Abstract
Background
The study of balance using stabilogram analysis is of particular interest in the study of falls. Although simple statistical parameters derived from the stabilogram have been shown to predict risk of falls, such measures offer little insight into the underlying control mechanisms responsible for degradation in balance. In contrast, fractal and non-linear time-series analysis of stabilograms, such as estimations of the Hurst exponent (H), may provide information related to the underlying motor control strategies governing postural stability. In order to be adapted for a home-based follow-up of balance, such methods need to be robust, regardless of the experimental protocol, while producing time-series that are as short as possible. The present study compares two methods of calculating H: Detrended Fluctuation Analysis (DFA) and Stabilogram Diffusion Analysis (SDA) for elderly and control subjects, as well as evaluating the effect of recording duration.
Methods
Centre of pressure signals were obtained from 90 young adult subjects and 10 elderly subjects. Data were sampled at 100 Hz for 30 s, including stepping onto and off the force plate. Estimations of H were made using sliding windows of 10, 5, and 2.5 s durations, with windows slid forward in 1-s increments. Multivariate analysis of variance was used to test for the effect of time, age and estimation method on the Hurst exponent, while the intra-class correlation coefficient (ICC) was used as a measure of reliability.
Results
Both SDA and DFA methods were able to identify differences in postural stability between control and elderly subjects for time series as short as 5 s, with ICC values as high as 0.75 for DFA.
Conclusion
Both methods would be well-suited to non-invasive longitudinal assessment of balance. In addition, reliable estimations of H were obtained from time series as short as 5 s.
Background
The study of balance deficits is of interest for many reasons, in particular for people with various pathological conditions affecting balance, and the elderly. In respect to an elderly population, falls are a major problem, in terms of both frequency and consequences. In France alone, more than two million falls are recorded among the elderly each year, leading to more than 9000 deaths [1]. Most prospective studies have attempted to identify risk factors, particularly in groups at high risk of falling [2-5]. The factors identified in these studies have often varied, mainly due to differences in methodology, diagnosis, and the study population [6]. Nevertheless, several factors are regularly cited, such as muscular weakness, a previous fall, or balance problems [2,4,7-9]. In addition, several factors that augment the risk of falling, such as visual, vestibular, or proprioceptive problems, will adversely affect balance [10-12].
Balance can be evaluated either clinically, using tests such as the "Timed Get-up-and-go" [13], "Berg Balance Scale" [14] and the "Tinetti Balance Scale" [15], or biomechanically, using a force plate to evaluate postural sway [16]. In order to measure postural sway, the movement of the centre of pressure (COP) over the support base of the subject can be evaluated [17], with the resulting stabilogram displaying the movement of the COP over time for anteroposterior (AP), mediolateral (ML), and resultant (R) directions. Simple statistical parameters derived from the stabilogram, such as the area and the shape covered by the displacement of the COP have been shown to predict risk of falls [3,18].
Although both clinical and biomechanical tests have been shown to be able to identify elderly at a greater risk of falling, such tests have yet to be used for long-term monitoring of balance. Recent technological advances might enable biomechanical tests to be used for home-based longitudinal study aimed at fall prevention. Before any such study could be envisaged there are several factors that need to be addressed. Firstly, the simple statistical parameters derived from the stabilogram offer little insight into the underlying control mechanism that is responsible for the degradation in balance observed. In addition, the duration of the testing remains problematic, with tests lasting longer than 10 s likely to decrease subject compliance. Finally, the testing equipment needs to be adapted for home-based non-invasive monitoring. The present study will address those issues related to the type of parameters that can be extracted from the stabilogram, as well as the shortest possible signal duration from which reliable parameters are able to be extracted. Information related to the development of a home-based assessment protocol can be found in [19].
In terms of the extraction of parameters that provide information related to underlying physiological control processes, over the last ten years, a number of authors have used more complex signal processing techniques to analyse the stabilogram (signal). These techniques have included Stabilogram Diffusion Analysis (SDA) [20-22], Detrended Fluctuation Analysis (DFA) [23,24], and Rescaled Range Analysis (R/S) [23]. Such methods have been used as the stabilogram has been shown to be a nonstationary time series [25,26] that displays fractal characteristics [21,27]. The advantage of such methods is that information related to the underlying motor control strategies governing postural stability could be extracted. For instance, the SDA, DFA, and R/S methods provide information on the long-term correlations contained within the time series. Despite the unpredictability of fractal signals, an element of order can exist. This order, although not evident for two successive values, implies that values depend on the global history of the series, and that long-term correlations exist. Furthermore, such long-term correlations exhibit scaling laws, first described by Mandelbrot and Van Ness [28] and termed fractional Brownian motion in the following equation [28]:
| ⟨Δx2⟩ ∝ Δt2H |
where Δx is the distance between two points separated in time by Δt, and where the Hurst exponent H is in the range 0 < H < 1.
When consecutive values are positively correlated (H > 1/2), the signal is said to show persistence, whereas negative correlations (H < 1/2) are termed anti-persistence. The special case of Brownian motion occurs when H = 1/2. The determination of the scaling exponent H of a stabilogram is of particular interest, as it can be inferred to relate to mechanisms of postural control [29].
The control of posture is very complex, involving input from the visual, vestibular, and proprioceptive systems. Collins and De Luca [30] suggested that both closed-loop and open-loop mechanisms of postural control are present in order to control postural sway. A closed-loop system implies that the system responds quickly to feedback concerning deviations from acceptable limits, and responds accordingly. In contrast, an open-loop system operates without feedback, and is therefore much less accurate than a closed-loop system. Collins and De Luca identified two distinct zones in their stabilogram diffusion plots, each of which had a different scaling exponent [30]. They interpreted the presence of short-range positive correlations (H > 1/2) in COP data as verifying the use of open-loop control mechanisms over short time periods (t < 1 s). Thereafter, long-range negative correlations were observed (H < 1/2). The explanation proposed was that posture is loosely controlled until acceptable limits are passed, upon which time a more rigid closed-loop system is applied, ensuring that postural sway values fall within more acceptable limits. The point at which these two strategies converge, the "critical time" gives an indication of the degree of laxity in control. In a subsequent study, Collins and colleagues observed longer critical times in elderly subjects, implying that a greater time spent in open-loop control could be a factor in falls in the elderly [29].
Since the pioneering work of Collins and De Luca, subsequent studies, in particular that of Delignieres and colleagues [23], have failed to find any evidence of two distinct zones of control. They suggested that the results of Collins and colleagues were due to the manner in which the biological time series was mapped as a stochastic process, and the resulting estimations of H. The method of Collins and De Luca did not take into account that biological time series have bounds imposed by physiological limits, as compared with fractional Brownian motion, which is unbounded and can therefore be expected to increase indefinitely with time. However, the upper limit imposed on the COP displacement by the support area of the feet acts as a ceiling which causes the second anti-persistent part of the stabilogram diffusion plot [23]. When there is a definite upper limit for a time series, scaling is restricted to short time intervals, beyond which values saturate at twice the variance of the data [31]. The two methods previously cited to calculate the Hurst exponent, DFA and R/S use an integrated signal, and therefore do not suffer from the bounded limitation of the second part of SDA. However, the choice of methods depends of the nature of series to which the methods are to be applied. The DFA method can be applied to both fractional Brownian motion (fBm) and fractional Gaussian motion (fGn) whereas R/S can only be applied to fGn series [32]. It is necessary, therefore to apply the DFA method first, from which the nature of the time series can be determined. If the slope α obtained from DFA is greater than 1, this indicates that the series is fBm; if α is less than 1, the series is fGn. In the present study, α obtained from DFA was greater than 1 for all subjects, thus all time series are fBm and the R/S method can not be used.
The aims of the current investigation are twofold: firstly, the SDA and DFA methods of estimating the Hurst exponent will be compared and applied to postural signals for elderly and control subjects. Secondly, the minimum recording duration needed in order to obtain reliable results will be identified for both methods.
Methods
Subjects
Ninety young control subjects and ten elderly subjects participated in the study. Anthropometric data for the two subject groups are presented in Table 1. All subjects who participated gave their written informed consent. No subjects reported any musculoskeletal or neurological conditions that precluded their participation in the study.
Table 1.
Anthropometric data for elderly and control groups.
| Gender | Number | Age | Height | Weight | |
| Control | Men | 57 | 19.8 ± 0.9 | 179.5 ± 8.2 | 71.6 ± 9.9 |
| Women | 33 | 19.6 ± 0.8 | 166.5 ± 4.9 | 58.9 ± 8.1 | |
| Elderly | Men | 4 | 80.0 ± 2.2 | 173 ± 4.5 | 81.9 ± 8.5 |
| Women | 6 | 80.8 ± 6.0 | 160.5 ± 1.2 | 68.4 ± 5.8 |
Values are means and standard deviations.
Centre of pressure data
Centre of pressure data were recorded using a Bertec 4060-08 force plate (Bertec Corporation, Columbus, OH, USA), which amplifies, filters, and digitises the raw signals from the strain gauge amplifiers inside the force plate. The resulting output is a six-channel 16-bit digital signal containing the forces and moments in the x, y, and z axes. The digital signals were subsequently converted via an external analogue amplifier (AM6501, Bertec Corporation). The initial COP signals were calculated with respect to the centre of the force-plate before normalization by subtraction of the mean.
Data acquisition and processing
Data were recorded using the ProTags™ software package (Jean-Yves Hogrel, Institut de Myologie, Paris, France) developed in Labview® (National Instruments Corporation, Austin TX, USA). Data were sampled at 100 Hz, with an 8th-order low-pass Butterworth filter with a cut-off frequency of 10 Hz. All calculations of COP data were performed with Matlab® (Mathworks Inc, Natick, MA, USA).
Experimental protocol
All subjects were tested either barefoot or wearing socks, and were instructed to stand upright with their arms by their sides in front of the force-plate, while looking at a target of a 10-cm cross fixed on the wall two meters in front of the force-plate. Upon a verbal command, subjects stepped onto the force plate, with no constraint given over foot position. Data were recorded for 30 seconds, which included both the step onto and off the force plate, and at least 20 seconds during which time subjects remained stationary in an upright posture. At the end of the trial another verbal command was given for subjects to step off the force-plate. Subjects performed the test four times, with a delay of 10 s between tests.
This protocol is similar to that which would be used for home monitoring, in that subjects were free to choose their foot position, the speed at which they stepped onto the force plate, and the length of their step onto the force plate.
Estimation of the Hurst exponent
Stabilogram Diffusion Analysis (SDA)
Collins and De Luca [30] hypothesized that the trajectory of the COP could be modelled as a correlated random walk. They proposed a simple method to calculate the scaling exponent H of a stabilogram, whereby the square of the displacement for a given time interval Δt is calculated for all possible pairs of points separated by Δt, and the average calculated as:
where N is the number of points in the vector x, and m is the interval between two values expressed as the number of data.
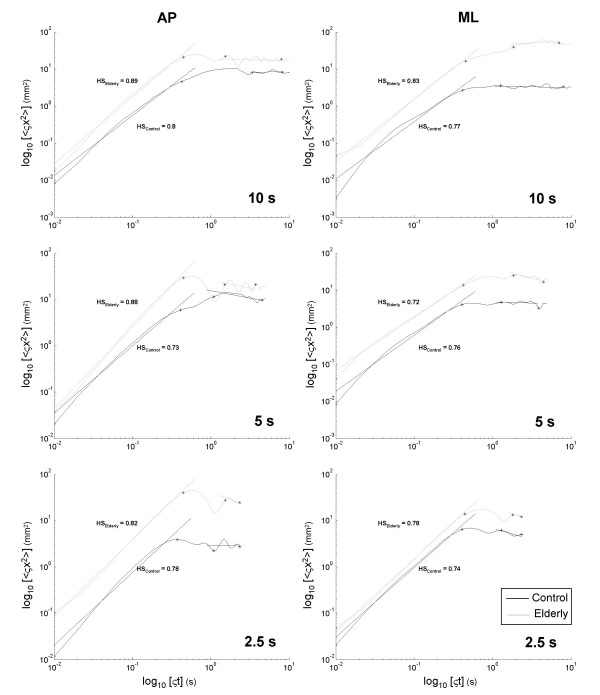
Estimations of the Hurst exponent are then obtained from the graph of Δt by <Δx>2 in log scale by calculating the slope of the short-term (HS) and long-term (HL) regions of the curve. The equations used by Collins and colleagues to estimate HS and HL contain several assumptions. The second derivative of the Δt by <Δx>2 data is used to locate four times (T1, T2, T3, T4) between which the slopes HS (T1, T2) and HL (T3, T4) are calculated. The first time, T1, is always taken as zero, while T2 is the first maximum that occurs before 1 s. The slope HS is then calculated between these points. Similarly, the slope HL is calculated between T3 and T4, where T3 is calculated as the second maximum, and T4 as the first maximum occurring after the first minimum when the signal is analyzed backwards from 9 s. If no maximum is found before 7 s, T4 is taken as 9 s. A copy of the Matlab® program, as well a detailed explanation are available at colleagues [33]. In order to estimate HL for the 5-s and 2.5-s windows, if the second maximum was not found, T3 was taken as 2.5 s for the 5-s window and 1.25 s for the 2.5-s window, while T4 was taken as 4.5 s and 2.5 s respectively, as these windows were too small to use the normal method of obtaining T4 between 7 and 9 s.
Detrended Fluctuation Analysis (DFA)
Peng and colleagues [34] introduced another method of estimating the Hurst exponent specifically for biological time series data, which they termed Detrended Fluctuation Analysis (DFA). The first step is to subtract the mean from the original series, which is then integrated:
This series is then divided into windows of equal length n. If the total length N is not divisible by n, the length N is adjusted to the largest multiple of n < N. The local trend of each window yn is obtained and subtracted from the summed series, using a line of least-squared fit to obtain the detrended fluctuation F(n) as:
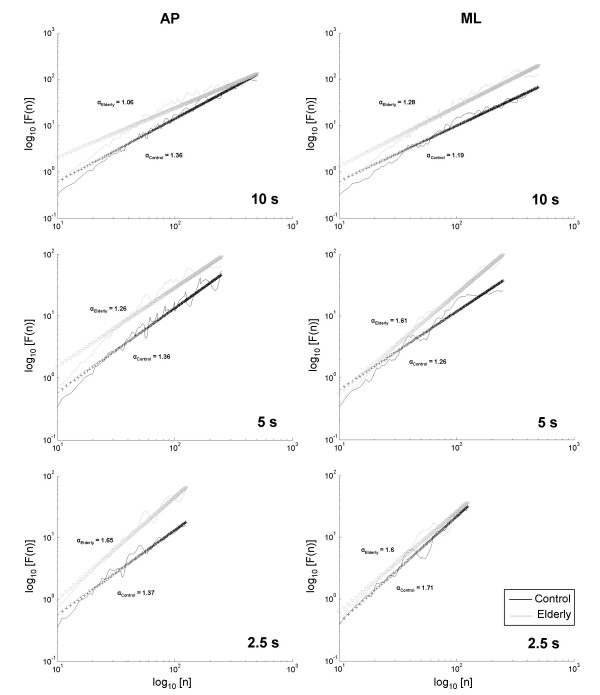
The slope of the regression line for F(n) on a log scale is calculated (α) and used to estimate the Hurst exponent, hereafter indicated as HDFA, with HDFA = α-1 for fractional Brownian motion [32].
Data analysis
Centre of pressure data were calculated from the moment the second foot contacted the force plate (FC2) for all displacement directions. The time FC2 occurred was calculated as time at which the maximum value of the second derivative of the ML signal occurred, which corresponded to the time the second foot touched the force plate when the largest acceleration of ML would occur when the COP moved rapidly towards the second foot. This instant in time was used for all AP, ML, and R displacements.
Variables were calculated for sliding windows of 10, 5, and 2.5 s, starting from FC2. The windows were slid by 1 s increments until nine windows in total were obtained. The number of windows was kept at nine in order to ensure that there were more subjects than windows for subsequent statistical analysis (10 elderly subjects were analysed). The overlap percentage for the three window sizes were 90, 80, and 60% for the windows of 10, 5, and 2.5 s respectively. Estimations of the Hurst exponent were then calculated using DFA and SDA (HS and HL) methods.
Examples of SDA and DFA plots calculated for a typical elderly and a typical control subject for all window lengths are presented in Figure 1 and Figure 2, respectively.
Figure 1.
Stabilogram diffusion analysis plots for elderly and control subjects for anteroposterior and mediolateral displacements. Data are typical values for an elderly and a control subject for 10, 5, and 2.5 s. Data are plotted in a log-log scale.
Figure 2.
Detrended fluctuation analysis plots for elderly and control subjects for anteroposterior and mediolateral displacements. Data are typical values for an elderly and a control subject for 10, 5, and 2.5 s. Data are plotted in a log-log scale.
All statistical analyses were performed with the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA). Measures of skewness and kurtosis, as well as the Kolmogorov-Smirnov test were used to check for normality [34]. Analysis of variance (ANOVA) was used to test for the effect of subject group on the estimations of the Hurst exponent, with a Bonferroni adjustment when evaluating contrasts. Repeated measures ANOVA was used to test for the effect of the sliding windows on the estimations of the Hurst exponent. The independent variables were subject group and time, with an interaction between subject group and time included. The dependent variables were estimations of the Hurst exponent using the SDA and DFA for the different displacement directions. The intra-class correlation (ICC) was used as a measure of reliability [36], with a two-way mixed model used in order to ensure an unbiased estimation of reliability [37]. Data were expressed as means and 95% confidence intervals. Alpha levels were set at p < 0.05.
Results
Sliding window effect
Stabilogram Diffusion Analysis (SDA)
There were no differences between the four trials for any of the parameters studied. Accordingly, mean values of all four trials were used for all subsequent statistical analysis, with the notable exception of the reliability analysis.
There were no significant results for HL for the effect of time nor were there any differences between window-lengths. In addition values were often less than zero, which would make interpretation difficult. Finally, HL was unable to differentiate between subject groups, therefore no further analyses were performed on HL and all subsequent references to SDA relate to HS.
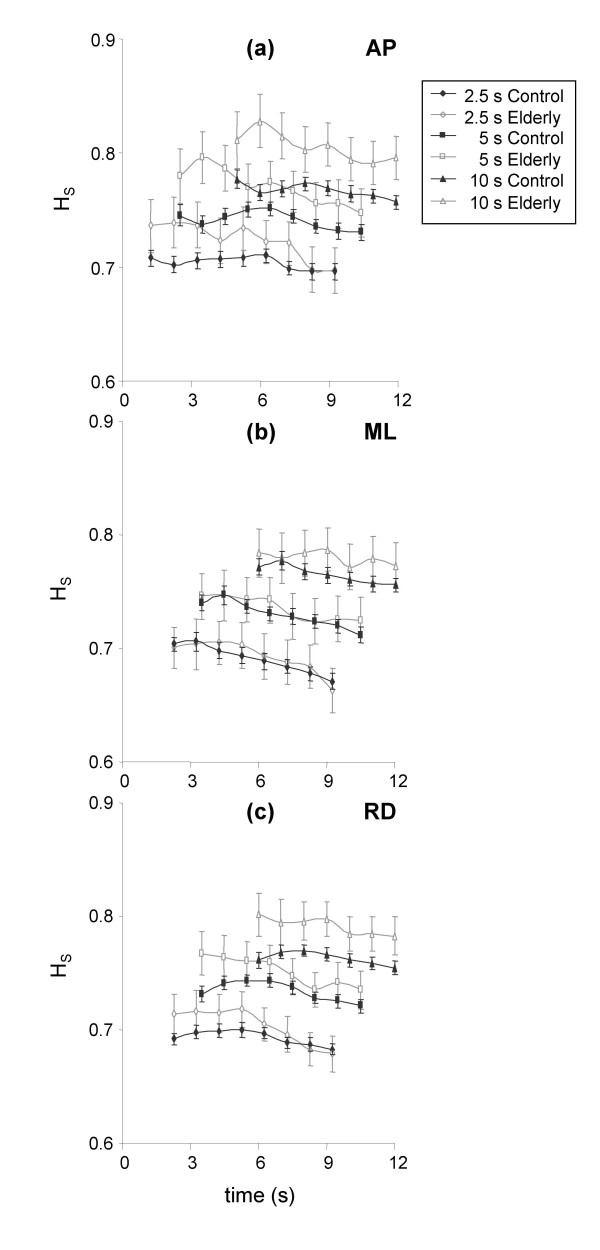
In terms of the effect of time on HS, there was a significant decrease for all window lengths for the control group for all displacement directions (Figure 3). In contrast, the effect of time on window length for the elderly group was significant only for the 2.5s window for AP and RD displacement (Figures 3a and 3c). For both the 5 s and 10 s window lengths, although HS tended to decrease, the effect was not significant. There were no interaction effects between time and subject group for HS for any window length.
Figure 3.
Evolution of HS for anteroposterior (a), mediolateral (b), and resultant (c) displacement. Data are means and 95% confidence intervals. The x axes represent time in seconds, while the y axes represent the estimation of HS. The zero values on the x axes correspond to FC2, while the x coordinate of each data point corresponds to the centre of the data window.
Detrended Fluctuation Analysis (DFA)
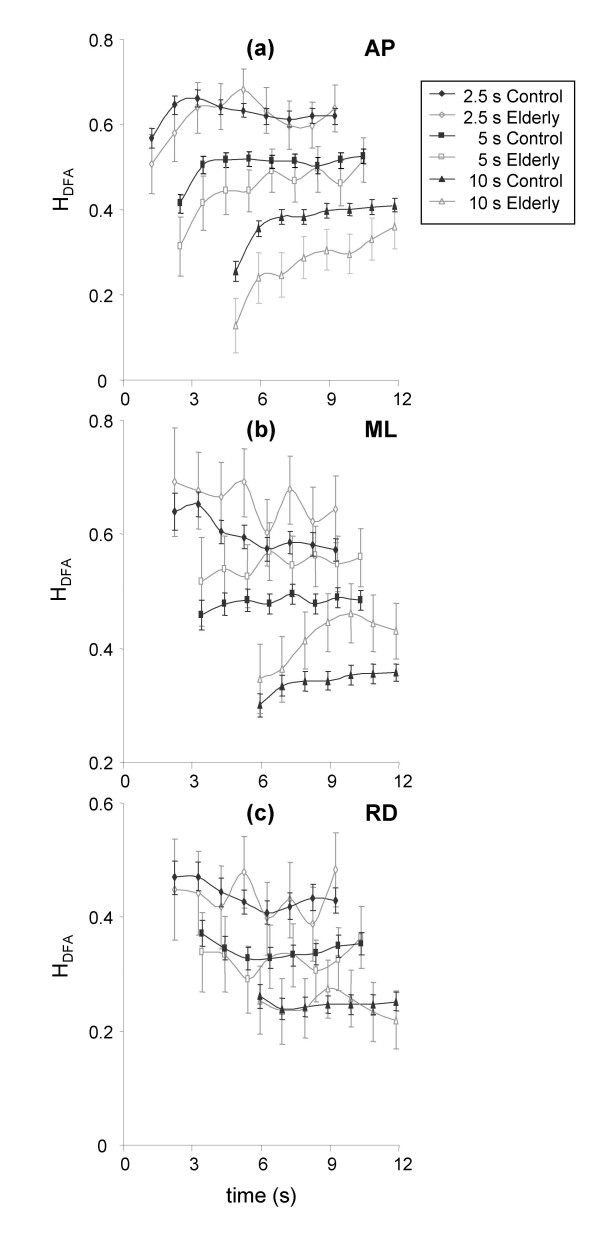
In terms of the effect of time on HDFA, there was a significant increase for all window lengths for the control group for AP displacement (Figure 4a). For ML displacement, HDFA decreased for the 2.5-s window, increased for the 10-s window, but did not change significantly for the 5-s window (Figure 4b). For the resultant displacement, HDFA decreased significantly for both the 2.5s and 5s window lengths, but not for the 10s window (Figure 4c). For the elderly subjects HDFA increased significantly for AP displacement for all window lengths (Figure 4a). In contrast, HDFA increased significantly for only the 10s window for ML displacement (Figure 4b), and had no significant change for resultant displacement (Figure 4c).
Figure 4.
Evolution of HDFA for anteroposterior (a), mediolateral (b), and resultant (c) displacement. Data are means and 95% confidence intervals. The x axes represent time in seconds, while the y axes represent the estimation of HDFA. The zero values on the x axes correspond to FC2, while the x coordinate of each data point corresponds to the centre of the data window.
There was also an interaction effect for all displacement directions for the 10s window, where the rate of increase in HDFA was greater for elderly subjects than for the controls.
Reliability analysis
The reliability analyses were performed separately for the control and elderly subject groups owing to the differences in the values of HS and HDFA between groups, which are reported in the next section of the results.
Stabilogram Diffusion Analysis (SDA)
There was no significant effect of time on the ICC values for any window length. As subsequent tests found no evidence of non-normality, ANOVA was performed on the individual ICC values for each sliding position for each window length in order to identify differences between groups and methods. In respect to differences between the control and elderly subjects, ICC values were significantly higher for the elderly for the 2.5-s window for AP and RD directions, for the 5-s window for all directions, and for the 10-s window for the RD direction (Table 2). In terms of differences between window lengths, the only significant difference was observed for control subjects for the AP direction, whereby the ICC value for the 10-s window was significantly greater than that of the 5-s window (Table 2).
Table 2.
Mean ICC values for Stabilogram Diffusion Analysis.
| Anteroposterior | Mediolateral | Resultant | ||||
| Window size (s) | Control | Elderly | Control | Elderly | Control | Elderly |
| 2.5 | 0.18 | 0.55* | 0.31 | 0.34 | 0.30 | 0.56* |
| 5 | 0.29 | 0.54* | 0.42 | 0.58* | 0.43 | 0.62* |
| 10 | 0.44† | 0.49 | 0.49 | 0.58 | 0.53 | 0.67* |
Values are means calculated for all windows of each size.
*Significantly different from control subjects.
†Significantly different from 5-s window.
Detrended Fluctuation Analysis (DFA)
As for the SDA, there was no significant effect of time on the ICC values for any window length. Statistical tests were therefore performed on the individual ICC values. Significant differences between the control and elderly subjects were observed for all window sizes for both AP and RD directions (Table 3). In respect to the effect of the window length, the only significant differences were that the ICC values for the 2.5-s window were lower than those for the 5-s window for both AP and ML directions (Table 3).
Table 3.
Mean ICC values for Detrended Fluctuation Analysis.
| Anteroposterior | Mediolateral | Resultant | ||||
| Window size (s) | Control | Elderly | Control | Elderly | Control | Elderly |
| 2.5 | 0.20† | 0.56*† | 0.27† | 0.34 | 0.24 | 0.54* |
| 5 | 0.40§ | 0.72*§ | 0.41 | 0.52 | 0.32§ | 0.56* |
| 10 | 0.48 | 0.75*§ | 0.52 | 0.62 | 0.43 | 0.68* |
Values are means calculated for all windows of each size.
*Significantly different from control subjects.
†Significantly different from 5-s window.
§Significantly different from the SDA values reported in Table 2.
When ICC values were compared between the SDA and DFA methods, significantly higher values were observed for DFA for both control and elderly subjects for the 5-s window for the AP direction, and for elderly subjects for the AP direction for the 10-s window (Table 2 and 3). In contrast, a significantly higher ICC value was observed for SDA for the RD direction for the 5-s window for control subjects (Table 2 and 3).
The effect of age on postural stability
Stabilogram Diffusion Analysis (SDA)
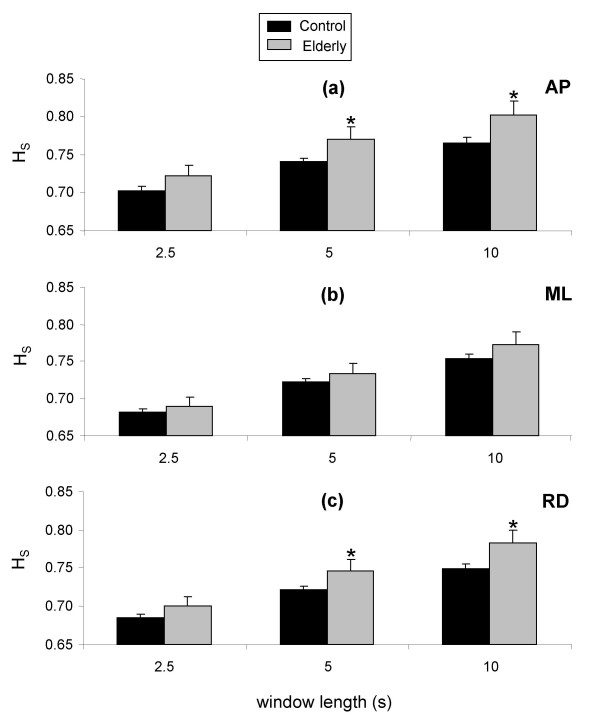
For the analysis of the effect of age on postural stability, mean values across all four tests and all window positions after were obtained for each subject for each window length. These mean values were then used in the subsequent analysis, the results of which are presented in figure 5. It can be seen that no significant differences were observed between groups for ML displacement, regardless of the window size (Figure 5b). However, HS calculated for both AP and RD displacement was significantly greater for elderly subjects for both 5 and 10-s windows (Figure 5a and 5c).
Figure 5.
Differences in HS between control and elderly subjects for 2.5-s, 5-s, and 10-s window lengths anteroposterior (a), mediolateral (b), and resultant (c) displacement. Data are means and 95% confidence intervals for all windows of each window length. *Significantly different from control subjects.
Detrended Fluctuation Analysis (DFA)
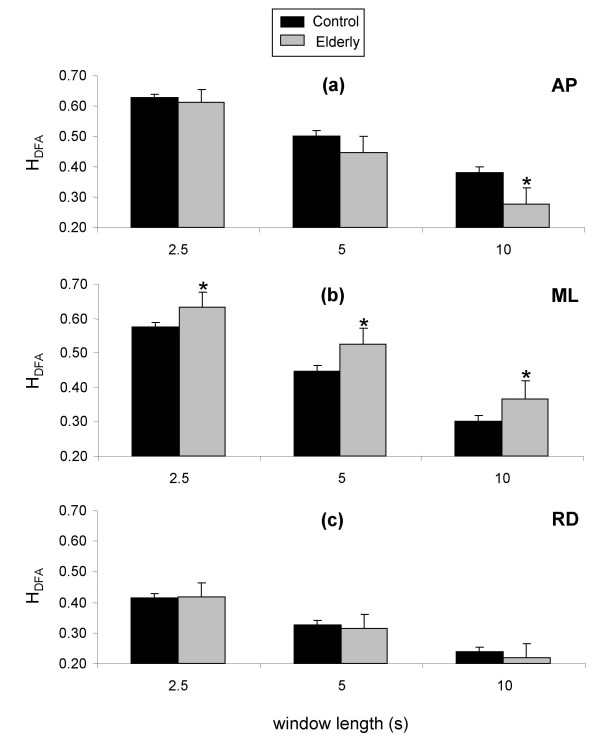
Once again, mean values were obtained across all four tests and all window positions were obtained for each subject for each window length. These mean values were then used in the subsequent analysis, with the results are presented in figure 6. In contrast to the results for SDA presented above, significant differences were observed between groups for ML displacement for all three window sizes, with significantly greater values of HDFA observed for elderly subjects (Figure 6b). In respect to AP and RD displacement, the only significant effect of age group on HDFA was a decrease in elderly subjects for AP displacement for the 10-s window length (Figure 6a and 6c).
Figure 6.
Differences in HDFA between control and elderly subjects for 2.5-s, 5-s, and 10-s window lengths for anteroposterior (a), mediolateral (b), and resultant (c) displacement. Data are means and 95% confidence intervals for all windows of each window length. *Significantly different from control subjects.
Discussion
Sliding window effect
The sliding window analysis was performed in order to identify the optimal time to start analysis. As shown in figure 3, HS decreased with time for all displacement directions for control subjects. A decrease in HS is indicative of a more precisely controlled movement in that the value of HS corresponds to the slope of the short-term of the log-log plot of Δt and <Δx2>. This decrease in HS is indicative of a more tightly controlled static posture where displacements are smaller for a given time interval. Thus subjects became more stable the longer they remained on the force plate. In contrast, HS for elderly subjects decreased only for AP and RD displacement direction, uniquely for the 2.5-s window. The absence of any effect for ML displacement for elderly subjects was due to the increased variability in HS for these subjects in comparison to control subjects. Such an explanation is also true for the other displacement directions.
The DFA method yielded similar results to those of the SDA method in that time has a significant effect on HDFA. However, in contrast to the results for HS, HDFA increased with time. In addition, these results were evident for both control and elderly subjects. These results were, however, due to the initial value at FC2, which was much lower than the subsequent values. Given this result it would be worth starting any analysis from FC2 + 1 s in order to remove any effect of time on HDFA. The interpretation of HDFA depends on the values detected. Values of HDFA greater than 0.5 are indicative of a persistent times series, with higher values due to a smoother time series, with a corresponding decrease in variability [38]. As values of H tend towards 1, the signal is smoother with a higher correlation between successive points [39]. High values of HDFA would, therefore, be indicative of increased postural stability. Another interpretation is possible for HDFA for lower values, whereby HDFA less than 0.5 is indicative of an anti-persistent signal. For such data the variation between successive points in the time series is more likely to change direction than to continue in the same direction, thus reflecting a more tightly controlled time series.
Reliability analysis
In terms of reliability, the values reported varied according to the window size, the displacement direction, and the subject group, but did not vary with time. In other words, the values of HS and HDFA for the initial part of the signal were just as reliable as those for the later part where greater postural stability was observed. In respect to the effect of window size, higher ICC values were observed for the 5-s and 10-s window lengths for both SDA and DFA methods. Such a finding was expected given that previous studies of diverse physiological and behavioral time series have typically shown greater variability when less data points are used. Eke and colleagues recommended using time series with at least 212 (4096) data points due to the unreliable results obtained with shorter time series [32]. However, recent results have demonstrated that, despite an increased variability, time series as short as 28 (256) points still produced acceptable results [40]. Furthermore, the mean of four short time series of 256 points was shown to provide a better estimate of H than a single time series of 1024 points. The results of the reliability analysis of the present study confirm the results of Delignières, in that ICC values as high as 0.72 were obtained for 5-s time series containing only 500 data points.
Given the low ICC values obtained for the 2.5-s window length, all subsequent comparisons of reliability are discussed for only the 5 and 10-s windows. In respect to differences in reliability for the two groups, it can be seen that elderly subjects were far more reliable than were the control subjects. The ICC values for elderly subjects were consistently considered to be "fair to good", bordering on "excellent" using the scale developed by Fleiss [41], with values varying from 0.49 to 0.75. In contrast, the ICC values for the control subjects were consistently lower than those for the elderly group, ranging from 0.29 to 0.53. Such differences could well be related to the calculation of the ICC:
For heterogeneous subject groups, the between subject variation would increase, thus increasing the ICC, as seen for the elderly subject group. A homogenous subject group would be expected to have less between subject variation, and a resulting decrease in the ICC, as observed for the control group.
In respect to the differences between the SDA and DFA methods in terms of reliability, the DFA method generally produced more reliable parameters for AP displacement, with both ICC values that approached the 0.75 "excellent" level of Fleiss [41] obtained for DFA for elderly subjects for AP displacement. It should be stressed that ICC values calculated for DFA and SDA analysis are only for very short time series. It is likely that higher ICC values would be obtained should longer time series be compared.
When the reliability results are compared with other studies, it is clear that clinical balance tests provide greater reliability, with values as high as 0.99 [42]. However, as the aim of the study is to develop a home-based assessment that does not require the intervention of a third party, a more realistic comparison is that made with other biomechanical measures of balance. In this respect, the ICC values observed are particularly encouraging, especially for elderly subjects, when it is considered that no constraints were imposed on the subjects in terms of foot position. In one study that compared the reliability of SDA parameters, ICC values ranged from 0.41 to 0.79 [43]. The lack of constraint used in the present study was needed in order to ensure that the results could be generalised to a home-based study where it would be impossible to closely control the experimental protocol.
The effect of age on postural stability
It was expected that there would be underlying differences between the two subject groups in terms of postural stability. The results of the present study confirmed this assumption for both SDA and DFA methods. Although the two methods both detected differences between the age groups, these differences were not the same for the two methods. The SDA method identified differences in AP displacement provided the window was at least 5-s long. Elderly subjects had increased values of HS, which are indicative of a less precisely controlled movement, as outlined at the beginning of the discussion. In contrast, no significant differences were observed for mediolateral displacement using SDA. Elderly subjects also had increased values of HS for resultant displacement for the 5-s and 10-s windows. These differences were no doubt due to the differences observed for AP displacement, which is the greatest component of resultant displacement due to the nature of the ankle and knee joints, which limit movement in the mediolateral direction.
In respect to the differences between elderly and control subjects identified by the DFA method, HDFA for mediolateral displacement was significantly higher for elderly subjects than for the controls irrespective of window length. As detailed at the start of the discussion section, values of HDFA less than 0.5 are indicative of anti-persistence, with lower values reflecting greater anti-persistence, and thus a more closely posture. Elderly subjects were, therefore, less stable than the control subjects for mediolateral displacement. In contrast, HDFA values for anteroposterior displacement were lower for elderly subjects than for control subjects, which is indicative of an increased postural stability for elderly subjects in the AP direction.
One interpretation of the two results could be that elderly subjects control their movement in the AP direction more precisely. A similar finding was reported by Norris and colleagues for AP displacement, who identified lower DFA values for elderly subjects at risk of falling [44]. Their interpretation centred around the fact that HDFA values were less than 0.5, and thus anti-persistent. The strategy adopted by the elderly subjects was highly anti-persistent, with the aim of reducing AP movement in order to maintain a stable posture.
In contrast to the results of the present study, Norris and colleagues reported no differences in ML displacement between control and elderly subjects. The contrasting findings of the two studies could be due to the different protocols used. In the present study subjects were free to choose their own foot position, values were calculated for analysis windows of 2.5, 5, and 10 s, and analysis commenced as soon as subjects had their two feet on the platform. In contrast Norris and colleagues imposed a standardised foot position, collected data for a 30-s time period, and waited for five seconds after subjects were positioned before beginning data collection. The lack of differences observed may therefore have been due to the imposed condition of a stable posture.
In respect to the differences observed between the SDA and DFA methods, the contrasting findings are due to the method used to analyse the time series. The SDA method provides two estimations of the Hurst exponent, for the short-term (HS) and long-term (HL) regions of the log-log plot of Δt and <Δx2>. Given that it was not possible to exploit the results for HL, the SDA method via HS provided information that was only related to short-term oscillations. These results indicated persistence, as all values for HS were greater than 0.5. In contrast, HDFA, which was obtained for the whole time series was less than 0.5, thus demonstrating anti-persistence. Thus, these two methods provide complimentary information related to different aspects of postural control for short-term and long-term auto-correlations.
Recording duration
In respect to the minimum recording duration needed, a 5-s window appears sufficient. The 2.5-s window was not sufficiently reliable, whereas reliability was similar for the 5-s and 10-s windows. Differences between subject groups were also evident for both the 5-s and 10-s windows. If the aim is to select the most non-invasive protocol, a 5-s window would be sufficient. It should be noted, however, that decreasing the window length introduces a bias into both HS and HDFA estimations whereby HS is underestimated and HDFA is overestimated for short window lengths. Such a finding means that comparison between different populations and different studies would not be possible for different window lengths. However, in terms of a longitudinal home-based study, although a bias would be present for 5-s recordings, the underestimation of HS and the overestimation of HDFA for short window lengths would not pose a problem for comparison of values between different testing sessions for the same individual given that the measures are reliable. The ideal start point for the analysis would one second after stepping onto the force plate, in order to remove the initial values that were markedly different from subsequent values due to the perturbation induced by the step.
Conclusion
The SDA and DFA methods were both able to identify differences in postural stability between control and elderly subjects for time series as short as 5 s. In addition, measurements proved to be reliable across testing sessions, with DFA the more robust method for AP displacement. Both methods, as well as providing evidence of underlying postural control strategies, appear to be well-suited to a non-invasive longitudinal assessment of balance.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
HA and MA carried out the data collection and data analysis. DH participated in the conception, design, and coordination of the study, performed the statistical analysis, and drafted the manuscript. VM participated in the design and coordination of the study. MD participated in the design and coordination of the study. JD participated in the conception of the study, and its design and coordination. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This study was undertaken as part of the PARAChute research project (Personnes Âgées et Risque de Chute), which was supported in part by the French Ministry of Research (Grant 03-B-254), the European Social Fund (Grant 3/1/3/4/07/3/3/011), the European Regional Development Fund (Grant 2003-2-50-0014), CRCA (Grant E200308251), and INRIA (Grant 804F04620016000081).
Contributor Information
Hassan Amoud, Email: hassan.amoud@utt.fr.
Mohamed Abadi, Email: mabadi@univ-ag.fr.
David J Hewson, Email: david.hewson@utt.fr.
Valérie Michel-Pellegrino, Email: valerie.michel@utt.fr.
Michel Doussot, Email: michel.doussot@utt.fr.
Jacques Duchêne, Email: jacques.duchene@utt.fr.
References
- Comité Français d'Education pour la Santé Les clés du " bien vieillir " : prévention des chutes chez les seniors. Caisse Nationale de l'Assurance Maladie des Travailleurs Salariés; 2001. p. 20.
- Brauer SG, Burns YR, Galley P. A prospective study of laboratory and clinical measures of postural stability to predict community-dwelling fallers. J Gerontol A Biol Sci Med Sci. 2000;55:M469–476. doi: 10.1093/gerona/55.8.m469. [DOI] [PubMed] [Google Scholar]
- Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol. 1994;49:M72–84. doi: 10.1093/geronj/49.2.m72. [DOI] [PubMed] [Google Scholar]
- Raiche M, Hebert R, Prince F, Corriveau H. Screening older adults at risk of falling with the Tinetti balance scale. Lancet. 2000;356:1001–1002. doi: 10.1016/S0140-6736(00)02695-7. [DOI] [PubMed] [Google Scholar]
- Tromp AM, Pluijm SM, Smit JH, Deeg DJ, Bouter LM, Lips P. Fall-risk screening test: a prospective study on predictors for falls in community-dwelling elderly. J Clin Epidemiol. 2001;54:837–844. doi: 10.1016/S0895-4356(01)00349-3. [DOI] [PubMed] [Google Scholar]
- Rubenstein LZ, Josephson KR. The epidemiology of falls and syncope. Clin Geriatr Med. 2002;18:141–158. doi: 10.1016/S0749-0690(02)00002-2. [DOI] [PubMed] [Google Scholar]
- Nevitt M, Cummings S, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. JAMA. 1989;261:2663–2668. doi: 10.1001/jama.261.18.2663. [DOI] [PubMed] [Google Scholar]
- Vellas BJ, Wayne SJ, Romero L, Baumgartner RN, Rubenstein LZ, Garry PJ. One-leg balance is an important predictor of injurious falls in older persons. J Am Geriatr Soc. 1997;45:735–738. doi: 10.1111/j.1532-5415.1997.tb01479.x. [DOI] [PubMed] [Google Scholar]
- Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson's disease: a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry. 2002;72:721–725. doi: 10.1136/jnnp.72.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allum JH, Bloem BR, Carpenter MG, Honegger F. Differential diagnosis of proprioceptive and vestibular deficits using dynamic support-surface posturography. Gait Posture. 2001;14:217–226. doi: 10.1016/S0966-6362(01)00142-4. [DOI] [PubMed] [Google Scholar]
- Gill J, Allum JH, Carpenter MG, Held-Ziolkowska M, Adkin AL, Honegger F, Pierchala K. Trunk sway measures of postural stability during clinical balance tests: effects of age. J Gerontol A Biol Sci Med Sci. 2001;56:M438–447. doi: 10.1093/gerona/56.7.m438. [DOI] [PubMed] [Google Scholar]
- Lord SR, Menz HB. Visual contributions to postural stability in older adults. Gerontology. 2000;46:306–310. doi: 10.1159/000022182. [DOI] [PubMed] [Google Scholar]
- Mathias S, Nayak U, Isaacs B. Balance in elderly patients: the "get-up and go" test. Arch Phys Med Rehabil. 1986;67:387–389. [PubMed] [Google Scholar]
- Shumway-Cook A, Baldwin M, Polissar NL, Gruber W. Predicting the probability for falls in community-dwelling older adults. Phys Ther. 1997;77:812–819. doi: 10.1093/ptj/77.8.812. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- Lord S, Clark R, Webster I. Postural stability and associated physiological factors in a population of aged persons. J Gerontol. 1991;46:M69–76. doi: 10.1093/geronj/46.3.m69. [DOI] [PubMed] [Google Scholar]
- Murray MP, Seireg AA, Sepic SB. Normal postural stability and steadiness: quantitative assessment. J Bone Joint Surg Am. 1975;57:510–516. [PubMed] [Google Scholar]
- Thapa PB, Gideon P, Brockman KG, Fought RL, Ray WA. Clinical and biomechanical measures of balance as fall predictors in ambulatory nursing home residents. J Gerontol A Biol Sci Med Sci. 1996;51:M239–246. doi: 10.1093/gerona/51a.5.m239. [DOI] [PubMed] [Google Scholar]
- Doussot M, Hewson D, Duchêne J. A remote monitoring system for assessing balance quality. 3rd European Medical and Biological Engineering Conference (EMBEC): Advancement of Medicine and Health Care through Technology; 20–25 November. 2005.
- Collins JJ, De Luca CJ. Upright, correlated random walks: A statistical-biomechanics approach to the human postural control system. Chaos. 1995;5:57–63. doi: 10.1063/1.166086. [DOI] [PubMed] [Google Scholar]
- Riley MA, Wong S, Mitra S, Turvey MT. Common effects of touch and vision on postural parameters. Exp Brain Res. 1997;117:165–170. doi: 10.1007/s002210050211. [DOI] [PubMed] [Google Scholar]
- Laughton CA, Slavin M, Katdare K, Nolan L, Bean JF, Kerrigan DC, Phillips E, Lipsitz LA, Collins JJ. Aging, muscle activity, and balance control: physiologic changes associated with balance impairment. Gait Posture. 2003;18:101–108. doi: 10.1016/S0966-6362(02)00200-X. [DOI] [PubMed] [Google Scholar]
- Delignières D, Deschamps T, Legros A, Caillou N. A methodological note on nonlinear time series analysis: is the open- and closed-loop model of Collins and De Luca (1993) a statistical artifact? J Mot Behav. 2003;35:86–97. doi: 10.1080/00222890309602124. [DOI] [PubMed] [Google Scholar]
- Duarte M, Zatsiorsky VM. Long-range correlations in human standing. Physics Letters A. 2001;283:124–128. doi: 10.1016/S0375-9601(01)00188-8. [DOI] [Google Scholar]
- Carroll JP, Freedman W. Nonstationary properties of postural sway. J Biomech. 1993;26:409–416. doi: 10.1016/0021-9290(93)90004-X. [DOI] [PubMed] [Google Scholar]
- Harris GF, Riedel SA, Matesi D, Smith P. Standing postural stability assessment and signal stationarity inchildren with cerebral palsy. IEEE Transactions on Rehabilitation Engineering. 1993;1:35–42. doi: 10.1109/86.242406. [DOI] [Google Scholar]
- Peterka RJ. Postural control model interpretation of stabilogram diffusion analysis. Biol Cybern. 2000;82:335–343. doi: 10.1007/s004220050587. [DOI] [PubMed] [Google Scholar]
- Mandelbrot B, Van Ness J. Fractional brownian motion, fractional noses and applications. SIAM Review. 1968;10:422–437. doi: 10.1137/1010093. [DOI] [Google Scholar]
- Collins JJ, De Luca CJ, Burrows A, Lipsitz LA. Age-related changes in open-loop and closed-loop postural control mechanisms. Exp Brain Res. 1995;104:480–492. doi: 10.1007/BF00231982. [DOI] [PubMed] [Google Scholar]
- Collins JJ, De Luca CJ. Open-loop and closed-loop control of posture: a random-walk analysis of center-of-pressure trajectories. Exp Brain Res. 1993;95:308–318. doi: 10.1007/BF00229788. [DOI] [PubMed] [Google Scholar]
- Kantz H, Schreiber T. Nonlinear time series analysis. 2. Cambridge, UK: Cambridge University Press; 2004. [Google Scholar]
- Eke A, Herman P, Kocsis L, Kozak L. Fractal characterization of complexity in temporal physiological signals. Physiol Meas. 2002;23:R1–38. doi: 10.1088/0967-3334/23/1/201. [DOI] [PubMed] [Google Scholar]
- International Society of Biomechanics http://www.isbweb.org/software/movanal/stamp
- Peng CK, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos. 1995;5:82–87. doi: 10.1063/1.166141. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 4. Needham Heights, MA, USA: Allyn and Bacon; 2001. [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;2:420–428. doi: 10.1037/0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Hopkins W. Measures of reliability in sports medicine and science. Sports Med. 2000;30:1–15. doi: 10.2165/00007256-200030010-00001. [DOI] [PubMed] [Google Scholar]
- Goldberger AL, Amaral LA, Hausdorff JM, Ivanov P, Peng CK, Stanley HE. Fractal dynamics in physiology: alterations with disease and aging. Proc Natl Acad Sci USA. 2002;99:2466–2472. doi: 10.1073/pnas.012579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke A, Herman P, Bassingthwaighte JB, Raymond GM, Percival DB, Cannon M, Balla I, Ikrenyi C. Physiological time series: distinguishing fractal noises from motions. Pflugers Arch. 2000;439:403–415. doi: 10.1007/s004240050957. [DOI] [PubMed] [Google Scholar]
- Delignières D, Torre K, Lemoine L. Methodological issues in the application of monofractal analyses in psychological and behavioral research. Nonlinear Dynamics in Psychology and Life Science. 2005. [PubMed]
- Fleiss JL. The design and analysis of clinical experiments. New York: Wiley; 1986. [Google Scholar]
- Karen W Hayes MEJ. Measures of adult general performance tests: The Berg Balance Scale, Dynamic Gait Index (DGI), Gait Velocity, Physical Performance Test (PPT), Timed Chair Stand Test, Timed Up and Go, and Tinetti Performance-Oriented Mobility Assessment (POMA) Arthritis Care & Research. 2003;49:S28–S42. doi: 10.1002/art.11411. [DOI] [Google Scholar]
- Chiari L, Cappello A, Lenzi D, Della Croce U. An improved technique for the extraction of stochastic parameters from stabilograms. Gait Posture. 2000;12:225–234. doi: 10.1016/S0966-6362(00)00086-2. [DOI] [PubMed] [Google Scholar]
- Norris JA, Marsh AP, Smith IJ, Kohut RI, Miller ME. Ability of static and statistical mechanics posturographic measures to distinguish between age and fall risk. J Biomech. 2005;38:1263–1272. doi: 10.1016/j.jbiomech.2004.06.014. [DOI] [PubMed] [Google Scholar]