The prevalence of peripheral neuropathy is about 2% in the general population, but it rises to 12% and 17% in people with one or two recognised risk factors.1 Diabetes is one such risk factor and the most common cause of this disorder—about half of patients who have had diabetes for 25 years have peripheral neuropathy. The early symptoms of diabetic neuropathy and other peripheral neuropathies are due to degeneration of small somatic nerve fibres, which may remain the only nerves involved.2 However, “small fibre neuropathy” may not be detected by traditional physical, neurophysiological, and neuropathological tests. In the past decade, skin biopsy has become a popular method for investigating small nerve fibres.3 It allows general practitioners and non-specialists—such as diabetologists and specialists in orthopaedics—to diagnose neuropathy (thereby avoiding delayed or incorrect diagnosis), to investigate its aetiology, and to focus treatment, in particular for neuropathic pain.
Sources and selection criteria
We searched the Medline database using combinations of the terms “skin biopsy”, “cutaneous nerve fibres”, “intraepidermal nerve fibres”, “neuropathy”, “painful neuropathy”, and “pain”. We also included evidence provided by the recent guidelines of the European Federation of the Neurological Societies.3
What are the clinical features of small fibre neuropathy?
Small fibre neuropathy can be associated with common metabolic disorders (such as diabetes and hyperlipidaemia), immune mediated conditions (such as Sjögren's syndrome, sarcoidosis, and coeliac disease), drug toxicity (such as that caused by antineoplastic drugs and antiretroviral drugs), and viral infections (such as HIV). It can be a feature of hereditary diseases (such as familial amyloidosis and Fabry's disease), or it may be idiopathic.
In the absence of known systemic disease, the diagnosis of small fibre neuropathy may not be easy. Pain or burning (or both) in the feet are prominent symptoms, and may be worse at night. Some patients report that contact with warm or cold water elicits discomfort or pain (thermal allodynia). Others may complain of nocturnal pain, restless leg syndrome, cramps, and fatigue. These symptoms reflect the degeneration of somatic small nerves, namely unmyelinated C and thinly myelinated Aδ fibres, which carry thermal and pain sensation from the skin to the spinal cord and eventually the brain. Pain induced by non-painful touch stimuli (dynamic mechanic allodynia) is due to large fibre dysfunction and thus is mild or absent in small fibre neuropathy.
Pinprick and thermal sensation may be reduced in the feet or soles, while light touch and vibratory sensation and deep tendon reflexes are retained, reflecting the preservation of large myelinated nerve fibres. In most patients the neurological examination is normal. Symptoms of autonomic impairment, such as altered sweating, flushing, and skin decolouration, may be reported in small fibre neuropathy.
What are the limitations of diagnostic tests for peripheral neuropathy?
Routine neurophysiological examinations do not provide diagnostic clues in small fibre neuropathy. Sensory nerve conduction studies—usually performed on the sural nerve when investigating polyneuropathy—evaluate large myelinated fibres, which are typically normal in small fibre neuropathy. Finally, in some regions of the body, such as the trunk and the proximal limbs, sensory nerves are not superficial enough to be investigated by neurophysiological tests.
Quantitative sensory testing to detect thermal and pain thresholds has been used extensively to assess small fibre impairment. Studies have shown that an increased warm threshold correlates with skin denervation in small fibre neuropathy.3 However, this test requires patients to collaborate, and results should not be used as the sole criteria for diagnosing neuropathy.4 Laser evoked potentials and contact heat evoked potentials selectively excite C and Aδ fibres, and are theoretically useful for assessing their function, but need further evaluation and have limited clinical usefulness at present.
Sural nerve biopsy has long been used for the histopathological diagnosis of most peripheral neuropathies but this too has its limitations. It is an invasive procedure performed in the operating room, and it carries potential risks such as pain and permanent sensory loss distal to the biopsy site. Quantitative analysis of unmyelinated fibres is possible in the biopsy specimen but requires the use of an electron microscope, making it a difficult and time consuming procedure. Furthermore, unmyelinated C and thinly myelinated Aδ axons, which have either somatic or autonomic function, are enclosed in the bundles of the nerve and cannot be differentiated from one another, which limits the usefulness of this technique for diagnosing small fibre neuropathy. Finally, the biopsy cannot be repeated to monitor progress of the neuropathy except by using the opposite sural nerve.
What can skin biopsy show that other methods can't?
Skin biopsy is a safe, almost painless, and cheap technique for evaluating small nerve fibres. The density of these fibres can be measured easily using bright field microscopy in sections cut from the specimen and appropriately immunostained with antibodies against markers expressed by peripheral nerve fibres (such as protein gene product 9.5, microtubules, and neuropeptides). Skin biopsy can also be repeated within the same nerve territory to evaluate the natural progression of the neuropathy and the effect of treatments, such as steroids or immunoglobulin. The positive predictive value of skin biopsy in diagnosing small fibre neuropathy is estimated at 93%; specificity is 97% and sensitivity ranges from 69% to 82%.3 This technique has recently been shown to detect morphological and quantitative changes in skin innervation earlier than neurophysiological tests, and it can predict the progression of neuropathy.5 6
Skin biopsy allows small fibres with different functions to be investigated separately. This has important implications in clinical practice. Intraepidermal nerve fibres have an exclusively somatic function and express the capsaicin receptor, indicating that they are peripheral nociceptors.7 Therefore, in painful neuropathy, skin biopsy can detect abnormalities of the target nerves, namely unmyelinated axons carrying pain sensation. Conversely, fibres innervating sweat glands, arrectors pilorum, and blood vessels have autonomic function, and their degeneration is indicative of an autonomic neuropathy, which can be subclinical in patients with peripheral neuropathy.8
Comparative studies have strengthened the role of skin biopsy in diagnosing small fibre neuropathy. An analysis of patients with small fibre neuropathy showed that the density of unmyelinated intraepidermal nerve fibres could be low despite normal morphometry of small nerve fibres from sural nerve samples on ultrastructural examination.2 A further study found that skin biopsy and sural nerve biopsy provide concordant results in about 75% of patients, but that skin biopsy can detect small fibre neuropathy in 25% of patients with normal sural nerve morphometry.9
Once the diagnosis of small fibre neuropathy is established, further tests can be performed to define its aetiology. One study found that in 42% of patients diagnosed with small fibre neuropathy by skin biopsy, oral glucose tolerance testing revealed an unknown impaired glucose tolerance.10 In diabetes, the extent of intraepidermal nerve fibre loss increases with the duration of disease and correlates with raised warm thresholds, suggesting that these two parameters may be the major indicators of small fibre neuropathy.11
The use of skin biopsy to measure intraepidermal nerve fibre density has enabled small fibre neuropathy to be diagnosed in other conditions, including HIV infection, neurotoxicity from antiretroviral and antineoplastic drugs, sarcoidosis, Sjögren's syndrome, and coeliac disease.2 6 12 13 14 15
What are the other clinical uses of skin biopsy?
Skin biopsy has shown that unmyelinated axons can be involved in neuropathies previously thought to affect large nerve fibres only, such as chronic demyelinating inflammatory polyradiculoneuropathy and Guillain-Barré syndrome, in which the loss of skin nerves predicts autonomic failure and worse outcome.16 17 Interestingly, skin biopsy was also useful in the diagnostic work-up of demyelinating neuropathies. In patients with immune mediated neuropathy caused by antibodies against myelin associated glycoprotein, specific deposits of IgM and complement have been detected in myelinated skin nerves.18 In vasculitic neuropathy, perivascular infiltrating cells can be demonstrated around dermal vessels.19 In inherited neuropathies, such as Charcot-Marie-Tooth disease, examining large myelinated fibres in the skin can provide the same pathological and molecular biological information as that provided by sural nerve biopsy.20 21
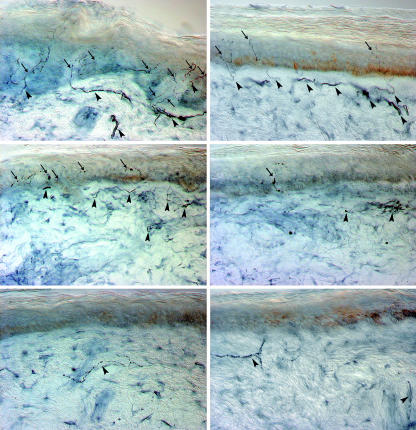
The distribution of skin nerve density can be useful in localising the site of pathology (fig 1). In distal and symmetrical peripheral neuropathies, such as in diabetic polyneuropathy, skin nerves are lost mainly at the distal rather than the proximal regions of the lower limbs, as the longest sensory axons degenerate first. This is consistent with the length dependent pattern of sensory disturbances, which start from the feet and progress proximally. Conversely, when sensory neurones of dorsal root ganglia are primarily affected, such as in paraneoplastic and immune mediated sensory neurone diseases, the loss of skin nerves may not differ between proximal and distal regions of the limbs, suggesting a process that does not depend on nerve length.22
Fig 1 Pattern of cutaneous innervation in the lower limb in a healthy subject (top row), in a patient with diabetic polyneuropathy (middle row), and in a patient with sensory neuronopathy (bottom row). Skin biopsies were taken from the proximal thigh (left) and distal leg (right) and immunostained with antibodies against protein gene product 9.5 for bright field microscopy. Arrows indicate intraepidermal nerve fibres; arrowheads indicate subpapillary nerve bundles (original magnification ×40). The healthy subject (top) shows the normal decreasing gradient of intraepidermal nerve fibre density from the proximal to distal leg. In the patient with diabetic polyneuropathy (middle), cutaneous nerves degenerate earlier in the distal leg, reflecting the length dependent degeneration process. Intraepidermal nerve fibres show large and diffuse swellings whereas dermal nerves are fragmented, indicating axonal degeneration. The patient with sensory neuronopathy (bottom) shows a complete loss of intraepidermal nerve fibres at both sites, reflecting the non-length dependent pattern of denervation caused by primary degeneration of sensory neurones of the dorsal root ganglion
Demonstrating subclinical peripheral neuropathy
In diabetic and HIV neuropathy, intraepidermal nerve fibres can show diffuse and large swellings that precede the degeneration of axons and predict the progression of symptoms to overt neuropathy.5 6 Skin biopsy has shown that diabetes causes subclinical defects of nerve fibre functions. The rate of regeneration of epidermal nerves after topical application of capsaicin, which causes transient denervation of the skin, is slower in diabetics with no evidence of neuropathy than in healthy people.23
Demonstrating autonomic neuropathy
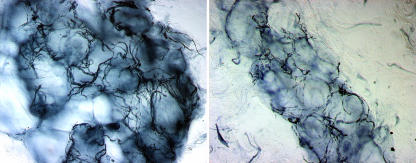
Skin biopsy can show the presence of autonomic neuropathy by providing a semiquantitative assessment of the denervation of sweat glands (fig 2).24 This approach is more sensitive than functional examination of autonomic nerves using quantitative sudomotor axon testing.3 This can be particularly useful when autonomic impairment is clinically silent, as is often the case in early diabetic neuropathy.
Fig 2 Normal innervation of a sweat gland at the distal leg in a healthy subject (left). Reduced innervation of a sweat gland in a patient with diabetes indicates autonomic neuropathy (right). Bright field immunohistochemical study with antibodies against protein gene product 9.5 (original magnification ×40)
Monitoring neuropathy
Patients with neuropathy can be evaluated over time by measuring intraepidermal nerve fibre density. Follow-up biopsies can be taken adjacent to the previous biopsy site within the same nerve distribution. In patients with diabetes, HIV infection, and idiopathic disease, serial skin biopsies have shown that loss of intraepidermal nerve fibres correlates with the progression of neuropathy.3 Collateral sprouting of intraepidermal nerve fibres from overlapping skin biopsies could be used to assess the efficacy of compounds in accelerating nerve regeneration.25
Regeneration of skin nerve fibres, which can occur spontaneously after nerve injury, is followed by recovery of heat-pain and pinprick sensations.26 In experimental models of neuropathy, skin biopsy proved to be a reliable tool for monitoring the efficacy of neuroprotective agents, such as erythropoietin and insulin-like growth factor-I.27 28 29 Thus, nerve fibre density in skin might be a useful outcome measure in clinical studies of human neuropathies.
How is a skin biopsy carried out?
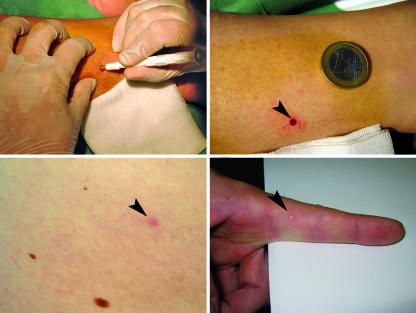
Skin biopsy can be performed at any site of the body using a disposable punch (usually 3 mm in diameter), under sterile technique, and after local anaesthesia with lidocaine. No suture is needed and no side effects have been reported. Healing is usually complete within one week and the scar is barely visible after three months (fig 3). Specimens are immediately fixed, cryoprotected, cut into vertical sections, and processed immunohistochemically to examine the innervation of epidermis, dermis, and sweat glands.
Fig 3 Skin biopsy performed using a 3 mm disposable punch (top left). Healed biopsy site (arrowhead; top right). Scar on hairy skin three months after biopsy (arrowhead; bottom left). Scar on glabrous skin eight weeks after biopsy (arrowhead; bottom right)
An even less invasive method, the “blister technique,” can be used to investigate the innervation of the epidermis alone. A suction capsule is applied to the skin to separate the epidermis from the dermis. No bleeding occurs and local anaesthesia is not needed.30 The drawback is that the morphology of the epidermal fibres cannot be examined and no information on the innervation of the underlying dermis and sweat glands is provided.
Additional educational resources
Johns Hopkins University (http://hiv.neuro.jhmi.edu/cutaneous/)—Provides detailed information for patients, clinicians, and researchers interested in skin biopsy for peripheral neuropathies
University of Minnesota Medical School (http://kennedylab.med.umn.edu/kennedyweb/biopsy/)—Provides information on methods to analyse skin biopsy and to quantify nerve fibres
The choice of skin to be biopsied depends on the types of fibre to be investigated. Hairy skin from the lower limbs is commonly taken to examine unmyelinated and thinly myelinated fibres, whereas glabrous skin from the fingers is best for examining large myelinated fibres.
For diagnostic purposes skin biopsies are taken from the distal leg (10 cm above the lateral malleolus). A further biopsy at the proximal thigh can help differentiate a length dependent process typical of axonopathies (such as in diabetic polyneuropathy) from nerve degeneration not dependent on length, which is typical of sensory neuronopathies.22
How can I measure the density of intraepidermal nerve fibres?
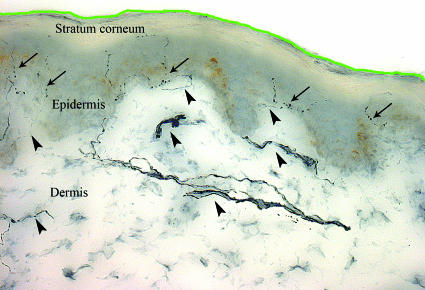
The two most common methods are bright field immunohistochemistry and indirect immunofluorescence with or without confocal microscopy. Using bright field microscopy, individual axons entering the epidermis are counted in at least three 50 µm thick sections at high magnification with the help of a video camera. The length of the section is measured with computerised software (freely available at http://rsb.info.nih.gov/nih-image/index.html) and the linear density of intraepidermal nerve fibres per millimetre is calculated (fig 4).31
Fig 4 Normal cutaneous innervation at the distal leg in a healthy subject visualised by bright field immunohistochemistry using antibodies against protein gene product 9.5 (original magnification ×40). Arrows indicate intraepidermal nerve fibres that are counted throughout the section. The green line highlights part of the surface of the section, which is measured through its entire length using a computerised system to obtain the linear density of intraepidermal nerve fibres. The epidermis is easily distinguished from the underlying dermis by its greyish colour. Arrowheads indicate the dermal nerve bundles from which intraepidermal nerve fibres arise
Confocal laser microscopy allows a three dimension reconstruction of images obtained using indirect immunofluorescence (fig 5). The density of intraepidermal nerve fibres is measured using dedicated software for image analysis. Two or more targets can be stained using different antibodies, the technique is particularly useful for studying the innervation of receptors, glands, and vessels.32 A comprehensive review on methods and rules for counting intraepidermal nerve fibres has recently been published.33
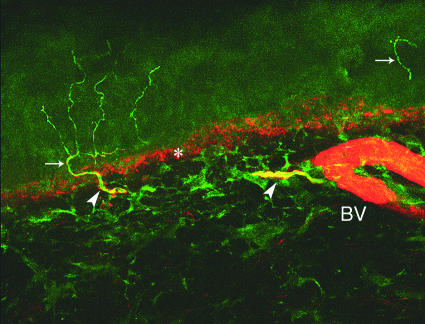
Fig 5 Skin biopsy taken at the distal leg in a healthy subject. Double staining confocal microscopy with antibodies against protein gene product 9.5 (green), which stain nerve fibres, and antibodies against collagen IV (red), which stain the dermal-epidermal junction (asterisk) and blood vessels (BV). Arrows indicate intraepidermal nerve fibres and arrowheads indicate dermal nerve bundles. Note the branched intraepidermal fibre arising from a dermal nerve fibre (original magnification ×40)
What can you see in a skin biopsy?
Skin biopsies taken with a 3 mm punch include the epidermis, the papillary dermis, and the reticular dermis. Several structures can be recognised in vertical sections, and some differences are seen between hairy and glabrous skin. The most superficial layer—the stratum corneum—is not innervated. It covers the epidermis, which contains keratinocytes, unmyelinated axons, and Langerhans' cells. The epidermis is separated from the dermis by the dermal-epidermal junction, and nerve fibres need to cross this junction to enter the epidermis. The dermis is organised into papillae. In glabrous skin, the apexes of the dermal papillae contains mechanoreceptors known as Meissner's corpuscles. The dermis contains nerve bundles, vessels, arrector pilorum muscles, hair follicles, and sweat glands.
Unmyelinated fibres
Both hairy and glabrous skin contain a rich supply of unmyelinated nerve fibres, which are the endings of small size sensory neurones of dorsal root ganglia, namely the nociceptors. The epidermis is exclusively innervated by naked somatic axons, which arise from subpapillary dermal nerve bundles and lose their Schwann cell ensheathment when crossing the dermal-epidermal junction. Intraepidermal nerve fibres run towards the surface of the skin; they have a linear course, few branches, and slight varicosities. They decrease in density from the proximal to the distal regions of the limbs—density is about 60% higher in the thigh than in the supramalleolar area. Unmyelinated fibres can be immunostained using antibodies against cytoplasmic markers, such as protein gene product 9.5, or against specific components of the cytoskeleton, such as microtubules and neurofilaments.34
Myelinated fibres
Thinly myelinated fibres (Aδ fibres) can be examined in the dermis of hairy and glabrous skin. Large myelinated fibres (Aβ fibres) can be examined in the dermis of glabrous skin taken from the lateral aspect of the finger or the fingertip. Myelinated fibres are immunostained using antibodies against the different components of the myelin sheath, such as myelin basic protein, peripheral myelin protein 22, and myelin associated glycoprotein.
Autonomic nerve fibres
The innervation and structure of dermal autonomic organs, such as sweat glands, arrector pilorum muscles, and vessels can be examined by skin biopsy. Autonomic fibres are specifically labelled by antibodies against neuropeptides, such as vasointestinal peptide. The density of sweat gland fibres can be assessed semiquantitatively.
What are the limitations of skin biopsy?
No study has specifically focused on cutaneous large fibres in vasculitic neuropathies, for which sural nerve biopsy is mandatory to demonstrate inflammatory infiltrating cells surrounding endoneurial and epineurial blood vessels. Further studies are needed to confirm the diagnostic usefulness of skin biopsy in immune mediated and inherited demyelinating neuropathies. Skin biopsy is available in a limited number of specialised centres and its use for diagnostic purposes must be preceded by training in an established laboratory.
Summary points
Skin biopsy is easy to perform and almost painless
It provides pathological information on small nerve fibres, which cannot be examined by routine neurophysiological tests
Skin biopsy is more sensitive than sensory nerve conduction studies and sural nerve biopsy in diagnosing small fibre neuropathy and can demonstrate subclinical impairment of nerve fibres in patients with diabetes
Follow-up biopsies can be performed to monitor the progression of neuropathy and to assess the efficacy of treatment
Conclusion
Skin biopsy should be considered in the diagnostic work-up of patients with symptoms suggestive of small fibre neuropathy (such as burning, prickling, or deep and aching pains in the feet) but with normal physical and neurophysiological test results, and in patients with autonomic neuropathy. Moreover, it can demonstrate subclinical neuropathy in patients at risk, such as those with diabetes. Skin biopsy can be used to diagnose sensory mononeuropathy when nerve conduction studies cannot be performed. Finally, follow-up skin biopsies allow the progression of neuropathy and the efficacy of treatments to be monitored.
Ongoing research
Collaborative international studies aim to standardise techniques for processing skin biopsies and measuring nerve fibre density
Studies in patients with painful neuropathies aim to compare the diagnostic efficacy of skin biopsy with clinical measures and non-conventional neurophysiological tests
Studies focused on large myelinated fibres aim to confirm the usefulness of skin biopsy in the diagnosis of immune mediated and inherited demyelinating neuropathies
Skin biopsy is being used to measure outcomes in clinical trials with neuroprotective agents
Competing interests: None declared.
Provenance and peer review: Commissioned; peer reviewed.
References
- 1.Beghi E, Ponticelli ML, the Italian General Practitioner Study Group (IGPST). Chronic symmetric symptomatic polyneuropathy in the elderly: a field screening investigation of risk factors for polyneuropathy in two Italian communities. J Clin Epidemiol 1998;51:697-702. [DOI] [PubMed] [Google Scholar]
- 2.Holland NR, Crawford TO, Hauer P, Cornblath DR, Griffin JW, McArthur JC. Small-fiber sensory neuropathies: clinical and neuropathology of idiopathic cases. Ann Neurol 1998;44:47-59. [DOI] [PubMed] [Google Scholar]
- 3.Lauria G, Cornblath DR, Johansson O, McArthur JC, Mellgren SI, Nolano M, et al. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol 2005;12:1-12. [DOI] [PubMed] [Google Scholar]
- 4.Shy ME, Frohman EM, So YT, Arezzo JC, Cornblath DR, Giuliani MJ, et al. Quantitative sensory testing. Report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology 2003;60:898-904. [DOI] [PubMed] [Google Scholar]
- 5.Lauria G, Morbin M, Lombardi R, Borgna M, Mazzoleni G, Sghirlanzoni A, et al. Axonal swellings predict the degeneration of epidermal nerve fibers in painful neuropathies. Neurology 2003;61:631-6. [DOI] [PubMed] [Google Scholar]
- 6.Herrmann DN, McDermott MP, Henderson D, Chen L, Akowuah K, Schifitto G, The North East Aids Dementia (Nead) Consortium. Epidermal nerve fiber density, axonal swellings and QST as predictors of HIV distal sensory neuropathy. Muscle Nerve 2004;29:420-7. [DOI] [PubMed] [Google Scholar]
- 7.Lauria G, Morbin M, Lombardi R, Capobianco R, Camozzi F, Pareyson D, et al. Expression of capsaicin receptor immunoreactivity in human peripheral nervous system and in painful neuropathies. J Peripher Nerv Syst 2006,11:262-71. [DOI] [PubMed]
- 8.Perretti A, Nolano M, De Joanna G, Tugnoli V, Iannetti G, Previtera V, et al. Is Ross syndrome a dysautonomic disorder only? An electrophysiologic and histologic study. Clin Neurophysiol 2003;114:7-16. [DOI] [PubMed] [Google Scholar]
- 9.Herrmann DN, Griffin JW, Hauer P, Cornblath DR, McArthur JC. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology 1999;53:1634-40. [DOI] [PubMed] [Google Scholar]
- 10.Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003;60:108-11. [DOI] [PubMed] [Google Scholar]
- 11.Shun CT, Chang YC, Wu HP, Hsieh SC, Lin WM, Lin YH, et al. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain 2004;127:1593-605. [DOI] [PubMed] [Google Scholar]
- 12.Hoitsma E, Marziniak M, Faber CG, Reulen JPH, Sommer C, De Baets M, et al. Small fibre neuropathy in sarcoidosis. Lancet 2002;359:2085-6. [DOI] [PubMed] [Google Scholar]
- 13.Chai J, Herrmann DN, Stanton M, Barbano RL, Logigian EL. Painful small-fiber neuropathy in Sjögren syndrome. Neurology 2005;65:925-7. [DOI] [PubMed] [Google Scholar]
- 14.Brannagan TH III, Hays AP, Chin SS, Sander HW, Chin RL, Magda P, et al. Small-fiber neuropathy/neuronopathy associated with celiac disease. Skin biopsy findings. Arch Neurol 2005;62:1574-8. [DOI] [PubMed] [Google Scholar]
- 15.De Sousa EA, Hays AP, Chin RL, Sander HW, Brannagan TH III. Characteristics of patients with sensory neuropathy diagnosed with abnormal small nerve fibres on skin biopsy. J Neurol Neurosurg Psychiatry 2006;77:983-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiang MC, Lin YH, Pan CL, Tseng TJ, Lin WM, Hsieh ST. Cutaneous innervation in chronic inflammatory demyelinating polyneuropathy. Neurology 2002;59:1094-8. [DOI] [PubMed] [Google Scholar]
- 17.Pan CL, Tseng TJ, Lin YH, Chiang MC, Lin WM, Hsieh ST. Cutaneous innervation in Guillain-Barré syndrome: pathology and clinical correlations. Brain 2003;126:386-97. [DOI] [PubMed] [Google Scholar]
- 18.Lombardi R, Erne B, Lauria G, Pareyson D, Borgna M, Morbin M, et al. Anti-MAG neuropathy patients show specific IgM deposits in cutaneous nerve fibers. Ann Neurol 2005;57:180-7. [DOI] [PubMed] [Google Scholar]
- 19.Lee JE, Shun CT, Hsieh SC, Hsieh ST. Skin denervation in vasculitic neuropathy. Arch Neurol 2005;62:1570-3. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Bai Y, Ghandour K, Qin P, Grandis M, Trostinskaia A, et al. Skin biopsies in myelin-related neuropathies: bringing molecular pathology to the bedside. Brain 2005;128:1168-77. [DOI] [PubMed] [Google Scholar]
- 21.Sabet A, Li J, Ghandour K, Pu Q, Wu X, Kamholz J, et al. Skin biopsies demonstrate MPZ splicing abnormalities in Charcot-Marie-Tooth neuropathy 1B. Neurology 2006;67:1141-6. [DOI] [PubMed] [Google Scholar]
- 22.Sghirlanzoni A, Pareyson D, Lauria G. Sensory neuron diseases. Lancet Neurol 2005;4:349-61. [DOI] [PubMed] [Google Scholar]
- 23.Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain 2004;127:1606-15. [DOI] [PubMed] [Google Scholar]
- 24.Sommer C, Lindenlaub T, Zillikens D, Toyka KV. Selective loss of cholinergic sudomotor fibers causes anhidrosis in Ross syndrome. Ann Neurol 2002;52:247-50. [DOI] [PubMed] [Google Scholar]
- 25.Hahn K, Sirdofsky M, Brown A, Ebenezer G, Hauer P, Miller C, et al. Collateral sprouting of human epidermal nerve fibers following intracutaneous axotomy. J Peripher Nerv System 2006;11:142-7. [DOI] [PubMed] [Google Scholar]
- 26.Simone DA, Nolano M, Johnson T, Wendelschafer-Crabb G, Kennedy WR. Intradermal injection of capsaicin in human produces degeneration and subsequent reinnervation of epidermal nerve fibres: correlation with sensory function. J Neurosci 1998;18:8947-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bianchi R, Buyukakilli B, Brines M, Savino C, Cavaletti G, Oggioni N, et al. Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc Natl Acad Sci U S A 2004;10:823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bianchi R, Brines M, Lauria G, Savino C, Giardini A, Nicolini G, et al. Protective effect of erythropoietin and of its carbamylated derivative in experimental cisplatin peripheral neurotoxicity. Clin Cancer Res 2006;12:2607-12. [DOI] [PubMed] [Google Scholar]
- 29.Toth C, Brussee V, Zochodne DW. Remote neurotrophic support of epidermal nerve fibres in experimental diabetes. Diabetologia 2006;49:1081-8. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy WR, Nolano M, Wendelschafer-Crabb G, Johnson TL, Tamura E. A skin blister method to study epidermal nerves in peripheral nerve disease. Muscle Nerve 1999;22:360-71. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, et al. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology 1995;45:1848-55. [DOI] [PubMed] [Google Scholar]
- 32.Nolano M, Provitera V, Crisci C, Stancanelli A, Wendelschafer-Crabb G, Kennedy WR, et al. Quantification of myelinated endings and mechanoreceptors in human digital skin. Ann Neurol 2003;54:197-205. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy WR, Wendelschafer-Crabb G, Polydefkis M, McArthur J. Pathology and quantitation of cutaneous nerves. In: Dyck PJ, Thomas PK. eds. Peripheral neuropathy 4th ed. Philadelphia: Saunders, 2005:869-96.
- 34.Lauria G, Borgna M, Morbin M, Lombardi R, Mazzoleni G, Sghirlanzoni A, et al. Tubule and neurofilament immunoreactivity in human hairy skin: markers for intraepidermal nerve fibers. Muscle Nerve 2004;30:310-6. [DOI] [PubMed] [Google Scholar]