Abstract
Iridescent structural colour is found in a wide variety of organisms. In birds, the mechanisms that create these colours are diverse, but all are based on ordered arrays of melanin granules within a keratin substrate in barbules. The feathers of the grackles and allies in the family Icteridae range in appearance from matte black to iridescent. In a phylogenetic analysis of this clade, we identified several evolutionary transitions between these colour states. To describe a possible mechanistic explanation for the lability of plumage coloration, we used spectrometry, transmission electron microscopy and thin-film optical modelling of the feathers of 10 icterid species from five genera, including taxa with matte black or iridescent feathers. In matte black species, melanin was densely packed in barbules, while in iridescent species, melanin granules were arranged in ordered layers around the edges of barbules. The structured arrangement of melanin granules in iridescent species created optical interfaces, which are shown by our optical models to be critical for iridescent colour production by coherent scattering. These data imply that rearrangement of melanin granules in barbules is a mechanism for shifts between black and iridescent colours, and that the relative simplicity of this mechanism may explain the lability of plumage colour state within this group.
Keywords: structural colour, sexual selection, cowbirds, plumage colour, thin-film
1. Introduction
Coloration in animals can arise through the deposition of carotenoids, melanins, or other pigments (i.e. pigmentary colours) and through the precise arrangement of pigments and tissues at a nanostructural scale (i.e. structural colours) (Fox & Vevers 1960; Gill 1995; Hill & McGraw 2006). Structural coloration of integumentary structures, including feathers, butterfly scales and invertebrate colours is caused by a wide variety of mechanisms (Parker 1998; Vukusic & Sambles 2003; Prum 2006) and can produce white, non-iridescent, or iridescent (i.e. varying in reflectance spectra at different viewing angles; Newton 1704) coloration. The mechanisms that produce iridescent plumage coloration, while diverse, have a common structural basis of ordered stacks of melanin granules within a keratin substrate in feather barbules (Durrer 1986). These stacks typically create colour through thin-film scattering (Greenewalt et al. 1960; Land 1972; Zi et al. 2003; Brink & van der Berg 2004), in which light is phase shifted at the interfaces of materials of different refractive indices (Vašíček 1960). All feather barbules contain keratin, and many non-iridescent feather barbules (such as those in matte black species) contain melanin (Dyck 1971, 1976; Prum 1999; Shawkey et al. 2003, 2005). Thus, the components needed for production of iridescent structural colour are present in many non-iridescent barbules.
This similarity of structural components between feathers with melanin-based pigmentary coloration and feathers with structural iridescent coloration suggests an evolutionary pathway from melanin to structural coloration via the rearrangement of structural components. Morphological evolution often occurs not through the appearance of new structures, but through the modification of existing ones (Jacob 1977), a type of evolutionary tinkering (Bockaert & Pin 1999; Saraste & Castresana 1999) classically illustrated by the wrist bones of the giant panda Ailuropoda melanoleuca that have been modified into a ‘thumb’ (Gould 1980). Several secondary sexual traits also clearly arose in this manner; for example, the tail streamers of barn swallows are elongated rectrices and the tusks of boars are enlarged canines (Andersson 1994). Given the widespread occurrence of this type of evolution, we might expect that the bright plumage coloration of birds also evolved through this process, as Prum (2006) proposed for iridescent structural colour.
We explore evolutionary patterns and describe the mechanisms underlying variation in plumage coloration in a group of closely related bird species. The family Icteridae is an ideal clade for the study of the evolution of structural coloration because it has a well-supported phylogeny (Lanyon 1992; Johnson & Lanyon 1999) and species in this family vary in colour from matte black to iridescent (Jaramillo & Burke 1999). While the function of these colours is virtually unstudied (but see McGraw et al. 2002), Webster (1992) suggested that patterns of sexual size dimorphism, and hence perhaps secondary sexual traits such as plumage colour, among icterids (as seen across Molothrus cowbirds: Hauber et al. 1999) arose through sexual selection. We did not address any hypotheses on the functional significance of plumage colour in this study and instead focused on broad-scale evolutionary patterns and anatomical mechanisms.
First, we mapped colour state (matte or iridescent) on to this phylogeny and identified patterns of colour evolution. We then used full-spectrum spectrometry, transmission electron microscopy (TEM) and thin-film optical modelling on the plumage of matte black and iridescent members of the family Icteridae to identify physical mechanisms of colour production and morphological differences between these types of barbules. To describe the relationship between the arrangement and density of melanin granules and the type of colour display produced, we took measurements of keratin and melanin layers of feather barbules and applied them in standard models of thin-film reflection (Jellison 1993) to predict the colour produced by different feathers. These models enabled us to understand how observed changes in morphology of barbules could cause a shift between matte and iridescent colour across species of this family.
2. Methods and materials
2.1 Colour measurement and classification
We first determined colour states of all species in the phylogeny of grackles and related taxa in the family Icteridae in Johnson & Lanyon (1999) by using visual inspection and full-spectrum spectrometry of museum specimens at the Louisiana State University Museum of Natural Science. When available (greater than 90% of cases), we measured five individuals of each species, but in some cases fewer specimens were present in the collection. We examined specimens visually under diffuse light and classified them as iridescent if any of their black colour patches had overtones of other colours and as matte if they did not.
To ensure the accuracy of our human eye-based classifications, we took reflectance measurements from the belly region of intact skins using an Ocean Optics S2000 spectrometer (range 250–880 nm, Dunedin, FL, USA). We chose this region because all species initially classified as ‘iridescent’ exhibited iridescence in this region. Using a block sheath that excluded ambient light, we held a bifurcated optic probe with a 1 μm diameter fibre at a 90° angle 5 mm from the feather surface, creating a measurement area of 2 mm in diameter. As expected of iridescent colours, reflectance curves changed with different angles of illumination and measurement. Exploratory investigation of different angles of measurement (using both identical and separate angles of illumination and measurement) revealed that a 90°-measurement geometry created the most saturated and repeatable curves, and thus we used it in all of the analyses presented here. However, it is important to recognize the potential biological and physical importance of changes in reflectance at different measurement and reflectance angles (see Osorio & Ham 2002; Fleishman et al. 2005 for more details). This measurement area was illuminated by both a UV (deuterium bulb) and a visible (tungsten-halogen bulb) light source. All data were generated relative to a white standard (WS-1, Ocean Optics). We used OOIbase software to record and average 20 spectra sequentially, and we have recorded and averaged measurements from five arbitrarily chosen points on each sample. Spectra were classified as iridescent if they had discrete reflectance peaks and as matte if they had no reflectance peaks. While some species may have peaks that are only visible at certain angles of measurement and/or illumination, the concurrence of our visual and spectrometric classification (see §3) suggests that this possibility is unlikely.
2.2 Phylogenetic analysis
To examine the patterns in the evolution of plumage coloration, we traced the character states of plumage coloration on to the phylogeny of Johnson & Lanyon (1999) using MacClade (Maddison & Maddison 2000). States of interior nodes were determined by parsimony optimization.
2.3 Structural imaging
To identify the mechanisms of colour production, we chose representative matte black and iridescent species. We first focused on the genus Molothrus because it contains both matte and iridescent taxa. We then chose two iridescent species of the genus Quiscalus, and one other iridescent and two other matte black species from the family Icteridae. We pulled belly feathers from each of the five species of the molothrine cowbirds (screaming cowbird Molothrus rufoaxillaris (matte), giant cowbird Scaphidura (Molothrus) oryzivora (iridescent), bronzed cowbird Molothrus aeneus (iridescent), shiny cowbird Molothrus bonariensis (iridescent) and brown-headed cowbird Molothrus ater (iridescent)) and from the red-winged blackbird Agelaius phoeniceus (matte), the boat-tailed grackle Quiscalus major (iridescent) and great-tailed grackle Quiscalus mexicanus (iridescent), as well as from the bobolink Dolichonyx oryzivorus (matte) and the western meadowlark Sturnella neglecta (matte) from study skins in the Museum of Vertebrate Zoology at the University of California, Berkeley. We also pulled feathers from scrub blackbird Dives warszewiczi from study skins in the Louisiana State University Museum of Natural Science.
We prepared feather barbules for TEM by standard methods (see Shawkey et al. 2003), and took all micrographs of barbules at 2500× magnification using a Phillips EM301 (Veeco FEI Inc., Hillsboro, OR) transmission electron microscope. We took micrographs of a waffle-pattern diffraction grating (Ted Pella, Redding, CA) accurate to 1 nm±5% at the same magnification for calibration of the images. After scanning TEM micrograph negatives at 400 dpi using an Epson Perfection 1240U flatbed scanner, we used the programme NIH Image v. 1.62 (available for download at http://rsb.info.nih.gov/nih-image) to measure structural components. We measured the thickness of the keratin cortex and outer melanin layer at six evenly spaced points on each barbule (see electronic supplementary material, §1, for an example). For the species without a clearly defined outer melanin layer, we measured the diameter of the outermost melanin granule at six points on the barbule. For simplicity, we subsequently refer to all of these as measurements of ‘outer melanin layers’. We counted the number of melanin granules traversed during measurement of the outer layer and divided its thickness by these values to calculate the mean size of the granules. To determine the density of melanin in the outer melanin layer and in the interior portion of the barbule bounded by this layer, we measured the total surface area of melanin granules within the outer melanin layer and barbule interior and divided these values by the total surface area of each section. We measured five different barbules from each individual species, and took the mean value of these barbules for our subsequent analyses.
2.4 Modelling colour production
We used thin-film optical modelling to determine the mechanisms of colour production in these species. We aimed to predict the reflectance spectra of feathers from the density of keratin and melanin layers to allow us to check the relative importance of the keratin and melanin layers in determining the reflectance characteristics, and how variation in each might affect coloration. For each species, we used the mean thickness of the keratin and melanin layers to construct theoretical reflectance curves based on thin-film optics. We used the standard matrix model summarized in Jellison (1993) to create models for reflected light from all possible two- and three-beam combinations of interfaces for each species (see electronic supplementary material, §2). In all cases, we used a perpendicular initial angle of incidence and the previously published real refractive indices of air (RI=1.00), keratin (RI=1.56) and eumelanin (RI=2.00) (Land 1972; Brink & van der Berg 2004), and the estimated lower limit extinction coefficients for keratin (k=0.03) and eumelanin (k=0.6) (Brink & van der Berg 2004) in our calculations. Despite some uncertainty, these values represent the best available estimates at this time (Brink & van der Berg 2004). Because we used perpendicular incidence, we only considered the p-component of polarization in our models (Vašíček 1960).
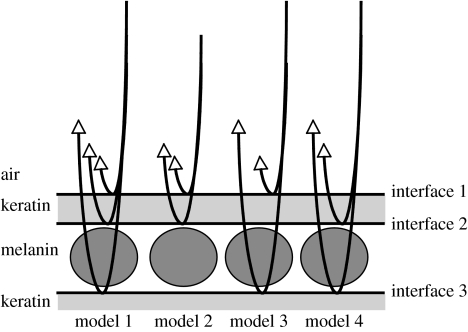
We created four reflectance models for each species (summarized in figure 2). Model 1 incorporated all three interfaces (air/keratin, keratin/melanin, melanin/keratin) and the thicknesses of the keratin and melanin layers. Model 2 only incorporated the outer two interfaces and the thickness of the keratin layer. Model 3 only incorporated the air/keratin and melanin/keratin interfaces, and the thickness of the melanin layer. Model 4 only incorporated the inner two interfaces and the thickness of the melanin layer. Following the methods of Vukusic et al. (1999), Parker et al. (2003), Zi et al. (2003) and Brink & van der Berg (2004), we visually compared predicted and measured reflectance curves.
Figure 2.
Cartoon illustrating the three-beam (model 1) and two-beam models of thin-film reflectance from feather barbules used in this study. The ambient layer is air and the top layer is keratin, while the circles represent melanin granules. The lines with arrows represent beams of light reflected and phase shifted by interfaces between materials of different refractive indices.
To identify the concurrent evolutionary transitions between barbule morphology and colour state, we mapped barbule morphology of these selected species onto the larger phylogeny.
3. Results
3.1 Colour measurement and classification
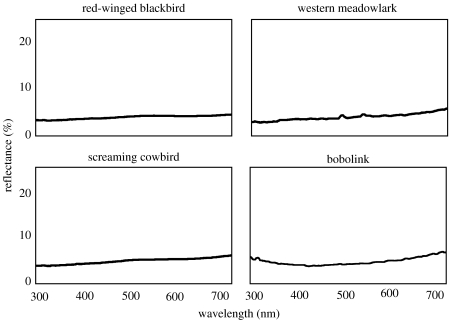
Spectrometric analyses confirmed our visual assessments. Reflectance spectra of all species initially classified as iridescent had discrete reflectance peaks, while those of matte black species had no discrete peaks (see figures 2 and 3 for examples). Three species were further visually classified as ‘matte brown’.
Figure 3.
Measured reflectance curves of matte black belly feathers of red-winged blackbird, screaming cowbird, western meadowlark and bobolink.
3.2 Phylogenetic analysis
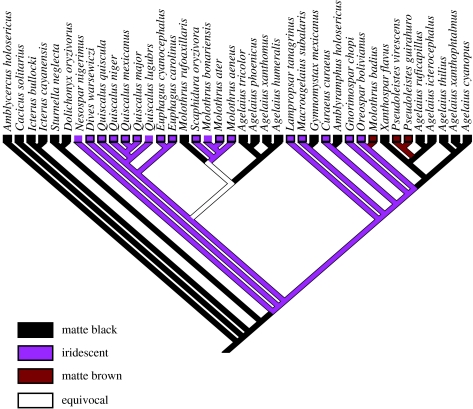
Our phylogenetic analysis indicated that matte black is the ancestral colour state of belly feathers of grackles and allies in the family Icteridae and that iridescent coloration is derived from matte black. It is worth noting, however, that a more complete reconstruction of colour within the larger icterid lineage may reveal a different evolutionary pathway. Transitions, including putative reversals, between matte black and iridescent colours occurred at least five times in the lineage (figure 4). In three cases, a matte black colour state was gained from an ancestral iridescent colour state while in one case iridescent colour was gained from a basal matte black state. In the fifth case, either matte or iridescent colour was gained from an equivocal ancestral state.
Figure 4.
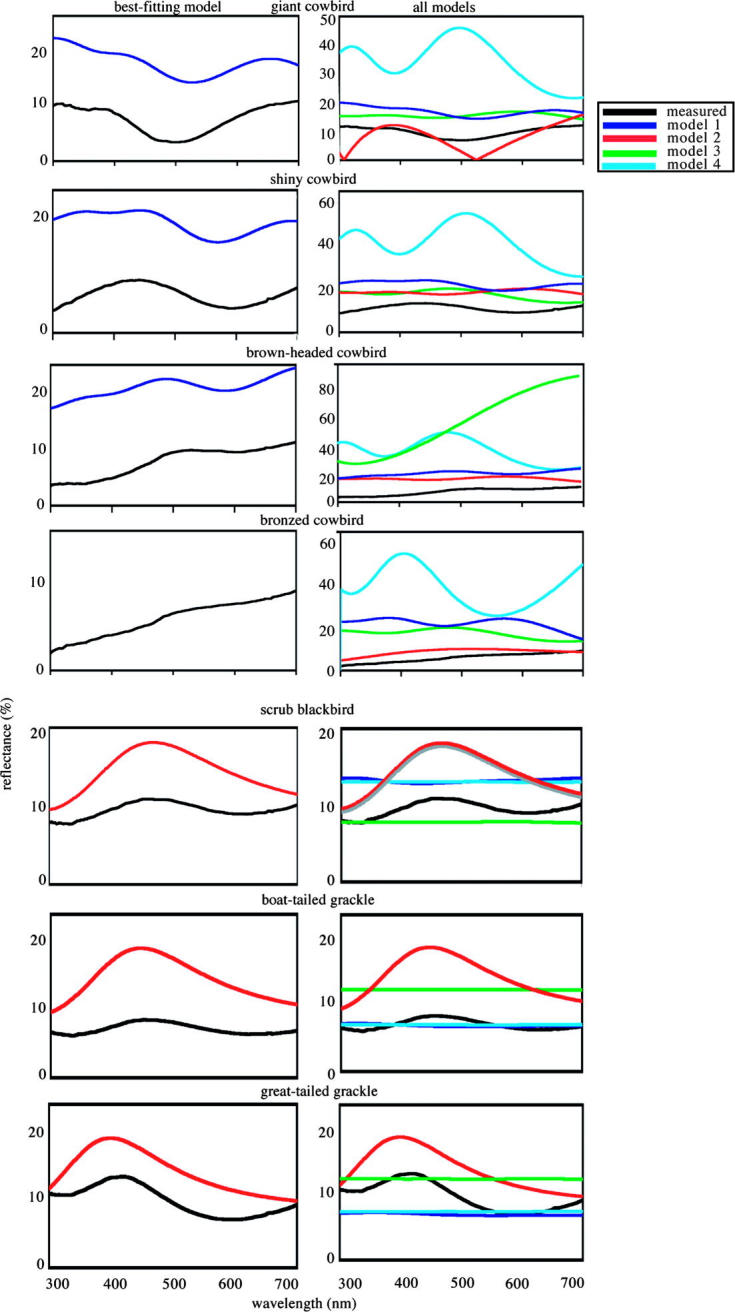
Measured and predicted reflectance spectra of belly feathers of seven species of grackles and allies with iridescent plumage in direct perpendicular light. Predicted reflectance spectra were based on a three-beam model (model 1, figure 1) generated using the matrix thin-film optical method of Jellison (1993). All three interfaces of materials of different refractive indices within the barbule (air/keratin, keratin/melanin and melanin/keratin) were incorporated into model 1. Model 2 only incorporated the outer two interfaces and the thickness of the keratin layer. Model 3 only incorporated the air/keratin and melanin/keratin interfaces, and the thickness of the melanin layer. Model 4 only incorporated the inner two interfaces and the thickness of the melanin layer. Figures on the right-hand side are measured curves and predicted curves based on all four models, while figures on the left-hand side are measured curves and predicted curves based on the best-fitting model.
3.3 Structural imaging
Cortex thickness varied from 61 to 241 nm (table 1). The cortex of giant cowbirds was thinnest, while the cortex of bobolinks was thickest. As noted earlier, barbules of the matte black red-winged blackbird, screaming cowbird, bobolink and western meadowlark did not have distinct outer melanin layers (figure 5a,b, electronic supplementary material, §3). In every iridescent cowbird species except the bronzed cowbird, this layer was composed of single relatively thick melanin granules, while in the iridescent grackle species, this layer was composed of 2–3 relatively smaller melanin granules. In the bronzed cowbird, this layer was composed of a greater number of smaller melanin granules than in the other cowbird species (figure 5a, table 1, electronic supplementary material, §3).
Table 1.
Nanostructural measurements of barbules from belly feathers of members of grackles and allies. (All values are in nanometres, except where otherwise indicated.)
| species | cortex thickness | outer melanin layer thickness | number of melanin granules | diameter of melanin granules | melanin density in outer layer (%) | melanin density in inner layer (%) |
|---|---|---|---|---|---|---|
| red-winged blackbird Agelaius phoeniceus | 111.52±3.37 | 188.96±5.25a | 1a | 188.96±5.25a | 70.24±7.53 | 44.73±2.50 |
| scrub blackbird Dives warszewiczi | 164.51±6.16 | 367.97±44.62 | 2.28±0.33 | 161.55±11.01 | 61.03±14.22 | 20.65±3.45 |
| bobolink Dolichonyx oryzivorus | 241.12±27.51 | 331.75±13.17 | 1a | 331.75±13.17 | 55.37±7.69 | 43.55±2.33 |
| bronzed cowbird Molothrus aeneus | 82.38±2.37 | 223.61±7.28 | 1.89±0.07 | 118.38±3.24 | 76.91±4.54 | 35.65±4.20 |
| brown-headed cowbird Molothrus ater | 121.29±1.98 | 265.92±8.43 | 1.17±0.03 | 227.93±6.90 | 63.33±1.59 | 20.66±1.95 |
| shiny cowbird Molothrus bonariensis | 84.22±2.56 | 281.50±10.05 | 1.33±0.04 | 211.12±11.74 | 78.99±4.98 | 28.27±2.05 |
| screaming cowbird Molothrus rufoaxillaris | 117.06±3.97 | 229.39±6.32a | 1a | 229.39±6.32a | 68.31±3.62 | 40.70±2.64 |
| great-tailed grackle Quiscalus major | 123.47±2.28 | 438.05±25.33 | 2.93±0.20 | 139.34±8.22 | 55.37±7.96 | 25.66±1.54 |
| boat-tailed grackle Quiscalus mexicanus | 111.94±5.48 | 391.05±20.01 | 2.90±0.12 | 134.84±10.02 | 48.52±5.13 | 17.82±3.98 |
| giant cowbird Scaphidura oryzivora | 61.14±1.21 | 274.18±8.94 | 1.08±0.01 | 253.70±4.03 | 80.45±9.58 | 34.21±2.71 |
| western meaddowlark Sturnella neglecta | 110.18±7.89 | 230.34±6.33 | 1a | 230.34±6.33 | 70.33±6.53 | 42.33±2.20 |
Clear outer melanin layers were not present in these species, and we therefore measured the diameter of the single outermost melanin granule at six points on the barbule.
Figure 5.
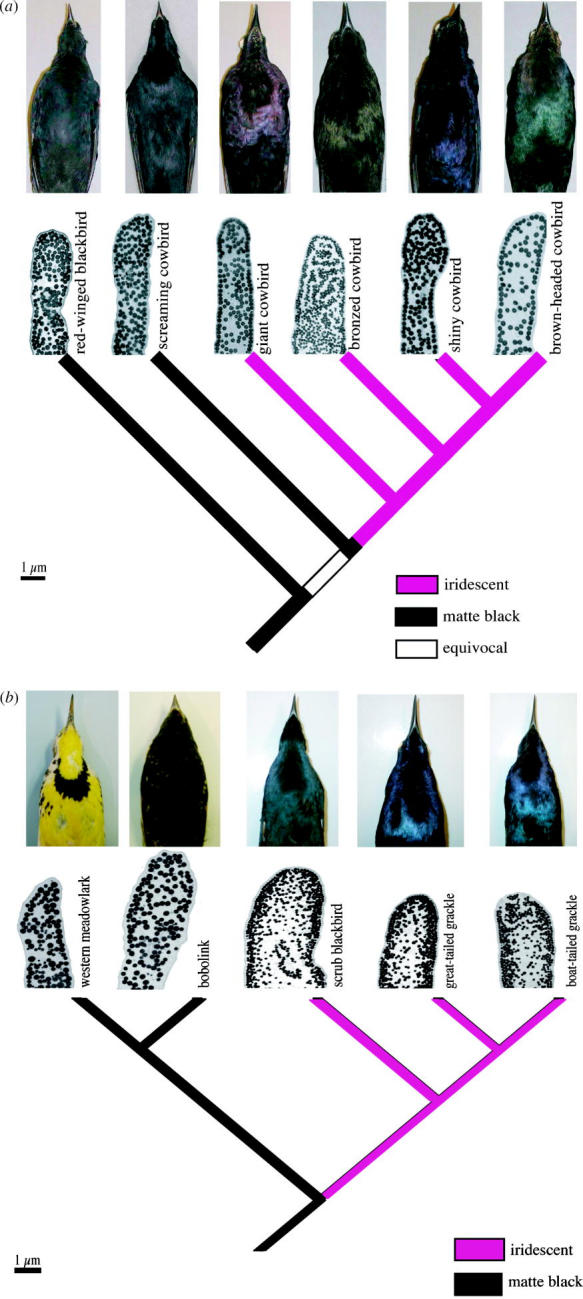
(a) Phylogeny of molothrine cowbirds based on Johnson & Lanyon (1999) with pictures of study skins to illustrate plumage colour differences and micrographs (2500×) of representative feather barbules from belly feathers. (b) Subset of the phylogeny of grackles and allies based on Johnson & Lanyon (1999) with pictures of study skins to illustrate plumage colour differences and micrographs (2500×) of representative feather barbules from belly feathers.
3.4 Modelling colour production
Model 1, incorporating all three interfaces, most successfully predicted the measured reflectance curve for the giant, shiny and brown-headed cowbirds (figure 3). The predicted curves were strikingly similar to reflected curves, but predicted values were shifted to higher reflectance. This difference in total reflectance is expected, as the models predict reflectance for a single idealized barbule, while we measured reflectance from whole feathers. Other aspects of feather morphology, such as the matte black barbs, may cause the measured reflectance to be lower than the predicted reflectance. The difference between our diffusively reflecting white standard and the non-diffusively reflecting iridescent feathers may also have caused these differences. For these species, eliminating any of these interfaces drastically reduced the models' predictive power (figure 3). Model 2, in which scattering by the melanin layer was eliminated, produced simple spectra that did not resemble the measured spectra (figure 3). Model 3, in which the keratin/melanin interface was eliminated, and Model 4, in which the keratin layer was treated as transparent, predicted double-peaked curves that varied little between species (figure 3). This similarity between species is probably a consequence of our model only considering the phase-change by the melanin layer, and the thickness of this layer being fairly uniform among the three species.
Model 2, incorporating only the outer two interfaces, most successfully predicted the measured reflectance curve for the great-tailed and boat-tailed grackles and scrub blackbird (figure 3). For these species, including scattering by the relatively thick melanin layer drastically reduced the models' predictive power. Coherent scattering appears to take place only in the outer keratin cortex, with little or no contribution by the melanin layer. None of the models accurately predicted the reflectance spectra of bronzed cowbirds.
3.5 Associations between morphology and colour
When we mapped both colour states (matte or iridescent) and melanin arrangement of belly feathers on the phylogeny, we found that iridescent colour was always associated with single-layer melanin deposition, while matte black colour was always associated with a dense, uniform melanin deposition (figure 5a,b, electronic supplementary material, §3). The screaming cowbird, bobolink, red-winged blackbird and western meadowlark are matte black and have a dense, fairly uniform arrangement of melanin granules within their barbules (figure 5a,b, table 1, electronic supplementary material, §3). Iridescent species, on the other hand, have cortexes of varying thickness and a thin layer of melanin granules around the edge of their barbules, with reduced numbers of melanin granules in the interior (figure 5a,b, table 1).
4. Discussion
This study presents the evolutionary context and describes the best-fitting mechanism for shifts from matte black pigmentary coloration to iridescent structural coloration in the grackles, cowbirds and related taxa. We demonstrated that matte black is the ancestral colour state for belly feathers of the family Icteridae. We also found that colour state is fairly labile in this group; iridescent coloration arose in the lineage from a matte ancestral state and was also subsequently reversed several times. The apparently small number of steps of morphological modification needed to bring about such a change in plumage reflectance may explain this lability. We used explicit optical models to explain how two shifts in the arrangements of melanin granules could cause a change from matte to iridescent colour. Iridescence was achieved by concentrating melanin granules into a uniform cortex layer around the edges of barbules. This melanin layer created two interfaces (air/keratin and keratin/melanin) that are critical to iridescent colour production in all six iridescent species, as is shown in our models. A reduction in the number of melanin granules in the centre of barbules of iridescent species created a third interface (melanin/keratin) that is used in colour production in half of the iridescent species. Thus, rearrangement of melanin granules in barbules created the proper physical conditions for iridescent colour production, and a shift from ‘pigmentary’ to ‘structural’ colour, in this group.
This colour production appears to occur through scattering at three different interfaces in feather barbules of cowbird species with iridescent plumage, and at two different interfaces in most grackle species. These are smaller numbers of interfaces than in the multi-layer stacks (7–15 layers) in the barbules of hummingbird feathers (Greenewalt et al. 1960). This small number of interfaces probably explains the low reflection of cowbirds and grackles in comparison to hummingbird feathers. Theory predicts a strong positive relationship between the number of interfaces and total reflectance (Dyck 1987). Indeed, the reflectance values of another species that uses a two-interface mode of colour production (satin bowerbird Ptilonorhynchus violaceus; Doucet et al. 2006) are relatively low compared to those of hummingbirds.
The greater thickness of their outer melanin layers may explain why iridescent grackle species only use two interfaces in colour production. At thicknesses beyond a certain range, the outer melanin layer may absorb most light before it reaches the underlying keratin interface. Thus, scattering does not occur at this third interface and scattering at the upper two interfaces alone creates the perceived colour. The satin bowerbird, another species that uses two interfaces in colour production, also has a thick melanin layer (approx. 900 nm; Doucet et al. 2006).
We were unable to accurately model colour production in the bronzed cowbird, but the thin outer melanin layer of the feathers of this species was composed of an average of close to two (versus one in the other glossy cowbird taxa) small melanin granules, and these were smaller than in the other four species. Nonetheless, the cortex of the feathers of bronzed cowbirds was thinner than that of the grackle species with outer melanin layers composed of several melanin granules. Perhaps this thin cortex is insufficient to create a strong colour display, or perhaps the melanin layer is composed in whole or in part of phaeomelanin, for which we do not have refractive index values. More extensive physical modelling may be necessary to precisely describe how it is produced.
Our phylogenetic analysis indicates that shifts between matte and iridescent plumage in the grackles and allies occurred through the rearrangement of melanin granules in barbules, as hypothesized by Prum (2006). A matte black colour display with a dense arrangement of melanin granules is present in all matte black species. The precise ordering of these granules along the edges of barbules is all that may be required for a shift from matte black to glossy or iridescent plumage. The ordering of granules creates a clearly defined, uniform cortex, while loss of granules from the centre creates a third interface from the outer melanin layer to the keratin on the interior of the barbule. Either two or all three of these interfaces are needed to create the chromatic, saturated curves in the iridescent species.
The evolution of structural iridescent coloration in belly feathers of icterids appears to have occurred through slight modifications of existing melanin structures. An opposite shift in plumage colour from non-iridescent blue to matte black between males in two populations of white-winged fairy-wrens Malurus leucopterus occurs owing to increased deposition of melanin in barbs (Doucet et al. 2004). That the mechanisms for producing black melanin coloration and iridescent structural coloration are so similar and interrelated argues against the traditional treatment of melanin- and structural-based coloration described as fundamentally different forms of ornamental colour display (reviewed in Hill & McGraw 2006). Recently described examples of colour displays produced by a combination of structural and pigmentary mechanisms (Grether et al. 2004; Rutowski et al. 2005; Shawkey & Hill 2005) further suggest that this dichotomous concept may not apply to all cases.
We have shown here that structural colour can evolve from pigmentary colour (matte black) through a loss and rearrangement of melanin granules into glossy iridescent structural colour. Similarly, matte black coloration appears to evolve through a loss of ordered arrangement of melanin granules. Structural colours may be further altered by additional changes in cortex thickness and in melanin layer thickness and composition. Such simple shifts in the arrangement of melanin granules and the cortex may be a widespread mechanism for the evolution of iridescent and matte black coloration in feathers (e.g. in hummingbirds). Furthermore, similar mechanisms may apply to patterns of colour patch evolution in other organisms. For example, simple changes in patterns of chitin deposition may explain the diversity of colour patches of butterfly wings (Kemp et al. 2005). The mechanism we have uncovered here thus may have broad implications for structural colour evolution in general.
Figure 1.
Phylogeny of grackles and allies from Johnson & Lanyon (1999) with colour state of belly feathers, as determined by visual inspection and full-spectrum spectrometry, mapped. Feathers of matte black and brown species have no overtones of other colours and lack discrete peaks in their reflectance curves, while iridescent species have overtones of other colours and have discrete peaks in their reflectance curves.
Acknowledgements
We would like to thank J. C. Reboreda and his laboratory group for sharing their insights into cowbird diversity, C. Cicero and the Museum of Vertebrate Zoology at the University of California, Berkeley, V. Remsen and the Museum of Natural Science at Louisiana State University for access to specimens, M. Toivio-Kinnucan for embedding and sectioning feather barbules, and D.J. Brink for assistance with optical modelling. This manuscript was greatly improved by comments from S. M. Doucet, E. H. DuVal, G. A. Heredia, A. Krakauer, H. L. Mays Jr, W. Monahan, R. L. Mueller, J. L. Parra, S. M. Rovito, G. E. H.'s laboratory group and many others. This work was funded in part by a Chapman Memorial Grant from the American Museum of Natural History and a Bleitz Research grant from the American Ornithologists' Union to M.D.S, the New Zealand Marsden Fund, the National Geographic Society, the Miller Institute for Basic Research in Science and the University of Auckland Research Council to M.E.H., and National Science Foundation grants DEB007804 and IBN0235778 to G.E.H. M.D.S. was supported while writing this manuscript by NSF grant IOB-0517549.
Footnotes
Present address: Department of Epidemiology and Public Health, Yale School of Medicine, 60 College Street, PO Box 208034, New Haven, CT 06520-8034, USA.
Supplementary Material
(1) Thin-film optical equations used to generate predicted reflectance curves; (2) an example of the nanostructural measurements used in these equations; and (3) larger images of TEM pictures of barbules from each species in the study.
References
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Bockaert J, Pin J.P. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J. 1999;18:1723–1729. doi: 10.1093/emboj/18.7.1723. doi:10.1093/emboj/18.7.1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink D.J, van der Berg N.G. Structural colours from feathers of the bird Bostrychia hagedash. J. Phys. D: Appl. Phys. 2004;37:813–818. doi:10.1088/0022-3727/37/5/025 [Google Scholar]
- Doucet S.M, Shawkey M.D, Rathburn M.D, Mays H.L, Jr, Montgomerie R. Concordant evolution of plumage colour, feather microstructure, and a melanocortin receptor gene between mainland and island populations of a fairy-wren. Proc. R. Soc. B. 2004;271:1663–1670. doi: 10.1098/rspb.2004.2779. doi:10.1098/rspb.2004.2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet S.M, Shawkey M.D, Hill G.E, Montgomerie R. Iridescent plumage in satin bowerbirds: structure, mechanisms and nanostructural predictors of individual variation in colour. J. Exp. Biol. 2006;209:380–390. doi: 10.1242/jeb.01988. doi:10.1242/jeb.01988 [DOI] [PubMed] [Google Scholar]
- Durrer H. The skin of birds: coloration. In: Bereiter-Hahn J, Matolsky A.G, Richards K.S, editors. Biology of the integument 2: vertebrates. Springer; Berlin: 1986. pp. 239–247. [Google Scholar]
- Dyck J. Structure and colour-production of the blue barbs of Agapornis roseicollis and Cotinga maynana. Z. Zellforsch. 1971;115:17–29. doi: 10.1007/BF00330211. doi:10.1007/BF00330211 [DOI] [PubMed] [Google Scholar]
- Dyck J. Structural colours. Proc. Int. Ornithol. Congr. 1976;16:426–437. [Google Scholar]
- Dyck J. Structure and light reflection of green feathers of fruit doves (Ptilinopus spp.) and an imperial pigeon (Ducula concinna) Biol. Skr. 1987;30:2–43. [Google Scholar]
- Fleishman L.J, Leal M, Sheehan J. Illumination geometry, detector position and the objective determination of animal signal colours in natural light. Anim. Behav. 2005;71:463–474. doi:10.1016/j.anbehav.2005.06.005 [Google Scholar]
- Fox H.M, Vevers G. Macmillan; New York: 1960. The nature of animal colours. [Google Scholar]
- Gill F. W.H. Freeman and Co; New York: 1995. Ornithology. [Google Scholar]
- Gould S.J. Penguin; London: 1980. The Panda's thumb. [Google Scholar]
- Greenewalt C.H, Brandt W, Friel D. The iridescent colours of hummingbird feathers. J. Opt. Soc. Am. 1960;50:1005–1013. [Google Scholar]
- Grether G.F, Kolluru G.R, Nersissian K. Individual colour patches as multicomponent signals. Biol. Rev. 2004;79:583–610. doi: 10.1017/s1464793103006390. doi:10.1017/S1464793103006390 [DOI] [PubMed] [Google Scholar]
- Hauber M.E, Clayton N.S, Kacelnik A, Reboreda J.C, DeVoogd T.J. Sexual dimorphism and species differences in HVC volumes of cowbirds. Behav. Neurosci. 1999;113:1095–1099. doi: 10.1037//0735-7044.113.5.1095. doi:10.1037/0735-7044.113.5.1095 [DOI] [PubMed] [Google Scholar]
- Hill G.E, McGraw K.J, editors. Bird coloration, vol. I: mechanisms and measurements. Harvard University Press; Boston, MA: 2006. [Google Scholar]
- Jacob F. Evolution and tinkering. Science. 1977;196:1161–1166. doi: 10.1126/science.860134. [DOI] [PubMed] [Google Scholar]
- Jaramillo A, Burke P. Princeton University Press; Princeton, NJ: 1999. New World blackbirds: the icterids. [Google Scholar]
- Jellison G., Jr Data analysis for spectroscopic ellipsometry. Thin Solid Films. 1993;234:416–422. doi:10.1016/0040-6090(93)90298-4 [Google Scholar]
- Johnson K.P, Lanyon S.M. Molecular systematics of the grackles and allies, and the effect of additional sequence (CYT B and ND2) Auk. 1999;116:759–768. [Google Scholar]
- Kemp D.J, Rutowski R.L, Mendoza M. Colour pattern evolution in butterflies: a phylogenetic analysis of structural ultraviolet and melanic markings in North American sulphurs. Evol. Ecol. Res. 2005;7:133–141. [Google Scholar]
- Land M.F. The physics and biology of animal reflectors. Prog. Biophys. Mol. Biol. 1972;24:77–106. doi: 10.1016/0079-6107(72)90004-1. [DOI] [PubMed] [Google Scholar]
- Lanyon S.M. Interspecific brood parasitism in blackbirds (Icterinae): a phylogenetic perspective. Science. 1992;255:77–79. doi: 10.1126/science.1553533. [DOI] [PubMed] [Google Scholar]
- Maddison W.P, Maddison D.R. Sinauer Associates; Sunderland, MA: 2000. MacClade 4: analysis of phylogeny and character evolution, version 4.0. [Google Scholar]
- McGraw K.J, Mackillop E.A, Dale J, Hauber M.E. Different colours reveal different information: how nutritional stress affects the expression of melanin- and structurally based ornamental plumage. J. Exp. Biol. 2002;205:3747–3755. doi: 10.1242/jeb.205.23.3747. [DOI] [PubMed] [Google Scholar]
- Newton I. Dover Publications; Mineola, NY: 1704. Opticks. [Google Scholar]
- Osorio D, Ham A.D. Spectral reflectance and directional properties of structural coloration in bird plumage. J. Exp. Biol. 2002;205:2017–2027. doi: 10.1242/jeb.205.14.2017. [DOI] [PubMed] [Google Scholar]
- Parker A.R. The diversity and implications of animal structural colours. J. Exp. Biol. 1998;201:2343–2347. doi: 10.1242/jeb.201.16.2343. [DOI] [PubMed] [Google Scholar]
- Parker A.R, Welch V.L, Driver D, Martini N. Structural colour: opal analogue discovered in a weevil. Nature. 2003;426:786–787. doi: 10.1038/426786a. doi:10.1038/426786a [DOI] [PubMed] [Google Scholar]
- Prum R.O. The anatomy and physics of avian structural colours. In: Adams N.J, Slotow R.H, editors. Proc. 22nd Int. Ornithological Congress. Bird Life South Africa; Durban, South Africa: 1999. pp. 295–353. [Google Scholar]
- Prum R.O. Anatomy, physics and evolution of avian structural colours. In: Hill G.E, McGraw K.J, editors. Bird coloration, vol. I: mechanisms and measurements. Harvard University Press; Boston, MA: 2006. pp. 295–353. [Google Scholar]
- Rutowski R.L, Macedonia J.M, Morehouse N, Taylor-Taft L. Pterin pigments amplify iridescent ultraviolet signal in males of the orange sulphur butterfly, Colias eurytheme. Proc. R. Soc. B. 2005;272:2329–2335. doi: 10.1098/rspb.2005.3216. doi:10.1098/rspb.2005.3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M, Castresana J. Cytochrome oxidase evolved by tinkering with denitrification enzymes. FEBS Lett. 1999;341:1–4. doi: 10.1016/0014-5793(94)80228-9. [DOI] [PubMed] [Google Scholar]
- Shawkey M.D, Hill G.E. Carotenoids need structural colours to shine. Biol. Lett. 2005;1:121–125. doi: 10.1098/rsbl.2004.0289. doi:10.1098/rsbl.2004.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawkey M.D, Estes A.M, Siefferman L.M, Hill G.E. Nanostructure predicts intraspecific variation in ultraviolet-blue plumage colours. Proc. R. Soc. B. 2003;270:1455–1460. doi: 10.1098/rspb.2003.2390. doi:10.1098/rspb.2003.2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawkey M.D, Estes A.M, Siefferman L, Hill G.E. The anatomical basis of sexual dichromatism in non-iridescent, ultraviolet-blue structural coloration of feathers. Biol. J. Linn. Soc. 2005;84:259–271. doi:10.1111/j.1095-8312.2005.00428.x [Google Scholar]
- Vašíček A. North Holland Publishing Company; Amsterdam: 1960. Optics of thin films. [Google Scholar]
- Vukusic P, Sambles J.R. Photonic structures in biology. Nature. 2003;424:852–855. doi: 10.1038/nature01941. doi:10.1038/nature01941 [DOI] [PubMed] [Google Scholar]
- Vukusic P, Sambles J.R, Lawrence C.R, Wootton R.J. Quantified interference and diffraction in single morpho butterfly scales. Proc. R. Soc. B. 1999;266:1403–1411. doi:10.1098/rspb.1999.0794 [Google Scholar]
- Webster M.S. Sexual dimorphism, mating system and body size in New World blackbirds (Icterinae) Evolution. 1992;46:1621–1641. doi: 10.1111/j.1558-5646.1992.tb01158.x. doi:10.2307/2410020 [DOI] [PubMed] [Google Scholar]
- Zi J, Yu X, Li Y, Hu S, Xu C, Wang X, Liu X, Fu R. Coloration strategies in peacock feathers. Proc. Natl Acad. Sci. USA. 2003;100:12 576–12 578. doi: 10.1073/pnas.2133313100. doi:10.1073/pnas.2133313100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(1) Thin-film optical equations used to generate predicted reflectance curves; (2) an example of the nanostructural measurements used in these equations; and (3) larger images of TEM pictures of barbules from each species in the study.