Abstract
Exogenous neuropeptide Y (NPY) reduces experimental anxiety in a wide range of animal models. The generation of an NPY-transgenic rat has provided a unique model to examine the role of endogenous NPY in control of stress and anxiety-related behaviors using paradigms previously used by pharmacological studies. Locomotor activity and baseline behavior on the elevated plus maze were normal in transgenic subjects. Two robust phenotypic traits were observed. (i) Transgenic subjects showed a markedly attenuated sensitivity to behavioral consequences of stress, in that they were insensitive to the normal anxiogenic-like effect of restraint stress on the elevated plus maze and displayed absent fear suppression of behavior in a punished drinking test. (ii) A selective impairment of spatial memory acquisition was found in the Morris water maze. Control experiments suggest these traits to be independent. These phenotypic traits were accompanied by an overexpression of prepro-NPY mRNA and NPY peptide and decreased NPY-Y1 binding within the hippocampus, a brain structure implicated both in memory processing and stress responses. Data obtained using this unique model support and extend a previously postulated anti-stress action of NPY and provide novel evidence for a role of NPY in learning and memory.
Keywords: anxiety, amygdala
Neuropeptide Y (NPY) (1) is highly expressed in the mammalian brain. Its pharmacological administration into the central nervous system reduces experimental anxiety in a wide range of animal models (2–5), but its involvement in memory function is less clear. NPY receptors cloned to date all belong to the superfamily of G protein-coupled receptors but differ in their ligand affinity profiles. The NPY-Y1 receptor (6–8) requires the intact NPY sequence for recognition and activation, and seems to be the subtype mediating anti-anxiety actions of NPY (3–5, 9, 10). The Y2 receptor subtype is also activated by C-terminal fragments of NPY, such as NPY13–36 (11). The highest number of NPY-binding sites, predominantly of the Y2 subtype, is found within the hippocampus. Activation of Y2 receptors within this structure has been shown to suppress hippocampal glutamatergic transmission through presynaptic mechanisms (12, 13), but the behavioral consequences of Y2 signaling in this area are unclear.
In agreement with anti-stress effects observed following central administration of NPY, a role for endogenous NPY in control of stress and anxiety-related behaviors is suggested by several findings. Acute physical restraint, which promotes experimental anxiety (14), suppresses NPY mRNA and peptide levels within the amygdala and cortex. In contrast, repeated exposure to the same stressor once daily for 10 days leads to a complete behavioral and endocrine habituation, accompanied by an up-regulation of amygdala NPY expression (15). We have therefore proposed that an up-regulation of NPY expression may contribute to the behavioral adaptation to stress. This extends a hypothesis that NPY may act to “buffer” behavioral effects of stress-promoting signals (16).
The postulated hypothesis predicts that an up-regulated expression of NPY might render a subject less sensitive to anxiety-promoting effects of stress. Transgenic NPY overexpression would offer an attractive system to test such a hypothesis but has until now only been available in mice (17). Because of species differences, a direct comparison with previously described effects of exogenous NPY on anxiety-related behaviors has not been possible. The generation of an NPY-transgenic rat has offered an attractive model for these studies. This model is unconventional in several respects, which may make it advantageous. First, this choice of species allows for a direct comparison with functional effects previously observed with exogenous NPY administration. Second, the construct used in generating these animals, a 14.5-kb fragment of the rat NPY genomic sequence, contains the normal intronic sequence elements and is flanked by an approximate 5-kb 5′ sequence thought to contain the major regulatory elements normally controlling NPY expression (18). The expression of this transgene may thus be regulated in a manner similar to that of endogenous NPY.
In the present study, we characterized the behavioral phenotype of rats carrying five copies of this transgene. To assess motor performance and exploratory motivation, locomotor activity was determined in a novel environment. Because of recent findings indicating an inverse relation between NPY expression and ethanol preference (17), voluntary ethanol consumption was examined using a two-bottle free-choice procedure. To address the anti-stress hypothesis outlined above, a previously described protocol (14, 15) was used to study experimental anxiety on the elevated plus maze both under baseline conditions and following restraint stress. In addition, experimental anxiety was studied in a conflict test that is stressful in itself, the punished drinking test. Finally, spatial learning was assessed in the Morris water maze (19). In search for possible anatomical substrates of the observed phenotype, prepro-NPY expression, as well as NPY-Y1 and -Y2 receptor binding, was mapped in NPY-transgene (tg) and wild-type control subjects.
Materials and Methods
Subjects and Design.
The generation of the NPY transgenic rats has been described in detail elsewhere (20). A 14.5-kbp lambda clone (18) containing the entire rat NPY gene (Fig. 1A) was subcloned after the addition of a polylinker with NotI and EcoRI restriction sites. Correct sequences of this plasmid were confirmed by restriction mapping, PCR amplification, and partial sequencing. The NPY transgene containing ≈5 kb 5′ and ≈1 kb 3′ of the natural rat NPY gene was released from the plasmid as a 14.5-kb NotI digest, and purified linear DNA was injected into the pronuclei of fertilized Sprague–Dawley rat eggs (Hilltop Lab Animals, Scottsdale, PA). Transgenic founders and progeny were identified by Southern analysis of tail DNA digested with EcoRI and hybridized to a 0.95-kbp probe (bp 377-1348 of rat NPY, GenBank M15792, amplified by PCR and labeled for chemiluminescence).
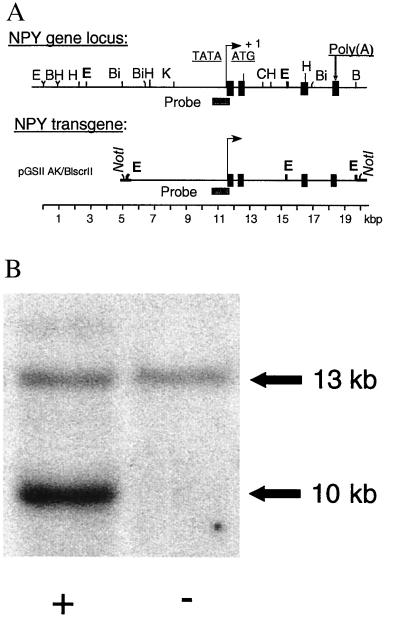
Figure 1.
(A) Restriction map of the NPY gene locus and the transgene used to generate the transgenic lines. The NPY transgene contains ≈5 kb 5′ and ≈1 kb 3′ of the natural rat NPY gene. (B) Transgenic founders and progeny were identified by Southern blot analysis of tail DNA. Digestion with EcoRI (defined as “E” in A) results in the detection of a 13-kb endogenous fragment and, in transgenic subjects (+), a 10-kb transgene fragment upon hybridization with a 0.95-kbp probe spanning bp 377 to 1348 of the rat NPY sequence. Based on intensity measures, five copies of the transgene are incorporated in the 400 line used in the behavioral experiments.
Founders (F0) were mated with Sprague–Dawley females. Mendelian inheritance rates (50.5%, n = 109) of the transgene were observed at this stage, indicating normal survival of transgenic subjects during embryogenesis. The offspring (F1) was bred into the same Sprague–Dawley background. Male subjects of line 400 hemizygous for the NPY-tg and wild-type littermate controls were used, the former carrying five copies of the NPY transgene as shown by Southern blotting (Fig. 1B).
NPY-tg subjects appeared normal and healthy, normal growth rates were observed, and no decrease in lifespan was found. Startle, orienting, and righting reflexes were normal. Subjects aged 17–24 weeks, weighing 325–375 g at the start of experiments, were used. Separate groups were used for each of the following experiments. Group 1: locomotor activity, followed 7 days later by plus maze test under baseline conditions; followed 8 days later by plus maze preceded by restraint stress; followed 2 weeks later by the punished drinking test; followed 3 weeks later by shock threshold titration; followed by a 3-week recovery, after which six subjects of each genotype were randomly taken for prepro-NPY in situ analysis. Group 2: Morris water maze, followed by a 2-week recovery, followed by the voluntary ethanol consumption procedure. Group 3: Peptide RIA. Group 4: NPY-Y1 and -Y2 quantitative autoradiography. The n for each experiment is given in Results. Animals (3–4 per cage, 12 h light/dark cycle, lights on at 7a.m., food and water ad libitum except when water deprived for punished drinking) were used according to the ethical guidelines of the Swedish Medical Research Council and under Ethical Permits S81–85/98.
Locomotion.
Infrared locomotor cages were used (20 × 38 × 16 cm; eight beams for horizontal and vertical movements, respectively; interbeam distance 8 cm horizontally and 6 cm vertically), and activity was recorded for 60 min in 10-min intervals (MedAssociates, St. Albans, VT).
Ethanol Two-Bottle Free Choice Procedure.
The two-bottle free choice procedure was as previously described (14).
Elevated Plus Maze.
The elevated plus maze is a pharmacologically validated model of anxiety (21–23). Here, it was used as described previously (14). Baseline testing was followed by a 10-day recovery, and retesting was 1 h following the completion of the restraint stress. Restraint stress was for 1 h in a Plexiglas tube, and plus maze testing was performed 1 h following the end of the restraint period. Using this procedure, we have previously shown that basal plus maze behavior is stable between session and sensitive to restraint stress (14). Venous tail blood for corticosterone analysis was obtained immediately following the insertion of the subject into the restraint tube (baseline) and immediately before removing the subject from the restrainer (stress).
Corticosterone Analysis.
Corticosterone was determined using a solid-phase RIA according to the manufacturer's instruction (Coat-A-Count; Diagnostic Products Corporation). Serum was obtained from venous tail blood by centrifugation and stored at −20°C until assayed. Duplicate 50-μl samples were analyzed.
Punished Drinking Test.
A modified Vogel's conflict procedure was used as described (14). Unpunished drinking was measured for 4 min before the onset of an 8-min punished drinking period, yielding an internal control for effects on drinking motivation and performance. To control for effects on nociception, shock thresholds were measured in separate sessions by placing each subject in the operant chamber and manually increasing the current through the grid floor until the animal displayed a reaction (jump, jerk, or similar) to the stimulus, as judged by an observer blind to treatment and shock level applied.
Morris Water Maze.
Training and testing in the Morris water Maze were carried out as described (24). Animals were trained to find a platform hidden under the water surface from four different starting positions. The accumulative latency time was calculated and used as a measure of learning. Training was for four consecutive days. Retention and performance were measured at 7 and 8 days posttraining using only one starting point.
In Situ Hybridization.
In situ hybridization for prepro-NPY and NPY receptors was as previously described (25). Coronal cryosections (12 μm) were fixed, dehydrated, dried, and stored at −70°C until use. Labeled riboprobe was added at 20 × 103 cpm/μl, and 0.1 ml of the solution was applied to each slide. Hybridization was at 55°C over night in a humidified chamber. Sections were washed, dehydrated, dried, and exposed to hyperfilm in the presence of radioactive standards. Total number of cells, number of labeled cells, and number of grains of each labeled cell were automatically determined from a digitized image using the Leica Q-Win program. Six measurements per hemisphere and a minimum of two sections per subject were measured for each brain region. Neurons were defined as having light cresyl violet staining and NPY-positive cells as those having concentrated overlying silver grains.
NPY RIA.
A 1.0-mm microcurette was used to obtain hippocampal tissue samples from consecutive 300-μm cryosections taken at approximately 2.60–3.60 mm posterior to bregma. RIA was carried out as described (26). Results are given as ng/mg supernatant protein. Resulting data did not fulfill criteria of homogeneity of variances, and nonparametric analysis was therefore used.
Quantitative Receptor Autoradiography.
Quantitative receptor autoradiography for NPY-receptor subtypes Y1 and Y2 was performed as previously described (27, 28). Briefly, brains were rapidly frozen. Coronal cryosections (20 μm) were mounted on slides, dried, and stored at −80°C until use. Sections were preincubated in Krebs–Ringer phosphate and then incubated in fresh Krebs–Ringer phosphate containing 0.1% BSA and 25 pM of either [125I]GR231118, in the presence and absence of 100 nM BIBO3304, or [125I]PYY3–36, in the presence and absence of 100 nM BIIE0246. Following a 1-h incubation for [125I]GR231118 and 2.5-h incubation for [125I]PYY3–36, sections were washed, desalted, and dried. Nonspecific binding was determined using 100 nM GR231118 for [125I]GR231118 and 1 μM PYY for [125I]PYY3–36. Sections were apposed against 3H hyperfilms for 4 days alongside radioactive standards. Y1 sites were determined as [125I]GR231118 binding sensitive to the Y1 antagonist BIBO3304 and Y2 sites as [125I]PYY3–36 binding sensitive to the Y2 antagonist BIIE0246.
Statistics.
For all data that followed criteria of parametric tests, analysis of variance was used and followed, when appropriate, by Tukey honest significant difference post hoc test. For data with nonhomogeneous variances, Mann–Whitney u test was used.
Results
Unless otherwise indicated, means ± SEM are given. No genotype differences or trends for differences were observed in body weight, exploratory locomotor activity, habituation to novelty, or ethanol consumption (data not shown).
Elevated Plus Maze (Fig. 2).
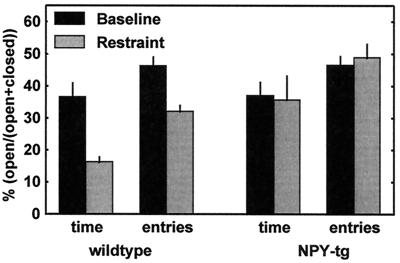
Figure 2.
Plus maze behavior (mean ± SEM for the respective parameter) of wild-type controls (left) and NPY-transgenic subjects (right). Animals were exposed to the plus maze twice, once during baseline conditions (solid bars) and once more 10 days later, 1 h following the completion of a 1-h restraint period (hatched bars). There was no genotype difference at baseline. A highly significant anxiogenic effect of the restraint was seen in the wild-type controls (% open time, P = 0.005; % open entries, P = 0.002) but was absent in the transgenic animals (% open time, P = 0.92; % open entries, P = 0.60. For detailed statistics, see Results.
Percentage open arm time.
A significant overall effect of the stressor [n = 9–13; F(1,38) = 4.69; P = 0.04] and a trend-level significant overall effect of genotype were found on this measure [F(1,38) = 3.72; P = 0.06]. A highly significant stress x genotype interaction term [F(1,38) = 9.33; P = 0.004] suggested stress to differentially affect the two genotypes. Accordingly, a highly significant effect of the stressor was seen within the wild-type group (P = 0.005) but was entirely absent within the NPY-tg group (P = 0.92). The two groups did not differ at baseline (P = 0.83) but did so following stress exposure (P = 0.01).
Percentage open arm entries.
A significant overall effect of the stressor [F(1,38) = 8.13; P = 0.007] and a trend-level significant overall genotype effect were also seen here [F(1,38) = 3.85; P = 0.06]. Again, a highly significant interaction term [F(1,38) = 13.79; P = 0.0001] suggested that stress differentially affected the two genotypes. A highly significant stress effect was seen within the wild-type group (P = 0.002), but not in NPY-tg subjects (P = 0.60). The two groups did not differ at the baseline session (P = 0.91), but did so to a highly significant degree following stress exposure (P = 0.0007).
Total entries (measure of activity).
No effects on this measure were detected [wild-type baseline, 13.4 ± 1.0; stress, 10.9 ± 1.1; NPY-tg baseline, 11.9 ± 1.1; restraint stress, 12.9 ± 1.6; overall stress effect, F(1,38) = 0.38; P = 0.54; overall genotype effect, F(1,38) = 0.052; P = 0.82; specific comparisons: wild-type at baseline vs. stress, P = 0.51; NPY-tg at baseline vs. stress, P = 0.94; baseline wild-type vs. NPY-tg, P = 0.81; stress wild-type vs. NPY-tg, P = 0.71].
Corticosterone.
Restraint stress produced a robust overall increase in serum levels of corticosterone [n = 8–13; stress-effect, F(1,39) = 250; P < <0.0001] in the absence of an overall genotype effect [F(1,39) = 0.04; P = 0.85). The stress effect was present in both groups [ng/ml; wild-type, baseline vs. stress, 118.5 ± 19.0 vs. 447.1 ± 18.1; P = 0.0002; NPY-tg, baseline vs. stress, 101.1 ± 20.3 vs. 429.6 ± 26.1; P = 0.0002), whereas no genotype effect was present under either condition (baseline, wild-type vs. NPY-tg, P = 0.94; stress wild-type vs. NPY-tg, P = 0.93).
Punished Drinking Test and Shock Thresholds (Fig. 3).
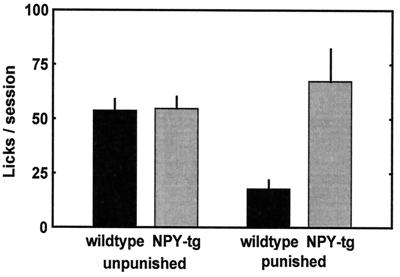
Figure 3.
Unpunished and punished drinking behavior in wild-type and NPY-tg subjects (mean ± SEM). Unpunished drinking was identical in both genotypes. Markedly elevated punished drinking was found in presence of 0.16-mA shock in NPY-tg rats (open bars) vs. wild-type littermate controls (solid bars, P = 0.009; for detailed statistics, see Results).
A markedly higher number of punished drinking episodes, and thus shocks accepted, was found in NPY-tg subjects. Unpunished drinking did not differ between the groups [n = 12–13; unpunished responding, F(1,24) = 0.065, P = 0.80, wild-type controls vs. NPY-transgenic subjects; punished responding, F(1,24) = 8.11, P = 0.009]. No difference in shock thresholds was found (P = 0.59, Mann–Whitney u test).
Morris Water Maze (Fig. 4).
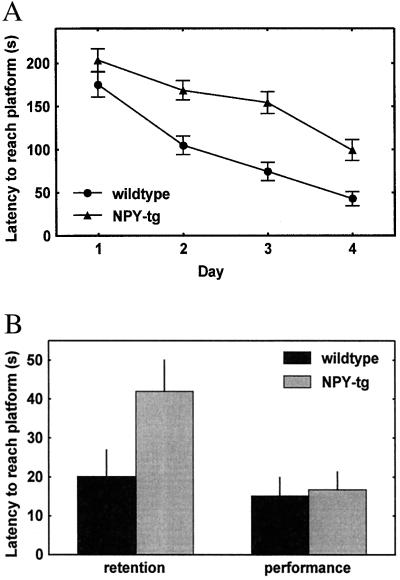
Figure 4.
(A) Markedly attenuated decline of latency to find the platform in NPY-tg subjects (overall genotype effect, P = 0.002). The groups did not differ at outset but did so on each of individual test days 2–4 (P = 0.66, 0.002, 0.0002, and 0.004 for days 1–4, respectively; for detailed statistics, see Results). (B) Memory retention (left) 7 days after the last acquisition session, measured as the latency to cross the former platform position from one starting position, was equally impaired in NPY-tg subjects (P = 0.012). Performance (right), measured as the time to find the platform when visible above fluid surface, was not affected by genotype (detailed statistics, see Results).
Over 4 days of training, latency to reach the platform decreased in wild-type controls and NPY-tg subjects, but markedly less so in NPY-tg subjects. At the end of training, the latency of NPY-tg subjects to find the platform was approximately double that of controls [n = 8–10; genotype effect, F(1,16) = 22.1; P = 0.0002]. The groups did not differ at outset but did so on each of individual test days 2–4 (P = 0.66, 0.002, 0.0002, and 0.004 for days 1–4, respectively). Escape latency of NPY-tg subjects was equally impaired when retested 7 days following the last training session [F(1,16) = 8.10; P = 0.012]. In contrast, the latency to reach the platform when made visible over the water surface did not differ between groups.
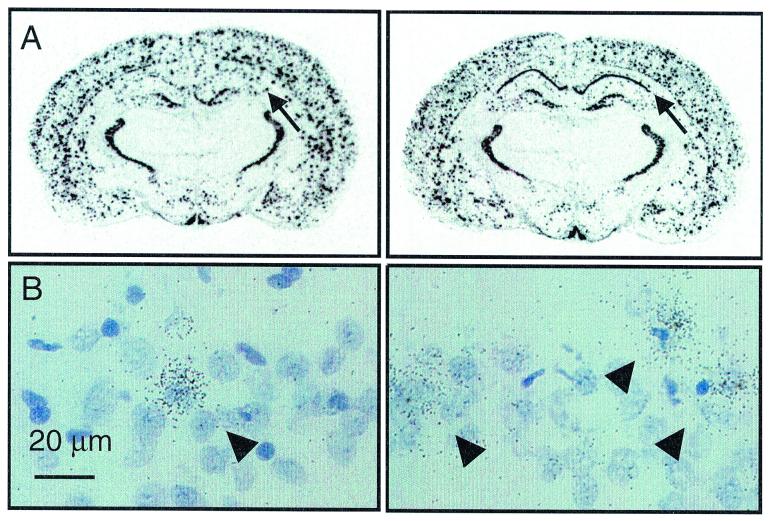
NPY Expression (Fig. 5).
Figure 5.
Coronal sections of two representative subjects at the level of the hippocampus (approximately 2.8 mm posterior to bregma; left, control; right, NPY-tg). Densitometry indicated overexpression of prepro-NPY mRNA in hippocampal fields CA1 and CA2 (P = 0.03). (B) Detailed analysis of emulsion-dipped sections revealed approximately a doubling in the number of NPY-positive cells in this area (P = 0.003; for detailed statistics, see Results).
Densitometric analysis of in situ hybridization for prepro-NPY was carried out in cingulate and retrosplenial cortex, caudate/putamen, nucleus accumbens, hippocampal CA 1–2 and CA3 regions, paraventricular nucleus of the hypothalamus, arcuate nucleus, medial amygdala, and basolateral amygdala. Using this methodology, no differences in overall anatomical distribution of NPY expression were observed between NPY-tg and wild-type subjects. Overexpression of prepro-NPY was indicated within hippocampal subfields CA1–2 [nCi/g, n = 6; 136.50 ± 2.6 vs. 154.49 ± 6.7; F(1,11) = 6.21; P = 0.03]. Subsequent quantitative image analysis of this area at a cellular level revealed the number of cells expressing NPY to be more than doubled (18.9 ± 2.4 vs. 9.0 ± 0.9; F(1,11) = 14.76; P = 0.003). This was paralleled by elevated NPY peptide levels in micropunches of this area, measured using a sensitive and specific RIA [ng/mg; n = 17–18; median (interquartile range), 1.9 (1.5–2.5) vs. 2.8 (1.9–3.2); Mann–Whitney u test; P = 0.03].
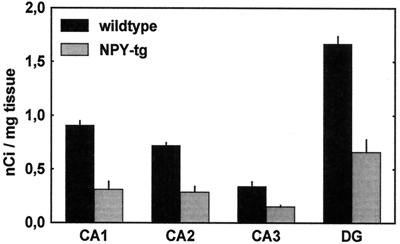
Quantitative Receptor Autoradiography for NPY-Y1 and -Y2 Receptors (Fig. 6).
Figure 6.
Quantitative receptor autoradiography demonstrated a marked down-regulation of Y1-binding ([125I]GR231118 binding sites sensitive to BIBO3304) within CA1, CA2, and dentate gyrus (DG); P = 0.0001 for wild-type vs. NPY-tg within CA1, CA2, and dentate gyrus, nonsignificant within CA3. Y2 binding ([125I]PYY3–36 labeling sensitive to BIIE0246) did not differ.
A marked decrease in binding sites for the Y1 receptor was found within the CA1, CA2, and dentate gyrus subfields of the hippocampus [n = 4; group effect F(1,6) = 81.7; P = 0.0001, P < 0.001 for wild-type vs. NPY-tg within CA1, CA2, and dentate gyrus, nonsignificant within CA3]. Y2-type binding was unaffected by the genotype.
Discussion
We report here robust phenotypic traits in NPY-tg rats, suggesting a behavioral insensitivity to stress and fear. These traits were observed in the absence of genotype effects on locomotor activity, unpunished drinking, or shock thresholds, and appear therefore to be highly specific. In addition, an impairment of spatial memory acquisition was suggested in the Morris water maze by a markedly smaller decline of latency to reach the hidden platform over 4 days of training. This memory effect also appears to be specific, as NPY-tg subjects reached the platform with latencies identical to controls when the platform was made visible in a control trial. A deficit to reach the former platform position was also present on retesting 7 days posttraining. Conclusions regarding possible additional effects on memory retention may, however, not be warranted, considering that little learning seems to have occurred in the NPY-tg subjects to begin with.
Effects on stress and memory might reciprocally affect each other. The motivation to complete the task in the Morris water maze is largely aversive, and altered sensitivity to stress might therefore affect outcome in this model. However, normal escape latencies were found in NPY-tg subjects under visible platform conditions, demonstrating that neither the motivation nor the ability to find the platform was affected by the transgene. Conversely, impaired learning and memory might affect tests of emotionality. Indeed, learning ability correlates with behavior in conditioned fear models (29). However, neither the elevated plus maze nor our punished drinking procedure relies on conditioning, and it has previously been demonstrated that learning and memory deficits per se, such as observed during aging (30) or following treatment with amnestic drugs, such as the noncompetitive N-methyl-d-aspartate (NMDA)-receptor antagonist MK-801 or the muscarinic cholinergic antagonist scopolamine (31), are not sufficient to produce anxiolytic-like behavior in this type of model. Based on this dissociation, we believe it unlikely that the anti-stress effects observed in NPY-tg subjects might be accounted for by effects on learning and memory.
Different anxiety disorders exist clinically, and experimental models of anxiety have been postulated to differentially reflect these (32, 33). However, exogenous administration of NPY produces anti-anxiety actions in all models tested, including ethologically derived paradigms, such as the elevated plus maze (2, 4) and the social interaction test (5); models based on fear suppression of behavior, including nonoperant punished drinking (2), and an operant, food-reinforced paradigm (3, 34), as well as fear potentiated startle (4). In addition, centrally administered NPY protects against stress-induced gastric erosions (35). Thus, exogenous NPY seems to counteract stress and fear responses independently of the model chosen and may therefore be acting upon a core mechanism common to these models.
Based on these observations, we have proposed that endogenous NPY may be expressed and released in the brain as a part of an integrated response to threats and stressors. Such an opposing process organization would serve to adequately terminate behavioral stress responses following their initiation by other mediators and protect against adverse consequences that might result upon extensive activation (16). Central administration of NPY-Y1 receptor targeting antisense oligonucleotides (9) or a receptor antagonist (36) supports such an anti-stress role for endogenous NPY. Also, in agreement with this model, an up-regulated NPY expression has been found within the amygdala in conjunction with behavioral and endocrine habituation to stress (15). These findings provide additional support for a “stress-buffering” action of NPY within the central nervous system.
In addition, expression of NPY in the adrenal medulla is regulated by stress, and its up-regulation might contribute to behavioral effects by modifying the salience of a stressor and/or through feedback actions on the brain (37). One possibility for this would be through described paracrine effects on the adrenal cortex. However, such a mechanism, or actions at other levels of the hypothalamic-pituitary-adrenal axis, is made unlikely by normal corticosterone levels found in NPY-tg subjects both at baseline and following a stress challenge. These results are consistent with previous studies that have shown that behavioral and endocrine stress responses can be dissociated (38).
To date, studies of transgenic NPY overexpression have only been feasible in mice (17). Whereas homologous recombination knockout studies have been consistent with an anti-anxiety role for NPY (39), data on effects of overexpression in this behavioral dimension have been lacking. Furthermore, experiments in mice prevent a direct comparison with previous results obtained with NPY in relation to stress and anxiety, which have all been carried out in rats. Some of the paradigms used for this purpose are not readily adapted to mice, and additional issues of genetic background can further complicate behavioral studies in this species (40).
The generation of an NPY-transgenic rat has enabled us to circumvent some of these complications and has made it possible to probe the role of NPY in stress/fear-related behaviors using some of the well established animal models used previously. The results are consistent with previous observations. The marked release of punished responding found in the present study directly parallels previous observations with central NPY administration (2). On the elevated plus maze, centrally injected NPY has been reported to produce anxiolytic-like effects (2), whereas in the present study, the same effect was found in NPY-tg subjects if the test session was preceded by a stressor. The requirement for pretest stress exposure, or a model which in itself is stressful, is likely to reflect that neuronal activation is needed for endogenous NPY release, but not for effects of exogenous NPY. In addition to an anti-stress effect of the NPY transgene, a learning deficit was detected.
Anatomical mapping indicates a restricted but highly significant hippocampal NPY overexpression in NPY-tg subjects. A correlate of this finding is a profound down-regulation of NPY-Y1 receptors, presumably a compensatory effect. Hippocampal glutamatergic transmission is crucial for spatial learning (41). In vitro studies have shown that NPY within the hippocampal formation acts mainly at presynaptic Y2 sites to reduce presynaptic Ca2+ entry, inhibit glutamatergic transmission, and suppress the formation of long-term potentiation (13, 42, 43). Our finding of memory impairment in NPY-tg subjects is therefore in agreement with these in vitro data and may be attributable to an enhanced Y2-mediated inhibition of excitatory synaptic activity. Interestingly, both at the level of hippocampal neurotransmission and of integrated memory function, the effects of NPY are similar to those observed when hippocampal glutamate release is inhibited by benzodiazepines or ethanol (44).
In contrast, previous studies have pointed to the amygdala as a structure mediating anti-anxiety effects of exogenous NPY (3), and no NPY overexpression was found in this structure in our study (5). However, the dorsal hippocampus is in fact an important component of neuronal circuitry controlling anxiety-related behaviors and stress responses (45, 46). Intrahippocampal administration of a metabotropic glutamate receptor antagonist, which is likely to mimic aspects of NPY-induced inhibition of hippocampal glutamatergic transmission, is anxiolytic in the type of model used in our study (47). The overexpression of NPY found within this area might therefore independently lead to anti-stress/anti-anxiety effects observed in our present study. In addition, it is possible that overexpression of NPY within the amygdala might be recruited in NPY-tg subjects following stress exposure.
In summary, the NPY-tg rat studied here seems to offer a unique model for studies of NPY mediated control of stress- and fear-related behaviors. Using this model, we present data that provide additional support for an important role of central NPY in adaptive responses to threats and stress and provide novel evidence for a role of NPY in learning and memory. In contrast, two other established effects of NPY, feeding and control of ethanol intake, were not detected, presumably because overexpression of our model is limited to areas outside those controlling these behaviors. Interestingly, in conjunction with previously reported anti-convulsant effects (48), the emerging effect profile of NPY seems to be highly similar to that of established anti-anxiety compounds, such as the benzodiazepines. If anti-anxiety and cognitive effects of NPY can be dissociated on the basis of the receptor subtypes mediating them, the NPY system may offer an attractive target for drug development in both areas.
Acknowledgments
Comments on the manuscript by Prof. D. Larhammar, T. Hökfelt, and C. Wahlestedt and the generous gift of the NPY clone by Prof. D. Larhammar are gratefully acknowledged, as is the skilful technical assistance by Siv Eriksson, T. Michal Kiewicz, and Stuart McDougall. This work was supported by Swedish Medical Research Council (M.H.), Medical Research Council of Canada (R.Q.), and National Institutes of Health Grant NIH-NHLBI 57921 (M.M.). GR231118 was a kind gift by Glaxo Wellcome, and BIBO3304 and BIIE0246 were kind gifts by Boehringer-Ingelheim (Germany).
Abbreviations
- NPY
neuropeptide Y
- tg
transgene
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.220232997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.220232997
References
- 1.Tatemoto K, Carlquist M, Mutt V. Nature (London) 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- 2.Heilig M, Söderpalm B, Engel J A, Widerlöv E. Psychopharmacology. 1989;98:524–529. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- 3.Heilig M, McLeod S, Brot M, Heinrichs S C, Menzaghi F, Koob G F, Britton K T. Neuropsychopharmacology. 1993;8:357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- 4.Broqua P, Wettstein J G, Rocher M N, Gauthier-Martin B, Junien J L. Behav Pharmacol. 1995;6:215–222. [PubMed] [Google Scholar]
- 5.Sajdyk T J, Vandergriff M G, Gehlert D R. Eur J Pharmacol. 1999;368:143–147. doi: 10.1016/s0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- 6.Eva C, Keinanen K, Monyer H, Seeburg P, Sprengel R. FEBS Lett. 1990;271:81–84. doi: 10.1016/0014-5793(90)80377-u. [DOI] [PubMed] [Google Scholar]
- 7.Herzog H, Hort Y J, Ball H J, Hayes G, Shine J, Selbie L A. Proc Natl Acad Sci USA. 1992;89:5794–5798. doi: 10.1073/pnas.89.13.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larhammar D, Blomqvist A G, Yee F, Jazin E, Yoo H, Wahlestedt C. J Biol Chem. 1992;267:10935–10938. [PubMed] [Google Scholar]
- 9.Wahlestedt C, Pich E M, Koob G F, Yee F, Heilig M. Science. 1993;259:528–531. doi: 10.1126/science.8380941. [DOI] [PubMed] [Google Scholar]
- 10.Heilig M. Regul Pept. 1995;59:201–205. doi: 10.1016/0167-0115(95)00103-i. [DOI] [PubMed] [Google Scholar]
- 11.Sheikh S P, Håkansson R, Schwartz T W. FEBS Lett. 1989;245:209–214. doi: 10.1016/0014-5793(89)80223-6. [DOI] [PubMed] [Google Scholar]
- 12.McQuiston A R, Colmers W F. J Neurophysiol. 1996;76:3159–3168. doi: 10.1152/jn.1996.76.5.3159. [DOI] [PubMed] [Google Scholar]
- 13.Qian J, Colmers W F, Saggau P. J Neurosci. 1997;17:8169–8177. doi: 10.1523/JNEUROSCI.17-21-08169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Möller C, Wiklund L, Sommer W, Thorsell A, Heilig M. Brain Res. 1997;760:94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
- 15.Thorsell A, Carlsson K, Ekman R, Heilig M. NeuroReport. 1999;10:3003–3007. doi: 10.1097/00001756-199909290-00024. [DOI] [PubMed] [Google Scholar]
- 16.Heilig M, Koob G F, Ekman R, Britton K T. Trends Neurosci. 1994;17:80–85. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 17.Thiele T E, Marsh D J, Ste M, Bernstein I L, Palmiter R D. Nature (London) 1998;396:366–369. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- 18.Larhammar D, Ericsson A, Persson H. Proc Natl Acad Sci USA. 1986;84:2068–2072. doi: 10.1073/pnas.84.7.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris R G. Learn Motivat. 1981;12:239–260. [Google Scholar]
- 20.Michalkiewicz M. In: Methods in Molecular Biology: Neuropeptide Y Protocols. Balasubramaniam A A, editor. Totowa, NJ: Humana Press; 2000. [Google Scholar]
- 21.Pellow S, Chopin P, File S E, Briley M. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 22.Pellow S, File S E. Pharmacol Biochem Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- 23.Handley S L, McBlane J W. J Pharmacol Toxicol Methods. 1993;29:129–138. doi: 10.1016/1056-8719(93)90063-k. [DOI] [PubMed] [Google Scholar]
- 24.Pham T M, Söderstrom S, Henriksson B G, Mohammed A H. Behav Brain Res. 1997;86:113–120. doi: 10.1016/s0166-4328(96)02252-8. [DOI] [PubMed] [Google Scholar]
- 25.Caberlotto L, Fuxe K, Overstreet D H, Gerrard P, Hurd Y L. Brain Res Mol Brain Res. 1998;59:58–65. doi: 10.1016/s0169-328x(98)00137-5. [DOI] [PubMed] [Google Scholar]
- 26.Michalkiewicz M, Huffman L J, Dey M, Hedge G A. Am J Physiol. 1993;264:E699–E705. doi: 10.1152/ajpendo.1993.264.5.E699. [DOI] [PubMed] [Google Scholar]
- 27.Dumont Y, Cadieux A, Doods H, Pheng L H, Abounader R, Hamel E, Jacques D, Regoli D, Quirion R. Br J Pharmacol. 2000;129:1075–1088. doi: 10.1038/sj.bjp.0703162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dumont Y, Quirion R. Br J Pharmacol. 2000;129:37–46. doi: 10.1038/sj.bjp.0702983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oler J A, Markus E J. Hippocampus. 1998;8:402–415. doi: 10.1002/(SICI)1098-1063(1998)8:4<402::AID-HIPO8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 30.Miyagawa H, Hasegawa M, Fukuta T, Amano M, Yamada K, Nabeshima T. Behav Brain Res. 1998;91:73–81. doi: 10.1016/s0166-4328(97)00105-8. [DOI] [PubMed] [Google Scholar]
- 31.Umezu T. Jpn J Pharmacol. 1999;80:111–118. doi: 10.1254/jjp.80.111. [DOI] [PubMed] [Google Scholar]
- 32.McCreary A C, McBlane J W, Spooner H A, Handley S L. Pol J Pharmacol. 1996;48:1–12. [PubMed] [Google Scholar]
- 33.File S E. Behav Brain Res. 1993;58:199–202. doi: 10.1016/0166-4328(93)90103-w. [DOI] [PubMed] [Google Scholar]
- 34.Heilig M, McLeod S, Koob G F, Britton K T. Regul Pept. 1992;41:61–69. doi: 10.1016/0167-0115(92)90514-u. [DOI] [PubMed] [Google Scholar]
- 35.Heilig M, Murison R. Eur J Pharmacol. 1987;137:127–129. doi: 10.1016/0014-2999(87)90191-9. [DOI] [PubMed] [Google Scholar]
- 36.Kask A, Rago L, Harro J. Eur J Pharmacol. 1996;317:R3–R4. doi: 10.1016/s0014-2999(96)00838-2. [DOI] [PubMed] [Google Scholar]
- 37.Nussdorfer G G, Gottardo G. Horm Metab Res. 1998;30:368–373. doi: 10.1055/s-2007-978900. [DOI] [PubMed] [Google Scholar]
- 38.Koob G F, Heinrichs S C, Pich E M, Menzaghi F, Baldwin H, Miczek K, Britton K T. Ciba Found Symp. 1993;172:277–289. doi: 10.1002/9780470514368.ch14. [DOI] [PubMed] [Google Scholar]
- 39.Palmiter R D, Erickson J C, Hollopeter G, Baraban S C, Schwartz M W. Recent Prog Horm Res. 1998;53:163–199. [PubMed] [Google Scholar]
- 40.Crawley J N. What's Wrong with My Mouse: Behavioral Phenotyping of Transgenic and Knockout Mice. New York: Wiley; 2000. [Google Scholar]
- 41.Vizi E S, Kiss J P. Hippocampus. 1998;8:566–607. doi: 10.1002/(SICI)1098-1063(1998)8:6<566::AID-HIPO2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 42.Colmers W F, Lukowiak K, Pittman Q J. J Neurosci. 1988;8:3827–3837. doi: 10.1523/JNEUROSCI.08-10-03827.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whittaker E, Vereker E, Lynch M A. Brain Res. 1999;827:229–233. doi: 10.1016/s0006-8993(99)01302-5. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu K, Matsubara K, Uezono T, Kimura K, Shiono H. Neuroscience. 1998;83:701–706. doi: 10.1016/s0306-4522(97)00339-4. [DOI] [PubMed] [Google Scholar]
- 45.Andrews N, File S E, Fernandes C, Gonzalez L E, Barnes N M. Psychopharmacology. 1997;130:228–234. doi: 10.1007/s002130050233. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez L E, File S E, Overstreet D H. Pharmacol Biochem Behav. 1998;59:787–792. doi: 10.1016/s0091-3057(97)00525-x. [DOI] [PubMed] [Google Scholar]
- 47.Chojnacka-Wojcik E, Tatarczynska E, Pilc A. Eur J Pharmacol. 1997;319:153–156. doi: 10.1016/s0014-2999(96)00941-7. [DOI] [PubMed] [Google Scholar]
- 48.Woldbye D P, Larsen P J, Mikkelsen J D, Klemp K, Madsen T M, Bolwig T G. Nat Med. 1997;3:761–764. doi: 10.1038/nm0797-761. [DOI] [PubMed] [Google Scholar]