted 1:200, which was removed by absorption. Neutralization assay was conducted by using the thrice absorbed serum from rabbit 4, shown in Fig. 3C. The absorption procedure caused no significant reduction of neutralizing activity against either the subtype B or C virus tested, strains SVPB11 and DU123, respectively, both of which were resistant to neutralization by antibodies induced by gp120R2.
Neutralization of Primary Viruses is Mediated by IgG.
Insufficient serum volumes were available from the post-fourth-dose bleeds to permit purification and neutralization testing of IgG fractions. Therefore, sera collected after two more immunizations, as described in Experimental Procedures, were used for this purpose. Sera and IgG fractions were tested in parallel for neutralization of the viruses shown in Fig. 3D. The IgG concentrations were adjusted to be approximately equivalent to the concentration of IgG in rabbit serum (i.e., 10 mg/ml of undiluted serum). The neutralizing activity of the serum and IgG were identical against the R2 strain, whereas the IgG was equivalent or superior to serum against five additional subtype B strains, two subtype C strains and single strains of subtypes A, D, and E. All of the strains shown in Fig. 3D, except R2, were resistant to neutralization by gp120-induced antibodies. No neutralizing activity was present in the IgG from the control rabbits.
HIV-1 specificity of the neutralizing activity in the post-sixth-dose serum was evaluated, as described in SI Text and SI Figs. 6 and 7. Both the serum and IgG from rabbit 4 contained 293T cell binding activity and VSV-neutralizing activity. Serial absorption with 293T cells removed most of the cell binding activity and all of the VSV-neutralizing activity from the IgG, and significantly reduced both in the serum. Absorption did not affect neutralization of the HIV-1 strains tested. Thus, the evidence indicated that the IgG contained antibodies that specifically neutralized HIV-1 strains that were generally neutralization resistant strains.
Induction of gp140-Binding Antibodies Measured by ELISA.
The levels of IgG were measured by using gp140s of three strains of HIV-1 in ELISA. The gp140s were from the strains R2, 14/00/4, and CM243 (10). Results are shown in SI Fig. 8. The antibodies induced by gp120 immunization reacted at higher titers against gp140R2, whereas the antibodies induced by gp140 reacted less potently with gp140R2 and equivalently with all three gp140s. Antibodies reached near-maximal levels after only three doses of either gp120 or gp140.
Discussion and Conclusion
The results of this study demonstrate the induction of neutralizing antibodies in an animal model that exhibit truly broad cross reactivity against primary strains of HIV-1 of multiple subtypes. The sera of immunized rabbits developed progressively increasing virus inhibitory activity that distinguished them from sera of control rabbits. The responses induced by gp120 and gp140 were also distinct. The neutralizing activity induced by gp120 displayed restricted cross reactivity, with ≈20% of strains recognized, whereas that induced by gp140 was cross reactive among all of the HIV-1 strains tested. Evidence presented supports the interpretation that the highly cross reactive neutralization was not mediated by anti-cell antibodies, was HIV-1 specific, and was mediated by IgG. The particular HIV-1 Env, strain R2, and the adjuvant, AS02A, have each been used previously, but the combination used in this study seems to be exceptionally effective in its capacity to induce cross reactive neutralization.
The kinetics of antibody development is of interest. The neutralizing responses against the nine HIV-1 strains that were neutralized similarly after gp120 and gp140 immunization tended to reach near-maximal levels after the second dose of immunogen. The total IgG responses, as measured by ELISA, also reached maximal titers after the second or third immunization. In contrast, the responses induced by gp140 that neutralized strains that were resistant to the gp120-induced responses continued to increase after the fourth dose of immunogen. Based on the differences in timing of the responses against the two groups of viruses we interpret that the neutralizing antibodies that are most extensively cross-reactive represent a small proportion of the total anti-Env antibodies induced by gp140, and that the levels and/or affinities of these antibodies continue to increase throughout the immunization regimen.
The HIV-1-neutralizing activity induced was clearly associated with the IgG fraction of serum. The immunization regimens also induced anti-cellular IgG, but this response did not account for the HIV-1-specific neutralizing activity. Preabsorption of the test sera or IgG with 293T cells in a manner that significantly reduced cell binding activity did not reduce HIV-1-neutralizing activity. Moreover, sera and IgG that neutralized HIV-1 strains did not neutralize heterologous viruses produced in the same manner as the HIV-1 strains. These considerations engender our confidence that the broad neutralizing activity we observed is the result of a viral protein-specific immune response.
Overall, our results demonstrate that induction of an HIV-1 specific neutralizing antibody response that has a truly broad cross-reactivity is achievable. The broad responses were moderate in potency, with neutralization of some strains being more potent than others. Nonetheless, the data provide an encouraging lead for further vaccine development. Raising the titers of the neutralizing sera even higher using novel immunization methods may result in broadly effective protection against infection. The HIV-1 strains we used for testing neutralizing responses were representative of current epidemics, and can now be grouped based on our results according to sensitivity to both narrowly and broadly cross-reactive neutralization or only broadly cross-reactive neutralization (20, 25, 26). Some proportion of the antibodies that are detected by using those strains sensitive to gp120 induced antibodies may be directed against linear epitopes in variable loops in gp120, as demonstrated by others (15). The nature of the epitope(s) that are recognized by the very broadly cross-reactive neutralizing antibodies are important targets for further study. Importantly, the spectrum of cross reactivity of the neutralizing antibodies induced by gp140R2 immunization is similar to that in the serum of the R2 donor. Whereas the R2 Env has interesting features, it remained uncertain until now whether it possessed the characteristics necessary for induction of antibodies with the broad spectrum of cross-reactivity observed in the donor.
Experimental Procedures
Production of gp140 and gp120.
The gp140R2, gp14014/00/4, and gp140CM243 coding sequences were prepared by insertion of two translational termination codons just before the predicted gp41 transmembrane region and arginine to serine substitutions to disrupt protease cleavage signals to increase the yield of oligomeric Env during production (8, 10). The gp120R2 coding sequence was prepared by insertion of a translational termination codon. The genes were subcloned into the vaccinia vector, pMCO2 (8). Recombinant vaccinia viruses were generated by using standard methodology (8, 10). Glycoproteins were produced and purified from culture supernatants, prepared with serum-free media, by using lentil lectin Sepharose 4B affinity, followed by size exclusion chromatography (8, 10). The oligomeric gp140R2 has been extensively analyzed, and has been shown based on size exclusion chromatography to be ≈40% trimer and 60% dimer (8, 10). Analysis by SDS/PAGE and Coomassie blue staining revealed electrophoretic migration typical of glycoprotein, and purity of 98%. Endotoxin concentration was 0.2–1.1 units/μg.
Immunization Regimen.
The rabbits were divided into three groups of three. The rabbits in Group 1 each received 30 μg gp120R2 per dose and in Group 2 each received 30 μg gp140R2 per dose each in 500 μl of AS02A adjuvant. Rabbits in the control group each received 500 μl of adjuvant only. Immunizations were administered as two 250-μl aliquots, each into a hind leg. Sera were collected by bleed from the ear vein before the first vaccination and 10 days after each vaccination. Adult New Zealand White rabbits were inoculated at 0, 3, 6, and 28 weeks. The rabbits in the gp140-immunized and control groups received two additional doses of immunogen, at 3 and 7 months after the fourth dose. Each of these doses consisted of the same materials as the previous doses, except that the last dose used the oil-emulsion adjuvant, AS03A. Post-sixth-dose sera were used for IgG purification.
Enzyme Linked Immunosorbent Assays (ELISA).
An antigen capture ELISA was used to determine serum Ig responses, as described (8, 10).
Cloning of Envelope Genes.
Viruses isolated from patients in Xinjiang Province, China, were passaged once in PBMC from HIV-1-negative donors. Genomic DNA was extracted from the cells, and env gene cloning was accomplished by using PCR, as described (9, 27). Sequence encoding the HIV-2 strain 7312A gp160 was cloned by using PCR from cell-free virus stock supplied by the AIDS Research and Reference Reagent Program [provided by B. Hahn and F. Gao (28)], by using methods described above for HIV-1. The pCG-VSV-G plasmid encoding the VSV-G glycoprotein was obtained from P. Cannon (Childrens' Hospital, Los Angeles, CA). Plasmids encoding the Nipah virus F and G glycoproteins were provided by D. Khetawat (unpublished).
Virus Strains Used In Neutralization Assays.
Env gene (env) encoding plasmids used for preparation of pseudotyped viruses in this study are described in SI Text and SI Tables 1 and 2. All are from primary viruses. The plasmids beginning with the letters SVPB or DU were obtained from D. Montefiori (Duke University Medical Center, Durham, NC), and encode Env of subtypes B and C considered to be representative of current epidemic strains (25, 29). Most of the subtype B strains and three of the subtype C strains in those panels have been shown to be highly resistant to neutralization (20). Neutralization of strains from Xinjiang Province, China, by sera from subtype C-infected Chinese donors is described in SI Table 2. The remaining strains were cloned in our laboratory; many are resistant to neutralization as indicated in SI Text and are described in refs. 7–10, 16, 27, and 30.
Neutralization Assays.
We used a pseudotyped reporter virus neutralization assay, as described (7–10, 17). Briefly, neutralization assays were carried out in triplicate in HOS-CD4+CCR5+ cells. To determine neutralization, luminescence obtained in the presence of three control sera diluted 1:5 was averaged and compared with the mean for each individual serum. Test sera that inhibited ≥50–75% were assigned titers of 1:5. Test sera that inhibited ≥75% were tested in serial dilutions in comparison with serial dilutions of concurrent control serum. This control serum was prepared by pooling serum from each of the control rabbits at the same sampling date. We have recently participated in a multicenter validation study comparing this assay with that described by Mascola et al. (25). The assays produce very similar results.
Absorption of Rabbit Sera with 293T Cells and FACS Analysis.
Cells obtained by trypsinization from a confluent 75 cm2 flask of 293T cells were resuspended in 400 μl of sera at final serum dilutions of 1:2.5. The suspensions were incubated at 4°C for 3 h with light rocking, cells were sedimented by centrifugation, and the absorption was repeated with new cells a second and third time. The third absorption was continued overnight. After each absorption 5 μl of each serum was removed, diluted 1:200 and 1:1,000 in PBS with 3% goat serum, and 100 μl of each was used to suspend 1.2 × 105 293T cells for FACS analysis. After 30 min on ice the cells were washed twice with PBS with 3% goat serum, and reacted with Biotin-SP-conjugated Anti Rabbit IgG (H+L) (Jackson ImmunoResearch, West Grove, PA), and then Streptavidin-PE (Sigma, St. Louis, MO). The cells were washed and resuspended in 2% paraformaldehyde in PBS. Cells were analyzed on Beckman Coulter EPICS XL-MCL flow cytometer.
Purification of Serum IgG.
Sera were clarified by centrifugation at 10,000 rpm for 15 min and then diluted 1:10 with PBS (pH 7.2). IgG was purified from diluted sera by using the HiTrap protein G HP column (GE Healthcare Biosciences, Piscataway, NJ), according to the manufacturer's instructions. After purification, IgG was concentrated by centrifugation at 1,500 × g for 25 min by using the centriprep centrifugal filter unit with Ultracel YM-30 membrane (Millipore, Billerica, MA). Concentration of purified IgG was determined by using the NanoDrop ND-1000 Spectrophotometer.
Supplementary Material
Acknowledgments
We thank Cloe Angello for assistance with env gene cloning, sequencing, and neutralization testing regarding the samples from Xinjiang. This work was supported by funding from GlaxoSmithKline Biologicals and by National Institutes of Health Grants AI37438 and AI064070.
Abbreviations
- Env
envelope
- SHIV
simian-human immunodeficiency virus.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Goepfert, P., Horton, H., McElrath, J., Tomaras, G., Montefiori, D., Ferrari, G., Chiu, J., Shea, T., Deers, M., Self, S., et al., AIDS Vaccine 2003 Conference, September 18–21, 2003, Hilton, NY, Abstr. 51.
Leroux-Roels, I., Clement, F., Leroux-Roels, G., Koutsoukos, M., Van Belle, P., Van Uffel, K., Vandepapelière, P., Hanon, E., Voss, G., Pedneault, L., AIDS Vaccine 2005 Conference, September 6–9, 2005, Montreal, QC, Canada, Abstr. 308.
This article contains supporting information online at www.pnas.org/cgi/content/full/0608635104/DC1.
References
- 1.Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, Nabel GJ, Sodroski J, Wilson IA, Wyatt RT. Nat Immunol. 2004;5:233–236. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- 2.Burton DR. Nat Rev Immunol. 2002;2:706–713. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- 3.Scanlan CN, Pantophlet R, Wormald MR, Ollmann Saphire E, Stanfield R, Wilson IA, Katinger H, Dwek RA, Rudd PM, Burton DR. J Virol. 2002;76:7306–7321. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zwick MB, Labrijn AF, Wang M, Spenlehauer C, Saphire EO, Binley JM, Moore JP, Stiegler G, Katinger H, Burton DR, Parren PW. J Virol. 2001;75:10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, Hendrickson WA, Sodroski JG. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 6.Labrijn AF, Poignard P, Raja A, Zwick MB, Delgado K, Franti M, Binley J, Vivona V, Grundner C, Huang CC, et al. J Virol. 2003;77:10557–10565. doi: 10.1128/JVI.77.19.10557-10565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang PF, Chen X, Fu DW, Margolick JB, Quinnan GV., Jr J Virol. 1999;73:5225–5230. doi: 10.1128/jvi.73.6.5225-5230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong M, Zhang PF, Grieder F, Lee J, Krishnamurthy G, VanCott T, Broder C, Polonis VR, Yu XF, Shao Y, et al. J Virol. 2003;77:3119–3130. doi: 10.1128/JVI.77.5.3119-3130.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang PF, Bouma P, Park EJ, Margolick JB, Robinson JE, Zolla-Pazner S, Flora MN, Quinnan GV., Jr J Virol. 2002;76:644–655. doi: 10.1128/JVI.76.2.644-655.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quinnan GV, Jr, Yu XF, Lewis MG, Zhang PF, Sutter G, Silvera P, Dong M, Choudhary A, Sarkis PT, Bouma P, et al. J Virol. 2005;79:3358–3369. doi: 10.1128/JVI.79.6.3358-3369.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pantophlet R, Burton DR. Annu Rev Immunol. 2006;24:739–769. doi: 10.1146/annurev.immunol.24.021605.090557. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert PB, Peterson ML, Follmann D, Hudgens MG, Francis DP, Gurwith M, Heyward WL, Jobes DV, Popovic V, Self SG, et al. J Infect Dis. 2005;191:666–677. doi: 10.1086/428405. [DOI] [PubMed] [Google Scholar]
- 13.Mooij P, van der Kolk M, Bogers WM, ten Haaft PJ, Van Der Meide P, Almond N, Stott J, Deschamps M, Labbe D, Momin P, et al. AIDS. 1998;12:F15–F22. doi: 10.1097/00002030-199805000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Voss G, Manson K, Montefiori D, Watkins DI, Heeney J, Wyand M, Cohen J, Bruck C. J Virol. 2003;77:1049–1058. doi: 10.1128/JVI.77.2.1049-1058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Svehla K, Mathy NL, Voss G, Mascola JR, Wyatt R. J Virol. 2006;80:1414–1426. doi: 10.1128/JVI.80.3.1414-1426.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quinnan GV, Jr, Zhang PF, Fu DW, Dong M, Margolick JB. AIDS Res Hum Retroviruses. 1998;14:939–949. doi: 10.1089/aid.1998.14.939. [DOI] [PubMed] [Google Scholar]
- 17.Zhang MY, Xiao X, Sidorov IA, Choudhry V, Cham F, Zhang PF, Bouma P, Zwick M, Choudhary A, Montefiori DC, et al. J Virol. 2004;78:9233–9242. doi: 10.1128/JVI.78.17.9233-9242.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pichyangkul S, Gettayacamin M, Miller RS, Lyon JA, Angov E, Tongtawe P, Ruble DL, Heppner DG, Jr, Kester KE, Ballou WR, et al. Vaccine. 2004;22:3831–3840. doi: 10.1016/j.vaccine.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Robert Putnak J, Coller BA, Voss G, Vaughn DW, Clements D, Peters I, Bignami G, Houng HS, Chen RC, Barvir DA, et al. Vaccine. 2005;23:4442–4452. doi: 10.1016/j.vaccine.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, et al. J Virol. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y, Pauza CD, Lu X, Montefiori DC, Miller CJ. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:6–18. doi: 10.1097/00042560-199809010-00002. [DOI] [PubMed] [Google Scholar]
- 22.Tan RC, Harouse JM, Gettie A, Cheng-Mayer C. J Med Primatol. 1999;28:164–168. doi: 10.1111/j.1600-0684.1999.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 23.Igarashi T, Endo Y, Englund G, Sadjadpour R, Matano T, Buckler C, Buckler-White A, Plishka R, Theodore T, Shibata R, Martin M. Proc Natl Acad Sci USA. 1999;96:14049–14054. doi: 10.1073/pnas.96.24.14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibata R, Maldarelli F, Siemon C, Matano T, Parta M, Miller G, Fredrickson T, Martin MA. J Infect Dis. 1997;176:362–373. doi: 10.1086/514053. [DOI] [PubMed] [Google Scholar]
- 25.Mascola JR, D'Souza P, Gilbert P, Hahn BH, Haigwood NL, Morris L, Petropoulos CJ, Polonis VR, Sarzotti M, Montefiori DC. J Virol. 2005;79:10103–10107. doi: 10.1128/JVI.79.16.10103-10107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louder MK, Sambor A, Chertova E, Hunte T, Barrett S, Ojong F, Sanders-Buell E, Zolla-Pazner S, McCutchan FE, Roser JD, et al. Virology. 2005;339:226–238. doi: 10.1016/j.virol.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Cham F, Zhang PF, Heyndrickx L, Bouma P, Zhong P, Katinger H, Robinson J, van der Groen G, Quinnan GV., Jr Virology. 2006;347:36–51. doi: 10.1016/j.virol.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Gao F, Yue L, Robertson DL, Hill SC, Hui H, Biggar RJ, Neequaye AE, Whelan TM, Ho DD, Shaw GM, et al. J Virol. 1994;68:7433–7447. doi: 10.1128/jvi.68.11.7433-7447.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M, Salazar-Gonzalez JF, Derdeyn CA, Morris L, Williamson C, Robinson JE, Decker JM, Li Y, Salazar MG, Polonis VR, et al. J Virol. 2006;80:11776–11790. doi: 10.1128/JVI.01730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinnan GV, Jr, Zhang PF, Fu DW, Dong M, Alter HJ. AIDS Res Hum Retroviruses. 1999;15:561–570. doi: 10.1089/088922299311088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.