Abstract
The objective of this research was to investigate stress-signaling patterns in response to two dimensional (2-D) and three dimensional (3-D) calcium phosphate (CP) materials using human embryonic palatal mesenchyme cells (HEPM, CRL-1486, ATCC, Manassas, VA), an osteoblast precursor cell line. Control discs and scaffolds were fabricated from hydroxyapatite (HA) and β tri-calcium phosphate (TCP) ceramics. Phospho-specific antibody cell-based ELISA (PACE) technique was utilized on members of the mitogen-activated protein kinase (MAPK) cascade including; the extracellular signal-regulated kinases (ERK1/2), p38, c-Jun N-terminal kinase (JNK), and the anti-apoptosis mediator protein kinase B (AKT). Quantification of these signals was evaluated during the early attachment phase of osteoblast precursor cells. In this study, it was observed that 3-D CP scaffolds significantly activated the stress mediators p38 and JNK but not ERK1/2. This signal trend was matched with an up-regulation in AKT, suggesting the ability of cells to manage high stress signals in response to 3-D CP architecture and that 3-D CP scaffolds are necessary for studies simulating a natural trabecular bone organization. The absence of these signals in 2-D CP surfaces indicated the importance of local architecture conditions on cell stress response. It was concluded from this study that osteoblast precursor cells cultured in 3-D CP scaffolds experience greater stress-signaling patterns when compared to 2-D CP surfaces.
1. Introduction
Regeneration of bone tissue assisted by ceramic scaffold grafts has shown excellent capability in recent therapeutic techniques [1–11]. Additionally, the development of artificial scaffolds for bone reconstruction offers several distinct advantages for cell studies. In particular, the use of calcium phosphate (CP) ceramics for scaffold development exhibits a variety of useful properties; strong mechanical characteristics closely matched to bone, negligible immunoreactivity and the availability of local calcium and phosphorous for surrounding cells. At the protein and cellular level, CP materials are directly bound to the collagen matrix forming a strong mechanical interlock between bone and implant [12]. In addition to material composition, research has also established the importance of specific surface area and shape of ceramic substrates for cell differentiation into the osteogenic lineage [13]. These scaffolds offer a reproducible platform for the identification and isolation of specific biological signals as well as their clinical use in regenerative orthopedics [14–16]. Their structures provide a stable support network for cell adhesion, migration, and proliferation as osteoconductive platforms [17]. Their strong resemblance of trabecular bone also permits investigation of cell signaling patterns in an environment analogous to natural tissue. As bone cells identify and react to the environment by means of a mechano-transduction system, it is of interest to distinguish changes in intracellular communication with respect to 3-dimensional (3-D) curvatures.
Intracellular pathways such as mitogen-activated protein kinase (MAPK) signaling drive many cell reactions to external stimuli including cytokines, G-protein coupled receptors, growth factors, and integrin-based cell adhesion [18,19]. This signaling system has also been identified in committing human mesenchyme stem cells to osteogenic or adipocyte lineages [20]. Within this family are three sub-pathways; the extracellular signal-regulated kinases (ERK1/2), c-Jun N-terminal kinases, (JNK), and p38 kinases [21], the latter two are collectively termed the stress-activated protein kinases (SAPKs). Cell survival signaling also involves a diverse mediator termed AKT/PKB, protein kinase B, originally named from an AKR/J thymoma mouse cell line [22]. A general outline of the various inputs and outputs from the MAPK and AKT members are shown in Figure 1.
Figure 1.

Representation of parallel MAPK cascade consisting of ERK1/2, P38 and JNK members and the cell survival signal AKT/PKB. Stimulations, mediators and generalized responses are illustrated in flow diagram form.
The objective of this study was to determine pre-osteoblast response to scaffold architecture during the cell adhesion-phase through the mitogen-activated protein kinase (MAPK) cascade and anti-apoptosis mediator AKT signaling. Testing was performed on hydroxyapatite (HA) and tri-calcium phosphate (TCP) scaffolds using human embryonic palatal mesenchyme cells (HEPM, CRL-1486, ATCC, Manassas, VA), an osteoblast precursor cell line. Specifically, phospho-activation of extracellular signal-regulated kinases (ERK1/2), p38, c-Jun N-terminal kinase (JNK), and AKT on 3-D and 2-dimensional (2-D) HA and TCP surfaces were measured.
2. Materials and methods
2.1. Sample preparation
Disc shaped specimens (controls) were produced from micro particulate HA and TCP (TAL Materials, Ann Arbor, MI) using a hydraulic press and stainless steel mold with diameter of 6 mm. The materials were sintered in air at 1230 °C for 3 hours. HA and TCP scaffolds were prepared using a foam-dipping technique. Polyurethane sponges (EN Murray, Denver, CO) were coated with HA or TCP slurry. Binders were used with the slurry to improve sintering and to stabilize the scaffold structure and included 3% high molecular weight polyvinyl alcohol, 1 % v/v carboxymethylcellulose, 1% v/v ammonium polyacrylate dispersant, and 3% v/v N,N-dimethylformamide drying agent. Coated sponges were vacuum-dried overnight before sintered. Scaffolds were twice coated with CP slurry and re-sintered to 1230 °C. Purity of the composition was validated using X-ray diffraction analysis. Final scaffold dimensions were ø 5 mm and length of 5 mm, discs were prepared with ø 6 mm and height of 1.5 mm. All samples were placed into non-binding 96 well plates (Corning, Acton, MA) and ethylene oxide gas sterilized before analysis.
2.2. Sample characterization
Calcium phosphate disc-controls and scaffolds were imaged by scanning electron microscopy. Histomorphometric measures of the scaffold were performed on cross-sectional slides. Scaffolds were embedded in one-component photo-curing resin (Exakt 7200 VLC, Oklahoma City, OK), and thin sectioned using a precision microsaw (Buehler, Lake Bluff, IL). Sections were progressively polished on 600, 800, 1000, 1200 grit paper and adhered to glass slides using a methyl methacrylate resin (Surgipath Medical Ind., Richmond, IL). Sections were imaged at 100X magnification with a digital camera (QImaging, Burnaby, Canada) on a Nikon TE300 microscope (Nikon, Melville, NY). An analysis of the scaffold was performed using bone histomorphometry software, Osteo v.7 (Bioquant, Nashville, TN). A total of four cross-sections were prepared from each scaffold and parameters were defined using traditional histomorphometry guidelines for trabecular bone structures as follows:
Percentage of scaffold volume [SV / TV = scaffold volume / total volume × 100%]
Ratio of scaffold surface perimeter to total volume [SS / TV = surface length / total volume]
Ratio of scaffold surface perimeter to scaffold volume [SS / SV = surface length / scaffold volume]
Trabecular thickness [Tb.th. = (4 / 1.199) × (SS / SV)]
Trabecular number [Tb.n. = ((4 / π) × (SV / TV))−0.5/(Tb.th.)]
Trabecular separation [Tb.sp. = ((4 / π) × (TV / BV) − 1) × (Tb.th.)]
The formulae shown above were derived with respect to trabecular bone tissue that includes both rod- and plate-like geometry. Consequently, these formulae may not be ideally suited to the isotropic character of these scaffolds. The incorporation of these properties herein permits a comparison with known values with the human lumbar spine, a predominantly rod-like trabecular structure [23].
2.3. Cell culture and adhesion
Human embryonic palatal mesenchyme cells (HEPM, CRL-1486, ATCC, Manassas, VA), an osteoblast precursor cell line, were cultured in Dulbecco’s modified eagle medium (DMEM, Invitrogen, Carlsbad, CA) supplemented with 7% fetal bovine serum (FBS, Invitrogen) and 1% antibiotic/antimycotic (PSA, 100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin B, MP Biomedicals, Solon, OH) at 37 °C with 5% CO2. Cells were cultured in TC-75 flasks with biweekly media changes and passaged at confluence using TrypLE, a trypsin-like enzyme (Invitrogen). HEPM cells were counted using a coulter counter (Beckman Coulter Z2, Fullerton, CA), and 20,000 cells were seeded onto tissue culture treated plates, CP discs or scaffolds.
Attachment behavior of HEPM cells to CP surfaces was evaluated in a time course study with cells plated and attached for 1, 2, 4 or 6 hours. The cell media was collected along with two washes of PBS to count unattached cells at the selected time points. The percent cell attachment was calculated as (%Att = (cells seeded − unattached cells) / cells seeded × 100 %). This methodology was confirmed by detaching the remaining cells from the material to ensure count equivalence. Percent attachment values were used to determine a suitable time point for remaining studies such that an equivalent cell number could be assumed between HA and TCP materials.
2.4. Material phospho-specific antibody cell-based ELISA (PACE)
HEPM cells were attached to CP surfaces for 6 hours followed by washing twice with ice-cold PBS. Cells were fixed with 4% Carson’s Millonig formaldehyde in PBS for 30 minutes at room temperature then endogenous peroxidase activity was quenched using 0.3% H2O2 in PBS -0.1% Triton X-100 (PBS-T) for 30 minutes. Cells were washed three times with PBS-T, followed by blocking with 10% fetal calf serum (FCS, Invitrogen) in PBS-T for 1 hour. Primary rabbit antibodies against phospho-p44/42 (ERK1/2, Thr202/Tyr204), phospho-p38 (Thr180/Tyr182), phospho-SAPK/JNK (Thr183/Tyr185), and phospho-AKT (Ser 473, Cell Signaling Technology, Danvers, MA) were added in 5% FCS-PBS-T and incubated overnight at 4 °C with 20 rpm rocking. Dilutions of primary antibodies were set at 1:1000 except for pp38, at 1:500. Antibody dilutions were established from titer-controls with a minimum absorbance signal to noise ratio of 8 using non-stimulated cells on tissue culture treated plastic. Signal inhibitors for ERK1/2, p38, JNK, and AKT; PD98059 (50μM), SB203580 (50μM), SP600125 (10μM), Wortmannin (2μM), respectively, were added 30 minutes before test completion. After primary antibody incubation, cells were washed three times with PBS-T for 5 minutes and incubated with secondary anti-rabbit IgG, HRP-linked antibody (horseradish peroxidase) with a 1:1000 dilution in 5% FCS-PBS-T for 1 hour at room temperature with rocking. The cells were then washed three times with PBS-T, and twice with PBS for five minutes. One-Step Ultra TMB substrate (Pierce Biotechnology, Rockford, IL) was added to the wells and color developed for 30 minutes at room temperature in the dark. The oxidation reaction was stopped and color changed and stabilized using an equal volume of 2M H2SO4. The colored products were transferred to new 96 well plates and absorbance measured at 450 nm with reference at 620 nm on a Beckman Coulter AD34C plate reader (Fullerton, CA).
2.5 Statistical analysis
Statistical calculations were performed with SigmaStat software (Systat, Point Richmond, CA). Validity of signal inhibition with each material was compared by paired t-test. Significance between groups was analyzed by one-way ANOVA with Tukey pairwise multiple comparisons. Significance levels were set at p < 0.05.
3. Results
3.1. Material characterization
Calcium phosphate control surfaces imaged by SEM are shown in Fig. 2a–b revealing the micro based surface structure. While surface roughness was normalized by bulk fabrication, differences in the chemical composition are reflected in the surface character of HA and TCP materials. Sintered HA surfaces were observed to consist of smaller crystals with tight junctions while TCP surfaces were observed to exhibit larger crystal size with loosely bound junctions and micro-scale pores. These characteristics were also preserved in 3-D where strut-surfaces reflect the morphology differences of HA and TCP materials.
Figure 2.
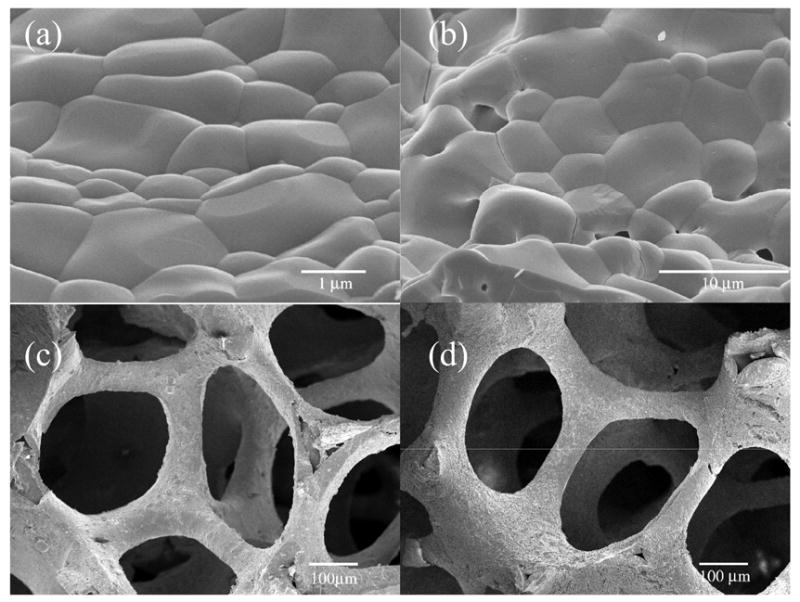
SEM photographs of (a) HA and (b) TCP surface micro structure with crystal size closely matched while scale bars differ demonstrating the smaller tightly bound crystals of HA and larger crystals of TCP with small micro-pores. Bulk scaffold architecture of (c) HA and (d) TCP materials show little differences in structure.
Bulk scaffold architecture, defined by the original polyurethane template, is nearly identical for the two CP materials studied (Fig. 2c–d). Small variations were seen in the percent shrinkage between HA and TCP after heat treatments, however, the differences were not significant as measured by histomorphometry. Average scaffold values were obtained for the following parameters shown in Table 1 with comparisons to vertebral bone structure. The architecture of the scaffold consisted of a rod-like arrangement with morphological similarity to human vertebral, trabecular bone [23].
Table 1.
Scaffold histomorphometry parameters compared to human vertebral bone of the second lumbar by Hildebrand et al* [41].
| Parameter | Abbreviation | Scaffold Value | Vertebral Value* | Units |
|---|---|---|---|---|
| Scaffold Volume/Total Volume | SV/TV | 32.51 ± 6.65 | 8.3 ± 2.4 | % |
| Scaffold Surface/Total Volume | SS/TV | 3.19 ± 0.27 | 1.87 ± 0.49 | mm−1 |
| Scaffold Surface/Scaffold Volume | SS/SV | 10.26 ± 2.32 | 23.73 ± 3.41 | mm−1 |
| Trabecular Number | Tb.N | 1.93 ± 0.25 | 0.93 ± 0.25 | mm−1 |
| Trabecular Separation | Tb.Sp | 1.00 ± 0.11 | 1.07 ± 0.33 | mm |
| Trabecular Thickness | Tb.Th | 0.34 ± 0.08 | 0.09 ± 0.01 | mm |
3.2. Cell Adhesion
Cells attached to HA and TCP at a rapid rate with approximately 80% binding within the first hour. As variations in attachment could affect the ELISA measurements, a time point was selected where differences were no longer significant between the two materials, but before changes in cell number by proliferation could influence the data. The cell attachment rate on HA and TCP surfaces, as shown in Fig. 3, indicated no significant differences between the two surfaces at each time point. By 4 to 6 hours of cell culture a stable plateau is reached where time differences are no longer significant (93% binding). The 6 hour time point was subsequently used for all ELISA testing. Scaffold attachment rates equaled or exceeded that of surfaces and also normalized to 95% by 6 hours with 2-D data representative of 3-D attachment behavior.
Figure 3.

HEPM cell attachment behavior on (white bars) HA and (black bars) TCP surfaces over 6 hours. More than 80% of cells attached to CP surfaces within 1 hour, and attachment stabilized to approximately 93% by 6 hours. Letters denote significant differences by Tukey pairwise multiple comparisons p<0.05, n=4/time point.
3.3. Activation of ERK1/2 on CP’s
The effects of surface material and architecture on the phosphorylation of ERK1/2 was investigated during the early attachment phase of HEPM cells to HA and TCP substrates as shown in Fig. 4. Phosphorylation of ERK1/2 was already present in basal cell culture on tissue culture plastic and was significantly reduced on 2-D TCP and 3-D HA materials. A significant increase in signal was also seen in 3-D TCP scaffolds over 2-D TCP and 3-D HA. Signal was inhibited using PD98059 (a binder of MEK1 inactive forms), limiting activation by upstream signalers. Inhibition of MEK1 significantly reduced pERK1/2 activity in all groups (p<.005).
Figure 4.

Phospho-immunoreactivity of ERK1/2 using HEPM cells on tissue culture plastic, calcium phosphate surfaces and scaffolds. White bars represent signal inhibition for 30 minutes with PD98059, black bars represent actual signal. Selective inhibition was significantly different from signal in each material by paired t-test, p<0.005. Letters denote significant differences by Tukey pairwise multiple comparisons p<0.05, n=6 from 2 independent experiments. ERK, extracellular-signal-regulated kinase.
3.4. Activation of p38 on CP’s
The activation of the stress signal mediator p38 was evaluated in response to material surface and architecture as shown in Fig. 5. A moderate difference was identified between tissue culture plastic and 2-D HA and TCP surfaces with both 3-D groups significantly greater than all other materials. The highest activation of p38 occurred in 3-D HA and was significantly greater than 3-D TCP. Selective inhibition was performed with SB203580, and significantly reduced all material responses (p<.05) except for tissue culture plastic indicating that p38 was not activated in response to this surface.
Figure 5.

Phospho-immunoreactivity of p38 using HEPM cells on tissue culture plastic, calcium phosphate surfaces and scaffolds. White bars represent signal inhibition for 30 minutes with SB203580, black bars represent actual signal. Selective inhibition was significantly different from signal in each material, except tissue culture plastic by paired t-test, p<0.05. Letters denote significant differences by Tukey pairwise multiple comparisons p<0.05, n=6 from 2 independent experiments. p38 from cytokine suppressive anti-inflammatory drug binding protein (CSBP).
3.5. Activation of JNK on CP’s
Phospho-activation of the JNK environmental stress mediator on CP materials is shown in Fig. 6. On 2-D surfaces, a difference was recognized between HA and elevated values on tissue culture plastic, however, this difference may not be a true signal change due to the high background of the inhibited signal on tissue culture plastic. Similar to the trend seen with p38, 3-D JNK signals were elevated with significant differences over all other materials but with highest signal strength in TCP rather than HA scaffolds. Selective signal inhibition was performed using SP600125, resulting in significant reductions (p<0.05) in all materials.
Figure 6.

Phospho-immunoreactivity of JNK using HEPM cells on tissue culture plastic, calcium phosphate surfaces and scaffolds. White bars represent signal inhibition for 30 minutes with SP600125, black bars represent actual signal. Selective inhibition was significantly different from signal in each material by paired t-test, p<0.05. Letters denote significant differences by Tukey pairwise multiple comparisons p<0.05, n=6 from 2 independent experiments. SAPK/JNK, stress-activated protein kinase/c-Jun-amino-terminal kinase.
3.6. Activation of AKT on CP’s
The cell survival signal AKT/PKB was evaluated in response to CP materials with results illustrated in Fig. 7. Signal strength was elevated in 2-D HA over tissue culture plastic and no difference was identified between 2-D and 3-D HA although the trend suggests higher activation in 3-D. TCP scaffolds elicited the greatest signal response significantly greater than all other groups. The pAKT pathway was wortmannin sensitive and selective inhibition of phosphatidylinositol 3-kinase significantly reduced (p<0.05) the values in all materials. Similarities were observed between the activation of AKT and JNK with regard to material dependant 3-D response and between AKT and p38 in 2-D response.
Figure 7.
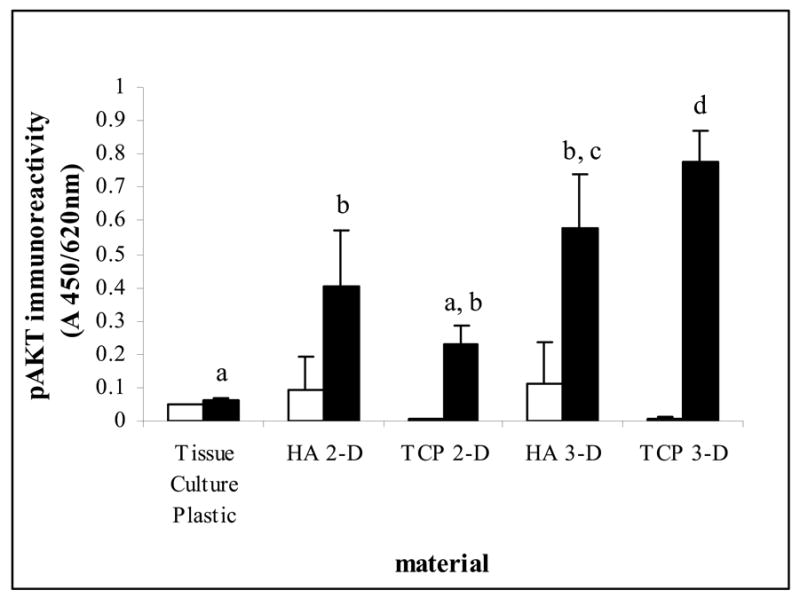
Phospho-immunoreactivity of AKT using HEPM cells on tissue culture plastic, calcium phosphate surfaces and scaffolds. White bars represent signal inhibition for 30 minutes with Wortmannin, black bars represent actual signal. Selective inhibition was significantly different from signal in each material by paired t-test, p<0.05. Letters denote significant differences by Tukey pairwise multiple comparisons p<0.05, n=6 from 2 independent experiments. AKT/PKB, AKR/J thymoma mouse cell line derived/protein kinase B.
4. Discussion
Cells translate environmental signals into physiological responses through intracellular pathways linking genetic regulation with changes in phenotype. The MAPK cascade plays a substantial role in environmental signaling. Herein, the importance of material substrate and 3-D architecture on the regulation of stress and apoptosis mediators were examined by PACE in-vitro testing of a mesenchyme cell line.
4.1 Scaffold architecture
Hydroxyapatite scaffolds mimicking the natural inorganic composition of bone were fabricated with careful attention to the local organization of trabecular bone. An interconnected rod-like structure was prepared closely matching the histological arrangement of the lumbar spine. It was postulated that cells respond differently to structures found in-vivo compared to traditional methods of maintaining cells on tissue culture treated plastic. The rationale for developing this structure as a model platform relates to its use for tissue-engineering. It is known that CP materials provide a local supply of calcium and phosphorous minerals [24] in addition to a large surface to volume area for cell adhesion. During scaffold manufacture, emphasis was placed on matching the local architecture of trabecular bone particularly the characteristics of the struts; trabecular number, separation and thickness [25]. The unique curvatures of these surfaces provide substantial micro and nano-based surface characteristics that are not represented by 2-D surfaces. It is known that during bone formation, osteoblasts deposit new matrix in circular curvatures, namely the lamellar organization of haversian osteons and trabeculae. The presence of these curvatures may influence adhesion-based integrin distribution in-turn altering sub-cellular signaling organization and biological response [26].
4.2 PACE testing for cell-biomaterial constructs
For statistical significance in biomaterial testing, a large number of samples are required which eliminates many techniques for signal-transduction analysis. Phospho-specific antibody cell-based ELISA (PACE) has previously been derived for investigations where large sample numbers prohibit comparative western blotting or time intensive kinase assays [27]. Additionally, traditional western blotting is hindered by fast signal saturation and non-linearity causing difficulty with samples where divergent response is anticipated. The enzymatic reaction in PACE testing was of a linear character permitting quantifiable comparisons when diverse signal ranges were tested. Application of this technique to biomaterials provided reproducible signals with low background noise. Selective inhibition at or upstream of the signal of interest allowed for significant reductions on every material tested with the exception of basal levels seen on tissue culture plastic with p38. The use of PACE was well-suited for high throughput biomaterial applications.
4.3 Intracellular stress pathways in a physiological simulated environment
Phospho-activation of p38, JNK but not ERK1/2 were increased when cultured on 3-D CP’s compared to 2-D surfaces, particularly tissue culture treated plastic. This leads us to postulate that traditional cell culture may be drastically underestimating the stress signal responses found in large tissue constructs. While the cross-talk between up-stream members in the MAPK cascade prohibit identification in this study of which or if a single pathway mediates the 3-D stress response, it appears that both p38 and JNK are principal players while ERK1/2’s role remains unclear. With the elevated levels of ERK1/2 observed on all materials and its known involvement with integrin-mediated cell attachment and migration [28], the data obtained from this study reinforces the idea that ERK1/2 is upregulated during the attachment phase [29–31] and may not demonstrate material or architecture dependence except in relation to adhesion sites. Additional evidence has suggested that with respect to osteogenic cell types, ERK1/2 may have a more significant role during differentiation and mineralization [32].
4.4 Anti-apoptosis signaling
Both ERK1/2 and AKT have been implicated in activation of cell survival pathways. As observed in this study, few differences were found between tested materials with respect to ERK1/2 activation. CP materials have not been shown to induce apoptosis [33], and it was not anticipated to see substantial changes in ERK1/2 or AKT. Thus, it was of interest to note the similarities in activation between the stress-activated protein kinases and AKT. Previous cell signaling studies involving the upstream MEKKs in the MAPK family demonstrated that apoptosis regulation could not be predicted exclusively by the activation of ERK or JNK pathways [34]. The high signal and correlation of AKT with p38 and JNK in this case suggest its involvement as an early anti-apoptosis regulator when cells are confronted with high stress signaling in response to curvatures not normally experienced in-vitro. As both HA and TCP materials do not induce cell death, the AKT signal could be considered a management pathway of elevated SAPK levels observed in 3-D cell culture. Future scaffolds designed to match the exact curvature of trabecular bone and additional time-based signaling analysis will help to isolate the mechanisms of bone-material interaction for regenerative orthopedics.
5. Conclusion
In summary, significant activation of stress mediators p38 and JNK was observed on 3-D CP scaffolds, whereas these signals were absent on 2-D CP surfaces. Additionally, a significant up-regulation of AKT, an anti-apoptosis signal was also observed on 3-D CP surfaces. It was concluded from this study that osteoblast precursor cells cultured in 3-D CP scaffolds experience greater stress-signaling patterns when compared to 2-D CP surfaces.
Acknowledgments
This study was supported by the National Institutes of Health (RO1AR46581).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arinzeh TL, Tran T, Mcalary J, Daculsi G. A comparative study of biphasic calcium phosphate ceramics for human mesenchymal stem-cell-induced bone formation. Biomaterials. 2005;26:3631–38. doi: 10.1016/j.biomaterials.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 2.Boyde A, Corsi A, Quarto R, Cancedda R, Bianco P. Osteoconduction in large macroporous hydroxyapatite ceramic implants: evidence for a complimentary integration and disintegration mechanism. Bone. 1999;24:579–89. doi: 10.1016/s8756-3282(99)00083-6. [DOI] [PubMed] [Google Scholar]
- 3.Dong J, Kojima H, Uemura T, Kikuchi M, Tateishi T, Tanaka J. In vivo evaluation of a novel porous hydroxyapatite to sustain osteogenesis of transplated bone marrow-derived osteoblast cells. J Biomed Mater Res. 2001;57:208–16. doi: 10.1002/1097-4636(200111)57:2<208::aid-jbm1160>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Dong J, Uemura T, Shiraasaki Y, Tateishi T. Promotion of bone formation using highly pure porous beta-TCP combined with bone marrow-derived osteoprogenitor cells. Biomaterials. 2002;23:4493–502. doi: 10.1016/s0142-9612(02)00193-x. [DOI] [PubMed] [Google Scholar]
- 5.Flautre B, Anselme K, Delecourt C, Lu J, Hardouin P, Descamps M. Histological aspects in bone regeneration of an association with porous hydroxyapatite and bone marrow cells. J Mater Sci Mater Med. 1999;10:811–4. doi: 10.1023/a:1008923625599. [DOI] [PubMed] [Google Scholar]
- 6.Gauthier O, Muller R, von Stechow D, Lamy B, Weiss P, Bouler JM, Aguado E, Daculsi G. In vivo bone regeneration with injectible calcium phosphate biomaterial: a three-dimensional mico-computed tomographic, biomechanical and SEM study. Biomaterials. 2005;26:5444–53. doi: 10.1016/j.biomaterials.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 7.Kon E, Muraglia A, Corsi A, Bianco P, Marcacci M, Martin I, Boyde A, Ruspantini I, Chistolini P, Rocca M, Giardino R, Cancedda R, Quarto R. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res. 1999;49:328–37. doi: 10.1002/(sici)1097-4636(20000305)49:3<328::aid-jbm5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 8.Livingston T, Ducheyne P, Garino J. In vivo evaluation of a bioactive scaffold for bone tissue engineering. J Biomed Mater Res. 2002;62:1–13. doi: 10.1002/jbm.10157. [DOI] [PubMed] [Google Scholar]
- 9.Lu JX, Gallur A, Flautre B, Anselme K, Descamps M, Thierry B, Hadouin P. Comparative study of tissue reactions to calcium phosphate ceramics among cancellous, cortical, and medullar bone sites in rabbits. J Biomed Mater Res. 1998;42:357–67. doi: 10.1002/(sici)1097-4636(19981205)42:3<357::aid-jbm3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Mastrogiacoma M, Scaglione S, Martinetti R, Dolcini L, Beltrame F, Cancedda R, Quarto R. Role of scaffold internal structure on in vivo bone formation in macroporous calcium phosphate bioceramics. Biomaterials. 2006;27:3230–37. doi: 10.1016/j.biomaterials.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 11.Uemura T, Dong J, Wang Y, Kojima H, Saito T, Iejima D, Kikuchi M, Tanaka J, Tateishi T. Transplantation of cultured bone cells using combinations of scaffolds and culture techniques. Biomaterials. 2003;24:2277–86. doi: 10.1016/s0142-9612(03)00039-5. [DOI] [PubMed] [Google Scholar]
- 12.Richard M, Aguada E, Cottrel M, Daculsi G. Ultrastructural and electron diffraction of the bone-ceramic interfacial zone in coral and biphasic CaP implants. Calcif Tissue Int. 1998;62:437–42. doi: 10.1007/s002239900456. [DOI] [PubMed] [Google Scholar]
- 13.Habibovic P, Yuan H, van der Valk C, Meijer G, van Blitterswijk C, de Groot K. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials. 2005;26:3565–75. doi: 10.1016/j.biomaterials.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 14.Koshino T, Murase T, Takagi T, Saito T. New bone formation around porous hydroxyapatite wedge implanted in opening wedge high tibial osteotomy in patients with osteoarthritis. Biomaterials. 2001;22:1579–1582. doi: 10.1016/s0142-9612(00)00318-5. [DOI] [PubMed] [Google Scholar]
- 15.Mangano C, Bartolucci EG, Mazzocco C. A new porous hydroxyapatite for promotion of bone regeneration in maxillary sinus augmentation: Clinical and histologic study in humans. Int J Oral Maxillofac Implants. 2003;18:23–30. [PubMed] [Google Scholar]
- 16.Zyman Z, Ivanov I, Glushko V, Dedukh N, Malyshkina S. Inorganic phase composition of remineralisation in porous CaP ceramics. Biomaterials. 1998;19:1269–1273. doi: 10.1016/s0142-9612(98)00024-6. [DOI] [PubMed] [Google Scholar]
- 17.Chang BS, Lee CK, Hong KS, Youn HJ, Ryu HS, Chung SS, Park KW. Osteoconduction at porous hydroxyapatite with various pore configurations. Biomaterials. 2000;21:1291–98. doi: 10.1016/s0142-9612(00)00030-2. [DOI] [PubMed] [Google Scholar]
- 18.Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin in Cell Biol. 1997;9:180–86. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 19.Morino N, Mimura T, Hamasaki K, Tobe K, Ueki K, Kikuchi K, Takehara K, Kadowaki T, Yazaki Y, Nojima Y. Matrix/integrin interaction activates the mitogen-activated protein kinase, p44ERK-1 and p42ERK-2. J Biol Chem. 1995;270:269–73. doi: 10.1074/jbc.270.1.269. [DOI] [PubMed] [Google Scholar]
- 20.Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275:9645–52. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- 21.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–80. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 22.Staal SP, Hartley JW, Rowe WP. Isolation of transforming murine leukemia viruses from mice with a high incidence of spontaneous lymphoma. Proc Natl Acad Sci. 1977;74(7):3065–67. doi: 10.1073/pnas.74.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hildebrand T, Laib A, Muller R, Dequekker J, Ruegsegger P. Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res. 1999;14:1167–1174. doi: 10.1359/jbmr.1999.14.7.1167. [DOI] [PubMed] [Google Scholar]
- 24.Gross KA, Berndt CC, Goldschlag DD, Iacono VJ. In vitro changes of hydroxyapatite coatings. Inter J Oral Maxillofac Implants. 1997;12:589–597. [PubMed] [Google Scholar]
- 25.Feldkamp LA, Goldstein SA, Parfitt AM, Jesion G, Kleerekoper M. The direct examination of three-dimensional bone architecture in-vitro by computed tomography. J Bone Miner Res. 1989;4:3–11. doi: 10.1002/jbmr.5650040103. [DOI] [PubMed] [Google Scholar]
- 26.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–28. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 27.Versteeg HH, Nijhuis E, van der Brink GR, Evertzen M, Pynaert GN, van Deventer SJ, Coffer PJ, Peppelenbosch MP. A new phosphospecific cell-based ELISA for p42/44 mitogen-activated protein kinase (MAPK), p38 MAPK, protein kinase B and cAMP-response-element-binding protein. J Biochem. 2000;350:717–22. [PMC free article] [PubMed] [Google Scholar]
- 28.Klemke RL, Cai S, Giannini SL. Regulation of cell motility by mitogen-activated protein kinase. J C Biol. 1997;137:481–93. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen P, Kinch MS, Lin TH, Burridge K, Juliano RL. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994;6:26602–05. [PubMed] [Google Scholar]
- 30.Schlaepfer DD, Hanks SK, Hunter T, van der Greer P. Integrin mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 31.Zhu X, Assoian RK. Integrin-dependant activation of MAP kinase: a link to shape-dependant cell proliferation. Mol Biol Cell. 1995;6:273–82. doi: 10.1091/mbc.6.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simmons CA, Matlis S, Thornton AJ, Chen S, Wang CY, Mooney D. Cyclic strain enhances matrix mineralization by adult human mesenchymal stem cells via the extracellular signal-regulated kinase (ERK1/2) signaling pathway. J Biomech. 2003;36:1087–96. doi: 10.1016/s0021-9290(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 33.Sepulveda P, Binner JG, Rogero SO, Higa OZ, Bressiani JC. Production of porous hydroxyapatite by the gel-casting of foams and cytotoxic evaluation. J Biomed Mater Res. 2000;50:27–34. doi: 10.1002/(sici)1097-4636(200004)50:1<27::aid-jbm5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 34.Bonvin C, Guillon A, van Bemmelen MX, Gerwins P, Johnson GL, Widmann C. Role of the amino-terminal domains of MEKKs in the activation of NFkB and MAPK pathways and in the regulation of cell proliferation and apoptosis. Cell Signalling. 2002;14:123–31. doi: 10.1016/s0898-6568(01)00219-4. [DOI] [PubMed] [Google Scholar]


