Abstract
Individuals with multiple smokers among first-degree relatives (FH+) are significantly more likely to be persistent smokers themselves. The mechanisms underlying this relationship are unknown. An independent line of research has suggested that persistent smoking is more common among smokers with heightened levels of cigarette craving after being exposed to smoking cues and stressors. The present study experimentally tested the hypothesis that FH+ smokers would exhibit stronger stress-and cue-induced craving reactions compared to FH− Smokers. We also explored gender and ethnicity-related differences in these effects. To that end, 160 smokers were recruited by advertisement and exposed to neutral (changing a light bulb), stressful (dental work), and smoking (lighting up after a meal) situations, using script-guided imagery under controlled laboratory conditions. Participants completed craving questionnaires before and after each condition. Supporting the hypotheses, even after controlling smoking history and strength of habit, FH+ Smokers (n=86) displayed stronger craving reactions to both dental and smoking imagery (ps<0.05) than FH− Smokers (n=74). Interestingly, women had higher stress-, but not smoking-cue-induced cravings, than men, with FH+ women exhibiting the highest levels of stress-induced craving. Findings suggest a mechanism through which a family history of smoking leads to poorer cessation success, especially among women.
Keywords: craving, family history, gender, smoking, stress, cues
1. Introduction
Tobacco smoking is the leading preventable cause of morbidity and mortality in the United States (American Cancer Society, 2006). According to a recent Surgeon General's report, an estimated 440,000 Americans die each year as a result of smoking-related illnesses. Despite the clear negative consequences of tobacco, a significant subset of the population continues to smoke (U.S. Department of Health and Human Services, 2000). Moreover, although the majority of smokers express a strong desire to quit, only about 5-15% are successful at maintaining abstinence durations of one year or more, even after employing some form of smoking cessation aid (Zhu et al. 2000).
Numerous studies have demonstrated that individuals with histories of cigarette smoking in first-degree relatives are at particular risk of persistent smoking compared to those without such histories (Heath & Martin 1993; Chassin et al. 1994, 1996; Kendler et al. 1999). Shared environmental influences, along with genetic factors, have been emphasized as important contributors to persistent smoking (Heath & Martin 1993; Kendler et al. 1999). Heritability estimates vary, ranging from 0.53 to 0.72 (Heath & Martin 1993; Kendler et al. 1999). Despite the strong data for increased risk of persistent smoking in this group, the mechanisms underlying the increased risk are not yet known.
Clinical reports, as well initial empirical evidence, have suggested that environmental cues (e.g., the sight or smell of one's preferred brand of cigarettes) can trigger strong craving reactions, which, in the context of a quit attempt, may pose a serious barrier to maintaining abstinence (e.g., Drummond, 2001). This phenomenon can be reliably modeled under laboratory conditions, in which smokers are exposed to smoking cues and report their craving levels (Carter & Tiffany, 1999). It has long been theorized that the magnitude of these cue-induced craving reactions may be predictive of smoking cessation failure (Drummond, 2001). While yet to be well established, there is some evidence in support of this theory. For example, Abrams et al. (1988), in a prospective study, found that participants with heightened cue-induced craving reactions were significantly less likely to succeed during a subsequent quit attempt. Similarly, exposure to stress has been suggested as a major contributor to cigarette craving and smoking cessation failure (Kassel et al., 2003; Stewart, 2003; Sinha, 2001). While data definitively linking stress-induced cigarette craving to smoking cessation outcomes is lacking, experimental animal (e.g., Shaham & Stewart, 1995) and human (e.g., Sinha et al., 2006) studies of other substances generally support this view.
Based on these lines of research, we (Erblich et al., 2003) have previously hypothesized that heightened levels of stress-induced cigarette craving might be one possible mechanism underlying familial risk of persisent smoking. Consistent with this possibility, we found that smokers with family histories including at least two first-degree relatives who smoked had significantly higher levels of laboratory stress-induced craving than smokers without such family histories. Evidence for a genetic contribution to this effect has also been reported (Erblich et al., 2004). Consistent with the role of dopamine in stress- and cue-induced drug seeking in animal (see Self & Nestler, 1998) and human (see McLernon & Gilbert, 2005) studies, we found that carriers of genetic polymorphisms in the dopamine signaling pathway had heightened stress-induced (Erblich et al., 2004) and cue-induced (Erblich et al., 2005) cigarette cravings.
Based on these results, the present study aimed to replicate our previous findings of heightened stress-induced craving among smokers with family histories of smoking in an independent sample, and to extend them, by concurrently examining contributions of family history to levels of cue-induced craving. In addition, given the paucity of literature on the potential effects of ethnicity and gender on cue- and stress-induced craving, we explored the possibility that these factors would further modify participants' response in this experimental model. Indeed, accumulating evidence suggests that quit rates are lower for women than for men (Wetter et al., 1999), and for African Americans, compared to other racial/ethnic groups (U.S. Department of Health and Human Services, 1998). The exploration of potential modifying effects of gender and race/ethincity, thus, is of critical interest.
2. Methods
2.1. Overview
We employed a classic imaginal exposure paradigm (Maude-Griffin & Tiffany, 1996), in which smokers were exposed to script-guided imagery of neutral, stressful, and smoking-related subject matter, separated by 3-minute rests. Brief assessments of cigarette craving and anxiety were taken immediately before and after each exposure. Changes in craving and acute anxiety as a function of the stress and smoking imagery were measured, controlling for changes as a function of neutral imagery.
2.2. Participants
One hundred seventy-four smokers were recruited by advertisements placed in and around a major medical center participated in the study. All participants qualified for a current DSM-IV diagnosis of nicotine dependence (American Psychiatric Association, 1994) ascertained during initial telephone contact with a trained interviewer and re-ascertained in-person upon arrival. To qualify as a smoker, participants who reported smoking at least 10 cigarettes per day for at least 5 years were included (Carter et al., 2006; Malaiyandi et al., 2006; Waters et al., 2004; Waters et al., 2003). To minimize sources of sample heterogeneity, we excluded participants if they reported: 1) currently being treated (or in a program) for smoking cessation, 2) having a history of other substance abuse, 3) having a history of hospitalization for mental illness, or 4) having a history of smoking-related illness (e.g., cancer, emphysema, chronic obstructive pulmonary disease). In addition, because a goal of this study was to examine possible racial/ethnic differences in effects, we only included participants who self-reported as African-American, Caucasian, and Hispanic, the three groups for which an adequate sample size was available. As a result, 14 participants who self-identified as other ethnicities were excluded, yielding a final sample size of 160. Mean age of the sample was 38.6 years (± 0.8; range 23-67). Fifty-five percent (n=88) of participants were female, and 41% reported earning below $20,000.00 annually. Eleven percent of participants reported not completing high school, 28% reported completing high school only, and 61% reported some education beyond high school. Consistent with the demographics of our catchment area, the ethnic background of the sample was diverse: 48% of the sample (n=77) self-identified as African-American, 26% (n=41) as Caucasian, and 26% (n=42) as Hispanic. Fifty-six percent of the sample reported never being married, 15% were currently married, 29% were separated, divorced, or widowed. Participants reported smoking an average of 19.5 (± 0.8) cigarettes per day for an average of 18.8 (± 0.8) years, with an average age at initiation of 16.5 (± 0.3), and had a mean Fagerstrom score of 5.6 (± 0.2).
2.3. Measures
Demographic and Smoking Questionnaire
Participants completed questionnaires assessing demographic information (e.g., age, gender, education, income, ethnicity, marital status) and personal smoking history (e.g., age at initiation, cigarettes per day, years smoked). They also completed a self-report familial smoking pedigree which included items assessing cigarettes per day and years smoked, in reference to first-degree relatives (i.e., parent, child, sibling). Assessments were conducted blind to participants' family history status. A positive smoking history in a family member was defined in the same fashion as for the participant [minimum 10 cigarettes per day for 5 years]. None of the participants reported not knowing family members' smoking status, although their accuracy could not be independently verified. Research has demonstrated excellent concordance between child and familial reports of familial smoking behavior (Marks et al., 2003; Sandler & Shore, 1986).
Fagerstrom Test of Nicotine Dependence (FTND)
A refinement of the well-known Fagerstrom Tolerance Questionnaire, this 6-item instrument (Heatherton et al., 1991) was developed to improve upon some of the psychometric properties of the original scale. The FTND was used to assess the strength of participants' addiction. The instrument has good reliability and validity (Heatherton et al., 1991).
Minnesota Nicotine Withdrawal Questionnaire (MNWQ)
This 8-item instrument was used to assess withdrawal symptoms over the last 24 hours. The MNWQ is considered the withdrawal questionnaire of choice (Patten & Martin, 1996), and has good reliability and validity (Hughes & Hatsukami, 1986; Hughes et al., 1991).
Imagery Vividness
A 4-item face-valid vividness questionnaire was administered after each of the exposures to control for variation in strength of imagery. The instrument has been used previously by our group and has demonstrated good psychometric properties (Erblich et al., 2004).
Acute Anxiety
To rapidly assess acute anxiety responses to stress exposures, we employed a visual analog scale (Bond & Lader, 1974) consisting of a 100 mm line anchored on the left by “Not at all anxious” and on the right by “As anxious as can be.” Participants responded to the question “How anxious are you feeling right now” and “How anxious did you feel during the scene” (pre- and post-imagery, respectively) by striking a line across the continuum. VAS measures have been found to be a rapid, reliable and valid way to assess transient changes in subjective feelings (Bond & Lader, 1974; Cella & Perry, 1986), and have been used extensively in other studies measuring changes in anxiety in response to experimental challenges (e.g., Krystal et al., 1993; Wright et al., 2006).
Cigarette Craving Questionnaire
Improving on the use of single-item craving assessments, we employed a brief, 5-item (“I have a desire for a cigarette right now,” “If it were possible, I would smoke now,” “All I want right now is a cigarette,” “I have an urge for a cigarette right now,” “I crave a cigarette right now”), 0-100 instrument is designed specifically to make rapid assessments of craving during experimental manipulations. The instrument has been used as an outcome measure in previous studies by our group (e.g., Erblich et al., 2004) and others (e.g., Hutchison et al., 1999). Cronbach's alpha in the present sample was very high (alphas ranged from 0.96-0.98 for the various administrations during the study), demonstrating the measure's excellent internal consistency. As above, pre- and post-imagery versions of the instrument assessed craving “right now” (as worded above) and “during the scene,” respectively.
2.4. Procedures
Potential participants who responded to advertisements were screened via telephone to determine eligibility. The study was described over the phone, and if eligible, participants were scheduled for the study. To avoid ceiling effects in cigarette craving, they were instructed to smoke a cigarette immediately before participating in the study session. Upon arriving at the study site, participants provided written informed consent. Participants were given a CO breath test using a MicroCO monitor (MicroDirect, Lewiston, ME), and to be conservative, CO levels were considered as a potential covariate in the analyses (see below). None of the participants was excluded on the basis of their CO levels. Participants then completed the demographic, smoking history, and familial pedigree questionnaires, the FTND, and the MNWQ. We then explained the classic script-guided imagery task: participants were to listen to a 60-second script and try to imagine the scene as vividly as possible, followed by a 30-second silent period, during which they were to continue imagining the scene, drawing on personal experiences if they wished. Before beginning the exposures, participants were read a practice script (a trip to the grocery store) to familiarize them with the task. They then listened to the neutral (changing a light bulb), stress (trip to a dentist), and smoking-related (lighting up after a meal) scripts, separated by 3-minute recovery periods, during which time participants viewed a nature video (Hannan, 1999). Immediately before and after each imagery exposure, participants completed the brief anxiety and craving questionnaires. To further reduce possible carryover effects, the neutral image was always administered first, and the smoking image, which we anticipated would be the more potent elicitor of craving, was always administered last. We recognize that this approach does not control for potential order effects, but previous research from our lab (Erblich & Bovbjerg, 2004) suggests no significant effect of stimulus order. Upon completion of the study, participants were thanked for their participation, offered referrals for smoking cessation interventions, and paid an honorarium of $50.00 for their time.
2.5. Data Analysis
To address the study hypotheses, we first categorized participants on the basis of their family histories. As in our previous study (Erblich et al., 2003) participants with at least 2 first-degree relatives who smoked (i.e., parent, child, sibling) were categorized as having a family history of smoking (FH+; n=86) and all others as FH− (n=74). Participants with only one first-degree relative (n=18), more likely to indicate a “sporadic” case of family history, were considered part of the FH− group. Preliminary analyses comparing these subgroups within the FH− Group confirmed no significant stress- or cue-induced craving differences. We considered deleting this subset of smokers or conducting a full set of 3-group analyses with subsequent post-hoc contrasts, but were concerned that these approaches would not be appropriate due to significant reductions in power.
We used the same criteria for familial smoking history (at least 10 cigarettes/day for at least 5 years) as we did for personal smoking history. It should be noted that some FH− smokers (n=9) reported multiple second-degree relatives who smoked. While power considerations precluded their independent examination, excluding this small subset from the analyses did not alter any results.
Family history, gender and ethnic groups were first compared on demographic and smoking variables to identify relevant covariates to include in the primary analyses. To address the study hypotheses, we calculated change scores for anxiety and craving for both the stress and the smoking imagery scenes. These scores served as the dependent variables in separate ANOVAs, with pre-imagery scores serving as a covariate (to control change-score reliability [Pedhazur, 1997]). FH status, gender, and ethnic background, and their interaction terms, served as the predictor variables. For each analysis, additional covariates included baseline-adjusted neutral imagery changes scores, and vividness, demographic characteristics and smoking variables, if found to be significantly related to the predictors in preliminary analyses.
3. Results
3.1. Demographic Variables
As displayed in Table 1, the FH+ group in our sample had more women; Χ2(1)=6.02, p<0.02, fewer high school graduates; Χ2(1)=12.07, p<0.0005, fewer never married participants; Χ2(2)=10.65, p<0.005, and was older; t(158)=1.98, p<0.05, than the FH− group. Family history groups did not differ on ethnicity or income level. No differences were observed between men and women as a group, with the exception that fewer women than men reported household incomes above $20,000 annually; Χ2(1)=4.09, p<0.05. Caucasian participants, as a group were more likely to report completing high school than African American or Hispanic participants; Χ2(2)=8.77, p<0.02; more Caucasian participants reported earning above $20,000 annually than African American participants; Χ2(1)=10.73, p<0.002, and African American participants were older than Hispanic participants; F(2,157)=4.92, p<0.01 (Duncan's post-hoc test of African American vs. Hispanic participants: p<0.05).
Table 1.
Bivariate Relations between Family History and Demographic Variables
| Variable % or Mean (SE) |
FH− (n=74) |
FH+ (n=86) |
Male (n=72) |
Female (n=88) |
African- American (n=77) |
Caucasian (n=41) |
Hispanic (n=42) |
Overall (n=160) |
|---|---|---|---|---|---|---|---|---|
| Gender (% Female) |
45%a | 64%a | - | - | - | - | - | 55% |
| Ethnicity | ||||||||
| Af. American | 40% | 55% | 37% | 57% | - | - | - | 48% |
| Caucasian | 34% | 19% | 31% | 22% | - | - | - | 26% |
| Hispanic | 26% | 27% | 32% | 22% | - | - | - | 26% |
| Education (% comp. HS) |
76%b | 49%b | 60% | 62% | 53%c | 80%c,d | 57%d | 61% |
| Income (% >$20K/yr) |
65% | 54% | 68%e | 52%e | 46%f | 78%f | 64% | 59% |
| Marital Status | ||||||||
| Never | 69%g | 44%g | 57% | 54% | 52% | 61% | 57% | 56% |
| Currently | 8% | 21% | 17% | 14% | 12% | 19% | 17% | 15% |
| Formerly | 23% | 35% | 26% | 32% | 36% | 20% | 26% | 29% |
| Age | 36.9h (1.3) |
40.1h (1.0) |
37.5 (1.1) |
39.5 (1.1) |
41.0i (1.1) |
37.8 (1.9) |
35.1i (1.3) |
38.6 (0.8) |
a-h Values with matching superscripts differ at p < 0.05
3.2. Descriptive statistics—Background smoking variables
Consistent with their higher risk of persistent smoking, FH+ participants smoked more cigarettes per day; t(158)=3.70, p<0.0005, had smoked for a greater number of years; t(158)=2.33, p<0.03, and were more highly dependent, as measured by the FTND; t(158)=3.65, p<0.0005, than FH− participants. In addition, women had higher FTND scores; t(158)=2.00, p<0.05, but slightly lower breath CO levels at the start of the study; t(158)=2.28, p<0.03, compared to men. Finally, Caucasian participants had lower FTND scores than either African American or Hispanic participants in our sample; F(2,157)=5.17, p<0.01 (Duncan's post-hoc test of Caucasian vs. African American and Hispanic groups: p's<0.05). Means and standard errors for these analyses can be found in Table 2.
Table 2.
Bivariate Relations between Family History, Demographics, and Smoking Variables
| Variable Mean ± (SE) |
FH− (n=74) |
FH+ (n=86) |
Male (n=72) |
Female (n=88) |
African- American (n=77) |
Caucasian (n=41) |
Hispanic (n=42) |
Overall (n=160 ) |
|---|---|---|---|---|---|---|---|---|
| Cigarettes per day |
16.6a (0.9) |
22.1a (1.1) |
19.3 (0.9) |
19.7 (1.2) |
19.6 (1.0) |
18.6 (1.3) |
20.3 (1.9) |
19.5 (0.8) |
| Years of Smoking |
16.9b (1.2) |
20.6b (1.1) |
18.1 (1.2) |
19.5 (1.1) |
19.9 (1.2) |
18.9 (1.8) |
16.9 (1.3) |
18.8 (0.8) |
| Age at initiation |
17.0 (0.4) |
16.0 (0.7) |
17.1 (0.6) |
16.0 (0.4) |
16.3 (0.5) |
16.9 (0.6) |
16.4 (0.7) |
16.5 (0.3) |
| FTND Score | 5.0c (0.2) |
6.2c (0.2) |
5.3d (0.2) |
6.0d (0.2) |
6.0e (0.2) |
4.7e,f (0.3) |
5.9 f (0.4) |
5.6 (0.2) |
| MNWQ Score | 17.3 (0.7) |
18.5 (0.7) |
17.3 (0.7) |
18.5 (0.7) |
17.8 (0.7) |
18.3 (1.0) |
17.9 (1.0) |
18.0 (0.5) |
| Breath CO (Start of Study) |
11.6 (0.9) |
12.3 (0.9) |
13.9g (1.0) |
10.9g (0.7) |
11.8 (0.9) |
12.6 (1.1) |
11.7 (1.2) |
12.0 (0.6) |
a-g Values with matching superscripts differ at p < 0.05
Results of the primary analyses are reported after adjusting for the demographic and smoking-related covariates; findings were equivalent, though, with or without their inclusion. Descriptive statistics for the response variables by family history, gender, and ethnicity can be found in Table 3.
Table 3.
Descriptive statistics for response variables (covariate-adjusted mean ± SE) by family history of smoking, gender, and ethnicity.
| FH− (n=74) |
FH+ (n=86) |
Male (n=72) |
Female (n=88) |
Af. Am. (n=77) |
Caucasian (n=41) |
Hispanic (n=42) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Neutral Imagery |
||||||||||||||
| Anxiety | 31.3 (3.4) |
35.9 (3.7) |
31.4 (3.5) |
28.1 (3.9) |
31.3 (3.5) |
35.7 (3.8) |
31.3 (3.2) |
28.3 (3.6) |
31.3 (3.5) |
31.7 (3.8) |
31.4 (4.9) |
31.0 (5.4) |
31.4 (4.3) |
33.4 (4.8) |
| Craving | 45.5 (3.4) |
29.5 (3.2) |
45.5 (3.6) |
25.2 (3.3) |
45.6 (3.5) |
22.0 (3.3) |
45.6 (3.2) |
32.8 (3.1) |
45.5 (3.5) |
28.7 (3.3) |
45.5 (4.9) |
20.3 (4.6) |
45.6 (4.3) |
33.0 (4.1) |
| Stress Imagery |
||||||||||||||
| Anxiety | 32.9 (4.0) |
57.7 (4.1) |
32.8 (4.3) |
60.1 (4.4) |
32.9 (4.3) |
55.4 (4.4) |
32.9 (4.1) |
62.4 (4.1) |
32.8 (4.2) |
58.6 (4.3) |
32.8 (5.7) |
59.0 (5.9) |
32.8 (5.3) |
59.0 (5.4) |
| Craving | 42.1 (3.7) |
51.5 (3.4) |
42.9 (3.9) |
63.4 (3.7) |
42.5 (3.9) |
52.1 (3.7) |
42.5 (3.7) |
62.8 (3.5) |
44.1 (3.8) |
61.5 (3.6) |
38.8 (5.2) |
54.0 (4.9) |
44.5 (4.8) |
56.8 (4.6) |
| Smoking Imagery |
||||||||||||||
| Anxiety | 37.8 (3.8) |
56.5 (4.0) |
37.8 (4.0 |
49.9 (4.2) |
37.8 (3.9) |
49.4 (4.1) |
37.8 (3.7) |
57.0 (3.8) |
37.8 (3.9) |
53.2 (4.1) |
37.8 (5.5) |
51.0 (5.7) |
37.8 (4.9) |
55.3 (5.1) |
| Craving | 48.7 (3.9) |
73.8 (2.9) |
49.4 (4.1) |
83.2 (3.1) |
47.9 (4.1) |
78.2 (3.1) |
50.1 (3.8) |
78.8 (2.9) |
50.1 (3.9) |
76.4 (3.1) |
47.5 (5.4) |
79.1 (4.1) |
52.4 (5.0) |
80.1 (3.8) |
3.3 Effects of Stress Imagery on Anxiety
The mean anxiety rating pre-imagery was 32.9 (± 2.8), compared to 57.4 (± 2.8) post imagery, for a mean increase of 24.5 (± 3.3) out of a possible 100 units, which was a significant increase, as indicated by a preliminary paired t-test t(159)=7.3, p<0.0001. As expected, the ANOVA revealed no effects of Family History, F(1, 159)=0.16, Gender, F(1, 159)=1.34, or Ethnicity F(2, 159)=0.00, p<0.99, on the anxiety change scores, confirming that the imaginal stressor was comparably anxiety provoking for the different groups in the sample.
3.4. Effects of Stress Imagery on Cigarette Craving
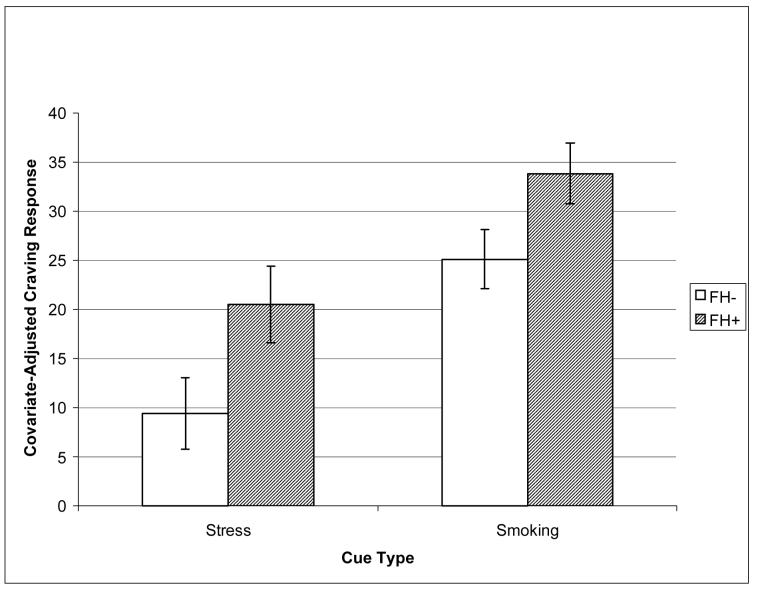
As expected, the mean craving rating pre-imagery was 43.0 (± 2.9), compared to 59.1 (± 3.0) post imagery, for a significant mean increase of 16.1 (± 2.7) out of a possible 100 units; t(159)=6.0, p<0.0001. Consistent with the study hypothesis and in line with our previous results (Erblich et al., 2003), in a factorial ANOVA, there was a significant effect of Family History, F(1, 159)=4.0, p<0.04, such that FH+ smokers exhibited stronger stress-induced craving reactions (Mean 20.5 ± 3.9) than did FH− smokers (Mean 9.4 ± 3.6). These differences between family history groups are depicted in Figure 1 (data in Table 4). Interestingly, there was a significant effect of Gender on stress-induced craving levels F(1, 159)=4.0, p<0.04, such that women (20.3 ± 3.5) had markedly higher levels than men (9.6 ± 3.8). Consistent with the additivity of the main effects of Family History and Gender, post-hoc comparisons revealed that FH+ women had significantly higher stress-induced craving levels (28.1 ± 4.7) than FH− men (3.6 ± 4.7) [p <0.001], with FH+ men (14.6 ± 5.7) and FH− women (13.5 ± 5.1) falling in between.
Figure 1.
Effects of Family History on Stress- and Smoking Cue-Induced Cigarette Craving
Table 4.
Relations between Family History, Demographics and Response Variables
| Variable [Covariate- Adjusted Mean ± (SE)] |
FH− (n=74) |
FH+ (n=86) |
Male (n=72) |
Female (n=88) |
African- American (n=77) |
Caucasian (n=41) |
Hispanic (n=42) |
Overall (n=160) |
|---|---|---|---|---|---|---|---|---|
| Stress-Induced Anxiety |
24.8 (4.1) |
27.3 (4.4) |
22.5 (4.4) |
29.5 (4.1) |
25.8 (4.3) |
26.2 (5.9) |
26.2 (5.4) |
24.5 (3.3) |
| Stress-Induced Craving |
9.4a (3.6) |
20.5a (3.9) |
9.6b (3.8) |
20.3b (3.5) |
17.4 (3.8) |
15.2 (5.5) |
12.3 (4.7) |
16.1 (2.7) |
| Smoking Cue- Induced Anxiety |
18.7 (4.0) |
12.1 (4.2) |
11.6 (4.1) |
19.2 (3.8) |
15.4 (4.1) |
13.2 (5.7) |
17.5 (5.1) |
15.1 (2.7) |
| Smoking Cue- Induced Craving |
25.1c (3.0) |
33.8c (3.1) |
30.3 (3.1) |
28.7 (2.9) |
26.3 (3.0) |
31.6 (4.3) |
30.6 (3.8) |
27.7 (2.6) |
a-c Values with matching superscripts differ at p < 0.05
There were, however, no effects of Ethnicity on stress-induced craving; F(2, 159)=0.3, p<0.72. All effects were consistent both with and without entering covariates in the ANOVA. In addition, adding the anxiety response as a covariate did not change results, demonstrating that differences between the family history groups in their craving responses to stress were not due to variability in anxiety responses (see below).
3.5. Effects of Smoking Imagery on Anxiety
The mean anxiety rating pre-smoking-imagery was 38.1 (± 2.9), compared to 53.2 (± 2.9) post imagery, for a significant increase of 15.1 (2.7) units; t(159)=5.6, p<0.0001. In a factorial ANOVA, however, there were no significant effects of Family History; F(1, 159)=1.2, p<0.28, Gender; F(1, 159)=1.7, p<0.19, or Ethnicity; F(2, 159)=0.2, p<0.86 on anxiety levels indicating that the smoking cue was comparably anxiety provoking across Groups.
3.6. Effects of Smoking Imagery on Cigarette Craving
The mean craving rating pre-imagery was 49.2 (± 3.0), compared to 77.0 (± 2.4) post imagery, for a significant increase of 27.7 (2.6) units; t(159)=10.8, p<0.0001. Consistent with the study hypothesis, we found a significant effect of family history on smoking cue-induced craving; F(1, 159)=3.8, p<0.05 (see Figure 1), such that FH+ smokers had stronger reactions (33.8 ± 3.1) than FH− smokers (25.1 ± 3.0). In contrast to our results with regard to stress-induced craving (and consistent with a previous report [Erblich & Bovbjerg, 2004]), men and women did not significantly differ in their levels of smoking-cue-induced craving; F(1, 159)=0.1, p<0.72. Finally, as was the case for stress-induced craving, there were no significant effects of ethnicity; F(1, 159)=0.6, p<0.55. As above, inclusion of the anxiety response as a covariate did not change results, demonstrating that differences were not due to variability in anxiety responses. It should be noted that in all analyses reported above, higher-order interactions between Family History, Gender, and Ethnicity, were nonsignificant (ps > 0.1).
4. DISCUSSION
The results of the current study replicated and extended findings from our previous study (Erblich et al., 2003) in a new sample of persistent smokers. Consistent with the study hypotheses, FH+ smokers exhibited both heightened levels of stress- and cue-induced cigarette cravings, compared to FH− smokers. Significant effects were observed even after covarying for baseline differences in smoking characteristics in the study sample (e.g., cigarettes per day, FTND). Together with our previous findings (Erblich et al., 2003), these results suggest a possible mechanism underlying familial risk for persistent smoking: heightened craving responses to environmental stimuli. It must be emphasized, however, that additional research is necessary to determine the extent to which the heightened craving responses in FH+ individuals have a genetic or environmental etiology. Recent studies from our lab (Erblich et al., 2004; 2005) suggest that genetic factors may contibute significantly to these effects.
A second important finding in this study is that women exhibited stronger stress-induced craving reactions than men, but did not differ on their levels of cue-induced craving. Although these findings need to be replicated across additional stress and cue stimuli, the results are consistent with an emerging literature on gender differences in the motivation for smoking. For example, Perkins (1996) argues that women smoke more for nonpharmacological reasons than men, and that negative affect may be a more important precursor to relapse for women than men. Additive effects of family history and gender in the present study suggest that FH+ women may have the highest levels of stress-induced craving, which raises the possibilty that this subgroup of women may be particularly vulnerable to the effects of stress on craving, which in turn might contribute to poorer success rates (Perkins, 2001). The gender differences in stress-induced craving response in the present study are also consistent with reports that nicotine replacement, which does not treat the nonpharmacological factors influencing smoking, is less effective for women than for men (Perkins et al., 1999). In addition, findings are consistent with a recent study (Byrne & Mazanov, 2003) indicating that stress was more strongly predictive of smoking behavior in women than men. Taken together, these data may ultimately be helpful in appropriately matching intervention strategies by gender (e.g., a greater focus on stress-management for women in addition to nicotine replacement). It of course, remains to be definitively established that cue- and stress-induced craving responses, measured under laboratory conditions, are predictive of smoking cessation outcomes, as cautioned above.
Examination of racial/ethnic background as a predictor of cue- and stress-induced craving failed to reveal any signifcant effects. To our knowledge, this is the first study to use a sufficiently powered sample to compare subgroups of smokers from different racial/ethnic backgrounds. In spite of data suggesting poorer cessation outcomes among African Americans (U.S. Department of Health and Human Services, 1998), findings revealed comparable levels of stress- and cue-induced craving responses between the 3 racial/ethnic groups. Findings demonstrating robust effects across the three ethnic groups in the current study, African Americans, Caucasians and Hispanics, do not provide support for a stress- or cue-induced craving mechanism explaining differences in cessation success rates.
It is important to note that in some instances, prestimulus anxiety and/or craving levels did not return completely to baseline (see Table 3; e.g., among FH+ smokers, pre-stress imagery craving levels were 42.9, but pre-smoking imagery craving levels were 49.4), suggesting, not surprisingly, a possible small carryover effect on craving levels within the study. However, substantial declines from the previous post-stimulus exposure (e.g., from 63.4 post-stress to 49.4 pre-smoking among FH+ smokers) and subsequent marked increases (e.g., to 83.2 post-smoking among FH+ smokers), argue that reactivity effects were well above and beyond any carryover effects. Moreover, as indicated above, the analyses controlled for prestimulus levels, yielding results that reflect reactivity independent of any effects of carryover into subsequent baseline periods.
Strengths of the study include the use of a well-established experimental design to help elucidate a possible mechanism for familial risk of persistent smoking. Limitations of the study include the use of self-report to identify family histories without independent confirmation, although studies have demonstrated the reliability of familial reports of smoking (e.g., Marks et al., 2003; Sandler & Shore, 1986). This study also relied on self-report to exclude individuals with histories of other drug use, which is likely to underestimate true numbers in the sample. Another limitation was the use of a single-item VAS measure to assess anxiety. While this approach is useful for rapid assessments during experimental probes with minimal participant burden, it is unlikely to capture the full breadth of a complex construct such as anxiety. In addition, while the study revealed significant differences between FH+ and FH− smokers, participants were not followed prospectively through a cessation attempt. Large scale prospective studies are now needed to definitively elucidate the pathways from familial risk to stress- and cue-induced craving, to cessation failure in the context of a quit attempt. Additional studies to more closely examine modifiers of gender effects (e.g., phase of menstrual cycle (Franklin et al., 2004) are also warranted. The results of the study described here, however, provide critical support for the effects of family history on stress-and cue-induced craving, and provide a strong rationale for larger, prospective studies of the effects of family history, as well as related genetic and environmental factors on smoking cessation success.
Acknowledgements
This work was supported in part by NIH grant nos. K07CA93387 (Erblich) and M01RR00071 (Mount Sinai General Clinical research Center), and American Cancer Society grant no. CRTG-01-153-04-CCE (Erblich). We would also like to acknowledge Francine Fernandez and Karen Feit, who assisted in data collection and manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams DB, Monti PM, Carey KB, Pinto RP, Jacobus SI. Reactivity to smoking cues and relapse: two studies of discriminant validity. Behav Res Ther. 1988;26:225–233. doi: 10.1016/0005-7967(88)90003-4. [DOI] [PubMed] [Google Scholar]
- American Cancer Society . Cancer Facts and Figures 2006. American Cancer Society; Atlanta, GA: 2006. [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47:211–218. [Google Scholar]
- Byrne DG, Mazanov J. Adolescent stress and future smoking behavior: a prospective investigation. J Psychosom Res. 2003;54:313–321. doi: 10.1016/s0022-3999(02)00411-7. [DOI] [PubMed] [Google Scholar]
- Carter BL, Robinson JD, Lam CY, Wetter DW, Tsan JY, Day SX, Cinciripini PM. A psychometric evaluation of cigarette stimuli used in a cue reactivity study. Nicotine Tob Res. 2006;8:361–369. doi: 10.1080/14622200600670215. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Cella DF, Perry SW. Reliability and concurrent validity of three visual analogue mood scales. Psychol. Rep. 1986;59:827–833. doi: 10.2466/pr0.1986.59.2.827. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson C, Sherman SJ, Mulvenon S. Family history of smoking. Health Psychol. 1996;15:478–484. doi: 10.1037//0278-6133.15.6.478. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson C, Sherman SJ, Mulvenon S. Family history of smoking and young adult smoking behavior. Psychol Addict Behav. 1994;8:102–110. [Google Scholar]
- Drobes DJ, Tiffany ST. Induction of smoking urge through imaginal and in vivo procedures: physiological and self-report manifestations. J of Abnorm Psychol. 1997;106:15–25. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- Drummond DC. Theories of drug craving, ancient and modern. Addiction. 2001;96:34–46. doi: 10.1046/j.1360-0443.2001.961333.x. [DOI] [PubMed] [Google Scholar]
- Erblich J, Bovbjerg DH. In vivo versus imaginal smoking cue exposures: is seeing believing? Exp Clin Psychopharmacol. 2003;12:208–215. doi: 10.1037/1064-1297.12.3.208. [DOI] [PubMed] [Google Scholar]
- Erblich J, Boyarsky Y, Spring B, Niaura R, Bovbjerg DH. A family history of smoking predicts heightened levels of stress-induced cigarette craving. Addiction. 2003;98:657–664. doi: 10.1046/j.1360-0443.2003.00351.x. [DOI] [PubMed] [Google Scholar]
- Erblich J, Lerman C, Self DW, Diaz GA, Bovbjerg DH. Effects of dopamine D2 receptor (DRD2) and transporter (SLC6A3) polymorphisms on smoking cue-induced cigarette craving among African American smokers. Mol Psychiatry. 2005;10:407–414. doi: 10.1038/sj.mp.4001588. [DOI] [PubMed] [Google Scholar]
- Erblich J, Lerman C, Self DW, Diaz GA, Bovbjerg DH. Stress-induced cigarette craving: effecs of DRD2TaqI RFLP and SLC6A3 VNTR polymorphisms. Pharmacogenomics J. 2004;4:102–109. doi: 10.1038/sj.tpj.6500227. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Napier K, Ehrman R, Gariti P, O'Brien CP, Childress AR. Retrospective study: influence of menstrual cycle on cue-induced cigarette craving. Nicotine Tob Res. 2004;6:171–175. doi: 10.1080/14622200310001656984. [DOI] [PubMed] [Google Scholar]
- Hannan D. Coral Sea Dreaming. DVD International; Mountain Lakes, NJ: 1999. [Google Scholar]
- Heath A, Martin N. Genetic models for the natural history of smoking: evidence for a genetic influence on smoking persistence. Addict Behav. 1993;18:19–34. doi: 10.1016/0306-4603(93)90005-t. [DOI] [PubMed] [Google Scholar]
- Heatherton T, Kozlowski LT, Frecker R, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Skoog K, Keenan R, Fenwick JW. Symptoms of tobacco withdrawal: A replication and extension. Arch Gen Psychiatry. 1991;48:52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hutchison K, Niaura R, Swift R. Smoking cues decrease prepulse inhibition of the startle response and increase subjective craving in humans. Exp Clin Psychoparmacol. 1999;7:250–256. doi: 10.1037//1064-1297.7.3.250. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kendler K, Neale M, Sullivan P, Corey L, Gardner C. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol Med. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Seibyl JP, Price LH, Woods SW, Heninger GR, Aghajanian GK, Charney DS. m-Chlorophenylpiperazine effects in neuroleptic-free schizophrenic patients. Evidence implicating serotonergic systems in the positive symptoms of schizophrenia. Arch Gen Psychiatry. 1993;50:624–635. doi: 10.1001/archpsyc.1993.01820200034004. [DOI] [PubMed] [Google Scholar]
- Malaiyandi V, Lerman C, Benowitz NL, Jepson C, Patterson F, Tyndale RF. Impact of CYP2A6 genotype on pretreatment smoking behaviour and nicotine levels from and usage of nicotine replacement therapy. Mol Psychiatry. 2006;11:400–409. doi: 10.1038/sj.mp.4001794. [DOI] [PubMed] [Google Scholar]
- Marks JL, Swan GE, Pomerleau CS, Pomerleau OF. Agreement between proband and parental self-report of smoking behavior and nicotine dependence. Nic Tob Res. 2003;5:527–533. doi: 10.1080/1462220031000118630. [DOI] [PubMed] [Google Scholar]
- Maude-Griffin P, Tiffany ST. Production of smoking urges through imagery: the impact of affect and smoking abstinence. Exp Clin Psychopharmacol. 1996;4:198–208. [Google Scholar]
- McLernan F, Gilbert D. Human functional neuroimaging in nicotine and tobacco research: basics, background, and beyond. Nicotine Tob Res. 2005;6:941–959. doi: 10.1080/14622200412331337394. [DOI] [PubMed] [Google Scholar]
- Patten CA, Martin JE. Measuring tobacco withdrawal: a review of self-report questionnaires. J Subst Abuse. 1996;8:94–113. doi: 10.1016/s0899-3289(96)90115-7. [DOI] [PubMed] [Google Scholar]
- Pedhazur E. Multiple Regression in Behavioral Research. 3rd Edition Harcourt Brace; Fort Worth, TX: 1997. [Google Scholar]
- Perkins KA. Sex differences in nicotine versus nonnicotine reinforcement as determinants of tobacco smoking. Exp Clin Psychopharmacol. 1996;4:166–177. [Google Scholar]
- Perkins KA. Smoking cessation in women: special considerations. CNS Drugs. 2001;15:391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1:301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Sandler DP, Shore DL. Quality of data on parents' smoking and drinking provided by adult offspring. Am J Epidemiol. 1986;124:768–778. doi: 10.1093/oxfordjournals.aje.a114453. [DOI] [PubMed] [Google Scholar]
- Self D, Nestler E. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119:334–41. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–31. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Stewart J. Stress and relapse to drug seeking: studies in laboratory animals shed light on mechanisms and sources of long-term vulnerability. Am J Addict. 2003;12:1–17. [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . Reducing Tobacco Use: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2000. [Google Scholar]
- US Department of Health and Human Services . Tobacco use among US racial/ethnic minority groups—African Americans, Pacific Islanders, and Hispanics: a report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 1998. [Google Scholar]
- Waters AJ, Shiffman S, Bradley BP, Mogg K. Attentional shifts to smoking cues in smokers. Addiction. 2003;98:1409–1417. doi: 10.1046/j.1360-0443.2003.00465.x. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. J. Cons. Clin. Psychol. 2004;72:1136–1143. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Fiore MC, Young TB, McClure JB, de Moor CA, Baker TB. Gender differences in response to nicotine replacement therapy: objective and subjective indexes of tobacco withdrawal. Exp Clin Psychopharmacol. 1999;7:135–144. doi: 10.1037//1064-1297.7.2.135. [DOI] [PubMed] [Google Scholar]
- Wright CE, Valdimarsdottir HB, Erblich J, Bovbjerg DH. Poor sleep the night before an experimental stress task is associated with reduced cortisol reactivity in healthy women. Biological Psychology. 2006 Sep 29; doi: 10.1016/j.biopsycho.2006.08.003. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Zhu S, Melcer T, Sun J, Rosbrook B, Pierce J. Smoking cessation with and without assistance: a population-based analysis. Am J Prev Med. 2000;18:305–311. doi: 10.1016/s0749-3797(00)00124-0. [DOI] [PubMed] [Google Scholar]