Abstract
Since the discovery of the vitamin D receptor (VDR) in mammary cells, the role of the vitamin D signaling pathway in normal glandular function and in breast cancer has been extensively explored. In vitro studies have demonstrated that the VDR ligand, 1,25(OH)2D3, modulates key proteins involved in signaling proliferation, differentiation and survival of normal mammary epithelial cells. Anti-proliferative and pro-differentiating effects of 1,25(OH)2D3 have also been observed in VDR positive breast cancer cells, indicating that transformation per se does not abolish vitamin D signaling. However, many breast cancer cell lines are less sensitive to 1,25(OH)2D3 than normal mammary epithelial cells. Reduced sensitivity to 1,25(OH)2D3 has been linked to alterations in vitamin D metabolizing enzymes as well as down regulation of VDR expression or function. In this report, we describe results from a proteomics screening approach used to search for proteins involved in dictating sensitivity or resistance to vitamin D mediated apoptosis in breast cancer cells. Several proteins not previously linked to 1,25(OH)2D3 signaling were identified with this approach, and a distinct subset of proteins was linked to 1,25(OH)2D3 resistance. Follow-up studies to determine the relevance of these proteins to vitamin D signaling in general are in progress.
Keywords: 1, 25-dihydroxyvitamin D, VDR, apoptosis, mammary
Overview: cell regulatory functions of 1,25(OH)2D3
Since the discovery of the VDR in mammary cells, the role of the vitamin D signaling pathway in normal glandular function and in breast cancer has been extensively explored. In most VDR positive epithelial cells, 1,25(OH)2D3 mediates anti-proliferative effects, and it may subsequently trigger differentiation or apoptosis. Expression profiling of breast, prostate, colon and squamous carcinoma cells by microarray technology has identified 1,25(OH)2D3 responsive gene clusters involved in regulation of cell cycle, differentiation, cell adhesion and immune responses [1–4], indicating a diverse and broad range of VDR target genes potentially involved in cell regulation. Anti-proliferative effects of 1,25(OH)2D3 have been linked to alterations in key cell cycle regulators which lead to de-phosphorylation of the retinoblastoma protein [5,6] and arrest of cells in G0/G1. The cyclin dependent kinase inhibitors p21 and/or p27 are genomic targets of the 1,25(OH)2D3 – VDR complex in many cell types [7,8]. 1,25(OH)2D3 also blocks mitogenic signaling, including that of estrogen, EGF and IGF-1, and up-regulates growth inhibitors such as TGFβ [9].
Induction of apoptosis by 1,25(OH)2D3
Our laboratory was the first to report the activation of apoptosis by 1,25(OH)2D3 [10], and the requirement of the VDR for this process [11]. Since our initial reports in MCF-7 breast cancer cells, cells derived from prostate cancer, squamous carcinoma, glioma and others have been shown to undergo apoptosis in response to 1,25(OH)2D3 [8,12–15]. The relevance of vitamin D mediated apoptosis in vivo has been confirmed in the context of normal mammary gland development and tumor biology. Mice lacking VDR exhibited delayed apoptosis in mammary epithelial tissue during post-lactational involution [16], indicating a role for vitamin D signaling during physiological apoptosis. Treatment of tumor bearing mice with low-calcemic vitamin D analogs induced tumor regression via activation of apoptosis [5,17,18], indicating the potential of vitamin D based drugs for manipulation of the cell death pathway in transformed cells.
These findings underscore the importance of dissecting the cellular mechanisms of VDR mediated apoptosis. In breast cancer cells, 1,25(OH)2D3 induced apoptosis involves generation of oxidative stress, dissipation of the mitochondrial membrane potential and cytochrome c release [5,10,19], features of the intrinsic (mitochondrial) pathway of apoptosis. These effects of 1,25(OH)2D3 involve redistribution of the pro-apoptotic protein Bax from cytosol to mitochondria [5,19,20], and can be prevented by overexpression of the anti-apoptotic protein Bcl-2 [21]. Downstream events involved in dismantling of the cell during 1,25(OH)2D3 induced apoptosis are mediated via several protease pathways, including caspases [15,19,22], μ-calpain [23] and cathepsins [6,24,25]. Collectively, these studies indicate that a wide variety of different signaling pathways, apoptotic regulatory proteins and proteases may contribute to 1,25(OH)2D3 mediated apoptosis depending on the specific cell type and/or context.
Models of resistance to 1,25(OH)2D3 mediated growth regulation
Although it is clear that the VDR is required for breast cancer cell responsiveness to vitamin D compounds [11] a number of breast cancer cell lines that express VDR fail to respond to the anti-proliferative effects of 1,25(OH)2D3. Data from mammary cell lines suggest that oncogenic transformation with SV40 or ras inhibits VDR signaling and induces resistance to the growth inhibitory effects of 1,25(OH)2D3 [26,27], supporting the concept that breast cancer progression may be facilitated by deregulation of the vitamin D pathway. Reduced sensitivity to 1,25(OH)2D3 has been linked to alterations in vitamin D metabolizing enzymes as well as down regulation of VDR expression or function. In an effort to understand the basis for vitamin D insensitivity, we selected resistant sub-clones by continuous culture of MCF-7 cells in 100nM 1,25(OH)2D3 [28]. The resulting MCF-7DRES cells express wild type VDR at lower levels that the parental MCF-7 cells, and do not undergo growth arrest or apoptosis in response to 1,25(OH)2D3. MCF-7DRES cells are selectively resistant to 1,25(OH)2D3 and its structural analogs, and respond to other anti-proliferative agents [28–30]. Similar results were subsequently reported in an independently derived 1,25(OH)2D3 resistant MCF-7 sub-clone labeled MCF-7/VDR [31]. Collectively, these MCF-7 sub-clones with selective 1,25(OH)2D3 resistance provide an excellent model for probing the mechanisms underlying vitamin D resistance.
Identification of novel mediators of vitamin D signaling and 1,25(OH)2D3 resistance in mammary cells
In recent studies, we used a proteomic screening approach (BD Powerblot, BD Biosciences, San Jose, CA) to identify additional mediators of vitamin D signaling and 1,25(OH)2D3 resistance in breast cancer cells. With this approach, we identified ten proteins (out of 270 proteins evaluated) that were differentially expressed between MCF-7 cells treated for 72 hours with 100nM 1,25(OH)2D3 and vehicle treated MCF-7 cells. As shown in Table 1, eight of these proteins (SHC, Stat6, cyclin A, cyclin D3, Rho-GDI, ILK, GSK3b and Grim 19) were significantly down regulated by 1,25(OH)2D3, whereas only two proteins (PTEN, Cathepsin D) were significantly up-regulated by 1,25(OH)2D3. The functions of most of the altered proteins are consistent with the anti-proliferative and pro-apoptotic effects of 1,25(OH)2D3 in the MCF-7 cell line. These include the down regulated proteins cyclin A and cyclin D3 (which drive cell cycle progression), ILK and Stat6 (which promote survival), SHC and GSK3b (proteins implicated in signal transduction cascades), and Rho-DGI (a regulator of ras signaling that is cleaved by caspases during apoptosis). Up-regulation of the lysosomal protease cathepsin D is consistent with our previous observations that 1,25(OH)2D3 and its analog EB1089 mediate lysosomal activation and up-regulate cathepsin B expression during apoptosis of MCF-7 cells [6,24]. Furthermore, EB1089 has recently been shown to induce autophagy, a form of apoptosis characterized by increased cathepsin activity, in MCF-7 cells [25]. Up-regulation of PTEN, an inhibitor of the AKT pathway, by 1,25(OH)2D3 in MCF-7 cells is consistent with up-regulation of this tumor suppressor protein during vitamin D analog induced terminal differentiation of leukemic cells [32].
Table 1. Identification of 1,25(OH)2D3 regulated proteins in MCF-7 cells.
MCF-7 cells were treated for 72 hours with either ethanol vehicle control (Con) or 100nM 1,25(OH)2D3. Each sample was analyzed in triplicate for expression of 270 apoptosis related proteins. Relative expression of each protein was normalized to several housekeeping genes. The normalized expression of each of the ten proteins listed was significantly different between vehicle and 1,25(OH)2D3 treated cells (p<0.05 by Students t test). Sign of change indicates whether the protein was up or down-regulated by 1,25(OH)2D3 in comparison to control.
| MCF-7 cells Con vs 1,25(OH)2D3 | Sign of Change | Function/Pathway |
|---|---|---|
| Grim19 | − | Mitochondrial oxidoreductase involved in electron transport, linked to apoptosis mediated by interferon β and retinoic acid. |
| Cyclin A | − | Cell cycle regulator that promotes both G1/S and G2/M transitions |
| Cyclin D3 | − | Cell cycle regulator required for G1/S transition |
| Stat 6 | − | Transcription factor activated by cytokine signaling through membrane receptors. Mediates anti-apoptotic activity of IL-4. |
| GSK-3b | − | Proline-directed serine-threonine kinase with multiple substrates, including β-catenin. |
| Integrin linked kinase(ILK) | − | Serine-threonine kinase, mediates integrin signaling to cell survival through AKT pathway |
| Rho-GDI | − | GDP dissociation inhibitor, stimulates signaling through Rho, a GTPase that regulates focal adhesions and stress fibers |
| SHC | − | Adaptor molecule in ras signal transduction |
| Cathepsin D | + | Lysosomal protease implicated in caspase independent cell death via autophagy |
| PTEN | + | Protein/lipid phosphatase that preferentially dephosphorylates phosphoinositides. Negative regulator of AKT. |
Grim-19, a protein down-regulated by 1,25(OH)2D3 in our initial screen, was of special interest since it had previously been linked to apoptosis induced by the combination of interferon β (IFN) and retinoic acid (RA) [33]. Grim-19 was significantly down regulated in MCF-7 cells treated with 1,25(OH)2D3 for 72 hours (Figure 1A). It is important to note that 1,25(OH)2D3 did not alter Grim-19 mRNA expression (data not shown), and therefore would not have been identified as a VDR target using genomic approaches. In MCF-7 cells, Grim-19 was re-distributed from mitochondria to nuclear bodies during apoptosis induced by 1,25(OH)2D3 (not shown). Expression of Grim-19 did not differ between MCF-7 and MCF-7DRES cells, which were selected for resistance to 1,25(OH)2D3 (Figure 1B). Furthermore, there was no effect of 1,25(OH)2D3 on Grim-19 in MCF-7DRES cells, but its expression was reduced in association with apoptosis triggered by IFN/RA in these cells. Surprisingly, Grim-19 was not down-regulated during apoptosis induced through cell surface TNFα death receptors (not shown). These data suggest that 1,25(OH)2D3 induced apoptosis is mechanistically distinct from that induced by TNFα, but mechanistically similar to that induced by the IFN/RA combination. These findings are worthy of follow-up considering the known cross-talk between retinoid receptors and VDR as well as the multiple effects of 1,25(OH)2D3 on cytokine signaling that have emerged from studies in other systems.
Figure 1. Expression of Grim-19 in MCF-7 cells treated with 1,25(OH)2D3 or selected for vitamin D resistance.
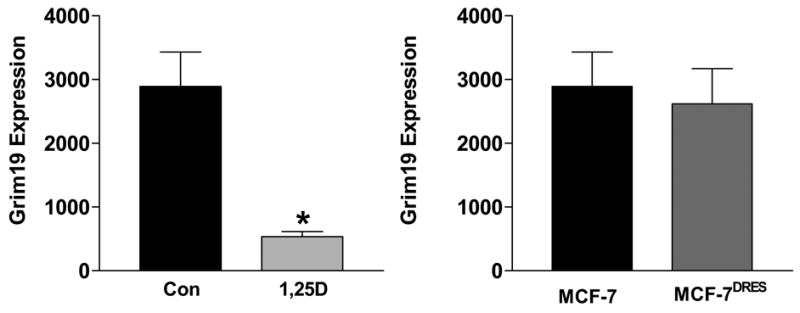
A. Lysates from MCF-7 cells treated with 100nM 1,25(OH)2D3 or ethanol vehicle for 72 hours were subjected to western blotting with an antibody directed against Grim-19. Western blots were scanned and expression of Grim-19 was normalized for protein loading. Data are expressed as mean ± standard error of triplicates. *p<0.05, control vs. 1,25(OH)2D3 treated.
B. Comparison of basal Grim-19 expression in MCF-7 cells and MCF-7DRES cells, which were selected for resistance to 100nM 1,25(OH)2D. Data was obtained as described in A.
Comparative analysis of protein expression in MCF-7 and MCF-7DRES cells under basal conditions (Table 2) identified 14 differentially expressed proteins (seven up-regulated and seven down-regulated). Proteins up-regulated in MCF-7DRES cells included those with known roles in cytoskeletal dynamics and motility (ACAP2, CapZ2), apoptosis (Bid) and mitogenic signaling (phospho-p38, MEK2), which could reasonably be expected to be the result of continued exposure to 1,25(OH)2D3 and/or the development of resistance. The potential roles of other differentially expressed proteins, such as Nip1 (a nuclear cap binding protein) and Sema 4C (a neuronal membrane receptor), are less obvious, and further studies will be needed to determine their relevance if any, to the phenotype of this sub-clone. Proteins down-regulated in MCF-7DRES cells relative to MCF-7 cells include those involved in induction of apoptosis (caspase 7, caspase 14, Rip2), those driving growth factor signal transduction pathways and/or cell cycle (cdk2, RAS-GAP) and, cytosolic proteins involved in remodeling of focal adhesions and stress fibers (Rho-GDI, ROCK-D1). Although some of these differentially expressed proteins appear to be consistent with the 1,25(OH)2D3 resistant phenotype of these cells (ie, down regulation of caspases, up-regulation of mitogenic signals), others may represent novel effectors of survival in MCF-7DRES cells. Additional work is needed to confirm differential expression of these proteins and to determine their relevance, if any, to 1,25(OH)2D3 resistance.
Table 2. Identification of proteins differentially expressed in MCF-7 versus MCF-7DRES cell.
Lysates from MCF-7 and MCF-7DRES cells were analyzed in triplicate for expression of 270 apoptosis related proteins. Relative expression of each protein was normalized to several housekeeping genes. The normalized expression of the fourteen proteins listed were significantly different between MCF-7 and MCF-7DRES cells (p<0.05 by Students t test). Sign of change indicates whether the protein was up or down-regulated in MCF-7DRES cells relative to MCF-7 cells.
| Protein | Sign of Change | Function/Pathway |
|---|---|---|
| ACAP2 | + | GTPase-activating protein, activates ARF6, a regulator of cell adhesion and motility |
| CapZ2 | + | F-actin capping protein |
| Bid | + | Pro-apoptotic Bcl-2 family protein |
| Nip1 | + | Nuclear cap binding protein |
| Phospho-p38 | + | Active form of p38 MAP kinase, mediates stress related transcription/cell cycle control |
| MEK2 | + | Dual specificity protein kinase, activates MAPK1/ERK2 and MAPK2/ERK3 |
| Sema4c | + | Semaphorin, linked to differentiation and development |
| Caspase 7 | − | Apoptotic caspase, activated by caspase 3 or caspase 10 |
| Caspase 14 | − | Apoptotic caspase, activated by caspase 8 or caspase 10 |
| Rip2 | − | Receptor interacting protein, has apoptosis inducing activity |
| Cdk2 | − | Cyclin dependent kinase, essential for G1/S transition |
| RAS-GAP | − | GTPase activating protein, down regulates ras signaling |
| Rho-GDI | − | GDP dissociation inhibitor, stimulates signaling through Rho, a GTPase that regulates focal adhesions and stress fibers |
| ROCK-D1 | − | Serine/threonine kinase, activated by Rho, a GTPase that regulates focal adhesions and stress fibers |
Summary and Future Directions
These studies have implicated several novel pathways in dictating breast cancer cellular sensitivity and resistance to the anti-proliferative and pro-apoptotic effects of 1,25(OH)2D3. Many of the proteins/pathways identified with our screening approach have now been linked to 1,25(OH)2D3 mediated apoptosis, including cathepsin mediated proteolysis, stat phosphorylation/signaling and Grim-19. Further work will clearly be necessary to clarify the role(s) of the remaining proteins in mediating the cellular actions of 1,25(OH)2D3 and how specific pathways intersect with VDR signaling in mammary cells. Complementary work in animal models, including mice with targeted ablation of the VDR, supports the concept that the vitamin D signaling pathway contributes to control of both proliferation and apoptosis in the mammary gland in vivo. Our pre-clinical studies have been complemented by emerging data from other groups suggesting that human breast cancer may be influenced by VDR genotype and vitamin D status. Collectively, these studies have reinforced the need to further define the regulation and function of the vitamin D pathway at the cellular and molecular level in relation to prevention and treatment of human breast cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Swami S, Raghavachari N, Muller UR, Bao YP, Feldman D. Vitamin D growth inhibition of breast cancer cells: gene expression patterns assessed by cDNA microarray. Breast Cancer Res Treat. 2003;80:49–62. doi: 10.1023/A:1024487118457. [DOI] [PubMed] [Google Scholar]
- 2.Lin R, Nagai Y, Sladek R, Bastien Y, Ho J, Petrecca K, Sotiropoulou G, Diamandis EP, Hudson TJ, White JH. Expression profiling in squamous carcinoma cells reveals pleiotropic effects of vitamin D3 analog EB1089 signaling on cell proliferation, differentiation, and immune system regulation. Mol Endocrinol. 2002;16:1243–1256. doi: 10.1210/mend.16.6.0874. [DOI] [PubMed] [Google Scholar]
- 3.Krishnan AV, Shinghal R, Raghavachari N, Brooks JD, Peehl DM, Feldman D. Analysis of vitamin D-regulated gene expression in LNCaP human prostate cancer cells using cDNA microarrays. Prostate. 2004;59:243–251. doi: 10.1002/pros.20006. [DOI] [PubMed] [Google Scholar]
- 4.Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de'Herreros AG, Lafarga M, Munoz A. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flanagan L, Packman K, Juba B, O'Neill S, Tenniswood M, Welsh J. Efficacy of Vitamin D compounds to modulate estrogen receptor negative breast cancer growth and invasion. J Steroid Biochem Mol Biol. 2003;84:181–192. doi: 10.1016/s0960-0760(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 6.Simboli-Campbell M, Narvaez CJ, vanWeelden K, Tenniswood M, Welsh J. Comparative effects of 1,25(OH)2D3 and EB1089 on cell cycle kinetics and apoptosis in MCF-7 breast cancer cells. Breast Cancer Res Treat. 1997;42:31–41. doi: 10.1023/a:1005772432465. [DOI] [PubMed] [Google Scholar]
- 7.Hager G, Formanek M, Gedlicka C, Thurnher D, Knerer B, Kornfehl J. 1,25(OH)2 vitamin D3 induces elevated expression of the cell cycle-regulating genes P21 and P27 in squamous carcinoma cell lines of the head and neck. Acta Oto-Laryngologica. 2001;121:103–109. doi: 10.1080/000164801300006353. [DOI] [PubMed] [Google Scholar]
- 8.Audo I, Darjatmoko SR, Schlamp CL, Lokken JM, Lindstrom MJ, Albert DM, Nickells RW. Vitamin D analogues increase p53, p21, and apoptosis in a xenograft model of human retinoblastoma. Invest Ophthalmol & Vis Sci. 2003;44:4192–4199. doi: 10.1167/iovs.02-1198. [DOI] [PubMed] [Google Scholar]
- 9.Colston KW, Hansen CM. Mechanisms implicated in the growth regulatory effects of vitamin D in breast cancer. Endocrine-Related Cancer. 2002;9:45–59. doi: 10.1677/erc.0.0090045. [DOI] [PubMed] [Google Scholar]
- 10.Welsh J, Simboli-Campbell M, Narvaez CJ, Tenniswood M. Role of apoptosis in the growth inhibitory effects of vitamin D in MCF-7 cells. Adv Exp Med Biol. 1995;375:45–52. doi: 10.1007/978-1-4899-0949-7_4. [DOI] [PubMed] [Google Scholar]
- 11.Zinser GM, McEleney K, Welsh J. Characterization of mammary tumor cell lines from wild type and vitamin D(3) receptor knockout mice. Mol Cell Endocrinol. 2003;200:67–80. doi: 10.1016/s0303-7207(02)00416-1. [DOI] [PubMed] [Google Scholar]
- 12.Pepper C, Thomas A, Hoy T, Milligan D, Bentley P, Fegan C. The vitamin D3 analog EB1089 induces apoptosis via a p53-independent mechanism involving p38 MAP kinase activation and suppression of ERK activity in B-cell chronic lymphocytic leukemia cells in vitro. Blood. 2003;101:2454–2460. doi: 10.1182/blood-2002-07-1984. [DOI] [PubMed] [Google Scholar]
- 13.Elias J, Marian B, Edling C, Lachmann B, Noe CR, Rolf SH, Schuster I. Induction of apoptosis by vitamin D metabolites and analogs in a glioma cell line. Rec Res Cancer Res. 2003;164:319–332. doi: 10.1007/978-3-642-55580-0_22. [DOI] [PubMed] [Google Scholar]
- 14.Reichrath J, Rech M, Seifert M. Vitamin D-induced apoptosis and melanoma: does calpain represent the major execution protease rather than caspases? The J Pathol. 2003;201:335–336. doi: 10.1002/path.1445. [DOI] [PubMed] [Google Scholar]
- 15.McGuire TF, Trump DL, Johnson CS. Vitamin D(3)-induced apoptosis of murine squamous cell carcinoma cells. Selective induction of caspase-dependent MEK cleavage and up-regulation of MEKK-1. J Biol Chem. 2001;276:26365–26373. doi: 10.1074/jbc.M010101200. [DOI] [PubMed] [Google Scholar]
- 16.Zinser GM, Welsh JE. Accelerated mammary gland development during pregnancy and delayed post-lactational involution in vitamin D3 receptor null mice. Mol Endocrinol. 2004;18:2208–2223. doi: 10.1210/me.2003-0469. [DOI] [PubMed] [Google Scholar]
- 17.VanWeelden K, Flanagan L, Binderup L, Tenniswood M, Welsh J. Apoptotic regression of MCF-7 xenografts in nude mice treated with the vitamin D3 analog, EB1089. Endocrinol. 1998;139:2102–2110. doi: 10.1210/endo.139.4.5892. [DOI] [PubMed] [Google Scholar]
- 18.James SY, Mercer E, Brady M, Binderup L, Colston KW. EB1089, a synthetic analogue of vitamin D, induces apoptosis in breast cancer cells in vivo and in vitro. British J Pharmacol. 1998;125:953–962. doi: 10.1038/sj.bjp.0702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narvaez CJ, Welsh J. Role of mitochondria and caspases in vitamin D-mediated apoptosis of MCF-7 breast cancer cells. J Biol Chem. 2001;276:9101–9107. doi: 10.1074/jbc.M006876200. [DOI] [PubMed] [Google Scholar]
- 20.Narvaez CJ, Byrne BM, Romu S, Valrance M, Welsh J. Induction of apoptosis by 1,25-dihydroxyvitamin D(3) in MCF-7 Vitamin D(3)-resistant variant can be sensitized by TPA. J Steroid Biochem Mol Biol. 2003;84:199–209. doi: 10.1016/s0960-0760(03)00029-3. [DOI] [PubMed] [Google Scholar]
- 21.Mathiasen IS, Lademann U, Jaattela M. Apoptosis induced by vitamin D compounds in breast cancer cells is inhibited by Bcl-2 but does not involve known caspases or p53. Cancer Res. 1999;59:4848–4856. [PubMed] [Google Scholar]
- 22.Guzey M, Kitada S, Reed JC. Apoptosis induction by 1alpha,25-dihydroxyvitamin D3 in prostate cancer. Mol Cancer Ther. 2002;1:667–677. [PubMed] [Google Scholar]
- 23.Mathiasen IS, Sergeev IN, Bastholm L, Elling F, Norman AW, Jaattela M. Calcium and calpain as key mediators of apoptosis-like death induced by vitamin D compounds in breast cancer cells. J Biol Chem. 2002;277:30738–30745. doi: 10.1074/jbc.M201558200. [DOI] [PubMed] [Google Scholar]
- 24.Simboli-Campbell M, Narvaez CJ, Tenniswood M, Welsh J. 1,25-Dihydroxyvitamin D3 induces morphological and biochemical markers of apoptosis in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 1996;58:367–376. doi: 10.1016/0960-0760(96)00055-6. [DOI] [PubMed] [Google Scholar]
- 25.Hoyer-Hansen M, Bastholm L, Mathiasen I, Elling F, Jaattela M. Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death. Cell Death Differ. 2005;12:1297–1309. doi: 10.1038/sj.cdd.4401651. [DOI] [PubMed] [Google Scholar]
- 26.Escaleira MT, Brentani MM. Vitamin D3 receptor (VDR) expression in HC-11 mammary cells: regulation by growth-modulatory agents, differentiation, and Haras transformation. Breast Cancer Res Treat. 1999;54:123–133. doi: 10.1023/a:1006198107805. [DOI] [PubMed] [Google Scholar]
- 27.Agadir A, Lazzaro G, Zheng Y, Zhang XK, Mehta R. Resistance of HBL100 human breast epithelial cells to vitamin D action. Carcinogenesis. 1999;20:577–582. doi: 10.1093/carcin/20.4.577. [DOI] [PubMed] [Google Scholar]
- 28.Narvaez CJ, Vanweelden K, Byrne I, Welsh J. Characterization of a vitamin D3-resistant MCF-7 cell line. Endocrinol. 1996;137:400–409. doi: 10.1210/endo.137.2.8593782. [DOI] [PubMed] [Google Scholar]
- 29.Narvaez CJ, Welsh J. Differential effects of 1,25-dihydroxyvitamin D3 and tetradecanoylphorbol acetate on cell cycle and apoptosis of MCF-7 cells and a vitamin D3-resistant variant. Endocrinol. 1997;138:4690–4698. doi: 10.1210/endo.138.11.5545. [DOI] [PubMed] [Google Scholar]
- 30.Nolan E, Donepudi M, VanWeelden K, Flanagan L, Welsh J. Dissociation of vitamin D3 and anti-estrogen mediated growth regulation in MCF-7 breast cancer cells. Mol Cell Biochem. 1998;188:13–20. [PubMed] [Google Scholar]
- 31.Hansen CM, Rohde L, Madsen MW, Hansen D, Colston KW, Pirianov G, Holm PK, Binderup L. MCF-7/VD(R): a new vitamin D resistant cell line. J Cell Biochem. 2001;82:422–436. doi: 10.1002/jcb.1162. [DOI] [PubMed] [Google Scholar]
- 32.Hisatake J, O'Kelly J, Uskokovic MR, Tomoyasu S, Koeffler HP. Novel vitamin D(3) analog, 21-(3-methyl-3-hydroxy-butyl)-19-nor D(3), that modulates cell growth, differentiation, apoptosis, cell cycle, and induction of PTEN in leukemic cells. Blood. 2001;97:2427–2433. doi: 10.1182/blood.v97.8.2427. [DOI] [PubMed] [Google Scholar]
- 33.Angell JE, Lindner DJ, Shapiro PS, Hofmann ER, Kalvakolanu DV. Identification of GRIM-19, a novel cell death-regulatory gene induced by the interferon-beta and retinoic acid combination, using a genetic approach. J Biol Chem. 2000;275:33416–33426. doi: 10.1074/jbc.M003929200. [DOI] [PubMed] [Google Scholar]


