Abstract
Though actin filaments running across the cell (transverse actin) have been occasionally reported for epithelial cells in groups and for cells growing on fibres, there has been no report heretofore of transverse actin in cells grown on planar substrata. This paper describes evidence in support of this possibility derived from actin staining, polarization microscopy and force measurements. The paper introduces two new methods for detecting the orientation and activity of contractile elements in cells. The orthogonal actin is most obvious in cells grown on groove ridge structures, but can be detected in cells grown on flat surfaces.
Keywords: transverse (orthogonal) actin, polarization microscopy, force measurement
Cytoskeletal arrangements of actin in many cells usually seem to reflect the general shape of the cell. In elongate cells, much of the actin cytoskeleton is aligned to the long axis of the cell, while in triangular cells the actin is often arranged oriented to the vertices of the cell. These actin fibres are often called ‘stress fibres’. In addition, there is usually an actin ‘cage’ surrounding the nucleus. These arrangements are sketched in figure 1. When cells are grown on a microgrooved structure, the cells respond by becoming highly oriented to the grooves and motile (Clark et al. 1987; Curtis et al. 1995; Wojciak-Stothard et al. 1996). The actin cytoskeleton also increases its polarization (Wojciak-Stothard et al. 1996). The actin cytoskeleton seems to play some role in the development of this very marked polarization, because treatment of the cells with cytochalasins results in the cells spreading laterally, though they are still elongate in the groove direction. Observations such as these could be explained by the tensegrity theory (Ingber & Jamieson 1985), or by postulating that the hydrostatic pressure within the cell is altered by growing cells in grooves or by postulating that there is a transverse cytoskeletal structure which leads to contraction in the transverse direction.
Figure 1.
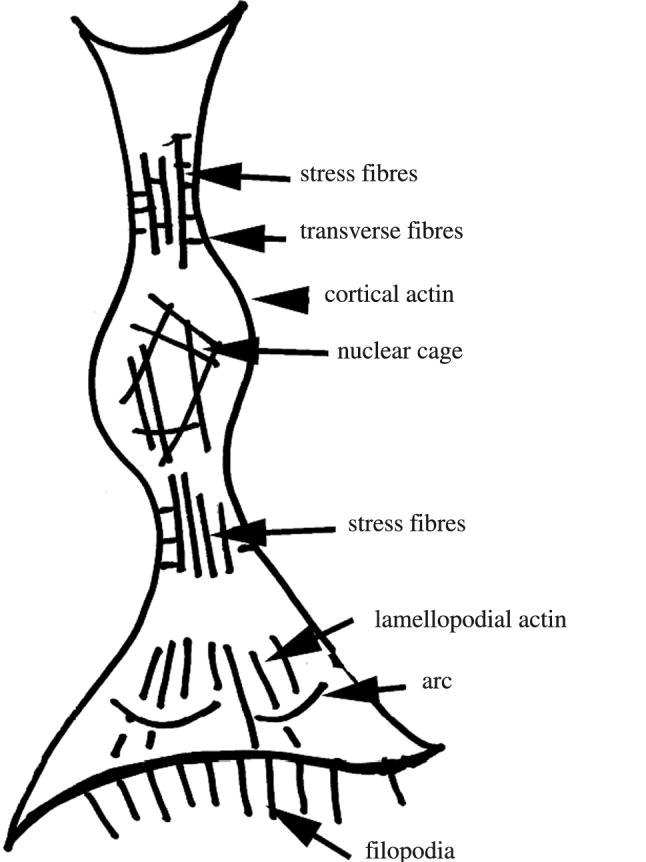
A sketch of actin organizations known. Based on the work by Resch et al. (2002), with addition of results from this paper.
There is already published evidence for a strong transverse contraction in cells grown in grooves. See figure 2 (published by Curtis & Wilkinson (1998a,b)) where the cells have managed to pull the polyurethane ridges towards themselves. This image does not appear to have been commented upon either by others or ourselves. This contraction could be explained by the existence of an actin/myosin system running from side to side of the cell. There have already been earlier papers describing evidence from microscopy for such a system, by Albrecht-Buehler (1979), Lo & Gilula (1980), Tucker (1981) and Svitkina et al. (1995), but only in cells in groups. We now offer further evidence from several different lines of evidence using both routine and new methods to suggest that such a transverse system may be relatively common and occur as isolated cells as well as grouped cells.
Figure 2.
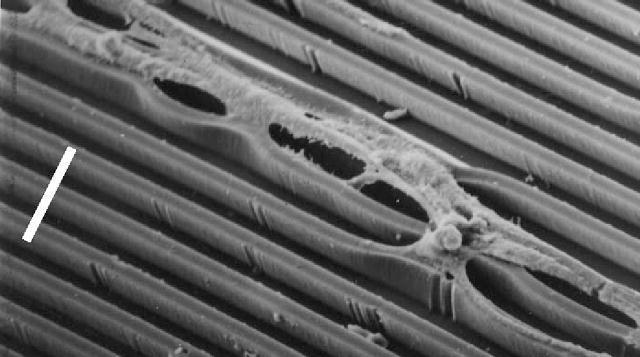
Taken from Curtis & Wilkinson (1998a,b) with part of SEM image extracted and rotated. Parts of epitenon cells growing on polyurethane grooves. Note how the polyurethane substrate has been distorted by cellular action. Scale bar, 25 μm.
In the course of work on reactions of cells to grooves and also by using the polscope polarization optical microscope, we obtained incidental evidence that there is a definite but subordinate transverse actin cytoskeleton transverse to the oriented main actin cytoskeleton. The polscope which uses circularly polarized light measures light intensity which has passed through the liquid crystal compensator and from this calculates the light retardation caused by the birefringence of the specimen and the azimuth of the slow ‘rays’. Extraction of the azimuth angle is especially useful in determining the orientation of polarizing structures.
On the basis of these observations, we decided to look for evidence for a transversely actin contraction system in fibroblasts and other cells. We used optical fluorescence methods for detecting actin, and polarization methods to detect orientations and contractile action of the actin.
1. Material and methods
Polydimethylsiloxane (PDMS) substrata were prepared in Anoptral (flat-bottomed) glass Petri dishes of 60 mm diameter or as thin sheets by polymerizing the monomer with an appropriate cross-linker at elevated temperature for 3 hours. The siloxane monomer (Sylgard 184) and polymerizing agent (both from Dow Corning Chemicals, Bad Soden, Germany) were mixed in a 6 : 1 proportion, degassed for 20–30 minutes under vacuum until no more bubbles form. The Sylgard mixture was then poured into a Petri dish (1/5 ml) or filled between two polycarbonate sheets or between two thin flexible polystyrene sheets and then cured at 90 °C for 14 hours. The polymerized PDMS has appreciable photoelasticity.
Cells: h-tert (human telomerase restricted) fibroblasts and Le-2 (B10, D2) mouse capillary endothelia are grown on the PDMS surfaces, coated with appropriate adhesion proteins, such as fibronectin for various periods (1–4 days) and the cultures are then examined at 37 °C with the polscope microscope.
1.1 Polarization microscopy
The polscope microscope (CRI International, Waltham, MA, US) was mainly used with 40× and 100× strain-free objectives. The microscope with its associated software can measure retardances down to 0.1 nm due to the photoelasticity of the PDMS substratum under strain (manufacturer's handbook) and azimuths, i.e. orientation of slow axis, as well. One nanometre retardance corresponds approximately to a straining force of 1×10−9 N mm−1. Pseudocolour images can be obtained which are useful for rapid survey observations and for revealing any mis-set up of the optical system, but for serious observation, images stamped with retardances at various points on the image are most useful. Images of the bare PDMS surface were obtained to check that there was no strain in the substratum in the absence of cells. The phase contrast images of the sampling areas were acquired, so that the exact boundaries of the cell or cells could be established. Three images of each area to be measured were taken in quick succession, one for the acquisition of ring for the raw birefringence data and two for further processing (for pseudocolouring and for stamping of calculated retardance values). Phase image and raw birefringence data image which can be used for further processing, e.g. retardance value stamping and pseudocolours, were acquired in quick succession for every measured area. This method can also be used for measuring forces over a time-series.
1.2 Cells
Actin staining and fluorescence microscopy. Cells were fixed in 4% formol saline for 5 minutes, permeabilized with 0.1% Triton X-100 in saline for 4 minutes and then washed with phosphate-buffered saline. The fixed cultures were then stained with rhodamine–phalloidin and examined by fluorescence microscopy.
2. Results
Typical images of actin organization in a cell grown on a grooved substrate are seen in figure 3a,b taken from earlier publications by one of the authors (A. S. G. Curtis; Wojciak-Stothard et al. (1995)). Note the concentrations of actin staining along the edge of the cell and also over the groove edges and the fine transverse staining joining the heavy staining. Also note that much of the actin staining over the groove edges is concentrated as a series of roughly regularly spaced dots.
Figure 3.
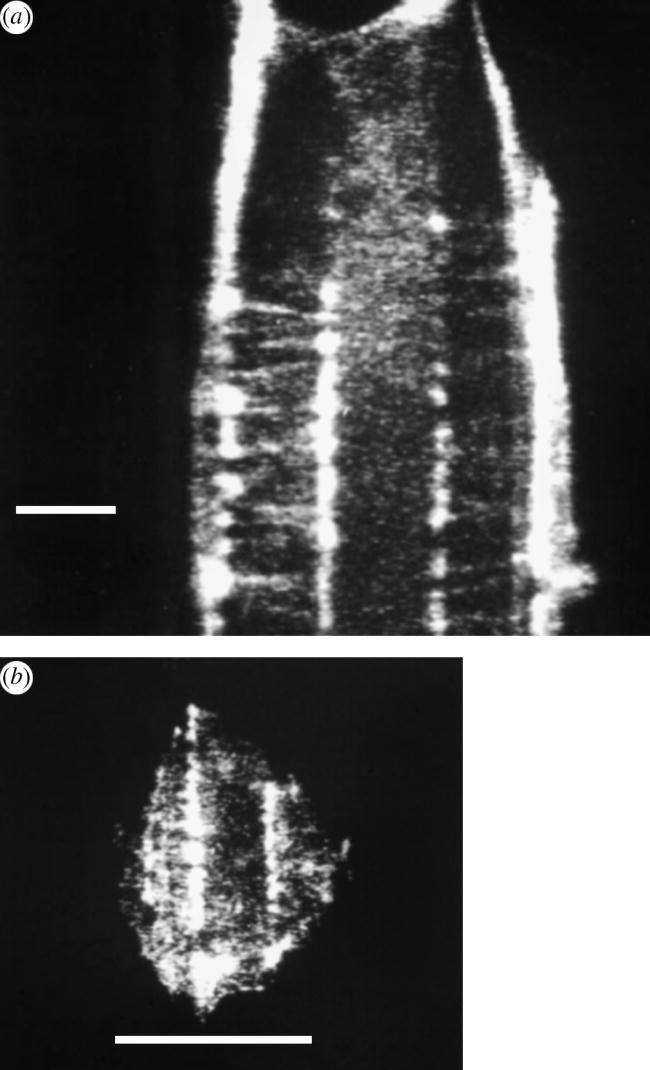
(a) Taken from Wojciak-Stothard et al. (1995): BHK fibroblast 20 minutes after plating out. On groove ridge structure. F-actin shown by rhodamine–phalloidin fluorescence. Taxol treated. Scale bar, 5 μm. (b) Taken from Wojciak-Stothard et al. (1995): BHK fibroblast 20 minutes after plating out. On groove ridge structure. F-actin shown by rhodamine–phalloidin fluorescence. Scale bar, 10 μm.
A similar image obtained by polarization microscopy of cells grown on flat PDMS (figure 4) shows that when the azimuth components of the image are extracted it can be seen that though much of the cell contains material aligned to the long axis of the cell, a well-known feature of such cells, there is also some material at about 90° to the long axis appearing as a short dash-like pieces. This is a birefringent structure arranged transversely across the cells,
Figure 4.
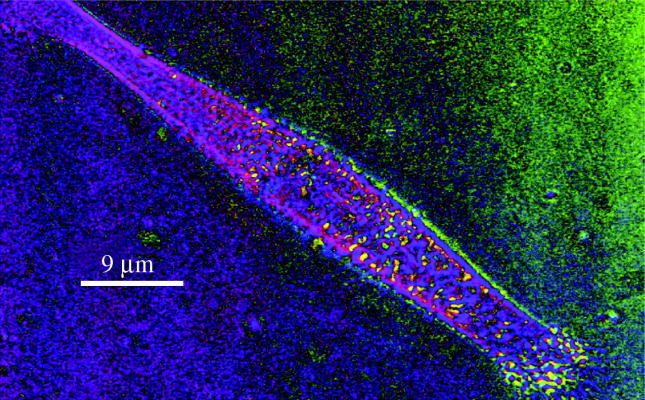
Polscope image of h-tert fibroblast growing on flat PDMS. Image in pseudocolour for azimuth of birefringence (orientation of slow ray). Yellow corresponds to 35°, blue to 310°. 100× objective.
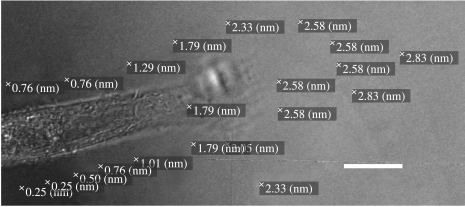
Further evidence for a contractile element running across the cells comes from force measurements by photoelastic measurements of the strain induced by cellular contraction in a surrounding flat PDMS substrate. Figure 5 shows retardance measurements at various points surrounding an elongate fibroblast. It can be seen that, as expected, the forces being exerted by the cell are greatest in front of the lamellopodium, but that appreciable forces of contraction are also found along the zone to the side of the cells. This is not surprising in view of the general finding that cells viewed by interference reflection microscopy show that the central region of the cell is often ‘raised’ away from the substratum. This would not happen if contraction were in one orientation. Such uniaxial strain would tend to flatten the cell onto the substratum. The presence of a pool of medium under the cell is consistent with contraction running across the cell.
Figure 5.
Polscope view of an h-tert fibroblast growing on a PDMS substrate. Retardances stamped on image. Stamped value refers to position at top left-hand corner of each stamp. Retardances measure strain in the PDMS around the cell. Note that there is both lateral and ‘end of cell’ directed strain. 100× objective. Scale bar, 8 μm.
3. Discussion
The fluorescence and polarization (polscope) images show the following.
Regularly spaced actin ‘clumps’ related to either the contact zone between a fibroblast (epitenon or h-tert cell) growing on a grooved surface.
Actin filaments running transversely across the cell from these clumps.
When the cells are grown on a photoelastic surface (PDMS), birefringence measurements show both an end to end strain which extends into the PDMS substratum and a smaller side to side strain.
The polscope has the unique advantage that azimuth (orientation angle) can be displayed for all the polarizing structures in the cell, so that by using the pseudocolour or azimuth overlay options to display orientation of separate structures, particular orientations can be detected. The polscope images show that there are in cells growing on flat PDMS lines of transversely oriented polarizing structures arranged down the sides of the cells close to the largely lengthwise actin in the cell cortex. The fluorescence images confirm the presence of actin in the same positions.
The polscope images are obtained from live cells, and thus perhaps less open to artefact than processed ones, but on the other side of the argument, it must be admitted that other polarizing structures in the cell would also be seen by this method of microscopy. The dimensions and contrast of the part of the polscope images suggest that we are detecting actin as does the close correlation with the fluorescent actin stained images. It is also interesting to note that such transverse structures have not been seen by cryoelectron microscopy (Resch et al. 2002), but that Verkhovsky et al. (2003) did see diagonal actin structures by enhanced phase microscopy in live cells. These observations are consistent with the concept of the cytoskeleton being a cross-linked network. Karl & Bereiter-Hahn (1998) described and discussed the contraction of cells under stretch. They interpreted their results in terms of the behaviour of a net of cytoskeletal components and, indeed, nets can be described as having both longitudinal and transverse components.
The polscope measurements of strain surrounding the cell (figure 5) show how the strain extends into the substratum. Methods of measuring strain used by Dembo et al. (1996) were done on more isodiametric cells, so that the distinction between lateral and end-related strains may have been less obvious.
Finally, it is worth remarking that having both transverse and longitudinal contractile systems explains the type of image of cell contacts seem by interference reflection microscopy (Curtis 1964) in flattened cells, where the cell forms paces for medium underneath it. Contraction in both directions would, if the periphery of the cell remains attached, tend to lift the centre of the cell off the substratum. These spaces bounded by focal contacts would allow local accumulation of cell products which could be available for mineralization or as signal sources for target cells.
Acknowledgments
We thank The University of Glasgow for general support and SHEFC for funding the purchase of the polscope microscope.
References
- Albrecht-Buehler G. Group locomotion of PtK1 cells. Exp. Cell Res. 1979;122:402–407. doi: 10.1016/0014-4827(79)90319-7. [DOI] [PubMed] [Google Scholar]
- Clark P, Connolly P, Curtis A.S.G, Dow J.A.T, Wilkinson C.D.W. Topographical control of cell behaviour. I. Simple step cues. Development. 1987;99:439–448. doi: 10.1242/dev.99.3.439. [DOI] [PubMed] [Google Scholar]
- Curtis A.S.G. The mechanism of adhesion of cells to glass. J. Cell Biol. 1964;20:199–215. doi: 10.1083/jcb.20.2.199. doi:10.1083/jcb.20.2.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis A.S.G, Wilkinson C.D.W. Topographical control of cells. Biomaterials. 1998a;18:1573–1583. doi: 10.1016/s0142-9612(97)00144-0. doi:10.1016/S0142-9612(97)00144-0 [DOI] [PubMed] [Google Scholar]
- Curtis A.S.G, Wilkinson C.D.W. Topographical control of cell migration. In: Soll D.R, Wessels D, editors. Motion analysis of living cells. Wiley-Liss; New York, NY: 1998b. pp. 141–155. ch. 7. [Google Scholar]
- Curtis A, Wilkinson C, Wojciak-Stothard B. Accelerating cell movement. J. Cell. Eng. 1995;1:35–38. [Google Scholar]
- Dembo M, Oliver T, Ibarra R. Imaging the traction stresses exerted by locomoting cells with the elastic substratum method. Biophys. J. 1996;70:2008–2022. doi: 10.1016/S0006-3495(96)79767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D.E, Jamieson J.D. Cells as tensegrity structures: architectural regulation of histo-differentiation by physical forces transduced over basement membrane. In: Andersson L.C, Gahmberg C.G, Ekblom P, editors. Gene expression during normal malignant differentiation. Academic Press; Orlando, FL: 1985. pp. 13–32. [Google Scholar]
- Karl I, Bereiter-Hahn J. Cell contraction caused by microtubule disruption is accompanied by shape changes and an increased elasticity measured by scanning acoustic microscopy. Cell Biochem. Biophys. 1998;29:225–241. doi: 10.1007/BF02737896. doi:10.1159/000028728 [DOI] [PubMed] [Google Scholar]
- Lo C.W, Gilula N.B. CC4 azal teratocarcinoma stem cell differentiation in culture. II. Morphological characterization. Dev. Biol. 1980;75:93–11. doi: 10.1016/0012-1606(80)90146-3. doi:10.1016/0012-1606(80)90146-3 [DOI] [PubMed] [Google Scholar]
- Resch G.P, Goldie K.N, Krebs A, Hoenger A, Small J.V. Visualisation of the actin cytoskeleton by cryoelectron microscopy. J. Cell Sci. 2002;115:1877–1882. doi: 10.1242/jcs.115.9.1877. [DOI] [PubMed] [Google Scholar]
- Svitkina T, Rovensky Y, Bershadsky A, Vasiliev J. Transverse pattern of microfilament bundles induced in epitheliocytes by cylindrical substrata. J. Cell Sci. 1995;108:735–745. doi: 10.1242/jcs.108.2.735. [DOI] [PubMed] [Google Scholar]
- Tucker J.B. Cytoskeletal coordination & intercellular signalling during metazoan embryogenesis. J. Embyol. Exp. Morphol. 1981;65:1–25. [PubMed] [Google Scholar]
- Verkhovsky A.B, Chaga O.Y, Schaub S, Svitkina T.M, Meister J.J, Borisy G. Orientational order of the lamellipodial actin network as demonstrated in living motile cells. Mol. Biol. Cell. 2003;14:4667–4675. doi: 10.1091/mbc.E02-10-0630. doi:10.1091/mbc.E02-10-0630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Curtis A.S.G, Monaghan W, McGrath M, Sommer I, Wilkinson C.D.W. Role of the cytoskeleton in the reaction of fibroblasts to multiple grooved substrata. Cell Motil. Cytoskeleton. 1995;31:147–158. doi: 10.1002/cm.970310207. doi:10.1002/cm.970310207 [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Curtis A, Monaghan W, MacDonald K, Wilkinson C. Guidance and activation of murine macrophages by nanometric scale tomography. Exp. Cell Res. 1996;223:426–435. doi: 10.1006/excr.1996.0098. [DOI] [PubMed] [Google Scholar]