Abstract
Using solid phase-assisted synthesis and purification, a 49 member library of analogs of the mammary tumor chemopreventive retinoid N-(4-hydroxyphenyl)retinamide (4-HPR) has been prepared. After prescreening for growth inhibitory activity in human mammary tumor cells (MCF-7) in culture, most of those analogs which showed activity (12 of them) were assayed for apoptosis-inducing activity in the MCF-7 cells. At least 3 of the analogs (13, 24, and 28) showed activity approaching that of 4-HPR.
The synthetic retinoid N-(4-hydroxyphenyl)retinamide (4-HPR; 1) was developed a number of years ago and has shown promise as a breast cancer chemopreventive agent in animals.1 This analog is a simple amide derivative of the naturally-occurring parent retinoid, all-trans retinoic acid (atRA; 2), but (1) is less toxic and substantially less teratogenic than 2.2 In addition to breast tumor cells, 4-HPR inhibits the growth of and induces apoptosis in a number of other tumor cell lines including prostate,3 bladder,4 head and neck squamous carcinoma,5 and neuroblastoma cell lines.6 This breadth of activity has led to its exploration in human clinical trials as a breast cancer chemopreventive agent7 and in animal studies as an antitumor agent.8
The mechanism through which 4-HPR acts remains unclear despite its structural resemblance to 2. Most retinoids are thought to act by binding to the nuclear retinoic acid receptors (RARs), which bind atRA. The receptors function as ligand-dependent transcription factors9 and some researchers have reported that 4-HPR can activate RARs in transactivation assays.10 We and others find, however, that 4-HPR has low affinity for the retinoid receptors.11–13 4-HPR has also been shown to induce apoptosis in both atRA -sensitive and -resistant cells, pointing to a mode of action that is independent of the RAR.
Despite continued interest in 4-HPR as a chemopreventive agent, relatively few 4-HPR analogs have been reported. The majority of these showed good activity in early studies of their ability to reverse squamous metaplasia in vitamin A-deficient hamster tracheae.15 A number of these analogs are being reinvestigated in an effort to find more potent retinamides given the relatively modest activity 1 has shown in a breast cancer prevention trial.7 In particular, it has been suggested that N-(2-carboxyphenyl)retinamide (2-CPR; 3) has more pronounced activity than 1 in some head and neck squamous carcinoma cell and lung cancer cell lines5 and N-(3-hydroxyphenyl)retinamide (3-HPR; 4) is more potent in bladder cancer cell lines.4 This suggests the possibility that in breast cancer cells, a similar improvement in activity might be obtainable.
Because of the limited range of 4-HPR related structures that have been prepared and uncertainties about the molecular target(s) for this molecule, preparation of a library of 1-like compounds appeared to be warranted. Due to the sensitivity of retinoids, synthesis of a series of arylamides of RA would be facilitated by a method of activating retinoic acid that generates little acid and could be adapted to solid phase methods to simplify isolation. To this end, it was reasoned that the mild, acid-free procedure of Villeneuve and Chan would suffice.16 In this method, acid chlorides are generated by treatment of a dry THF solution of the carboxylic acid with one equivalent of triphenylphosphine, 0.5 equivalents of hexachloroacetone and 6 equivalents of pyridine at −78°C. It is thought that triphenylphosphine and hexachloroacetone react to form the chlorinating reagent 5 which converts the carboxylic acid (RA here) to the acid chloride in the absence of any HCl generation, which would otherwise isomerize RA. Polymer supported phosphines and CCl4 can also be used to form acid chlorides at an elevated temperature in high yield.16 Adaptation of this latter strategy for use with hexachloroacetone appeared feasible as did removal of unreacted starting materials using solid phase trapping reagents. Parlow and coworkers have used the reactive tetrafluorophthalic anhydride (TFPA) to trap unreacted aniline as a polyamine resin removable phthalic acid monoamide.17 Others have used similar anion exchange resins to remove chloride ion and unreacted carboxylic acid from library mixtures.18–20 Thus, it was hoped that library purification would be accomplished by filtering off the phosphine resin, treating with TFPA, stirring with amine anion exchange resin, and evaporation of volatiles.
Initial efforts to optimize reaction and purification conditions were conducted on a 0.1 mmol scale for the synthesis of 4-HPR. The conditions investigated included reagent stoichiometry, order of reagent addition, temperature, time, and choice of anion exchange resin with the following conditions found to be optimal: 1) 1.0 RA/3.0 Ph3P-resin/THF, then 0.5 HCA; 0°C, 1 hr; 2) 1.5 ArNH2/6.2 pyridine; 0°C→rt, 1–2 hr; 3) 1.25 TFPA, then H2O, then 600 mg (2.8 meq) of Amberlite® A-21 resin. A goal of this method was to avoid any other purification of product retinamides prior to activity screening. Unfortunately, in all instances, whether solution phase16 or solid phase methods were employed, a small quantity (0.5–1%) of a non-polar retinoid impurity was formed. After numerous NMR spectroscopic and mass spectrometric experiments, the structure of this impurity was determined to be the retinoate ester 6. Extensive experiments established that formation of this undesired ester requires the generation of retinoyl chloride and the presence of activated hexachloroacetone and presumably resulted from reaction of the enolate of tetrachloroacetone dianion (see 5) with retinoyl chloride. Since the presence of 6 could confound activity screens and no method was found to suppress its formation, all retinamides were subjected to a rapid silica gel chromatography (EtOAc/hexanes) to give the final products.
Using these synthetic and purification methods summarized in the previous pharagraph, and commercially available anilines or their simple derivatives, a total of 49 retinamides (43 previously unreported) were synthesized. Table 1 shows the majority of the prepared retinamides. Table 2 shows the small number of naphthylamine amides that were synthesized and the N-methyl analog of 4-HPR (47) was also prepared. All structures were assigned based on the analysis of mass spectra, 1H and 13C NMR spectra, and COSY and/or 1H-13C correlation spectra. Product purity was assessed by reverse-phase HPLC (82 or 86% methanol/H2O) and found to be 91–99% (mean = 95±2%) for all analogs except 12, 25, and 45 (85–89%) and 38, which decomposed prior to analysis. Yields varied greatly (20–94%) and were generally the poorest for those analogs with electron withdrawing ring substituents, especially if the substituents sterically crowded the amine moiety. In particular, synthesis of 24 using 2-amino-5-nitrophenol also resulted in formation of substantial amounts of the retinoate ester which was removed chromatographically, while use of 2-amino-3-nitrophenol provided no amide and led exclusively to formation of the ester, which was not biologically evaluated.
Table 1.
Anilinamide analogs prepared
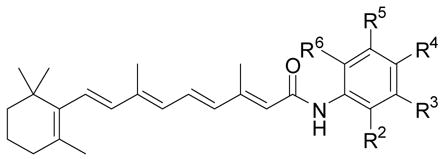
| Compound | R2 | R3 | R4 | R5 | R6 | % yield | Growtha Inhibition |
|---|---|---|---|---|---|---|---|
| 1b | H | H | OH | H | H | 94 | +++ |
| 7 | CH3 | H | OH | H | H | 71 | − |
| 8 | CH3 | H | OH | CH3 | H | 85 | − |
| 9 | OH | H | CH3 | H | H | 66 | ++ |
| 10 | OH | H | H | CH3 | H | 80 | − |
| 11 | OH | H | H | C(CH3)3 | H | 75 | − |
| 12 | OH | H | H | H | CH3 | 83 | − |
| 13 | H | OH | CH3 | H | H | 80 | +++ |
| 14 | CH3 | OH | H | H | H | 71 | − |
| 15 | H | OH | OCH3 | H | H | 66 | − |
| 16 | Cl | H | OH | H | H | 75 | − |
| 17 | H | Cl | OH | H | H | 70 | ++ |
| 18 | OH | H | H | Cl | H | 59 | ++ |
| 19 | H | Cl | OH | Cl | H | 75 | − |
| 20 | H | Br | OH | Br | H | 75 | − |
| 21 | OH | Cl | CH3 | Cl | H | 31 | − |
| 22 | NO2 | H | OH | H | H | 70 | − |
| 23 | H | NO2 | OH | H | H | 74 | − |
| 24 | OH | H | NO2 | H | H | 28 | +++ |
| 25 | OH | H | H | NO2 | H | 20 | + |
| 26 | OH | CO2CH3 | H | H | H | 64 | − |
| 27 | OH | CO2H | H | H | H | 77 | − |
| 28b | OH | H | CO2CH3 | H | H | 86 | + |
| 29 | OH | H | CO2H | H | H | quant. | − |
| 30c | OH | H | H | H | CO2CH3 | 59 | − |
| 31d | OH | H | H | H | CO2H | 36 | − |
| 32c | H | OH | CO2CH3 | H | H | 61 | − |
| 33 | H | OH | CO2H | H | H | 61 | − |
| 34 | H | CO2CH3 | OH | H | H | 81 | + |
| 35 | H | CO2H | OH | H | H | 76 | − |
| 36 | OH | H | H | CO2CH3 | H | quant. | − |
| 37 | OH | H | SO2CH2CH3 | H | H | 75 | − |
| 38 | CH2OH | H | H | H | H | 57 | NDf |
| 39 | H | CH2OH | H | H | H | 80 | + |
| 40 | H | H | NH2 | H | H | 76 | ++ |
| 41b | CO2CH3 | H | H | H | H | 64 | ND |
| 3b | CO2H | H | H | H | H | 74 | + |
| 42b,g | H | H | OCH2CH3 | H | H | 63 | − |
activity vs. 4-HPR standard, all at 10−5M:+++ = ≥ 100% the activity of standard, ++ = >50% the activity of standard, + = <50% the activity of standard, − = activity equivalent to vehicle;
previously reported analog;
at 5.9 x 10−6M;
at 3.9 x 10−6M;
at 6.6 x 10−6M;
not determined;
at 7.4 x 10−6M.
Table 2.
Naphthylamine amide analogs
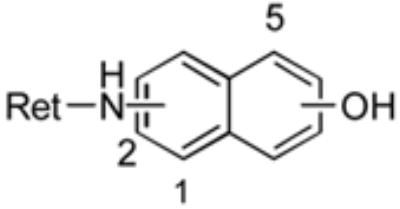
| position
|
||||
|---|---|---|---|---|
| Compound | Ret NH | OH | % yield | growtha inhibition |
| 43 | 1 | 2 | 37 | - |
| 44 | 1 | 4 | 26 | - |
| 45 | 1 | 5 | 43 | - |
| 46 | 2 | 3 | 88 | - |
as in Table 1.
All of the analogs except 38 and 41 were initially evaluated for their ability to inhibit growth of the human MCF-7 breast cancer cells in culture using a fluorescein diacetate cell viability assay.21 The number of live cells was assessed 48 hrs after the administration of a single dose of the analogs shown in Tables 1 and 2, analog 47 or 4-HPR (McNeil Pharmaceuticals), all at a final concentration of 10−5M, or vehicle (0.2% ethanol). The resulting effect on MCF-7 cell growth is summarized in Tables 1 and 2. Analog 47, which was prepared in 68% yield, showed modest activity (+ at best; see Table 1 legend) in this assay.
Since 4-HPR is known to induce apoptosis in these MCF-7 breast cancer cells, compounds showing cell growth inhibitory activity (Tables 1 and 2: + to +++, or those analogs with >25% of 4-HPR’s activity) were evaluated for apoptotic activity after 48 hr of treatment using a terminal dUTP nick-end labeling (TUNEL) assay.22 Cells were treated with vehicle or a single dose of standard 4-HPR, or one of 11 of the library analogs and synthetic 4-methoxyphenyl retinamide (4-MPR; 50) which is a known active 4-HPR metabolite, all at 10−5M. The % of TUNEL-labeled cells was assessed 48 hrs later. As shown in Figure 1, analog 24 showed activity comparable to 4-HPR while 13 and the previously known analog 28 showed lesser but significant apoptotic activity. To further evaluate the activity of 24 relatlve to 4-HPR, an 8 day cell viability assay was conduced at several doses of compound in MCF-7 cells, Figure 2 shows that the activity of 24 is similar to that of 1, with no live cells remaining after 8 days of exposure of cells to either compound at 10−5M. While no obvious pattern has yet emerged to build a hydroxyphenylretinamide structure-activity relationship, it is encouraging that other analogs of 4-HPR (notably 24, 13, and perhaps 28) can be prepared which retain significant mammary tumor cell growth inhibitory and apoptotic activity. It is notable that only several of the analogs prepared have any significant activity in inhibiting cell growth, demonstrating that the effect of these analogs must be specific, and not simply due to a non-specific cytotoxicity of this general class of agents.
Figure 1.

Structure of retinoids and chlorinating agent.
Figure 2.
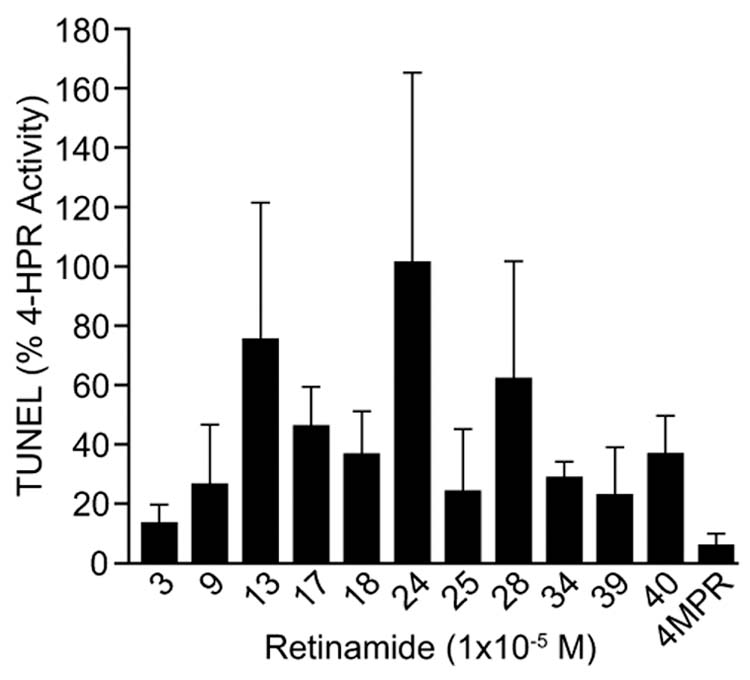
TUNEL staining in MCF-7 cells 48 h after exposure to 10−5M of compound. The results shown represent the mean values (+SEM) from three independent cell culture experiments (intra-assay variability); samples with the highest proportion of dead cells always showed the greatest assay variability (see supplementary materials).
In an effort to enhance activity and explore greater structural diversity, the solid phase assisted amide synthesis was used to prepare another series of retinamides (Table 3). The principal strategy used to select these targets was the batchwise Topliss tree approach, 24 which is a manual Hansch approach designed to rapidly improve the activity of lead compounds that contain monosubstituted aromatic rings (compound 48). In this method, different tree “branches” are followed based on the analogs they are being compared to. The branch choice depends on whether the analog has lower, equivalent, or more activity than the preceding compound in the branching scheme. Recommended substitutions are based on a systematic variation of the physico-chemical properties of the substituent and thus the molecule. After establishment of structure by the methods described above, reverse-phase HPLC (85% methanol/H2O) showed product purity of 91–99% (mean = 97± 3%) except for analog 53 (86%). Growth inhibition assessment in MCF-7 cells as above gave the results shown in Table 3. Given that analog 52 only showed half the activity of the known 4-HPR metabolite 50 (details not shown), the Topliss method leads us back to 1 as the most effective analog (see supplementary material for abbreviated tree and path followed based on activity results), suggesting this specific approach may not rapidly produce new, more active mammary tumor inhibitory 4-HPR analogs.
Table 3.
Topliss tree developed anilinamides

Using the methods developed here we have been able to smoothly and rapidly prepare research quantities (20–50 mg) of a 49-member library of retinamides using mainly aminophenols. While some of these compounds showed mammary tumor cell growth inhibitory activity in vitro, only one of them (nitrophenol 24) appears to be comparable to 4-HPR in its ability to induce cell death. As of yet we have no information on the toxicity to activity ratio of 24 vs. 1 in vivo. Nonetheless, these synthetic and rapid screening methods should be readily adaptable by us to prepare a much expanded library in efforts to find interesting mammary tumor targeted 4-HPR analogs.
Supplementary Material
Figure 3.
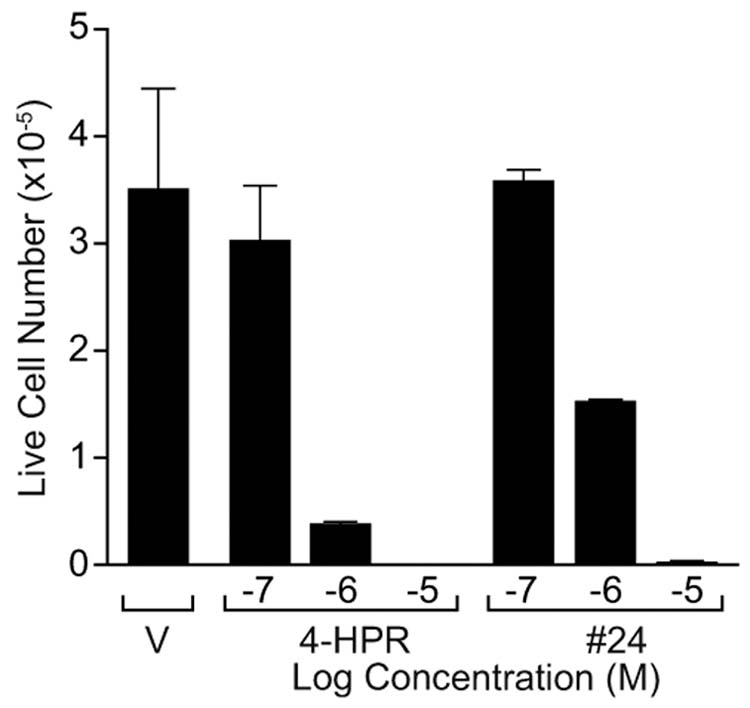
The number of live MCF-7 cells remaining after 8 days of treatment with either compound 24 or 1 is similar over a range of concentrations (10−7 to 10−5 M).
Acknowledgments
Financial support in the form of a grant (CA 49837) from the National Cancer Institute is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moon RC, Thompson HJ, Becci PJ, Grubb CJ, Gander RJ, Newton DL, Smith JM, Phillips SL, Henderson WR, Mullen LT, Brown CC, Sporn MB. Cancer Res. 1979;39:1339. [PubMed] [Google Scholar]
- 2.Kenel MF, Krayer JH, Merz EA, Pritchard JF. Teratog Carcinog Mutag. 1988;8:1. doi: 10.1002/tcm.1770080102. [DOI] [PubMed] [Google Scholar]
- 3.Igawa J, Tanabe T, Chodak GW, Rukstails DB. Prostate. 1994;24:299. doi: 10.1002/pros.2990240605. [DOI] [PubMed] [Google Scholar]
- 4.Clifford JL, Sabichi AL, Zou C, Yang X, Steele VE, Kelloff GJ, Lotan R, Lippman SM. Cancer Epidemiol Biomarkers Prevention. 2001;10:391. [PubMed] [Google Scholar]
- 5.Sun SY, Yue P, Kelloff GJ, Steele VE, Lippman SM, Hong WK, Lotan R. Cancer Epidemiol Biomarkers Prevention. 2001;10:595. [PubMed] [Google Scholar]
- 6.DiVinci A, Geido E, Infusini E, Giaretti W. Int J Cancer. 1994;59:422. doi: 10.1002/ijc.2910590322. [DOI] [PubMed] [Google Scholar]
- 7.Veronesi U, DePalo G, Marubini E, Costa A, Formelli F, Mariani L, Decensi A, Camerini T, Rosselli Del Turco M, Gaetana Di Mauro M, Grazia Muraca M, Del Vecchio M, Pinto C, D’Aiuto G, Boni C, Campa T, Magni A, Miceli R, Perloff M, Malone WF, Sporn MB. J Natl Cancer Inst. 1999;91:1847. doi: 10.1093/jnci/91.21.1847. [DOI] [PubMed] [Google Scholar]
- 8.Abou-Issa H, Curley RW, Jr, Panigot MJ, Tanagho SN, Sidhu BS, Alshafie GA. Anticancer Res. 1997;17:3335. [PubMed] [Google Scholar]
- 9.Chambon PA. FASEB J. 1996;10:940. [PubMed] [Google Scholar]
- 10.Fanjul AN, Delia D, Pierotti MA, Rideout D, Ya JQ, Pfahl M. J Biol Chem. 1996;271:22441. doi: 10.1074/jbc.271.37.22441. [DOI] [PubMed] [Google Scholar]
- 11.Abou-Issa HM, Alshafie GA, Curley RW, Jr, Wong MF, Clagett-Dame M, Repa JJ, Sikri V. Anticancer Res. 1999;19:999. [PubMed] [Google Scholar]
- 12.Sani BP, Shealy YF, Hill DL. Carcinogenesis. 1995;16:2531. doi: 10.1093/carcin/16.10.2531. [DOI] [PubMed] [Google Scholar]
- 13.Sheikh MS, Shao ZM, Li XS, Ordonez T, Conley BA, Wu S, Dawson MI, Han ZX, Chao WR, Quick T, Niles RM, Fontana JA. Carcinogenesis. 1995;16:2477. doi: 10.1093/carcin/16.10.2477. [DOI] [PubMed] [Google Scholar]
- 14.Delia D, Aiello A, Lombardi L, Pelicci PG, Grignani F, Formelli F, Menard S, Costa A, Veronesi U, Pierotti MA. Cancer Res. 1993;53:6036. [PubMed] [Google Scholar]
- 15.Newton DL, Henderson WR, Sporn MB. Cancer Res. 1980;40:3413. [PubMed] [Google Scholar]
- 16.Villeneuve GB, Chan TH. Tet Lett. 1997;38:6489. [Google Scholar]
- 17.Parlow JJ, Naing W, South MS, Flynn DL. Tetrahedron Lett. 1997;38:7959. [Google Scholar]
- 18.Flynn DL, Crich JZ, Devraj RV, Hockerman SL, Parlow JJ, South MS, Woodard SJ. J Am Chem Soc. 1997;119:4874. [Google Scholar]
- 19.Gayo LM, Suto MJ. Tetrahedron Lett. 1997;38:513. [Google Scholar]
- 20.Nilson UJ. J Chromatogr. 2000;885:305. doi: 10.1016/s0021-9673(99)01095-x. [DOI] [PubMed] [Google Scholar]
- 21.Jones PA, Baker VA, Irwin A, Earl LK. Toxicol In Vitro. 1997;11:769. doi: 10.1016/s0887-2333(97)00032-5. [DOI] [PubMed] [Google Scholar]
- 22.Engeland MV, Nieland LJW, Ramackers FCS, Schutte B, Reutelingsperger CPM. Cytometry. 1998;31:1. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 23.Narvaez CJ, Welsh J. J Biol Chem. 2001;276:9101. doi: 10.1074/jbc.M006876200. [DOI] [PubMed] [Google Scholar]
- 24.Topliss JG. JMedChem. 1972;15:1006. doi: 10.1021/jm00280a002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


