Abstract
Lysophosphatidic acid (LPA) is present in abundance in serum resulting from platelet activation and is also found in other biological fluids. LPA controls numerous cellular responses and plays a role in specific functions such as wound healing, especially in the skin. Nevertheless, its presence in the skin has never been investigated during wound healing, or in other situations. Since re-epithelialization occurs after blister rupture, the presence of LPA in blister fluids was investigated.
Using a radioenzymatic assay, LPA was detected in 33 blister fluids originating from 24 bullous dermatoses, and at higher concentrations than in plasma. LPA concentration was independent of the type of dermatoses. In parallel, blister fluids contained a lysophospholipase D (LPLD) activity but no detectable phospholipase A2 activity. The expression of the LPLD autotaxin (ATX) and of LPA1-receptor were greatly increased in blister skin when compared to normal skin. Finally, LPA was found to have a positive effect on the migration of cultured keratinocytes.
These results show that LPA is present in blister fluid synthesized by the LPLD ATX. Due to its ability to enhance keratinocyte migration, LPA in blister fluid could, via the LPA1-receptor, play an important role in re-epithelialization occuring after blister rupture.
Keywords: Adult; Aged; Aged, 80 and over; Autocrine Motility Factor; genetics; metabolism; Blister; enzymology; genetics; metabolism; Cell Movement; Cells, Cultured; Female; Glycoproteins; genetics; metabolism; Humans; Keratinocytes; drug effects; Lysophospholipids; analysis; biosynthesis; pharmacology; Male; Middle Aged; Multienzyme Complexes; genetics; metabolism; Phosphodiesterase I; Phosphoric Diester Hydrolases; metabolism; Pyrophosphatases; Receptors, Lysophosphatidic Acid; genetics; metabolism; Skin; chemistry; enzymology; metabolism; Wound Healing
INTRODUCTION
Lysophosphatidic acid (LPA) is the simplest phospholipid found in nature. It is present in abundance in serum resulting from platelet activation. LPA is also found in other biological fluids such as plasma (Baker et al, 2002) and ascite fluids (Xu et al, 1998) of patients suffering from ovarian cancer, aqueous humor and lacrimal gland fluid (Liliom et al, 1998), follicular fluid (Tokumura et al, 1999), saliva (Sugiura et al, 2002), extracellular fluid of adipose tissue (Valet et al, 1998) and arthritis inflammatory fluids (Fourcade et al, 1995). It has been demonstrated that LPA may be generated by various cells including cancer cells (Baker et al, 2002; Xu et al, 1998; Merchant et al, 1991), fibroblasts (Fukami and Takenawa, 1992) and adipocytes (Valet et al, 1998). Nevertheless, the precise cellular origin of LPA in biological fluids still remains unclear (Gaits et al, 1997; Pages et al, 2001). Furthermore, the pathways involved in LPA production are still a matter of debate. There are two main pathways: the phospholipase A2 (PLA2)-dependent deacylation of phosphatidic acid (PA) and the lysophospholipase D (LPLD)-dependent hydrolysis of lysophosphatidylcholine (LPC) (Gaits et al, 1997; Pages et al, 2001; Aoki et al, 2002). It has been previously proved that LPLD activity was involved in LPA production in fluids such as rat plasma (Tokumura et al, 1998), human follicular fluid (Tokumura et al, 1999), and in the extracellular medium of adipocytes (Gesta et al, 2002). Umezo-Goto et al (Umezu-Goto et al, 2002), Tokumura et al (Tokumura et al, 2002) and Ferry (Ferry et al, 2003) purified LPLD activity from bovine serum, human plasma and adipocytes respectively, and demonstrated that it corresponded to a soluble form of autotaxin (ATX). ATX is a tumor cell mobility factor, originally isolated from melanoma cell supernatants, and belonging to the ecto-nucleotide pyrophosphatase/phosphodiesterase family (Moolenaar, 2002). LPA acts via interaction with specific G-protein-coupled receptors belonging to the endothelium differentiation gene family: LPA1-receptor (LPA1-R: Edg-2), LPA2-receptor (LPA2-R: Edg-4) and LPA3-receptor (LPA3-R: Edg-7) (Chun et al, 2002). Pharmacological specificity and tissue distribution may differ from one subtype to another (Contos et al, 2000). LPA controls numerous cellular responses such as proliferation, differentiation, migration and apoptosis; and plays a role in specific functions such as wound healing (Liliom et al, 1998; Sturm et al, 1998; Hines et al, 2000). LPA is involved in the pathophysiology of arteriosclerosis (Siess W, 2002), and different situations, such as cancers, are associated with LPA production (Baker et al, 2002; Xu et al, 1998; Merchant et al, 1991). However, its precise mechanism of action is currently unknown. In the skin, it has been demonstrated that LPA plays a role in tissue repair and regeneration processes. Using cultured human keratinocytes, Piazza et al (Piazza et al, 1995) demonstrated that LPA induced proliferation and differentiation. The fact that LPA is released from activated platelets (Eichholtz et al, 1993), as well as the presence of active phospholipases in the skin (Mazereeuw-Hautier et al, 2000; Maury et al, 2000) strongly supports this hypothesis. This is also supported by the fact that topical application of LPA to a wound model of mouse or rat skin promotes wound healing (Demoyer et al, 2000; Balazs et al, 2001). Nevertheless, the presence of LPA has never been investigated in the skin, during wound healing, or in other situations. Since re-epithelialization of the underlying wound bed occurs after blister rupture, we decided to investigate LPA in blister fluid.
Skin blisters (Diaz and Giudice, 2000) are formed as a result of a breakdown of tissue integrity, with detachment of cellular junctions and fluid accumulation. There are several aetiologies of blister formation (hypersensitivity, physical injury, autoimmunity, hereditary, virus infection), and the blisters can occur at different levels within the epidermis. The blisters can be intraepidermal (eczema, burn, varicella-zoster virus infection) or subepidermal (bullous pemphigoid, toxic epidermal necrolysis, dystrophic epidermolysis bullosa). Some biochemical features of blister fluid have already been documented in succion blister. This fluid has been qualified as a “filtrate” of serum since the concentration of each compound was smaller than in serum and was dependent on its molecular weight (Volden et al, 1980). Blister fluid also contains local products of cell injury and inflammation (Grando et al, 1989a; Ono et al, 1995; D’Auria et al, 1999). In the present study, we report the presence of LPA in human skin blister fluid and show evidence for the involvement of a LPLD activity, corresponding to ATX, in the synthesis of blister fluid LPA. We also show that the expression of LPA receptors was regulated during bullous formation. In parallel, we demonstrate an effect of LPA on keratinocyte migration, that might contribute to the effect of LPA in skin wound healing.
MATERIALS AND METHODS
Collection of blister fluid and blood
33 samples from 24 patients (13 females and 11 males, mean age was 53.96 ± 30.44 years, range 2–90 years) suffering from different bullous dermatosis were investigated between December 2001 and November 2002. There were 6 different aetiologies of bullous dermatoses: bullous pemphigoid (n:10), second-degree burn (n:5), eczema (n:3), toxic epidermal necrolysis (n:3), varicella–zoster virus infection (n:2), dystrophic epidermolysis bullosa (n:1). The subjects of our study gave informed consent and investigations were carried out in accordance with the Declaration of Helsinki as revised in 2000 (http://www.wma.net/e/policy/b3.htm). Some fluids were collected at several clinical stages from some patients suffering from toxic epidermal necrolysis (n:2) or eczema (n:1). Final diagnosis was based on anamnesis, clinical manifestations, routine histology, detection of autoantibodies using direct and indirect imunofluorescence, and viral culture and serologies. Collection of fluid was realized before the beginning of any treatment, except for 7 of the 10 patients with bullous pemphigoid who were already under steroid therapy (topical application of DiproleneR (clobetasol) 30 g/j, n:2 or oral route of SolupredR (prednisolone) 0.50 to 1.50 mg/kg/j, n:5). The blisters originating from patients under steroid therapy were only used for LPA quantification experiments and not for the determination of the metabolic origin of LPA. The blisters were punctured with a needle and the fluid was collected in a syringe. In order to determine whether LPA was soluble or associated with cell particles, fluids were subjected to ultra-centrifugation (100.000 g for 5 min at 37°C). Fluids were immediately stored at −20°C until further analysis.
In cases of requirement of blood sample for medical reasons, human plasma was collected in EDTA conditioned tubes.
Collection of skin biopsies
Eight biopsies were taken between January and June 2003 from 4 other patients (3 females and 1 male, mean age 81 years, range 77–91 years) suffering from bullous dermatosis (bullous pemphigoid (n:3), second-degree burn (n:1)). For each 4 patient, two biopsies were performed: one containing the entire blister and one containing the uninvolved skin. Biopsies were immediately frozen in liquid nitrogen and stored at −80°C until further analysis.
LPA quantification in blister fluid
Quantification of LPA was performed using a radioenzymatic assay as previously described (Saulnier-Blache et al, 2000). In short, lipids contained in blister fluids were extracted with 1 volume of 1-butanol followed by evaporation of the solvent under nitrogen. In the presence of [14C] oleoyl-CoA, recombinant rat LPA acyl-transferase selectively catalyses the transformation of LPA into [14C] PA. Products of the reaction were separated by one-dimensional thin-layer chromatography and autoradiographed. Each spot of [14C] PA was scraped off and counted with 3 ml scintillation cocktail. Radioactivity was converted to nanomoles and the concentration of LPA was calculated.
Quantification of LPA was also performed after 6 hours of incubation of blister fluid at 37°C with 100 μM of quinacrine (Biomol, Plymouth Meeting, PA)), or phenanthrolin (Sigma-Aldrich, St Louis, MO).
PA and LPC quantification in blister fluid
As previously described (Gesta et al, 2002), the amount of PA and LPC present in blister fluid was determined by quantifying the amount of LPA generated after treatment by pancreatic PLA2 or bacterial PLD respectively. Incubation was realized at 37°C for 90 min with pancreatic PLA2 at 1,92 UI/ml (Sigma-Aldrich, St Louis, MO), or bacterial PLD from Streptomyces chromofuscus at 1UI/ml (Sigma-Aldrich, St Louis, MO).
Assay for PLA2 activity in blister fluids
PLA2 activity was determined by measuring the rate of release of pyrenyldecanoic acid from the pyrene-labelled phospholipid 1-hexadecanoyl-2-(1-pyrene-decanoyl-)-sn-glycero-3-phosphoglycerol, ammonium salt (Invitrogen, Carlsbad, California), as previously described (Radvanyi et al, 1989), with minor modifications. Blister fluid samples of 10 μl were incubated for 1 hour at room temperature with 1 ml reaction buffer containing 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 10 % (w/v) fatty acid free bovine serum albumin, and the substrate (final concentration 2 μM). The reaction was initiated by the addition of 10 mM CaCl2. Fluorescence measurements were performed with a spectrofluorometer (SFM 25 Kontron) at excitation and emission wavelengths of 345 and 398 nm, respectively.
Measurement of LPLD activity in blister fluid
LPLD activity was measured by conversion of radiolabeled LPC into radiolabeled LPA as previously described (Gesta et al, 2002) with minor modifications. A solution of [14C] palmitoyl-lysophosphatidylcholine (NEN, 55.8 mCi/mmole) at 0.0025 μCi/μl in DMEM supplemented with 1% fatty acid free BSA was first prepared, and 20 μl of this solution was incubated with 500 μl of blister fluid plus 1 μl of sodium orthovanadate 0.5 mM for 90 min at 37°C. At the end of the incubation period, phospholipids were extracted with 500 μl of 1-butanol, evaporated, spotted on a silica gel 60 TLC glass plate (VWR, West Chester, PA), and separated using CHCI3/MeOH/NH4OH (60/35/8) as a migration solvent. The plate was autoradiographed overnight at −80°C using a Biomax-MS film (Kodak, New York) in order to localize [14C]LPA spots, which were scraped off and counted with 3 ml of scintillation cocktail. Activity was expressed as pmol [14C]LPA released per min per mg of protein.
Expression of LPA-receptors in skin biopsies by Real Time RT-PCR
Total RNA were isolated from skin biopsies using the RNA STAT-60 kit (AMS Biotechnology, UK) and Rneasy mini protocol for RNA Cleanup (Quiagen, Valencia, CA). Total RNA (1 μg) were reverse transcribed for 60 min at 37°C using the Superscript II reverse transcriptase (Invitrogen, Carlsbad, California) in the presence of random hexamers. A minus RT reaction was performed in conjunction, as a control, to ensure the absence of genomic DNA contamination. Real time RT-PCR was performed starting with 26 ng cDNA with 300 nM (human LPA2-R) or 900 nM (human ATX, human LPA1-R, human LPA3-R and human hypoxanthine phosphoribosyl transferase as a control) concentrations of both sense and antisense primers in a final volume of 25 μl, using the SYBR green TaqMan Universel PCR Master Mix (Applied Biosystems, Forster city, CA). Fluorescence was monitored and analyzed in a GeneAmp 7000 detection system instrument (Applied Biosystems, Forster city, CA). Analysis of the 18S ribosomal RNA was performed in conjunction using the ribosomal RNA control taqman assay kit (Applied Biosystems, Forster city, CA) in order to normalize gene expression. Results were expressed according to the δCT method as 2(Ct18S-Ctgene), where Ct corresponds to the number of cycles needed to generate a fluorescent signal above a predefined threshold.
Primer design was optimized by using the Primer Express software (Perkin Elmer Life Sciences, Boston, MA). The oligonucleotides used were: human ATX: sense: 5′-GGACCAACATCTCCGGATCTT-3′, antisense: 5′-GGAGGTCCAGCCTCTTGAAGT-3′; human LPA1-R: sense: 5′-TGGGCCATTTTCAACTTGGT-3′, antisense: 5′-TCTGGCGAACATAGCCAAAGA-3′, human LPA2-R: sense: 5′-TCATCATGGGCCAGTGCTACT -3′, antisense: 5′-GTGGGAGCTGAGCTCTTTGC-3′, human LPA3-R: sense: 5′-TGGGCCATCGCCATTTT -3′, antisense: 5′-GAGCAGGCAGAGATGTTGCA-3′; human hypoxanthine phosphoribosyl transferase: sense: 5′-TGACACTGGCAAAACAATGCA-3′, antisense: 5′-GCTTGCGACCTTGACCATCT-3′.
Cell Migration assay of keratinocytes
HaCaT cells, a spontaneously transformed, non-tumorigenic human keratinocyte cell line, were cultured in RPMI 164 (Gibco, Langley, OK) with 10% fetal bovine serum at 37°C, with 5% CO2. After confluence, the medium was changed to a medium without serum for 24 h. Cell migration assays were performed in modified Boyden chambers containing 8 to 12-μm pore size uncoated polycarbonate membranes (Poretics Osmonic, California). The lower chambers were filled with various concentrations of LPA (0 to 5 μM) in RPMI containing 1% bovine serum albumin, or with human epidermal growth factor (Sigma-Aldrich, St Louis, MO) (35 ng/ml in RPMI 1% bovine serum albumin) as a positive control, or with the medium alone (RPMI 1% bovine serum albumin) as a negative control. The upper chambers were filled with cells in suspension in RPMI (1 × 106 per ml). After 45 min of incubation at 37°C the filter was removed and the top surface of the membrane was wiped to remove non migrating cells. The membrane was fixed in ethanol and stained with RAL 555 fast staining kit (VWR, West Chester, PA). The migration was evaluated by counting the stained migrating cells at the bottom surface of the membrane by light microscopy (Axioskop 20, Zeiss (Thornwood, NY)). At least, three different fields were evaluated and the average was calculated. Stimulation by LPA was expressed as a migration-index and represented the percentage of migrated cells with LPA divided by the negative control. All experiments were carried out in triplicate and repeated at least three times.
Protein determination
Protein concentration was determined by the DC Protein assay (Biorad, Hercules, CA) with bovine serum albumin as standard.
Statistical analysis
Data were expressed as mean ± SEM. Comparison was performed by means of Student’s test. Statistical significance was considered when p < 0.05.
RESULTS
LPA is present in blister fluids
In order to determine the presence of LPA in blister fluid, LPA was quantified using a radioenzymatic assay. Micromolar (mean ± SEM, 0.60 ± 0.0087 μM, range 0 – 1.90) concentrations of LPA were detected in blister fluids originating from all patients except for one, suffering from toxic epidermal necrolysis. The results for LPA concentrations are shown in Table 1. No statistical correlation could be established between LPA concentration and the following characteristics: age, sex, aetiology of bullous diseases, blister duration, indirect immunofluorescence, eosinophilia or steroid treatment for bullous pemphigoid.
Table I.
Lysophosphatidic acid (LPA) concentrations in blister fluids. Quantification of LPA was performed using a radioenzymatic assay as described in Materials and Methods.
| Bullous dermatoses | No. patients | No. samples | LPA (μM) Mean (± SEM) | Median (Range) |
|---|---|---|---|---|
| Eczema | 3 | 4 | 0.8 ± 0.3 | 0.2 – 1.6 |
| Burn | 5 | 5 | 0.9 ± 0.27 | 0.4 –1.9 |
| Bullous pemphigoid | 10 | 10 | 0.4 ± 0.09 | 0.2 – 1.2 |
| -No treatment | 3 | 3 | 0.68 ± 0.26 | 0.41 – 1.2 |
| -Under steroid treatment | 7 | 7 | 0.32 ± 0.04 | 0.16 – 0.5 |
| Varicella-zoster virus infection | 2 | 2 | 1.1 ± 0.42 | 0.7 – 1.5 |
| Toxic epidermal necrolysis | 3 | 11 | 0.5 ± 0.15 | 0 – 1.6 |
| Dystrophic epidermolysis bullosa | 1 | 1 | 0.2 |
We wanted to determine whether LPA present in blister fluid originated from plasma or was produced locally within the blister. We therefore compared LPA concentration in blister fluid to LPA concentration in plasma (n:2). We found that plasma concentrations were 9-fold lower than blister fluid concentrations (plasma: 0.07 μM vs. blister 0.4 μM and 0.064 μM vs. 1.2 μM for the two patients respectively) (data not shown). This result clearly shows that LPA is produced in blister fluid and does not originate from plasma. In blister fluid LPA was present in the supernatants and absent in the pellet after ultra-centrifugation and cell removal (data not shown), indicating LPA solubility.
Since LPA is present in blister fluid, we decided to investigate the presence of two possible precursors: PA and LPC. The amount of PA and LPC present in blister fluids (n:5 and n:10 respectively) was evaluated to 1.1 ± 0.25 and 2.8 ± 0.18 μM respectively. These results indicate that blister fluid not only contained LPA but also its precursors: PA and LPC.
Metabolic origin of LPA in blister fluid
We then decided to elucidate the metabolic origin of LPA produced in blister fluid. It has been demonstrated in other tissues that LPA could be produced by a soluble extracellular LPA synthesis activity (Tokumura et al, 1999; Gesta et al, 2002). In order to test the presence of such an activity in blister fluids, incubation of the fluids was performed at 37°C for 6 hours. After incubation, the concentration of LPA had doubled (mean ± SEM: 1.70 ± 0.72 vs. 3.40 ± 1.05 μM) (n: 5) (p< 0.005) (Fig 1), showing the presence of a soluble extracellular LPA synthesis activity. We then studied the two major pathways possibly involved in soluble LPA synthesis: the PLA2-dependent deacylation of PA, and the LPLD-dependent hydrolysis of LPC (Gaits et al, 1997; Pages et al 2001; Aoki et al, 2002). From the same 5 patients, we showed that the intrinsic LPA synthesis activity was not modified after 6 hours of incubation at 37°C with quinacrine, an inhibitor of PLA2 (McCrea et al, 1985) (Fig 1). In conjunction, no PLA2 activity could be detected in blister fluids (n:32), except for 1 patient suffering from toxic epidermal necrolysis (data not shown). In contrast, after 6 hours of incubation at 37°C with phenanthrolin, previously demonstrated to inhibit LPLD activity (Tokumura et al, 1998; Gesta et al, 2002), LPA synthesis activity was completely abolished (n:2) (Fig 1). In parallel, LPLD activity was 0.018 ± 0.005 pmol/min/mg protein, range 0–0.9, (n:10) (data not shown).
Fig 1.
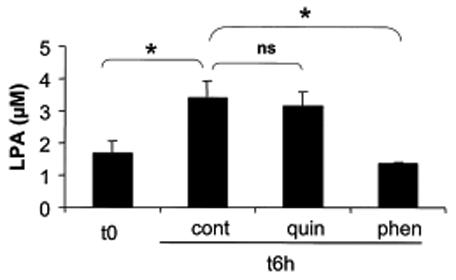
Soluble extracellular lysophosphatidic acid (LPA) synthesis activity in blister fluid and influence of quinacrine and phenanthrolin. LPA was quantified in blister fluids before (t0) and after 6 hours (t6h) incubation at 37°C without (cont) or with quinacrine (quin) at 100 μM (n:5) or phenanthrolin (phen) at 100 μM (n:2). Values are means ± SEM from n separate experiments. *: p < 0.05.
Since LPLD activity can be catalysed by ATX (Umezu-Goto et al, 2002; Tokumura et al, 2002; Ferry et al, 2003), the expression of ATX transcripts was investigated in blister skin using real time RT-PCR, and was compared to normal skin. As shown in Figure 2, ATX mRNAs were found in blister skin and were approximately 4-times more abundant than in normal skin (p<0.05). This result strongly suggested that ATX might be responsible for LPLD activity in blister fluid, and that skin expression of ATX was induced during the formation of the blister.
Fig 2.
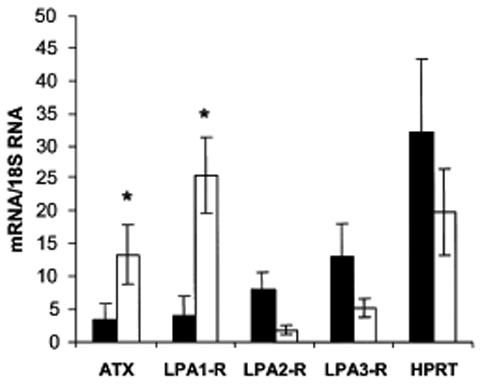
Expression of lysophosphatidic acid (LPA) receptor subtypes and autotaxin (ATX) in skin biopsies.
Blister skin and normal skin were both obtained from patients suffering from bullous dermatoses. Total RNA were isolated and mRNA encoding LPA1- (LPA1-R), LPA2- (LPA2-R) and LPA3- (LPA3-R) receptors, and autotaxin (ATX), and hypoxanthine phosphoribosyl transferase (HPRT) were quantified using real time PCR as described in Materials and Methods. Values are means ± SEM from 4 separate experiments. *: p < 0.05.
Skin expression of LPA-receptors
The biological responses of LPA being mediated by specific receptors (Chun et al, 2002; Contos et al, 2000) the expression of these receptors was also investigated in blister skin and was compared to normal skin. As shown in Figure 2, mRNA of the 3 LPA receptor subtypes (LPA1-R, LPA2-R, LPA3-R) were detected in blister skin. When compared to normal skin, LPA1-R mRNA from blister skin were 6.42 times more abundant (p<0.05), while LPA2-R mRNA and LPA3-R mRNA were 4.30 and 2.50 times (not statistically significant) less abundant, respectively. In parallel, no significant alteration in the level of hypoxanthine phosphoryl transferase mRNA (used as an housekeeping gene) was observed. These results showed that blister formation is accompanied by regulation of LPA receptors expression, and particularly by a consistent up-regulation of the LPA1-R subtype.
Effects of LPA on HaCaT cells migration
In the literature LPA has been involved in wound healing by inducing keratinocyte proliferation and differentiation. Since migration takes part in wound healing and LPA induces epithelial cells migration (Liliom et al, 1998; Sturm et al, 1998; Hines et al, 2000), we decided to investigate the effect of LPA on keratinocyte migration.
LPA was found to have a positive dose-dependent effect on keratinocyte migration. The effect increased according to LPA concentrations and was statistically different from control at concentrations of 1 μM or above, showing a plateau at 2 μM (Fig 3). This result suggests that LPA in blister fluid enhances keratinocyte migration.
Fig 3.
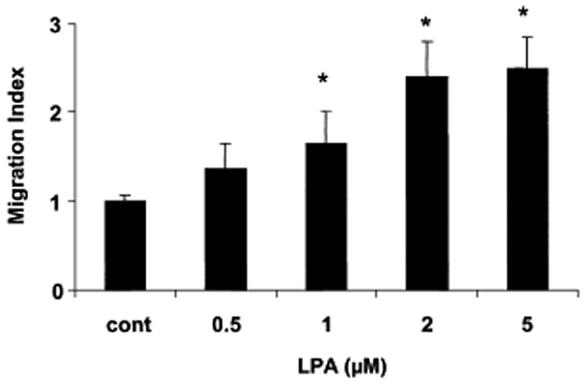
Effect of lysophosphatidic acid (LPA) on keratinocyte migration. HaCaT cells were treated or not (cont) to increased concentrations of LPA and cell migration was assessed using modified Boyden chambers as described in Materials and Methods. Values are means ± SEM from 3 separate experiments. *: p < 0.05.
DISCUSSION
The present work shows the presence of LPA in blister fluid. LPA is a bioactive phospholipid mediating its activity via stimulation of specific G-protein coupled receptors.
Several authors have concluded that LPA plays a role in skin wound healing (Piazza et al, 1995; Demoyer et al, 2000; Balazs et al, 2001) by enhancing proliferation and differentiation of keratinocytes. Sauer (Sauer et al, 2004) showed that LPA mediates keratinocyte growth arrest and chemotaxis. The LPA effects on wound healing have also been studied in intestinal epithelial cells (Liliom et al, 1998; Sturm et al, 1998; Hines et al, 2000) where LPA induced migration and proliferation. After blister formation, re-epithelialization of the underlying wound bed occurs after blister rupture. Re-epithelialization involves a sequential program of events in order to close a wound as quickly as possible (Yamaguchi and Yoshikawa, 2001). An increase in the rate of migration of keratinocytes over the wounded area, as well as an increase in the rate of proliferation of basal cells occurs. Simultaneously, cells migrate into the upper epidermal layers, ultimately undergoing terminal differentiation to form the stratum corneum and repairing the barrier function of the skin. LPA concentrations found in blister fluid were in accordance with the reported concentrations responsible for interactions between LPA and its receptors. Therefore, LPA may take part in wound healing via effects on cell proliferation, differentiation and migration. This hypothesis is supported by our data showing that LPA increases keratinocytes migration in culture, and that skin expresses LPA receptor subtypes (LPA1-R, LPA2-R, LPA3-R). Since Amano (Amano et al, 2004) demonstrated that LPA stimulates laminin 5 expression, the effect of LPA on migration could be mediated by this protein, known to be involved in the adhesion and migration of keratinocytes. Whereas the specific contribution of each LPA-receptor subtype in the biological activity of LPA in keratinocytes remains to be determined, its is interesting to notice that the expression of the LPA1-R subtype was substantially increased in skin from blisters when compared to normal skin, whereas the expression of LPA2-R and LPA3-R was not altered or was even decreased. Even if the underlying mechanisms remain unclear, up-regulation of the LPA1-R suggests that this receptor plays a predominant role in the biological activity of LPA in skin during blister formation. Therefore LPA1-R might represent a pharmacological target for skin wound healing therapy.
In our study LPA was present in blister fluids originating from all bullous dermatoses but no correlation could be established between the level of LPA and the characteristics of the blister. This result may be explained by the small number of cases and their heterogeneity, or could mean that LPA is a consequence of the blister rather than directly involved in pathogenesis of the bullous disease.
LPA concentration in blister fluid (0.6 μM) was close to that found in other biological fluids such as saliva (Sugiura et al, 2002), lacrimal gland and aqueous humour (Liliom et al, 1998) (0.785, 1 and 0.2 μM, respectively), and lower than in plasma (0.08 to 0.2 μM) (Tokumura et al, 1998). This last observation suggests that, conversely to what was described for other blister fluid constituents, such as fatty acids, organic acids and aminoacids, LPA does not result from a filtration of plasma but rather from a local synthesis. This hypothesis is supported by the demonstration of the presence of a LPA synthesis activity, as well as of LPA precursors (PA and LPC) in blister fluid.
Two major pathways might be involved in LPA synthesis: the PLA2-dependent deacylation of PA and the LPLD-dependent hydrolysis of LPC. Since LPA synthesis activity in blister fluid is not blocked by quinacrine, we excluded the possibility of the involvement of PLA2 in LPA synthesis in blister fluid. This hypothesis was also supported by the absence of detectable PLA2 activity in blister fluid. In contrast, our data strongly support the fact that LPA synthesis activity corresponds to a LPLD activity, since it is blocked by phenanthrolin, an ion chelator previously demonstrated to inhibit LPLD activity in plasma and adipose tissue (Tokumura et al, 1998; Gesta et al, 2002). Furthermore, an easily measurable LPLD activity was found in blister fluid. Based on the literature, the only enzyme demonstrated to be able to catalyze a soluble LPLD activity is ATX, suggesting that this enzyme could be responsible for LPA synthesis in blister fluid. This is in accordance with the demonstration of a relatively high level of expression of ATX in skin from blister.
Interestingly, ATX expression was more abundant in blister skin than in normal skin. Blister formation can be considered as tissue injury where cell composition and functions are altered, leading to the release of several components including mediators of inflammation (Grando et al, 1989a; Ono et al, 1995; D’Auria et al, 1999), enzymes such as tryptases, myeloperoxydases, or proteases and their inhibitors (D’Auria et al, 2000; Grando et al, 1989b). This type of skin injury could be responsible for the up-regulation of ATX expression and therefore lead to the synthesis of LPA. Such a relation between tissue injury and LPA production has been previously reported. LPA was indeed found to be released by epithelial cells in aqueous humor after corneal injury (Liliom et al, 1998) or during intra cranial hematoma in a pig model (Tigyi et al, 1995).
In conclusion, LPA is produced in blister fluid by a LPLD activity that probably corresponds to ATX. LPA in blister fluid might act via LPA-receptors, especially LPA1-R and have a role in skin wound healing, in particular via an effect on keratinocyte migration.
Since ATX is involved in LPA production, this enzyme could represent an interesting target to enhance skin wound healing.
Acknowledgments
We thank Marie Charveron and Isabelle Ceruti from Pierre-Fabre (Hôtel-Dieu Saint-Jacques, 2, rue Viguerie, 31025 Toulouse) for assistance with keratinocyte migration.
Abbreviations
- LPA
Lysophosphatidic acid
- PLA2
Phospholipase A2
- PA
Phosphatidic acid
- LPLD
Lysophospholipase D
- LPC
Lysophosphatidylcholine
- ATX
Autotaxin
- LPA1-R
LPA1 receptor
- LPA2-R
LPA2 receptor
- LPA3-R
LPA3 receptor
References
- Amano S, Akutsu N, Ogura Y, Nishiyama T. Increase of laminin 5 synthesis in human keratinocytes by acute wound fluid, inflammatory cytokines and growth factors, and lysophospholipids. J Invest Dermatol. 2004;151:961–70. doi: 10.1111/j.1365-2133.2004.06175.x. [DOI] [PubMed] [Google Scholar]
- Aoki J, Taira A, Takanezawa Y, et al. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J Biol Chem. 2002;277:48737–44. doi: 10.1074/jbc.M206812200. [DOI] [PubMed] [Google Scholar]
- Baker DL, Morrison P, Miller B, et al. Plasma lysophosphatidic acid concentration and ovarian cancer. JAMA. 2002;287:3081–2. doi: 10.1001/jama.287.23.3081. [DOI] [PubMed] [Google Scholar]
- Balazs L, Okolicany J, Ferrebee M, Tolley B, Tigyi G. Topical application of the phospholipid growth factor lysophosphatidic acid promotes wound healing in vivo. Am J Physiol Regul Integr Comp Physiol. 2001;280:R466–72. doi: 10.1152/ajpregu.2001.280.2.R466. [DOI] [PubMed] [Google Scholar]
- Chun J, Goetzl EJ, Hla T, et al. International Union of Pharmacology XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2002;54:265–9. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- Contos JJ, Fukushima N, Weiner JA, Kaushal D, Chun J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci U S A. 2000;97:13384–9. doi: 10.1073/pnas.97.24.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Auria L, Cordiali Fei P, Ameglio F. Cytokines and bullous pemphigoid. Eur Cytokine Netw. 1999;10:123–34. [PubMed] [Google Scholar]
- D’Auria L, Pietravalle M, Cordiali-Fei P, Ameglio F. Increased tryptase and myeloperoxydase levels in blister fluids of patients with bullous pemphigoid: correlations with cytokines, adhesion molecules and anti-basement membrane zone antibodies. Exp Dermatol. 2000;9:131–7. doi: 10.1034/j.1600-0625.2000.009002131.x. [DOI] [PubMed] [Google Scholar]
- Demoyer JS, Skalak TC, Durieux ME. Lysophosphatidic acid enhances healing of acute cutaneous wounds in the mouse. Wound Repair Regen. 2000;8:530–7. doi: 10.1046/j.1524-475x.2000.00530.x. [DOI] [PubMed] [Google Scholar]
- Diaz LA, Giudice GJ. End of the century overview of skin blisters. Arch Dermatol. 2000;136:106–12. doi: 10.1001/archderm.136.1.106. [DOI] [PubMed] [Google Scholar]
- Eichholtz T, Jalink K, Fahrenfort I, Moolenaar WH. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J. 1993;291:677–80. doi: 10.1042/bj2910677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry G, Tellier E, Try A, et al. Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation. Up-regulated expression with adipocyte differentiation and obesity. J Biol Chem. 2003;278:18162–9. doi: 10.1074/jbc.M301158200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcade O, Simon MF, Viode C, et al. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell. 1995;80:919–27. doi: 10.1016/0092-8674(95)90295-3. [DOI] [PubMed] [Google Scholar]
- Fukami K, Takenawa T. Phosphatidic acid that accumulates in platelet-derived growth factor-stimulated Balb/c 3T3 cells is a potential mitogenic signal. J Biol Chem. 1992;267:10988–93. [PubMed] [Google Scholar]
- Gaits F, Fourcade O, Le Balle F, et al. Lysophosphatidic acid as a phospholipid mediator: pathways of synthesis. FEBS Lett. 1997;410:54–8. doi: 10.1016/s0014-5793(97)00411-0. [DOI] [PubMed] [Google Scholar]
- Gesta S, Simon MF, Rey A, et al. Secretion of a lysophospholipase D activity by adipocytes: involvement in lysophosphatidic acid synthesis. J Lipid Res. 2002;43:904–10. [PMC free article] [PubMed] [Google Scholar]
- Grando SA, Glukhenky BT, Drannik GN, Epshtein EV, Kostromin AP, Korostash TA. Mediators of inflammation in blister fluids from patients with pemphigus vulgaris and bullous pemphigoid. Arch Dermatol. 1989a;125:925–30. [PubMed] [Google Scholar]
- Grando SA, Glukhenky BT, Drannik GN, Kostromin AP, Chernyavsky AI. Cytotoxic proteinases in blister fluid of pemphigus and pemphigoid patients. Int J Tissue react. 1989b;11:195–201. [PubMed] [Google Scholar]
- Hines OJ, Ryder N, Chu J, McFadden D. Lysophosphatidic acid stimulates intestinal restitution via cytoskeletal activation and remodeling. J Surg Res. 2000;92:23–8. doi: 10.1006/jsre.2000.5941. [DOI] [PubMed] [Google Scholar]
- Liliom K, Guan Z, Tseng JL, Desiderio DM, Tigyi G, Watsky MA. Growth factor-like phospholipids generated after corneal injury. Am J Physiol. 1998;274:C1065–74. doi: 10.1152/ajpcell.1998.274.4.C1065. [DOI] [PubMed] [Google Scholar]
- Maury E, Prevost MC, Simon MF, et al. Identification of two secreted phospholipases A2 in human epidermis. J Invest Dermatol. 2000;114:960–6. doi: 10.1046/j.1523-1747.2000.00965.x. [DOI] [PubMed] [Google Scholar]
- Mazereeuw-Hautier J, Redoules D, Tarroux R, et al. Identification of pancreatic type I secreted phospholipase A2 in human epidermis and its determination by tape stripping. Br J Dermatol. 2000;142:424–31. doi: 10.1046/j.1365-2133.2000.03351.x. [DOI] [PubMed] [Google Scholar]
- McCrea JM, Robinson P, Gerrard JM. Mepacrine (quinacrine) inhibition of thrombin-induced platelet responses can be overcome by lysophosphatidic acid. Biochim Biophys Acta. 1985;842:189–94. doi: 10.1016/0304-4165(85)90202-8. [DOI] [PubMed] [Google Scholar]
- Merchant TE, Kasimos JN, de Graaf PW, Minsky BD, Gierke LW, Glonek T. Phospholipid profiles of human colon cancer using 31P magnetic resonance spectroscopy. Int J Colorectal Dis. 1991;6:121–6. doi: 10.1007/BF00300208. [DOI] [PubMed] [Google Scholar]
- Moolenaar WH. Lysophospholipids in the limelight: autotaxin takes center stage. J Cell Biol. 2002;158:197–9. doi: 10.1083/jcb.200206094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono I, Gunji H, Zhang JZ, Maruyama K, Kaneko F. A study of cytokines in burn blister fluid related to wound healing. Burns. 1995;21:352–5. doi: 10.1016/0305-4179(95)00005-4. [DOI] [PubMed] [Google Scholar]
- Pages C, Simon M, Valet P, Saulnier-Blache JS. Lysophosphatidic acid synthesis and release. Prostaglandins. 2001;64:1–10. doi: 10.1016/s0090-6980(01)00110-1. [DOI] [PubMed] [Google Scholar]
- Piazza GA, Ritter JL, Baracka CA. Lysophosphatidic acid induction of transforming growth factors alpha and beta: modulation of proliferation and differentiation in cultured human keratinocytes and mouse skin. Exp Cell Res. 1995;216:51–64. doi: 10.1006/excr.1995.1007. [DOI] [PubMed] [Google Scholar]
- Radvanyi F, Jordan L, Russo-Marie F, Bon C. A sensitive and continuous fluorometric assay for phospholipase A2 using pyrene-labeled phospholipids in the presence of serum albumin. Anal Biochem. 1989;177:103–9. doi: 10.1016/0003-2697(89)90022-5. [DOI] [PubMed] [Google Scholar]
- Sauer B, Vogler R, Zimmermann K, et al. Lysophosphatidic acid interacts with transforming growth factor-beta signaling to mediate keratinocyte growth arrest and chemotaxis. J Invest Dermatol. 2004;123:840–9. doi: 10.1111/j.0022-202X.2004.23458.x. [DOI] [PubMed] [Google Scholar]
- Saulnier-Blache JS, Girard A, Simon MF, Lafontan M, Valet P. A simple and highly sensitive radioenzymatic assay for lysophosphatidic acid quantification. J Lipid Res. 2000;41:1947–51. [PMC free article] [PubMed] [Google Scholar]
- Siess W. Athero- and thrombogenic actions of lysophosphatidic acid and sphingosine-1-phosphate. Biochim Biophys Acta. 2002;1582:204–15. doi: 10.1016/s1388-1981(02)00173-7. [DOI] [PubMed] [Google Scholar]
- Sturm A, Becker A, Schulte KM, Goebell H, Dignass AU. Lysophosphatidic acid modulates intestinal epithelial cell function in vitro. Ann N Y Acad Sci. 1998;859:223–6. doi: 10.1111/j.1749-6632.1998.tb11134.x. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Nakane S, Kishimoto S, Waku K, Yoshioka Y, Tokumura A. Lysophosphatidic acid, a growth factor-like lipid, in the saliva. J Lipid Res. 2002;43:2049–55. doi: 10.1194/jlr.m200242-jlr200. [DOI] [PubMed] [Google Scholar]
- Tigyi G, Hong L, Yakubu M, Parfenova H, Shibata M, Leffler CW. Lysophosphatidic acid alters cerebrovascular reactivity in piglets. Am J Physiol. 1995;268:H2048–55. doi: 10.1152/ajpheart.1995.268.5.H2048. [DOI] [PubMed] [Google Scholar]
- Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, Fukuzawa K. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem. 2002;277:39436–42. doi: 10.1074/jbc.M205623200. [DOI] [PubMed] [Google Scholar]
- Tokumura A, Miyake M, Nishioka Y, Yamano S, Aono T, Fukuzawa K. Production of lysophosphatidic acids by lysophospholipase D in human follicular fluids of in vitro fertilization patients. Biol Reprod. 1999;61:195–9. doi: 10.1095/biolreprod61.1.195. [DOI] [PubMed] [Google Scholar]
- Tokumura A, Miyake M, Yoshimoto O, Shimizu M, Fukuzawa K. Metal-ion stimulation and inhibition of lysophospholipase D which generates bioactive lysophosphatidic acid in rat plasma. Lipids. 1998;33:1009–15. doi: 10.1007/s11745-998-0299-2. [DOI] [PubMed] [Google Scholar]
- Ono I, Gunji H, Zhang JZ, Maruyama K, Kaneko F. A study of cytokines in burn blister fluid related to wound healing. Burns. 1995;21:352–5. doi: 10.1016/0305-4179(95)00005-4. [DOI] [PubMed] [Google Scholar]
- Umezu-Goto M, Kishi Y, Taira A, et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158:227–33. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valet P, Pages C, Jeanneton O, et al. Alpha2-adrenergic receptor-mediated release of lysophosphatidic acid by adipocytes. A paracrine signal for preadipocyte growth. J Clin Invest. 1998;101:1431–8. doi: 10.1172/JCI806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volden G, Thorsrud AK, Bjornson I, Jellum E. Biochemical composition of suction blister fluid determined by high resolution multicomponent analysis (capillary gas chromatography--mass spectrometry and two-dimensional electrophoresis) J Invest Dermatol. 1980;75:421–4. doi: 10.1111/1523-1747.ep12524077. [DOI] [PubMed] [Google Scholar]
- Xu Y, Shen Z, Wiper DW, et al. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA. 1998;280:719–23. doi: 10.1001/jama.280.8.719. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Yoshikawa K. Cutaneous wound healing: an update. J Dermatol. 2001;28:521–34. doi: 10.1111/j.1346-8138.2001.tb00025.x. [DOI] [PubMed] [Google Scholar]


