Abstract
GFP-Vpr labeled HIV-1 virions have provided a method to visually examine the interactions between the virus and target cell during infection. However, existing methods to discriminate between virions that have been non-specifically endocytosed from those that have productively entered the host cell cytoplasm have remained problematic. Therefore, we examined the ability of a series of membrane targeted fluorescent fusion protein constructs to be incorporated into virions. We find that a fluorescent protein fusion targeted to the plasma membrane by the addition of the N-terminal 15 amino acid sequence of c-Src (S15) is efficiently packaged into HIV virions. Using fluorescent proteins fused to this sequence, we have generated virions dually labeled with S15-mCherry and GFP-Vpr. Importantly, we can detect the loss of this S15-mCherry membrane signal following fusion.
After infection with VSV-g pseudotyped HIV virions, we find a measurable, specific loss of membrane label during infection. This loss of fluorescence is not observed when fusion is prevented using bafilomycin A. This increased ability to discriminate between non-productively endocytosed virions and those actively undergoing steps in the infectious process will facilitate efforts to examine early steps in infection microscopically.
Introduction
Infection of a host cell by HIV-1 requires fusion of the viral membrane with the membrane of the host cell, thereby releasing the viral core into the host cell cytoplasm. Once in the cytoplasm, the viral core undergoes a series of trafficking and replicative events required to establish infection. To access nuclear host cell DNA, the HIV-1 core must be directionally trafficked towards the nucleus. During this time, the virus undergoes the poorly understood process of uncoating, which is the sequential loss of viral proteins from the preintegration complex (PIC) containing the viral genome. During this time, the viral RNA genome is converted to DNA, and this DNA is ultimately imported into the nucleus of the target cell and integrated into the host cell chromosome (Reviewed in (Dvorin and Malim, 2003)). However, studies examining these events have lagged behind other areas of HIV research such as viral fusion, gene regulation and virus production because the latter have been more easily studied using classical biochemical and molecular biology approaches. In recent years fluorescent microscopy has become an invaluable tool to examine the intracellular trafficking and early replicative events of viruses during infection (reviewed in (Campbell and Hope, 2005; Dohner, Nagel, and Sodeik, 2005). However, it has been shown that, in the case of HIV-1, nonspecific, receptor independent uptake of HIV virions occurs at high frequency. (Marechal et al., 1998; McDonald et al., 2002; Schaeffer, Geleziunas, and Greene, 2001). Therefore, analysis of the intracellular trafficking of enveloped virions requires the ability to discriminate between virions that have fused with the host cell membrane from virions that have nonproductively entered the host cell via endocytosis.
Our lab has previously examined the intracellular behavior of HIV by fluorescently labeling the HIV core with a GFP-Vpr fusion protein and labeling the virion membrane with the fluorescent, membrane intercalating dye DiD (McDonald et al., 2002). However, the efficiency of DiD labeling of virus can vary considerably between different viral preparations. We therefore sought to develop another method to label the membrane of virions with increased specificity and consistency. To this end, we examined the incorporation of a series of GFP fusion proteins targeted to the plasma membrane or otherwise known to be incorporated into HIV virions. We find a fluorescent fusion protein containing the 15 N-terminal amino acids of Src (Rodgers, 2002) is efficiently incorporated into HIV virions and is lost following fusion of the viral membrane with the host cell membrane, thus providing a powerful tool to identify fluorescently labeled HIV virions that have productively entered the host cell.
Results
Incorporation of fluorescent fusion proteins into HIV virions
We first wanted to examine the ability of specific GFP-fusion proteins to be incorporated into HIV virions. A series of fusion proteins were chosen for their ability to associate with the host cell plasma membrane or for their ability to otherwise be incorporated into HIV virions. These fusion proteins were then coexpressed in virus producing 293T cells also expressing an mRFP-Vpr fusion protein to label HIV-1 cores. Specifically, the membrane associated proteins examined were GFP derivatives containing the farnesylation sequence of p21(Ras) (EGFP-F) (Harvey, Lukovic, and Ucker, 2001), another containing the 10 N-terminal, membrane targeting amino acids of the Src tyrosine kinase p56lck fused to GFP (L10-GFP) (Rodgers, 2002; Rodgers, Crise, and Rose, 1994; Shenoy-Scaria et al., 1993), and one containing the 15 N-terminal membrane targeting amino acids of p60c-Src fused to GFP (S15-GFP) (Rodgers, 2002; Shenoy-Scaria et al., 1993). GFP fusions to the viral accessory protein Nef (Nef-GFP) and the cellular protein Cyclophilin A (CypA-GFP) were also examined since each has been reported to be specifically incorporated into HIV virions (Bukovsky et al., 1997; Franke, Yuan, and Luban, 1994; Thali et al., 1994; Welker et al., 1998). A GFP fusion targeted to the outer membrane of cells via a glycosyl-phosphatidyl-inositol (GPI) anchor was also examined but strongly inhibited virus production, and was therefore not studied further (data not shown). Unfused GFP was included as a control to determine the specificity of the virion incorporated fluorescence observed in these experiments. Virus collected from cells expressing the above GFP fusions was bound to glass coverslips, stained for p24 and imaged. mRFP-Vpr signal was observed to overlap with p24 staining, revealing that mRFP-Vpr is incorporated into virions (Fig 1B). When the GFP fluorescent intensity of mRFP-Vpr +, p24 + HIV-1 virions was examined, minimal incorporation of L10-GFP and CypA-GFP was detected. However, measurable incorporation of Nef-GFP, EGFP-F and S15-GFP into HIV virions was observed. Of these 3 proteins, S15-GFP incorporation was highest in terms of average virion fluorescence (Fig 1A) and the percentage of virions labeled (Fig 1B). Similar results were obtained when the S15 leader sequence was attached to the recently developed red fluorescent protein mCherry (Shaner et al., 2004) and virion cores were labeled with the better characterized GFP-Vpr protein (McDonald et al., 2002) (Fig 1C). Virion labeling with either S15-GFP or S15-mCherry typically resulted in detectable fluorescence in greater than 90% of virions as determined by p24 staining. By contrast, labeling with GFP-Vpr or mRFP-Vpr typically generated detectable fluorescence in 40–80% of virions as determined by p24 staining under these conditions (data not shown). In the absence of HIV transfection, supernatants from S15-mCherry transfected 293T cells did not release appreciable amounts of labeled membrane into the supernatant when these supernatants were exposed to glass coverslips as in figure 1B, 1C (data not shown). S15 expression in the virus producing 293T cells did not negatively affect virus production (data not shown). Moreover, when equivalent amounts of a GFP reporter virus were used to infect HeLa cells, virus preparations labeled with GFP-Vpr, S15-mCherry or both proteins remained as infectious as unlabeled VSV-g pseudotyped virus (Fig 1D).
Figure 1. Incorporation of fluorescent fusion proteins into HIV-1 virions.
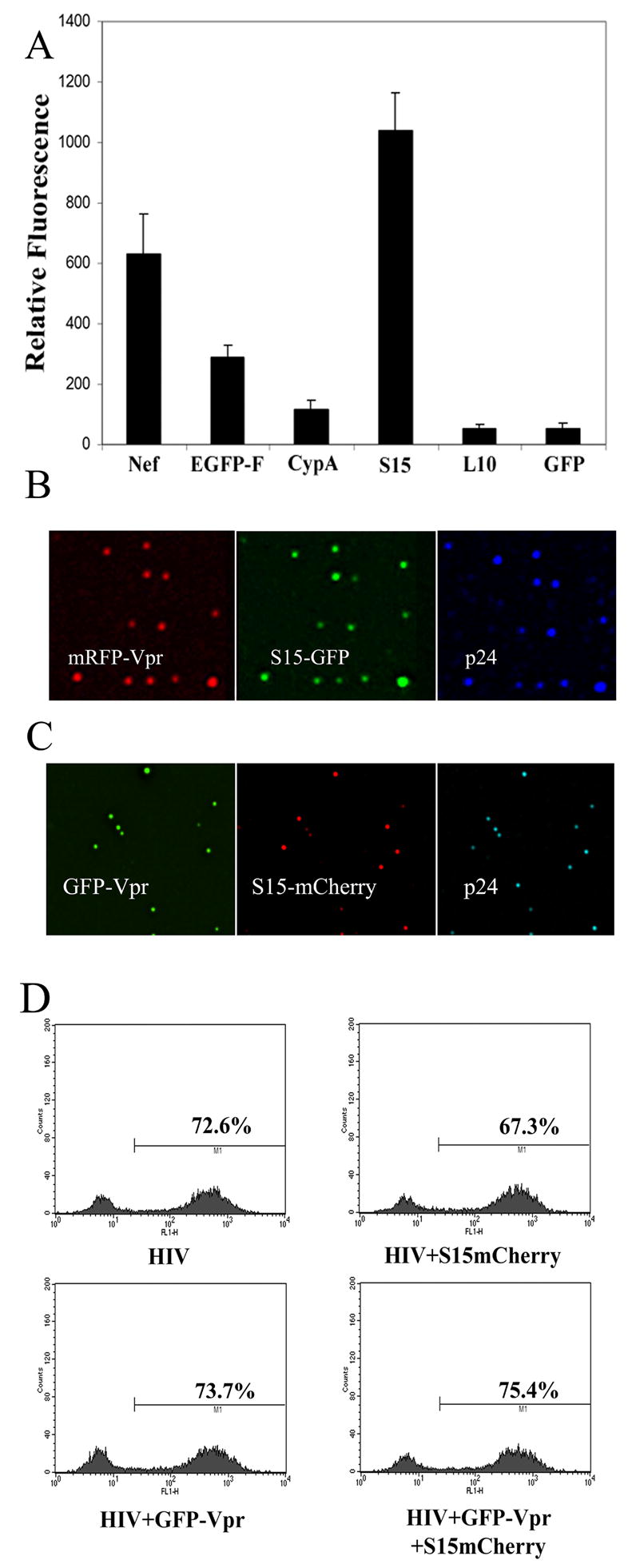
A. mRFP-Vpr labeled virions produced from 293T cells cotransfected with the indicated construct were filtered through a 0.45 μm filter and bound to glass coverslips for 4 hours, fixed and immunostained with mAb specific for p24CA. GFP fluorescence of mRFP-Vpr positive virions was quantified using the data inspector function of Applied Precision software. Error bars represent the SEM of the GFP intensities measured in 20 or more mRFP-Vpr+ virions. Fusions containing the 15 N-terminal amino acids of c-Src were found to efficiently label HIV-1 virions whether an S15-GFP (B) or S15-mCherry (C) was used. (D) HeLa cells were infected with 40 ng VSV-g pseudotyped GFP reporter virus lacking fluorescent label or labeled with the indicated fluorescent proteins. GFP expression was measured 48 hours later by flow cytometry.
S15-mCherry localizes to areas of microvilli and cell projection
We next sought to examine the intracellular localization of S15-mCherry in transfected cells. S15-mCherry expressing 293T cells were fixed and stained for ezrin. Ezrin is a member of the ezrin radixin moesin family of protein that links the actin cytoskeleton to the plasma membrane (Bretscher, Edwards, and Fehon, 2002). Our lab has found that ezrin is present on microvilli and other cell protrusions where CD4 and CCR5 accumulate (Steffens and Hope, 2003) as do constructs expressing HIV matrix and matric capsid fused to GFP (Gomez and Hope, 2006). As expected, S15mCherry was observed to localize primarily to the plasma membrane, accumulating in ezrin containing structures in 293T cells (Fig 2), while a small amount localized to discreet intracellular vesicular compartments.
Figure 2. S15-mCherry localizes to dynamic areas of the plasma membrane in 293T cells.
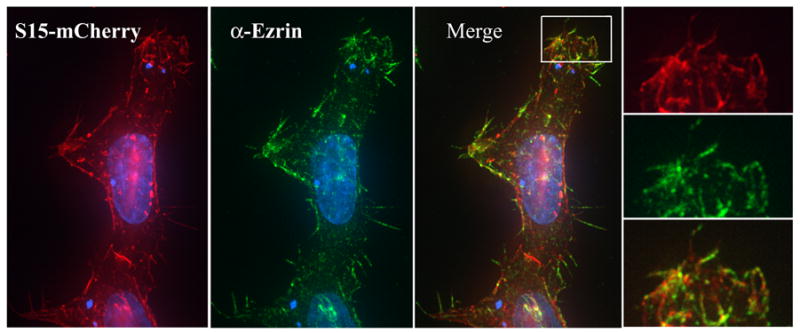
293T cells in a 6 well plate were transfected with 3 μg S15-mCherry using PEI. 24 hours post transfection, cells were stained with a monoclonal antibody specific for ezrin and analyzed by fluorescent microscopy. (INSERT) Membrane areas containing S15-GFP also stained positively for ezrin.
Loss of S15-mCherry from HIV virions correlates with fusion with target cells
Since S15-mCherry was able to label virion membranes with high efficiency (Fig 1), we next sought to determine if the S15-mCherry signal was lost from GFP-Vpr labeled virions upon fusion with target cells. We therefore infected HeLa cells with VSV-g pseudotyped HIV virions dually labeled with S15-mCherry and GFP-Vpr. We chose VSV-g envelope because it fuses with target cells at high efficiency and can be effectively inhibited by drugs such as bafilomycin A1 (BafA) that inhibit endosome acidification (Bowman, Siebers, and Altendorf, 1988; Yoshimori et al., 1991). To synchronize entry of virus into target cells, HeLa cells were spinoculated at 17ºC to allow virion binding but prevent virion endocytosis (O’Doherty, Swiggard, and Malim, 2000). Following spinoculation, cells were transferred to 37ºC in the presence or absence of BafA. These cells were then fixed at various times post-spinoculation and imaged. In cells pretreated with Bafilomycin A (BafA), which prevents fusion of VSV-g pseudotyped virions, most GFP-Vpr+ virions were still labeled with S15-mCherry 2 hours post infection (PI)(Fig 3A). In the absence of drug, however, while some S15-mCherry labeled virions were still clearly visible 2 hours post infection, a population of GFP-Vpr+ virions that lacked S15-mCherry labeling had emerged (Fig 3B). We conclude that productive entry into target cells results in the loss of S15-mCherry signal from HIV-1 virions.
Figure 3. Virion incorporated S15-mCherry signal is lost following productive entry into target cells.
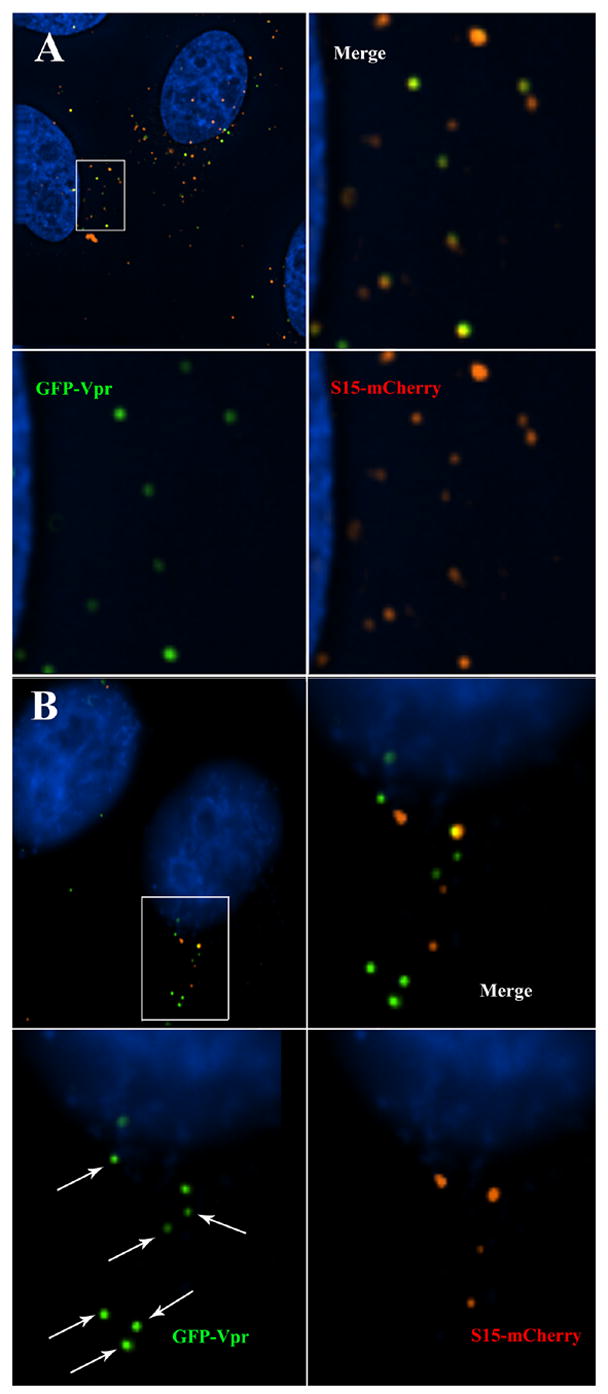
HeLa cells were spinoculated (O’Doherty, Swiggard, and Malim, 2000) with VSV-g pseudotyped, S15-mCherry and GFP-Vpr labeled R7ΔEnv virions for 2 hours at 17º C. Virus was removed and replaced with 37ºC media and then additionally incubated for 2 hours at 37ºC in the presence (A) or absence (B) of BafA. Cells were then fixed, stained for DNA and imaged. Arrows indicate virions that do not have detectable S15-mCherry labeling.
Kinetic analysis of HIV-1 infection of target cells using S15-mCherry
The ability to discriminate between virions that have productively entered the target cell from those that have not allowed us to examine the fate of both of these viral populations over time. When infection was mediated by spinoculation at 17ºC, cells fixed immediately after spinoculation maintained their S15-mCherry signal in the presence or absence of BafA (Fig 4A). The viral supernatant was removed from the remaining coverslips, replaced with fresh media and temperature was shifted to 37ºC. When these cells were fixed 1–4 hours PI, the overall number of GFP-Vpr + virions was observed to decrease over time. In contrast there was no decrease in the number of GFP-Vpr labeled particles after 4 hours in the presence of BafA (Fig 4A). BafA treatment prevented both the reduction of virion number and loss of S15-mCherry labeling (Fig 4A), suggesting that endosomal acidification is required both for fusion of VSV-g pseudotyped virions and degradation of virions which do not achieve productive entry to the target cell. After 1 hour of incubation at 37°C the total number of GFP-Vpr positive particles decreased by approximately 50%. Analysis of associated S15-mCherry signal at the 1 hour timepoint revealed a decrease in the number of S15-mCherry + virions. This was mirrored by an increase in the number of virions lacking S15-mCherry signal representing viral cores that had entered the cytoplasm (Fig 4A, 4B). Following this increase in the number of cytoplasmic virions observed at 1 hour PI, this viral population remained largely intact 2 hours PI and was then observed to decline by 4 hours PI (Fig 4A, 4B). The unfused, S15-mCherry + population, however, was observed to steadily decline between 1 and 4 hours PI, so that by 4 hours, the vast majority of cellular virions had lost their S15-mCherry membrane label (Fig 4A, 4B). The analysis of the ratio of the S15-mCherry labeled GFP-Vpr signal to those that had lost their membrane label in the remaining viral population is shown in the insert in figure 4B. This analysis reveals that initially, less than 5% of the virions were S15-mCherry negative. In contrast after 4 hours the S15-mCherry negative virions represented more than 80% of the remaining GFP-Vpr labeled population. Similarly, when HIV-1 virions lacking envelope were used to infect target cells, the S15-mCherry signal was not lost. However, in this case, the number of virions was observed to decrease over time (data not shown). We conclude that the loss of S15-mCherry signal from GFP-Vpr labeled virions correlates with the ability of virions to fuse with target cells and therefore identifies virions that have productively entered the target cell.
Figure 4. Temporal analysis of S15-mCherry loss following infection.
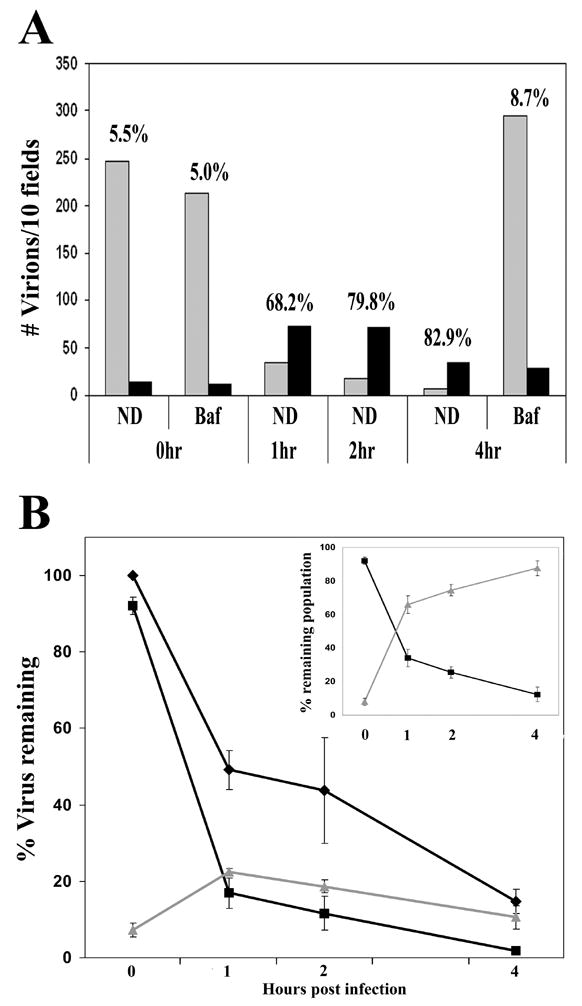
HeLa cells were spinoculated with VSV-g pseudotyped, S15-mCherry GFP-Vpr labeled R7ΔEnv virions for 2 hours at 17º C. Virus was removed and replaced with 37ºC media. Cells were incubated at 37ºC, fixed at the indicated time PI and imaged. GFP positive pucnta were than quantified and individually examined for the presence of above background mCherry signal. Panel A shows a representative experiment in which the number of virions observed per 10 Z-stack fields was calculated at the indicated time points in the presence or absence of BafA. Grey bars indicate the number of S15-mCherry positive virions and black bars indicate the number of S15-mCherry negative virions. Numbers above each time point indicate the percentage of GFP-Vpr positive virions lacking S15-mCherry signal. Panel B shows the total number of virions compiled from pooled data derived from 3 independent experiments. The input virion level was normalized to 100% in order to accommodate differences in initial observed viral input. The changes in the populations of total virions (◆) S15-mCherry positive virions (■) and S15-mCherry negative (▲) virions over time is shown. (Panel B inset) The relative amount of S15-mCherry positive virions (■) and S15-mCherry negative (▲) virions as a percentage of the remaining viral population. Error bars represent the standard error of the mean of 3 experiments.
Discussion
In this study, we find that the attachment of the 15 N-terminal amino acids of c-Src can drive incorporation of fluorescent fusion proteins into budding HIV virions. Targeting GFP to the plasma membrane by other sequences had limited ability to facilitate virion incorporation (Fig 1), suggesting that the specific membrane regions targeted by S15 fusion constructs is necessary for virion incorporation. This stretch of amino acids contains a myristoylated Gly2 residue followed by a string of basic amino acids known to be responsible for the membrane association of c-Src (reviewed in (Resh, 1994)). It has been shown that association of Nef with the plasma membrane and subsequent incorporation into HIV virions depends on a similar stretch of basic residues flanking a myristoylated glycine (Welker et al., 1998). It was therefore not surprising that both of these fluorescent fusions were specifically incorporated into HIV virions to similar degrees (Fig 1A). However, it has been reported that the S15-GFP protein is associated exclusively with the detergent soluble membrane (DSM) fraction (Rodgers, 2002). Alternatively, it has been reported that the HIV structural precursor protein Pr55Gag localizes initially to the DS fraction but subsequently localizes to the detergent resistant membrane (DRM) fraction containing glycolipid enriched membrane, or lipid rafts, prior to virion release (Ono and Freed, 2001). Despite this minor discrepancy, our microscopic analysis indicates that S15-mCherry localizes to ezrin containing membrane structures (Fig 2), in which Gag-GFP is also known to accumulate during assembly (Gomez and Hope, 2006), demonstrating that the S15 sequence directs proteins to the area of the plasma membrane that supports viral assembly.
To date, the preferred method to induce incorporation of non-viral proteins into HIV virions has been the generation of fusion proteins tethered to the viral Vpr protein (Cavrois, De Noronha, and Greene, 2002; McDonald et al., 2002; Sato et al., 1995; Wu et al., 1995). The attachment of the S15 leader sequence onto non-viral proteins similarly provides an alternative mechanism to drive virion incorporation. In fact, this method may prove to be more effective than Vpr fusions. In these experiments, membrane labeling directed by the S15 sequence always labeled a higher percentage of virions than was observed by the Vpr fusion protein, regardless of the combination of fluorescent proteins used (data not shown). This is likely due to the fact that high levels of Vpr expression in virus producing cells can negatively affect virus production (data not shown). Therefore, inducing virion incorporation of non-virion proteins by generation of fusion proteins containing the S15 sequence may prove more effective than Vpr in some cases, especially when there is no need for the protein to remain associated with the HIV preintegration complex following fusion. Such a mechanism for driving virion incorporation of non-virion proteins by inducing their membrane association may likely be achievable using other membrane targeting sequences and is likely not specific to HIV. Others have observed that GFP incorporation into virions can be achieved by tethering GFP to the membrane targeting sequence of HIV-1 Nef (Welker et al., 1998), consistent with the results observed in figure 1A. Morover, a palmitylated form of yellow fluorescent protein (YFP) has been shown to be efficiently incorporated into murine leukemia virus (Melikyan et al., 2005). In this regard, fluorescent proteins targeted to different sites in the membrane may therefore provide insight into the specific membrane microdomains used as assembly platforms by different viruses.
The ability of dually labeled virions to serve as a marker for viral fusion represents a significant step in our ability to track the interactions of the viral preintegration complex with the host cell. S15-mCherry signal is retained when virion fusion is prevented with BafA, which inhibits fusion mediated by pH dependent envelopes such as VSV-g, and following endocytosis of HIV-1 virions lacking an envelope protein, demonstrating that loss of S15 associated membrane signal occurs only when fusion occurs. Using this system to monitor fused and unfused viral populations over time following synchronized infection, we observed the disappearance of detectable cellular virions. Most of this loss occurs rapidly and represents the loss of the S15-mCherry positive viral population in the first hour post infection. This loss cannot completely be accounted for by conversion of this population to a cytoplasmic S15-mCherry negative population, but can be prevented with BafA. This suggests that many virions are rapidly degraded following endocytosis before they can enter the target cell, even when virions are pseudotyped with VSV-g.
Further work is required to determine the fate of GFP-Vpr labeled virions following fusion. By 4 hours PI, we observed a significant reduction in the number of cytoplasmic virions compared to earlier time points. Three factors could be contributing to the loss of cytoplasmic GFP-Vpr labeled cores that we observed over time in these experiments. Most obviously, the loss of GFP-Vpr signal could represent the fact that the viral DNA has been imported into the nucleus. This would include the loss of GFP-Vpr association from the PIC, as we do not observe GFP-Vpr positive puncta in the nucleus of target cells (data not shown). This is consistent with other work, which has measured nuclear viral DNA as early as 3 hours following an unsynchronized infection (Bukrinsky et al., 1992). Second, there may be a proteasome dependent mechanism by which cytoplasmic virions are degraded. This is consistent with previous reports showing an antiviral activity of the proteasome in HIV-1 infection (Schwartz et al., 1998), as well as our own data showing that proteasome inhibition does increase the stability of GFP-Vpr labeled virions over time (data not shown). Third, GFP-Vpr association with the viral PIC may be lost or become reduced during the process of uncoating and cDNA generation. Lastly, the loss of fluorescent signal we measure may be a result of pH quenching of fluorescent proteins within the lysosomal compartment. Such quenching has been reported previously, although it was also found that this quenching was rapidly reversible upon return to neutral pH (Melikyan et al., 2005). This effect would result in an underestimation of the number of unfused virions, although these virions would be subjected to conditions that would rapidly lead to their degradation in the absence of VSV-g mediated fusion. More work is required to understand how much each of these components contributes to the loss of GFP-Vpr labeled virions we observed in these experiments. The ability to discriminate between virions that have fused with the target cell, combined with the ability to synchronize infection by spinoculation, will allow these issues to be examined in more detail. Moreover, these methods will also facilitate the direct observation of early events during infection, as well as allow analysis of the protein composition of the viral preintegration complex, which should lead to an in situ characterization of the still poorly understood process of uncoating.
Materials and Methods
Cells and pharmaceuticals
293T and HeLa cells (American Type Culture Collection) were cultured at 37ºC, 7% CO2 in Dulbecco’s modified Eagle’s medium (Hyclone) containing 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 292 μg/ml L-glutamine and 10 μg/ml ciprofloxacin. Bafilomycin A1 (Sigma) was prepared in DMSO and diluted to a final concentration of 20nm in DMEM.
Virus and infections
To screen virion incorporation of fusion proteins, 293T cells in 6 well plates were transfected with 1.75 μg of the indicated fusion protein expression plasmid was cotransfected with 1.75 μg R7 env GFP, 1μg VSV-g and 0.1 μg mRFP-Vpr using polyethylenimine (PEI) (MW 25000, Polysciences). To generate virus uses in infection, 10 cm plates of 293T cells were transfected with 7.4 μg R7 env GFP, 4.2μg VSV-g, 0.5μg GFP-Vpr, and 6.4 μg S15-mCherry using PEI. Virus was harvested 48 hours post-transfection. To synchronize infection, target cells were seeded on fibronectin treated coverslips and spinoculated (O’Doherty, Swiggard, and Malim, 2000) with 0.5 ml of virus at a concentration of 50–300 ng/ml for 2 hours at 17ºC in the presence or absence of the indicated drug. Virus was then removed and replaced with 37º media in the presence or absence of the indicated drug and fixed at the indicated time point post infection. In figure 1D, the viral inoculum was replaced with fresh media 14 hours PI and GFP expression was measured 48h later using a FACS Calibur flow cytometer analyzing 5000 events per infection (Becton Dickinson).
Immunofluorescence and microscopy
Virions or cells on glass coverslips were fixed with 3.7% formaldehyde (Polysciences) in 0.1 M Pipes buffer, pH 6.8. Coverslips were stained with α-p24 mAb AG3.0 (NIH AIDS Research and Reference Reagent Program) or α-ezrin (Cytoskeleton Inc.) in blocking solution (10% normal donkey serum (Jackson ImmunoResearch Laboratories), 0.1% Triton X-100, 0.01% NaN3) for 1 hour at RT for primary stain and secondarily stained with labeled (AMCA, Cy5) donkey anti-mouse antibodies (Jackson ImmunoResearch Laboratories). Coverslips were mounted with Gel Mount (Biomedia). Images were collected and deconvolved with a Deltavision microscope and software (Applied Precision).
Statistical Image analysis
Following deconvolution, the number of GFP positive virions was assessed and each virion was individually inspected for punctate mCherry fluorescent signal. Between 9–25 images were analyzed for each data point provided. During analysis, channel intensity was increased above the level of the included figures to detect low level fluorescence.
Acknowledgments
We thank William Rodgers (Molecular Immunogenetics Program, Oklahoma Medical Research Foundation, OK) for S15-GFP and L10-GFP expression constructs. We thank David Ucker (University of Illinois at Chicago, College of Medicine,) for EGFP-F, John Guatelli (University of California, San Diego, CA) for Nef-GFP and Philippe Gallay for CypA-GFP. This work was supported by a National Institutes of Health grant R01 AI47770 and AI52051 to T.J.H. T.J.H. is an Elizabeth Glaser Scientist.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988;85(21):7972–6. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3(8):586–99. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- Bukovsky AA, Dorfman T, Weimann A, Gottlinger HG. Nef association with human immunodeficiency virus type 1 virions and cleavage by the viral protease. J Virol. 1997;71(2):1013–8. doi: 10.1128/jvi.71.2.1013-1018.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky MI, Sharova N, Dempsey MP, Stanwick TL, Bukrinskaya AG, Haggerty S, Stevenson M. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci U S A. 1992;89(14):6580–4. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EM, Hope TJ. Gene therapy progress and prospects: viral trafficking during infection. Gene Ther. 2005;12(18):1353–9. doi: 10.1038/sj.gt.3302585. [DOI] [PubMed] [Google Scholar]
- Cavrois M, De Noronha C, Greene WC. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat Biotechnol. 2002;20(11):1151–4. doi: 10.1038/nbt745. [DOI] [PubMed] [Google Scholar]
- Dohner K, Nagel CH, Sodeik B. Viral stop-and-go along microtubules: taking a ride with dynein and kinesins. Trends Microbiol. 2005;13(7):320–7. doi: 10.1016/j.tim.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Dvorin JD, Malim MH. Intracellular trafficking of HIV-1 cores: journey to the center of the cell. Curr Top Microbiol Immunol. 2003;281:179–208. doi: 10.1007/978-3-642-19012-4_5. [DOI] [PubMed] [Google Scholar]
- Franke EK, Yuan HE, Luban J. Specific incorporation of cyclophilin A into HIV-1 virions. Nature. 1994;372(6504):359–62. doi: 10.1038/372359a0. [DOI] [PubMed] [Google Scholar]
- Gomez CY, Hope TJ. Personal Communication. 2006. [Google Scholar]
- Harvey KJ, Lukovic D, Ucker DS. Membrane-targeted green fluorescent protein reliably and uniquely marks cells through apoptotic death. Cytometry. 2001;43(4):273–8. doi: 10.1002/1097-0320(20010401)43:4<273::aid-cyto1059>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Marechal V, Clavel F, Heard JM, Schwartz O. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J Virol. 1998;72(3):2208–12. doi: 10.1128/jvi.72.3.2208-2212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D, Vodicka MA, Lucero G, Svitkina TM, Borisy GG, Emerman M, Hope TJ. Visualization of the intracellular behavior of HIV in living cells. J Cell Biol. 2002;159(3):441–52. doi: 10.1083/jcb.200203150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikyan GB, Barnard RJ, Abrahamyan LG, Mothes W, Young JA. Imaging individual retroviral fusion events: from hemifusion to pore formation and growth. Proc Natl Acad Sci U S A. 2005;102(24):8728–33. doi: 10.1073/pnas.0501864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty U, Swiggard WJ, Malim MH. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J Virol. 2000;74 (21):10074–80. doi: 10.1128/jvi.74.21.10074-10080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono A, Freed EO. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc Natl Acad Sci U S A. 2001;98(24):13925–30. doi: 10.1073/pnas.241320298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh MD. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994;76(3):411–3. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Rodgers W. Making membranes green: construction and characterization of GFP-fusion proteins targeted to discrete plasma membrane domains. Biotechniques. 2002;32(5):1044–6. 1048–1050, 1. doi: 10.2144/02325st05. [DOI] [PubMed] [Google Scholar]
- Rodgers W, Crise B, Rose JK. Signals determining protein tyrosine kinase and glycosyl-phosphatidylinositol-anchored protein targeting to a glycolipid-enriched membrane fraction. Mol Cell Biol. 1994;14(8):5384–91. doi: 10.1128/mcb.14.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Isaka Y, Kodama M, Yoshimoto J, Kawauchi S, Kuwata T, Adachi A, Hayami M, Yoshi O, Fujiwara T. Targeting of chrolamphenicol acetyltransferase to human immunodeficiency virus particles via Vpr and Vpx. Microbiol Immunol. 1995;39(12):1015–9. doi: 10.1111/j.1348-0421.1995.tb03293.x. [DOI] [PubMed] [Google Scholar]
- Schaeffer E, Geleziunas R, Greene WC. Human immunodeficiency virus type 1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J Virol. 2001;75(6):2993–3000. doi: 10.1128/JVI.75.6.2993-3000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O, Marechal V, Friguet B, Arenzana-Seisdedos F, Heard JM. Antiviral activity of the proteasome on incoming human immunodeficiency virus type 1. J Virol. 1998;72(5):3845–50. doi: 10.1128/jvi.72.5.3845-3850.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22 (12):1567–72. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Shenoy-Scaria AM, Gauen LK, Kwong J, Shaw AS, Lublin DM. Palmitylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosyl-phosphatidylinositol-anchored proteins. Mol Cell Biol. 1993;13(10):6385–92. doi: 10.1128/mcb.13.10.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens CM, Hope TJ. Localization of CD4 and CCR5 in living cells. J Virol. 2003;77(8):4985–91. doi: 10.1128/JVI.77.8.4985-4991.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thali M, Bukovsky A, Kondo E, Rosenwirth B, Walsh CT, Sodroski J, Gottlinger HG. Functional association of cyclophilin A with HIV-1 virions. Nature. 1994;372(6504):363–5. doi: 10.1038/372363a0. [DOI] [PubMed] [Google Scholar]
- Welker R, Harris M, Cardel B, Krausslich HG. Virion incorporation of human immunodeficiency virus type 1 Nef is mediated by a bipartite membrane-targeting signal: analysis of its role in enhancement of viral infectivity. J Virol. 1998;72 (11):8833–40. doi: 10.1128/jvi.72.11.8833-8840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Liu H, Xiao H, Kim J, Seshaiah P, Natsoulis G, Boeke JD, Hahn BH, Kappes JC. Targeting foreign proteins to human immunodeficiency virus particles via fusion with Vpr and Vpx. J Virol. 1995;69(6):3389–98. doi: 10.1128/jvi.69.6.3389-3398.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991;266(26):17707–12. [PubMed] [Google Scholar]


