Abstract
Affective stimulus pictures that differed in valence (unpleasant, neutral, pleasant) were repeated as targets in an oddball task to elicit event-related potentials (ERPs) in young female adults. Each picture target was repeated consecutively four times, with picture order counterbalanced and time-on-task influences assessed across subjects. Response time decreased from the first to second stimulus presentation and remained stable. Stimulus repetition was associated with voltage increases for N1, P2, N2, and P3, from initial to subsequent presentations. Arousal effects did not interact with stimulus repetition at any latency range. Time-on-task was associated with decreased voltages for the N2 and P3 potentials but was unaffected by stimulus valence. The findings suggest affective arousal, stimulus repetition, and time-on-task independently modulate ERP outcomes at overlapping time ranges. Theoretical implications are discussed.
Keywords: P300, affect, emotion, arousal, repetition, habituation, event-related potentials
1. Introduction
Assessment of affective brain responses in healthy and clinical populations has received considerable recent attention (Carretié et al., 2004, Cuthbert et al., 2000; Delplanque et al., 2006; Herrmann et al., 2006). Recordings of event-related potentials (ERPs) to randomly presented, unexpected, and unfamiliar picture stimuli have revealed rapid electrophysiological dissociations between affective stimulus categories. However, in contrast to common experimental practice, affective events in real life are not always unexpected or unfamiliar. Given that affective responses reflect a biological warning system, the selective neural processing of important emotional stimuli are likely to be altered across repeated exposure. For example, neuroelectric responsivity may be processed in a different temporal fashion if the stimuli become familiar and expected across trials to promote habituation effects. This theoretical perspective was assayed in the present study by assessing affective stimulus repetition and time-on-task processing, with the temporal changes in ERP structure characterized by systematically evaluating the resulting components. The empirical background for this approach is reviewed next.
1.1. Affective stimuli and ERPs
ERPs elicited by emotional pictures index affective stimulus characteristics, with valence (positive-negative) and arousal (relaxing-arousing) as the primary affective dimensions (Cuthbert et al., 1995, 2000; Palomba et al., 1997; Schupp et al., 2004). Affective influences are present from around 100 ms after stimulus onset and can persist for several seconds. At early latencies (<250 ms), pictures of negative valence elicit a more positive-going ERP than positive and neutral valence images (Carretié et al., 2004; Smith et al., 2003). High-arousing pictures elicit a more positive-going ERP beginning at 200 ms and a peak in the 400–1000 ms range (Cuthbert et al., 2000). Valence effects are less often reported than arousal effects, although pictures with positive valence have been associated with increased frontal P300 amplitudes relative to pictures with negative valence (Conroy and Polich, 2006; Delplanque et al., 2004, 2005). ERPs are sensitive to arousal level, with similar ERP arousal effects found for positive and negative valence categories (Schupp et al., 2003). Affective modulations of ERPs are relatively consistent under various task requirements, stimulus durations, and presentation rates (Cuthbert et al., 2000; Schupp et al., 2004). These findings suggest that the rapid affective ERP effects are fairly automatic responses to stimulus content and context, and less dependent on conscious stimulus evaluation and task structure (Bernat et al., 2001; Schupp et al., 2000).
1.2. Stimulus repetition effects
When a stimulus is presented repeatedly, processing changes occur with respect to habituation, priming, and novelty phenomena. How stimulus repetition may contribute to affective ERP effects is unknown, but neural reactivity can be altered by changes in stimulus novelty/familiarity or through the cumulative effects of sustained affective stimulation that could increase the vigilance of the participant (Polich and Kok, 1995; Ranganath and Rainer, 2003). Several affective ERP studies have presented stimuli multiple times but collapsed over presentations to obtain ERP averages, so that repetition effects cannot be assessed (Aftanas et al., 2001; Carretié et al., 2001, 2003, 2004; Ito et al., 1998; Keil et al., 2001; Schupp et al., 2000, 2004). Recognition memory ERP studies using neutral stimuli have found increased amplitudes for late positive components when a stimulus is recognized as having been presented previously in the stimulus series (Bentin et al., 1992; Friedman, 1990; Rugg, 1990; Segalowitz et al., 1997; Smith and Halgren, 1994). Taken together, these findings suggest that effects commonly attributed to affective arousal and valence might be modulated by stimulus repetition.
1.3. Time-on-task effects
In this context, when an intense emotional stimulus occurs repetitively affective significance declines with increased time-on-task. For example, autonomic measures exhibit rapid decreases in skin conductance and heart rate responsivity when affective visual stimuli are presented for an extended time (Bradley et al., 1996, 1993; Codispoti et al., 2006; Houtween et al., 2001; Martin-Soelch et al., 2006). Moreover, the relatively early P1 and N1 components demonstrate small decreases in amplitude after several minutes of stimulus repetition compared to large amplitude changes observed for pleasant or neutral stimuli (Carretié et al., 2003, 2004; Smith et al., 2003). These studies findings were derived from limited sets of recurring stimuli, and whether time-on-task changes occur when stimuli are equally novel across an experimental session is unclear. Although P300 amplitude also declines with target stimulus trial-by-trial sequence repetition (Duncan-Johnson and Donchin, 1982; Gonsalvez and Polich, 2002), many trial blocks must occur before component amplitude declines from time-on-task (Polich, 1989; Ravden and Polich, 1998). P300 amplitude decreases also have been observed across unpleasant, neutral and pleasant stimulus categories (Codispoti et al., 2006). How relatively early and later occurring potentials differ with respect to affective stimulus repetition and time-on-task factors is unknown
1.4. Present study
The oddball task produces a P300 from a two-stimulus oddball discrimination task is produced when target stimulus detection engages frontal attention and temporal-parietal memory operations that are affected by arousal (Corbetta et al., 2000; Kiehl et al., 2005; Polich and Kok, 1995). P300 theory suggests that component amplitude increases when a change in the neural representation of the stimulus environment occurs, with component size determined by the amount of attentional resources engaged for stimulus processing (Donchin, 1981; Polich, 2003). Given the stimulus repetition and time-on-task effects outlined above, characterizing the likely affective interaction with the memory operations elicited in a target-detection paradigm is of practical and theoretical significance.
The present study therefore was designed to: (1) extend previous findings on affective stimulus content by evaluating ERPs elicited by unpleasant, neutral, and pleasant stimuli, (2) assess how sequential target stimulus repetition may contribute to elicitation of affective ERPs, and (3) assay possible ERP time-on-task effects for affective stimuli. Affective target picture stimuli that varied in valence were presented in an oddball task with nontarget standard stimuli of the same size and spatial frequency characteristics as the targets. Each target stimulus was presented four successive times with two to nine standards occurring between each target. Hence, each stimulus picture was novel during the first presentation, but expected for the following three presentations. Based on previous findings, it was hypothesized that early (<250 ms) and late (>250 ms) ERP components would differentially reflect repeated affective processing. Valence/arousal effects should be more pronounced for the later components and yield larger amplitude changes over stimulus repetition relative to earlier potentials.
2. Method
2.1. Participants
A total of 18 female young adult undergraduates (18–27 years) received course credit for their participation. Subject gender was held constant to minimize affective variation. All were fluent English speakers, had normal or corrected-to-normal eyesight, and provided written informed consent. Participants were informed as to the contents of the stimuli and viewed several sample pictures not included in the study. Three individuals declined to participate and were replaced.
2.2. Stimuli and procedure
The target stimuli consisted of 72 unique pictures from the International Affective Picture System (IAPS; Lang et al., 1999). These stimuli were similar to those employed in previous affective ERP studies (e.g., Aftanas et al., 2001; Cuthbert et al., 2000; Delplanque et al., 2005; Mini et al., 1996; Schupp et al., 2000). Pictures that were ambiguous as to content, included frames, or only depicted human facial expressions were excluded. The non-picture standard was the same size and composed of a red/white pattern of 2 cm triangles, which was designed to vary spatial frequency characteristics similar to the picture stimuli (Conroy and Polich, 2006; Delplanque et al., 2004). The targets occurred on 18% of the trials, and the standards occurred on 82% of the trials.
Target stimulus items were selected on the basis of their normative valence and arousal ratings (1–9 scale, female judges). Pictures were selected from the IAPS such that their ratings defined negative (M=2.03, SD=0.67), neutral (M=5.06, SD=0.32), and positive (M=6.98, SD=0.67) valence categories. The corresponding arousal ratings were as followings: negative (M=6.75, SD=0.44), neutral (M=2.87, SD=0.59), and positive (M=6.47, SD=0.59). Picture stimuli were 12 × 9 cm, enclosed in a black line frame, occurred with a 1000 ms duration, and 2000 ms inter-stimulus interval.
Participants were seated in an experimental booth facing a computer screen 100 cm in front of them. They were instructed to press a keyboard button whenever a target stimulus picture occurred and to refrain from responding to the standard non-picture. Detection accuracy and response time were recorded. Each target stimulus picture was presented consecutively four times, with two to nine standard stimuli occurring between each target. Picture stimuli from each category were randomly assigned to three different stimulus blocks. Each block consisted of eight pictures from each valence category for a total of 24 pictures, with no valence category shown more than twice consecutively (Lang et al., 1999). Order of the stimulus blocks was counterbalanced across subjects. Thus, the independent variables were three affective categories, four stimulus repetitions, and three stimulus blocks.
2.3. Neuroelectric recording
Electroencephalographic (EEG) data were recorded using Grass Model 12 amplifiers and a 21-channel electrode cap that included Fz, Cz, Pz, F3/4, F7/8, C3/4, P7/8, O1/2, referenced to linked earlobes, and a forehead ground. Impedances of 10 kΩ or less were obtained with the reference electrode impedances balanced. Additional electrodes were placed at the outer canthi as well as above and below the left eye to measure electro-ocular (EOG) activity with a bipolar recording. The bandpass filter was 0.01–30.0 Hz (6 dB/octave), and the EEG was digitized at 4.0 ms per point for 1024 ms, with a 100 ms pre-stimulus baseline. Waveforms were averaged off-line, such that trials on which the EEG or EOG exceeded ±100 μV were rejected, and single-trial data were subjected to an EOG correction procedure to remove remaining artifact (Semlitsch et al., 1986). Target trials with no behavioral response in the interval of 100–900 ms were excluded.
3. Results
3.1 Response time
Figure 1 illustrates the mean response time (upper) and P300 latency (lower) from target stimuli from each affective category as a function of repetition. Response times from each subject were analyzed with a three-factor (3 affective categories × 4 repetitions × 3 time-on-task blocks) repeated measures analysis of variance. Greenhouse-Geisser corrections to the df were applied as needed, with the corrected probabilities reported. Response time did not differ among the stimulus affect categories, (F<1, p>0.10), but declined with stimulus repetition, F(3,51)=20.4, p<0.0001 (η2=0.55), with no effect of time-on-task or any interactions among these factors. Performance accuracy was uniformly high, with few errors obtained (<1.0%) and will not be considered further.
Figure 1.
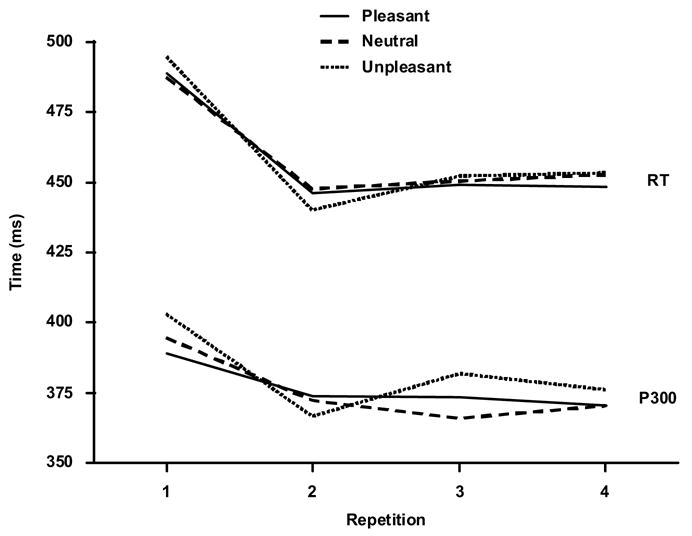
Mean response time (RT) and P3 peak latency (Pz) as a function of stimulus repetition.
3.2. ERP data
Laboratory software developed locally was used to assess the ERPs. After excluding artifactual trials or those lacking behavioral response, the ERP averages for repetition trials one to four included 90.5%, 89.8%, 88.3%, 86.5 % of the stimulus trials, respectively. A two-factor (3 valence categories × 4 repetitions) analysis of variance performed on the number of trials found no differences for affective category (F<1, p>0.10) or repetition order (F<2, p>0.10). Thus, an equivalent number of trials was available for each valence and repetition condition.
Figure 2 illustrates the grand averages from the midline electrodes, with each valence category overlapped for each repetition block. The ERP components were analyzed by measuring area amplitude relative to the mean of the pre-stimulus baseline. The latency windows employed for each component were: P1=80–120 ms, N1=120–160 ms, P2=160–220, N2=220–300, P3=300–450 ms, early slow wave=550–700, late slow wave=700–850 ms. In a separate analysis, component latencies were measured at point of maximum amplitude within each interval. Preliminary analysis of just the lateral electrodes found no reliably informative hemispheric differences, and these results will not be considered further.
Figure 2.
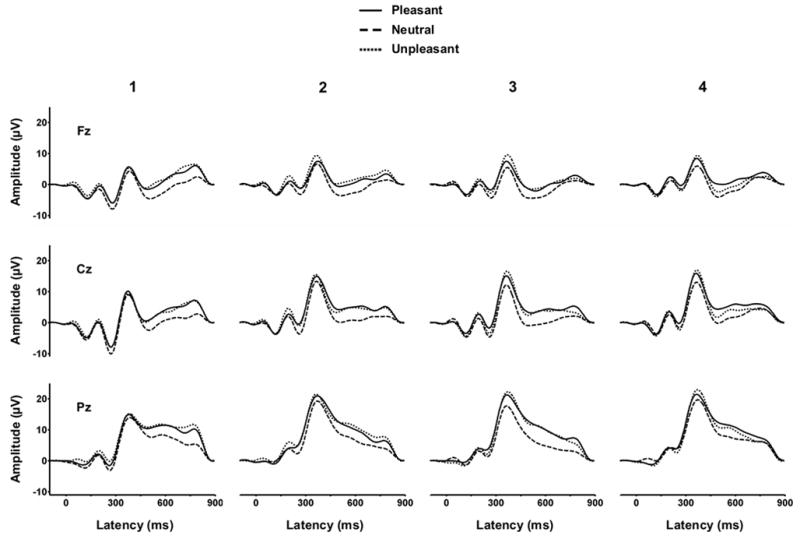
Grand average ERPs from each stimulus valence category and repetition condition for the midline electrodes (N=18).
Figure 3 illustrates the mean amplitude for the early (N1, P2, N2—top panel) and later (P3, Early SW, Late SW—lower panel) components from the Pz electrode. P1 yielded primarily electrode placement differences and is not shown. A three-factor repeated measures analysis of variance was applied to each dependent variable from each component (3 affective categories × 4 stimulus repetitions × 3 time-on-task stimulus blocks × 3 midline electrodes). Greenhouse-Geisser corrections to the df were applied as needed, with the corrected probabilities reported. The Newman-Keuls post-hoc means comparison procedure was employed to assess interactions. Table 1 summarizes the analysis of variances outcomes, which are discussed below for each component, and the corresponding η2 values presented in the text for the primary experimental variables.
Figure 3.
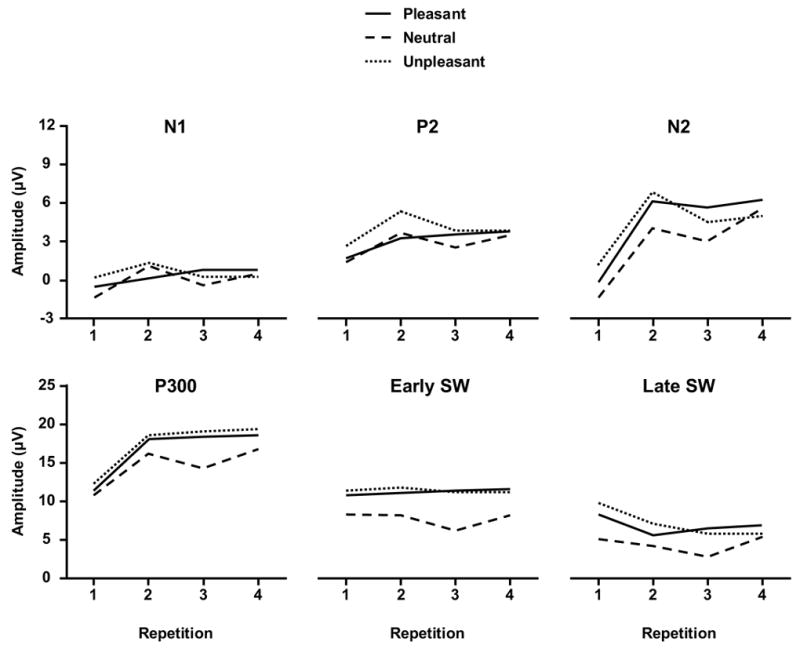
Mean amplitude voltage for the major components from each stimulus valence category as a function of repetition block from the Cz electrode for the early potentials (upper panel, 3 μV/tic mark) and Pz for the later potentials (lower panel, 5μV/tic mark). Different ordinate scales were employed for each panel to highlight the outcomes for the earlier compared to later potentials.
Table 1.
Summary of the four-factor (3 affect levels × 4 stimulus repetitions × 3 time-on-task blocks × 3 electrodes) repeated measures analyses of variation applied to amplitude data from each electrode for each component.
| P1 | N1 | P2 | N2 | P3 | Early Slow wave | Late Slow Wave | |
|---|---|---|---|---|---|---|---|
| Affect (2,34) | --- | --- | 5.42* | 6.11** | 12.24*** | 21.62*** | 12.57*** |
| Repetition (3,51) | --- | 4.22* | 9.40*** | 41.08*** | 31.28*** | --- | 3.69* |
| Time-on-Task (2,34) | --- | --- | --- | 3.52* | 4.52* | --- | --- |
| Electrode (2,34) | 28.66*** | 32.33*** | 14.20*** | 34.43*** | 80.17*** | 64.43*** | 18.57*** |
| Aff x Rep (6,102) | --- | --- | --- | --- | --- | --- | --- |
| Aff x Time (4,68) | 2.29# | 2.74* | --- | --- | --- | --- | --- |
| Rep x Time (6,102) | --- | --- | --- | --- | --- | --- | --- |
| Aff x Elec (4,68) | --- | --- | --- | --- | --- | --- | 5.34** |
| Rep x Elec (6,102) | --- | --- | 2.89* | 12.78*** | 16.17*** | 4.84** | --- |
| Time x Elec (4,68) | --- | --- | --- | --- | 4.91* | --- | --- |
p<0.10,
p<0.05,
p<0.01,
p<0.001,
P1 component (80–120 ms)
No effects of valence on P1 amplitude were obtained, although the component was larger at Pz compared to the other midline electrodes. P1 amplitude increased for the neutral stimuli but decreased for the unpleasant stimuli across repetition, with the strongest differences occurring over the parietal site. This outcome yielded a marginally significant interaction among the three variables (p<0.10). No peak latency effects were obtained.
N1 component (120–160 ms)
No effects of valence on N1 amplitude were obtained. Component amplitude changed over repetition trials, with the strongest decrease generally observed from the first to second repetition (η2=0.20). Stimulus valence and time-on-task yielded a reliable interaction, with somewhat more consistent amplitude increases observed for pleasant relative to the neutral and unpleasant stimuli (η2=0.14). N1 amplitude was largest at Pz. No peak latency effects were obtained.
P2 component (160–220 ms)
The different valence stimuli produced different P2 amplitude patterns, with unpleasant pictures yielding larger components than the neutral and pleasant pictures (η2=0.24). Repetition trials generally produced the strongest increase (becoming more positive) from the first to second repetition (η2=0.36). Amplitudes also were larger over Pz compared to other midline sites. The interaction between the repetition and electrode factors reflected stronger overall amplitude increases for the frontal and central compared to parietal. No peak latency effects were obtained.
N2 component (220–300 ms)
Component amplitude was more negative for the neutral compared to the unpleasant or pleasant stimulus conditions (η2=0.26) and became more positive with repetition (η2=0.71). Amplitudes were largest over the Pz electrode. The repetition and electrode factors yielded a significant interaction, such that larger amplitude increases were observed for the frontal and midline electrodes relative to the parietal site. N2 amplitude became more negative from the first to the third time-on-task blocks (η2=0.17). Peak latency effects were very similar to those obtained for the P3 component as described below (η2=0.22).
P3 component (300–450 ms)
Component amplitude was more positive for the unpleasant and pleasant compared to the neutral stimuli (η2=0.42). P3 amplitude increased with repetition and from the frontal to parietal electrode locations (η2=0.65). Valence category did not change with stimulus repetition. P3 amplitude declined from the first to the third time-on-task stimulus blocks (η2=0.21). This decrease was largest for the frontal/central relative to the parietal electrode to yield a reliable time-on-task x electrode interaction.
Figure 1 (lower) illustrates the mean P3 peak latency from Pz for each affect condition as a function of repetition. Peak latency did not differ among valence conditions p>0.40 (η2=0.05). Latency decreased overall across stimulus repetitions, F(3,51)=10.9, p<0.0002 (η2=0.39). The typical increase in peak latency from the frontal to parietal recording sites also was obtained, F(2,34)=5.58, p<0.02 (η2=0.25). Peak latency decreased marginally less from the first to subsequent repetitions for the frontal/central compared to the parietal midline recording site, F(6,102)=2.49, p<0.10 (η2=0.13).
Early slow wave (550–700 ms)
Area voltage was more positive for the unpleasant and pleasant compared to the neutral condition (η2=0.56). No repetition effects were obtained. Early slow wave voltage increased from the frontal to parietal electrode locations. Early slow wave area tended to decrease at the frontal electrode site and increase at the central/parietal sites across stimulus repetition to yield a significant interaction between the repetition and electrode factors.
Late slow wave (700–850 ms)
Similar to the early slow wave, area voltage was more positive for the unpleasant and pleasant stimulus conditions compared to the neutral condition (η2=0.43). However, as repetition increased, voltage areas decreased significantly (η2=0.18). Late slow wave voltage also increased from the frontal to parietal electrode locations (η2=0.52). The late slow wave area voltage tended to decrease less for the unpleasant and pleasant compared to the neutral stimuli, with this difference increasing from the frontal to the parietal recording sites to yield a significant interaction between valence and electrode.
4. Discussion
The present study employed a visual oddball task with IAPS images to assess affect, stimulus repetition, and time-on-task effects for unpleasant, neutral, and pleasant stimuli. In general, arousing stimuli demonstrated larger amplitudes from the P2 interval until stimulus offset. Stimulus repetition was associated with decreases in response time and increases for most ERP amplitudes, which were smaller for low-arousing neutral stimuli compared to high-arousing emotional stimuli and increased with stimulus repetition. Time-on-task was associated with decreases in N2 and P3 amplitude. Taken together, the findings suggest that affective arousal modulates the ERP through an extended positive shift, repetition of picture stimuli does not modulate affective processing, and time-on-task similarly reduces ERP amplitude across affective stimulus categories for the middle-latency (N2 and P3) components.
4.1. ERP effects of affect and repetition
Emotional arousal was associated with positive modulation of several ERP segments, encompassing P2, N2, P3, and the early and late slow waves. The onset of emotional arousal effects at 160–220 ms corresponds well with previous reports (Amhrein et al., 2004; Codispoti et al., 2006; Cuthbert et al., 2000; Delplanque et al., 2004; Palomba et al., 1997; Schupp et al., 2003, 2006). Repetition of target pictures influenced ERPs and response times in a way that differentiated the initial presentation from subsequent presentations. The finding of a repetition-related amplitude changes for the N1 (120–160 ms) to P3 (300–450 ms) intervals corroborates previous reports using verbal stimuli in a recognition memory paradigm (Bentin et al., 1992; Segalowitz et al., 1997; Smith and Halgren, 1989). However, affective ERP effects emerged most strongly for the relatively later components at N2 and longer latencies. The reasons for these outcomes are unclear, but they could reflect an interaction between affect and stimulus repetition that influences the overall ERP waveform by altering how components might overlap. The pattern of statistical effects across component area measures for the affective, repetition, and time-on-task independent variables support this view, as the different independent variables demonstrate their strongest effects across different ERP components.
The present findings of smaller P300 amplitudes and delayed latencies for the initial stimulus occurrence are commonly obtained when the target stimuli imposes high perceptual demands (García-Larréa and Cézanne-Bert, 1998; Wijers et al., 1989). Facilitated target discrimination results in an increased P300 amplitude, as well as a decrease in latency and response time (Magliero et al., 1984; McCarthy and Donchin, 1981). It is likely that the memory representation for the affective stimulus is modified with repetition as has been suggested by ERP priming studies (Bentin et al., 1992; Friedman, 1990; Rugg, 1990; Segalowitz et al., 1997; Smith and Halgren, 1994). However, no evidence was obtained for differential repetition modulation among the affective stimulus categories, since ERP amplitudes differed between low-arousing neutral and high-arousing emotional stimuli independently of stimulus repetition. Thus, novelty/expectancy of an affective target stimulus does not seem to influence the magnitude or onset time or arousal-related ERP effects, such that the present P300 repetition effects may result from a memory-induced increase in perceptual efficiency for expected target stimulus events.
Novel stimuli elicit a more extended network of brain regions than do repeated stimuli (Gonsalvez et al., 2005), which has been postulated to originate from neurophysiological responsivity wherein repeated stimuli engage a smaller set of neurons that have become “tuned” to specific stimulus features (Desimone, 1996). This interpretation has been suggested by neuroimaging of rapid repetition effects and behavioral facilitation for repeated stimuli (Dale et al., 2000; Wig et al., 2005). In addition, affective picture stimuli divided into 1 cm squares that have been scrambled randomly and are not recognizable elicit larger P300 components than the original pictures. Hence, that target processing of relatively complex but meaningful affective pictures may engage attentional resources to reduce P300 (Cano and Polich, 2006; Class and Polich, 2006). The increasing positive amplitudes over stimulus repetition may therefore index facilitation for the previously presented item as memory for the stimulus decreases attentional demands. The present late slow wave results are consonant with this view, as they demonstrated decreasing amplitude from the first to the subsequent presentations. Similar changes in positive slow wave activity during effortful processing conditions have been observed previously (García-Larrea and Cézanne-Bert, 1998; Lutzenberger et al., 1993; Rösler and Heil, 1991; Ruchkin et al., 1988; Strüber and Polich, 2002).
4.2. Time-on-task effects
P3 amplitudes declined primarily at fronto-central sites as a function of time-on-task, which is consistent with ERP habituation studies using simple, non-affective targets (Polich, 1989; Ravden and Polich, 1998). However, stimulus affect and time-on-task effects were also independent, which suggests that fatigue from time-on-task does not differentially diminish the arousal-related positivity in the late ERP components (Codispoti et al., 2006). This empirical outcome is important for the sometimes long-running nature of affective ERP studies.
4.3. Conclusion
The present results suggest that repeated picture targets facilitate neural and behavioral processing within an oddball paradigm. Although modulating ERPs in overlapping processing stages, affective arousal, stimulus repetition, and time-on-task do not appear to interact. The overall findings therefore suggest that arousal-related ERP effects are generated irrespective of stimulus novelty/familiarity, expectedness/unexpectedness of the picture content, and are resistant to time-on-task processing. Additional assessment of affective stimulus repetition is needed to determine how P300 amplitude (and other components) are affected by ERP memory effects, possible neural tuning operations, and affective stimulus context.
Acknowledgments
The first author was supported by a fellowship from the American-Scandinavian Foundation and Thord-Gray Memorial Fund. This study was supported by NIDA grant RO1-DA018262 and NIAAA P50-G10604. We thank Joshua Berg for help with data analysis and figure construction. This paper is publication number 18306 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aftanas LI, Varlamov AA, Pavlov SV, Makhnev NV, Reva NV. Affective picture processing: event-related synchronization within individually defined human theta band is modulated by valence dimension. Neuroscience Letters. 2001;303:115–118. doi: 10.1016/s0304-3940(01)01703-7. [DOI] [PubMed] [Google Scholar]
- Ahmrein C, Muhlberger A, Pauli P, Wiedemann G. Modulation of event-related brain potentials during affective picture processing: a complement to startle reflex and skin conductance response? International Journal of Psychophysiology. 2004;54:231–240. doi: 10.1016/j.ijpsycho.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Bentin S, Moscovitch M, Heth I. Memory with and without awareness: performance and electrophysiological evidence of savings. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1992;18:1270–1283. doi: 10.1037//0278-7393.18.6.1270. [DOI] [PubMed] [Google Scholar]
- Bernat E, Bunce S, Shevrin H. Event-related brain potentials differentiate positive and negative mood adjectives during both supraliminal and subliminal visual processing. International Journal of Psychophysiology. 2001;42:11–34. doi: 10.1016/s0167-8760(01)00133-7. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ, Cuthbert BN. Emotion, novelty, and the startle reflex: habituation in humans. Behavioral Neuroscience. 1993;107:970–980. doi: 10.1037//0735-7044.107.6.970. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Picture media and emotion: effect of a sustained affective context. Psychophysiology. 1996;33:662–670. doi: 10.1111/j.1469-8986.1996.tb02362.x. [DOI] [PubMed] [Google Scholar]
- Cano M, Polich J. Color, affect, and the P300 event-related potential. Poster presentation, Cognitive Neuroscience Society; San Francisco. 2006. p. 49. [Google Scholar]
- Carretié L, Mercado F, Tapia M, Hinojosa JA. Cerebral patterns of attentional habituation to emotional visual stimuli. Psychophysiology. 2003;40:381–388. doi: 10.1111/1469-8986.00041. [DOI] [PubMed] [Google Scholar]
- Carretié L, Hinojosa JA, Martin-Loeches M, Mercado F, Tapia M. Automatic attention to emotional stimuli: neural correlates. Human Brain Mapping. 2004;22:290–299. doi: 10.1002/hbm.20037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretié L, Mercado F, Tapia M, Hinojosa JA. Emotion, attention, and the “negativity bias” studied through event-related potentials. International Journal of Psychophysiology. 2001;41:75–85. doi: 10.1016/s0167-8760(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Class QA, Polich J. Affective pictures, stimulus characteristics, and the P300 event-related potential. Poster presentation, Cognitive Neuroscience Society; San Francisco. 2006. p. 49. [Google Scholar]
- Codispoti M, Ferrari V, Bradley MM. Repetitive picture processing: autonomic and cortical correlates. Brain Research. 2006;12:213–220. doi: 10.1016/j.brainres.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Conroy M, Polich J. Implicit memory, affective valence, and the P300 event-related brain potential. Cognition and Emotion. 2006;2006 in press. [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, McManis M, Hilman C, Bradley MM, Lang PJ. Cortical slow waves: emotional perception and processing. Psychophysiology. 1995;32:S26. [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, Halgren E. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;26:55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- Delplanque S, Lavoie ME, Hot P, Silvert L, Sequeira H. Modulation of cognitive processing by emotional valence studied through event-related potentials in humans. Neuroscience Letters. 2004;356:1–4. doi: 10.1016/j.neulet.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Delplanque S, Silvert L, Hot P, Sequeira H. Event-related P3a and P3b in response to unpredictable emotional stimuli. Biological Psychology. 2005;68:107–120. doi: 10.1016/j.biopsycho.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Delplanque S, Silvert L, Hot P, Rigoulot S, Sequeira H. Arousal and valence effects on event-related P3a and P3b during emotional categorization. International Journal of Psychophysiology. 2006;60:315–322. doi: 10.1016/j.ijpsycho.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Desimone R. Neural mechanisms for visual memory and their role in attention. Proceedings of the National Academy of Science USA. 1996;93:13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin E. Surprise!…surprise? Psychophysiology. 1981;18:493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 a manifestation of context updating? Behavioral Brain Sciences. 1988;11:355–372. [Google Scholar]
- Duncan-Johnson CC, Donchin E. The P300 component of the event-related brain potential as an index of information processing. Biological Psychology. 1982;14:1–52. doi: 10.1016/0301-0511(82)90016-3. [DOI] [PubMed] [Google Scholar]
- Friedman D. ERPs during continuous recognition memory for words. Biological Psychology. 1990;30:61–87. doi: 10.1016/0301-0511(90)90091-a. [DOI] [PubMed] [Google Scholar]
- García-Larrea L, Cézanne-Bert G. P3, positive slow wave and working memory load: a study on the functional correlates of slow wave activity. Electroencephalography and Clinical Neurophysiology. 1998;108:260–273. doi: 10.1016/s0168-5597(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Gonsalvez CJ, Polich J. The target-to-target interval is the critical determinant of the P3. Psychophysiology. 2002;39:388–396. doi: 10.1017/s0048577201393137. [DOI] [PubMed] [Google Scholar]
- Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD. Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron. 2005;47:751–761. doi: 10.1016/j.neuron.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Reif A, Jabs BE, Jacob C, Fallgatter AJ. Facial affect decoding in schizophrenic disorders: A study using event-related potentials. Psychiatry Research. 2006;141:247–252. doi: 10.1016/j.psychres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Houtween J, Reitveld S, Schoutrop M, Spiering M, Brosschot J. A repressive coping style and affective, facial, and physiological responses to looking at emotional pictures. International Journal of Psychophysiology. 2001;42:265–277. doi: 10.1016/s0167-8760(01)00150-7. [DOI] [PubMed] [Google Scholar]
- Ito TA, Larsen JT, Smith NK, Cacioppo JT. Negative information weighs more heavily on the brain: the negativity bias in evaluative categorizations. Journal of Personality and Social Psychology. 1998;75:887–900. doi: 10.1037//0022-3514.75.4.887. [DOI] [PubMed] [Google Scholar]
- Keil A, Muller MM, Gruber T, Wienbruch C, Stolarova M, Elbert T. Effects of emotional arousal in the cerebral hemispheres: a study of oscillatory brain activity and event-related potentials. Clinical Neurophysiology. 2001;112:2057–68. doi: 10.1016/s1388-2457(01)00654-x. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Stevens MC, Laurens KR, Pearlson G, Calhoun VD, Liddle PF. An adaptive reflexive processing model of neurocognitive function: supporting evidence from a large scale (N=100) fMRI study of an auditory oddball task. Neuroimage. 2005;25:899–915. doi: 10.1016/j.neuroimage.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical report A-4, The Center for Research in Psychophysiology. University of Florida; 1999. International Affective Picture System (IAPS): Instruction manual and affective ratings. [Google Scholar]
- Lutzenberger W, Roberts LE, Birbaumer N. Memory performance and area-specific regulation of slow cortical potentials: dual-task interference. International Journal of Psychophysiology. 1993;15:217–226. doi: 10.1016/0167-8760(93)90005-a. [DOI] [PubMed] [Google Scholar]
- Magliero A, Bashore T, Coles MGH, Donchin E. On the dependency of P300 latency on stimulus evaluation. Psychophysiology. 1984;21:171–186. doi: 10.1111/j.1469-8986.1984.tb00201.x. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Stöcklin M, Dammann G, Opwis K, Seifritz E. Anxiety trait modulates physiological reactions, but not habituation processes related to affective auditory stimuli. International Journal of Psychophysiology. 2006;61:87–97. doi: 10.1016/j.ijpsycho.2005.07.009. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Donchin E. A metric of thought: a comparison of P300 latency and reaction time. Science. 1981;211:77–80. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- Mini A, Palomba D, Angrilli Bravi S. Emotional processing and visual evoked brain potentials. Perceptual and Motor Skills. 1996;83:143–152. doi: 10.2466/pms.1996.83.1.143. [DOI] [PubMed] [Google Scholar]
- Palomba D, Angrilli A, Mini A. Visual evoked potentials, heart rate responses and memory to emotional pictorial stimuli. International Journal of Psychophysiology. 1997;27:55–67. doi: 10.1016/s0167-8760(97)00751-4. [DOI] [PubMed] [Google Scholar]
- Polich J. Habituation of P300 from auditory stimuli. Psychobiology. 1989;17:19–28. [Google Scholar]
- Polich J, Kok A. Biological and cognitive determinants of P3: an integrative review. Biological Psychology. 1995;41:113–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Polich J. Overview of P3a and P3b. In: Polich J, editor. Detection of Change: Event-Related Potential and fMRI Findings. Kluwer Academic Press; Boston: 2003. pp. 83–98. [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. 2006. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Rainer G. Neural mechanisms for detecting and remembering novel events. Nature Reviews Neuroscience. 2003;4:193–202. doi: 10.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- Ravden D, Polich J. Habituation of P300 from visual stimuli. International Journal of Psychophysiology. 1998;30:359–365. doi: 10.1016/s0167-8760(98)00039-7. [DOI] [PubMed] [Google Scholar]
- Ravden D, Polich J. On 300 measurement stability: habituation, intra-trial block variation, and ultradian rhythms. Biological Psychology. 1999;51:59–76. doi: 10.1016/s0301-0511(99)00015-0. [DOI] [PubMed] [Google Scholar]
- Romero R, Polich J. P3(00) habituation from auditory and visual stimuli. Physiology and Behavior. 1996;59:517–522. doi: 10.1016/0031-9384(95)02099-3. [DOI] [PubMed] [Google Scholar]
- Rösler F, Heil M. Toward a functional categorization of slow waves: taking into account past and future events. Psychophysiology. 1991;28:344–364. doi: 10.1111/j.1469-8986.1991.tb02205.x. [DOI] [PubMed] [Google Scholar]
- Ruchkin DS, Sutton S. Positive slow wave and P300: association and dissociation. In: Gaillard AWK, Ritter E, editors. Tutorials in ERP Research: Endogenous Components. North-Holland Publishing Company; Amsterdam: 1983. pp. 233–250. [Google Scholar]
- Ruchkin DS, Johnson R, Jr, Mahaffey D, Sutton S. Toward a categorization of slow waves. Psychophysiology. 1988;25:339–353. doi: 10.1111/j.1469-8986.1988.tb01253.x. [DOI] [PubMed] [Google Scholar]
- Rugg MD. Event-related brain potentials dissociate repetition effects of high and low frequency words. Memory and Cognition. 1990;18:367–379. doi: 10.3758/bf03197126. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. Attention and emotion: an ERP analysis of facilitated emotional stimulus processing. Neuroreport. 2003;14:1107–1110. doi: 10.1097/00001756-200306110-00002. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: an ERP analysis. Psychophysiology. 2004;41:441–449. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Stockburger J, Codispoti M, Junghöfer M, Weike AI, Hamm AO. Stimulus novelty and emotion perception: the near absence of habituation in the visual cortex. Neuroreport. 2006;17:365–369. doi: 10.1097/01.wnr.0000203355.88061.c6. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Van Roon P, Dywan J. The ERP late positivity: a graduated response to stimulus repetition. Neuroreport. 1997;3:757–760. doi: 10.1097/00001756-199702100-00035. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Smith ME, Halgren E. Dissociation of recognition memory compoments following temporal lobe lesions. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1989;15:50–60. doi: 10.1037//0278-7393.15.1.50. [DOI] [PubMed] [Google Scholar]
- Smith NK, Cacioppo JT, Larsen JT, Chartrand TL. May I have your attention please: electrocortical responses to positive and negative stimuli. Neuropsychologia. 2003;41:171–183. doi: 10.1016/s0028-3932(02)00147-1. [DOI] [PubMed] [Google Scholar]
- Strüber D, Polich J. P300 and slow wave from oddball and single-stimulus visual tasks: inter-stimulus interval effects. International Journal of Psychophysiology. 2002;45:187–196. doi: 10.1016/s0167-8760(02)00071-5. [DOI] [PubMed] [Google Scholar]
- Wig GS, Grafton ST, Demos KE, Kelley WM. Reductions in neural activity underlie behavioral components of repetition priming. Nature Neuroscience. 2005;8:1228–1233. doi: 10.1038/nn1515. [DOI] [PubMed] [Google Scholar]
- Wijers AA, Otten LJ, Feenstra S, Mulder G, Mulder LJM. Brain potentials during selective attention, memory search and mental rotation. Psychophysiology. 1989;26:452–467. doi: 10.1111/j.1469-8986.1989.tb01951.x. [DOI] [PubMed] [Google Scholar]


