Abstract
The chorion of Aedes aegypti eggs undergoes a hardening process following oviposition and individual chorion proteins become insoluble thereafter. Our previous studies determined that peroxidase-catalyzed chorion protein crosslinking and phenoloxidase-mediated chorion melanization are primarily responsible for the formation of a hardened, desiccation resistant chorion in A. aegypti eggs. To gain further understanding of peroxidase- and phenoloxidase-catalyzed biochemical processes during chorion hardening, we analyzed chorion proteins, identified three low molecular weight major endochorion proteins that together constituted more than 70% of the total amount of endochorion proteins, and assessed their insolubilization in relation to phenoloxidase- and peroxidase-catalyzed reactions under different conditions. Our data suggest that the three low molecular weight endochorion proteins undergo disulfide bond crosslinking prior to oviposition in A. aegypti eggs, and that they undergo further crosslinking through dityrosine or trityrosine formation by peroxidase-catalyzed reactions. Our data suggest that chorion peroxidase is primarily responsible for the irreversible insolubilization of the three major endochorion proteins after oviposition. The molecular mechanisms of chorion hardening are also discussed.
Keywords: Aedes aegypti, chorion proteins, chorion hardening, peroxidase
1. Introduction
Although the chorion of mosquito eggs is a densely packed proteinaceous structure (Chapman, 1998), it is susceptible to desiccation and unresistant to detergents or reducing agents. For example, when oviposited eggs were moved to an extremely dry environment immediately following oviposition, they rapidly dehydrated. Essentially all of the chorion proteins were solubilized when dissected mature eggs were treated in a solution containing SDS and a strong reducing agent. However, in a moist environment, the chorion turns black becomes highly resistant to desiccation within 2 hours after oviposition, a process that has been termed chorion hardening. Proteins are the major components in the chorion and they become insoluble after chorion hardening; consequently, chorion hardening likely is due primarily to structural modifications of individual chorion proteins, leading to their insolubilization. Our previous studies have demonstrated that both chorion peroxidase (CPO)- and phenoloxidase-catalyzed reactions are critical for the formation of an insoluble, desiccation-resistant chorion in Aedes aegypti (Li et al., 1993; Li et al., 1996). Conceivably, some chorion proteins may serve directly or indirectly as substrates for CPO and chorion phenoloxidase during chorion hardening. To understand the biochemical processes and mechanisms leading to chorion hardening, it is essential to know the identity of individual chorion proteins and their structural modification by CPO or chorion phenoloxidase during chorion hardening.
Ultrastructural studies revealed that there are two recognizable layers in the mosquito chorion, termed endochorion and exochorion, respectively. In mosquitoes, the endochorion is a homogenous electron-dense layer and the exochorion consists of a lamellar layer with protruding tubercles or a fibrilar network (Monnerat et al., 1999; Soumare and Ndiaye, 2005; Valle et al., 1999). These ultrastructural data suggest that the protein composition and assembly between the two layers might be quite different. Our recent data dealing with the protein profiles of the solubilized chorion proteins revealed that there are three low molecular weight major chorion proteins that are present in the endochorion and that constitute more than 70% of the total amount of endochorion proteins in Ae. aegypti. These major chorion proteins undergo disulfide bond crosslinking prior to oviposition, which is reversible in the presence of a strong reducing agent, and undergo further crosslinking reactions after oviposition, which leads to their irreversible insolubilization. The high abundance of the three low molecular weight major endochorion proteins makes them ideal targets for following the process of insolubilization. This study concerns the identification of these three major endochorion proteins and an assessment of the biochemical reactions resulting in their irreversible insolubilization.
2. Materials and methods
2.1 Materials
CHAPS, diethyldithiocarbamic acid (DETC), dithiothreitol, 2,5-dihydroxylbenzoic acid, phenyl methyl sulfonyl fluoride, sodium deoxycholate, trifluoroacetic acid, chymotrypsin and proteinase K were purchased from Sigma-Aldrich (St. Louis, MO, USA). Triton X 100 and HPLC-grade acetonitrile was from Fisher (Pittsburgh, PA, USA). Modified sequence-grade trypsin was from Promega (Madison, WI, USA). C18 cartridge was from Alltech (Deerfield, IL, USA). ZipTip C18 was from Millipore (Bedford, MA, USA). All buffers were prepared with fresh Milli-Q water.
2.2 Egg collection
A. aegypti mosquitoes used in this study were reared according to methods described by Christensen and Sutherland (1984). Adult females were blood-fed on anesthetized guinea pigs. Under the applied rearing conditions, eggs became mature 3 days after taking a blood meal.
Mosquito eggs were collected either through dissecting female mosquitoes at 72 hours after a blood meal or by providing a filter paper saturated with different buffers as substrate for mosquitoes to lay eggs. To collect eggs through dissection, female mosquitoes were first cold anesthetized on ice and eggs were dissected into cold insect Ringer’s solution (130 mM NaCl, 4.7 mM KCl, 1.9 mM CaCl2, 10 mM HEPES, pH 7.0). After gently washing with the same cold Ringer, the eggs were stored at −80°C before use.
To assess the role peroxidase and phenoloxidase play in chorion hardening, filter papers, saturated with 10 mM DETC (a phenoloxidase/peroxidase inhibitor) or 10 mM Na2EDTA (an inhibitor of metallic enzymes) in Ringer’s solution, were supplied as substrates for female mosquitoes to lay eggs. Filter paper saturated with Ringer’s solution served as a control. After 30 min (when the chorion remains white) and 2 hours or 3 hours (when the chorion turns complete black) incubation at 26°C, the eggs on filter papers were rapidly collected into Ringer’s solution and frozen at −80°C.
2.3 Chorion isolation and solubilization
Chorion isolation and solubilization were based on described methods in literature (Powell et al, 1986; Trougakos and Margaritis, 1998). 200 individual eggs were placed into 5 ml of ice-cold distilled water containing 1% Triton X100, and 1 mM PMSF. Eggs were first broken mechanically and then sonicated for 1 min to release oocytic materials. Chorion was collected by sedimentation on ice for 15 min and solubilized oocytic materials were poured off. The chorion sediments were washed three more times with the same solution. The isolated chorion sediments, free from oocytic components under microscopic examination, were solubilized in 0.1 ml Leammli buffer (0.125 mM Tris, pH 6.8, 2% SDS, 10% glycerol and 0.1 M DTT) at 70°C for 30 min. After centrifugation at 20,000 g for 10 min, the solubilized proteins were subjected to SDS-PAGE.
2.4 SDS-PAGE, electro-elution and in-gel digestion
The solubilized proteins were separated by SDS-PAGE with 12% acrylamide gel (Leammli, 1970). After electrophoresis, the gel with separated proteins was stained with Coomassie blue. The protein bands on the gel were excised, and then eluted at 50 mA for 2 hours using an electroelution device (Millipore, Bedford, MA, USA). The eluted protein was concentrated using an Amicon YM-10 centrifugal filter (Millipore). Aliquots of the samples were applied to MALDI/TOF/MS to determine the molecular mass of the intact proteins.
After reduction and alkylation, the aliquots of the protein samples were digested with trypsin (0.01μg/μl) or chymotrypsin (0.01μg/μl) in 50 mM Tris-HCl (pH 8.0) at 37°C for 16 hours, or digested with proteinase K (0.01 μg/μl) in 0.2 M Na2CO3 (pH 11.0) at 37°C for 5 hours (Wu et al, 2003). The samples were than applied to LC/MS/MS for de novo sequencing.
2.5 MALDI/TOF/MS and LC/ESI/MS/MS
MALDI spectra were acquired on a Voyager-DE STR (Applied Biosystems, Norwalk, CT, USA) in positive ion mode with a delayed extraction ion source. A capillary LC interfaced through a nanospray ESI source with a Q-TOF micro™ mass spectrometer (Waters Micromass, Manchester, UK) was used for LC/MS/MS. Peptide separation was achieved through gradient elution with mobile phase A (5% acetronitrile containing 0.1% formic acid) and B (90% acetonitrile containing 0.1% formic acid). The following gradient profile was applied: 5% B from 0 to 5 min, 5% – 40% B from 6 to 40 min, and 40 – 90% B from 40 to 65 min. Peptide sequence data were derived through de novo sequencing and database searching.
2.6 Separation of exochorion and endochorion layers
Under an electron microscope (EM), the mosquito chorion has two layers, termed endochorion and exochorion, respectively (Monnerat et al., 1999; Soumare and Ndiaye, 2005; Valle et al., 1999). To separate endochorion from exochorion, dissected mature eggs were first treated with a low concentration of neutral buffer (10 mM phosphate, pH 7.0) containing 0.1% of Triton X100 and 1 mM PMSF, which broke the chorion and released oocytic materials. The chorion sediments, after extensive washing in the same solution, were treated with 1% SDS, which separates endochorion from exochorion. The separated endochorion and exochorion pieces were then manually collected and solubilized in the Leammli buffer (0.125 mM Tris, pH 6.8, 2% SDS, 10% glycerol and 0.1 M DTT) at 70°C for 30 min for SDS-PAGE analysis.
2.7 Chorion hydrolysis and determination of tyrosine derivatives
The chorion from aliquots of 1000 eggs was isolated as described in “2.3” and gas-phase acid hydrolyzed (Gieseg et al, 1993; Fu et al, 1998; Belli et al, 2003). Briefly, extensively washed chorion sediments were mixed with 50 μl of 3% sodium deoxycholate and 100 μl of tricholoacetic acid (TFA). After centrifugation at 20,000g for 10 min, the pellets were resuspended and washed with 200 μl of ice-cold acetone three times. The samples were dried under vacuum in a Speed Vac (Labconco), mixed with 0.5 ml of 6 M HCl containing 1% phenol (w/v) and 50 μl mecaptoacetic acid, and hydrolyzed at 110°C for 20–24 hours. The hydrolysates were dried under vacuum, redissolved in 100 μl 0.1% TFA, and filtered through a 0.45 μm nylon filter (Whatman). This solution was analyzed by reverse-phase HPLC using a LaChrom D7000 HPLC system with fluorescent detector (Hitachi, Tokyo, Japan). The corresponding fractions were also collected and identified by high-resolution ESI/MS on a Q-TOF Ultima mass spectrometer. Reference dityrosine and trityrosine were prepared and purified according to methods described by Malencik et al (1996).
3. Results
3.1 Chorion melanization
The chorion of newly oviposited mature Ae. aegypti eggs was white (Fig. 1A) and remained the same for 50 min after oviposition (Fig. 1B). The chorion turned grayish around 60 min after oviposition (Fig. 1C) and became completely black about 1.5 hours after oviposition (Fig. 1D).
Fig. 1.
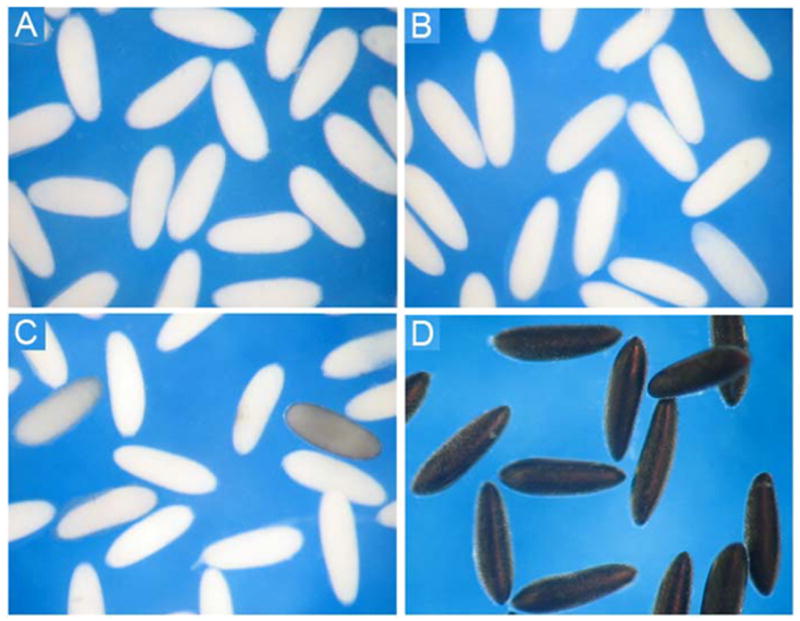
Chorion melanization in Aedes aegypti eggs. (A) Eggs at 0 – 5 min, (B) 45 ± 5 min, (C) 60 ± 5 min, and (D) 90 ± 5 min after oviposition. The chorion of oviposited eggs remained white for 40–50 min, and turned black 1.5 hours after oviposition.
3.1 Effect of SDS and reducing agent on chorion protein solubilization
When chorion sediments, isolated from dissected mature eggs, were treated with 2% SDS plus sonication in a Tris-HCl buffer at 70°C for 30 min and the supernatant was analyzed by SDS-PAGE, a number of protein bands were observed on the gel (Fig. 2A, lane 1). When supernatant, obtained from DTT-treated chorion sediments, was analyzed by SDS-PAGE, the number of protein bands decreased, but 3 low molecular weight bands, not observed in SDS-solubilized chorion proteins, appeared as the dominant bands on the gel (Fig. 2A, lane 2). More protein bands (especially the three low molecular weight bands) were observed on the gel when supernatant, obtained from both SDS- and DTT-treated chorion sediments, was analyzed by SDS-PAGE (Fig. 2A, lane 3). In addition, considerable amounts of insoluble components remained after the chorion sediments were solubilized with either SDS or DTT. In contrast, essentially no insoluble residues were observed after the chorion sediments were solubilized in a solution containing both SDS and DTT. It was apparent that the three low molecular weight bands were the major chorion proteins and that a strong reducing agent was essential for their solubilization. The requirement of a strong reducing agent for the solubilization of the three major low molecular weight proteins suggests that these proteins undergo intermolecular crosslinking through disulfide bond formation prior to oviposition. The three major low molecular weight protein bands showed a relative molecular weight of 16 KD, 15 KD and 13 KD, respectively, so they were preliminarily termed band c-16, c-15 and c-13, respectively.
Fig. 2.
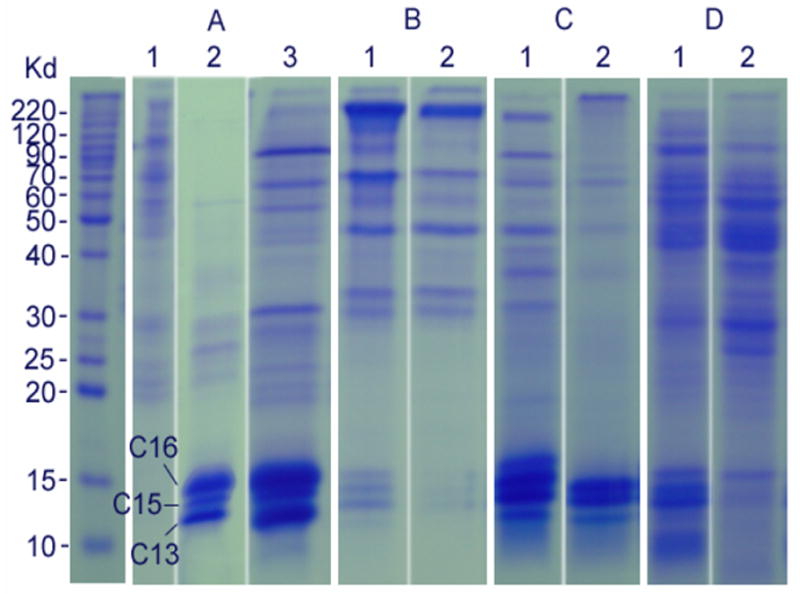
SDS-PAGE profiles of solubilized chorion proteins. Panel A: chorion protein profiles solubilized from the chorion of dissected mature eggs using 2% SDS (lane 1), 100 mM DTT (lane 2), and both 2% SDS and 100 mM DTT (lane 3); panel B: chorion protein profiles solubilized from chorion of 30 min (lane 1) and 2 hours (lane 2) oviposited control eggs using both 2% SDS and 100 mM DTT; panel C: Chorion protein profiles solubilized from the chorion of DETC-treated 30 min (lane 1) and 3 hours (lane 2) oviposited eggs using 2% SDS and 100 mM DTT; and panel D: chorion protein profiles solubilized from the chorion of EDTA-treated 30 min (lane 1) and 3 hours (lane 2) oviposited eggs using 2% SDS and 100 mM DTT. Proteins on each lane were solubilized from the chorion of approximately 200 eggs.
3.2 Insolubilization of major chorion proteins during chorion hardening
When chorion sediments from 30 min and 2 hour oviposited eggs were solubilized by SDS and DTT, the relative amounts of the major chorion proteins released from the chorion sediments were greatly decreased in eggs collected at 30 min after oviposition (Fig. 2B, lane 1) and essentially absent in the solubilization buffer when the chorion turned completely black (Fig. 2B, lane 2). These data suggest that the three low molecular weight major chorion proteins, in addition to possible crosslinking through disulfide bridges, undergo further structural modification, leading to their irreversible insolubilization during chorion hardening.
3.3 Effects of DETC and EDTA on chorion protein insolubilization
When filter papers, soaked with 10 mM DETC or EDTA were used to collect oviposited eggs, the duration required for the chorion to turn completely black was increased for eggs oviposited on EDTA and DETC soaked filter paper (2.5 – 3.5 hours). When eggs on DETC or EDTA treated filter paper were collected at 30 min (white eggs) and 3 hours (black eggs) after oviposition and their chorion was isolated and solubilized in the same manner as that of control eggs, considerable amounts of the major chorion proteins and other minor proteins remained soluble at 30 min and 3 hours after oviposition on DETC treated filter paper (Fig. 2C, lanes 1 & 2), and similar results were observed in EDTA treated eggs (Fig. 2D, lanes 1 & 2). Compared to DETC, EDTA seemed less effective in preventing the insolubilization of the three major chorion proteins. In addition, the profiles of the released chorion proteins between DETC and EDTA treated black eggs were somewhat different on the SDS-PAGE gel with considerable amounts of the three major chorion proteins solubilized from DETC treated black eggs and a number of high molecular weight proteins solubilized from EDTA treated black eggs (Fig. 2D, lane 2).
3.4 Identification of major chorion proteins
MALDI/TOF/MS showed the molecular weights of c-13, c-15, and c-16 were 9,671 Da, 10,667 Da, and 10,976 Da, respectively. They were about 20% smaller than their estimated molecular weight based on their migration positions on SDS-PAGE gel.
To identify the primary structure of these major proteins, each protein band was cut from the polyacrylamide gels and in-gel digested. MALDI/TOF/MS mapping of trypsin digested c-13, c-15 and c-16 did not lead to the detection of strong peptide signals (data not shown), suggesting that the three major chorion proteins were extremely resistant to trypsin digestion. Further proteinase K digestion of the three proteins and subsequent LC/MS/MS of their peptides resulted in the collection of a number of quality MS/MS spectra and de novo sequencing of these spectra obtained partial sequence data for the three proteins. Fig. 3 illustrates 4 MS/MS spectra and their de novo sequences derived from proteinase K digested c-13, c-15 and c-16 protein bands, respectively.
Fig. 3.
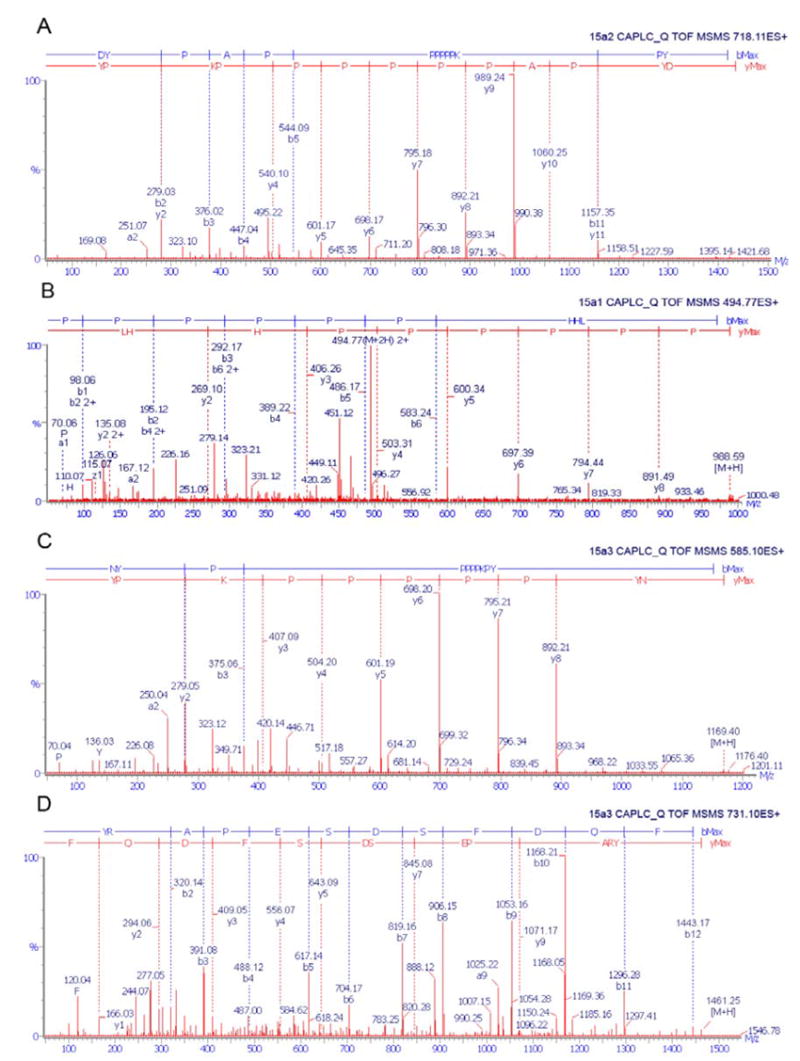
Typical ESI/MS/MS spectra and de novo sequences of proteinase K-digested peptides of the major chorion proteins: 15a2, 15a1, and 15a3. Spectra A, B and C correspond to a distinctive peptide from 15a2, 15a1, and 15a3, respectively. Spectrum D corresponds to the carboxyl end peptide from 15a3.
Blast search of the partial sequences against the NCBI protein database matched the c-13, c-15, and c-16 to “vitelline envelope proteins” 15a2, 15a1 and 15a3, respectively. Ultrastructural studies have shown that there was no vitelline envelope in the mosquito chorion (Monnerat et al., 1999; Valle et al., 1999). Therefore, we will henceforth refer to c-13, c-15 and c-16 as low molecular weight chorion proteins 15a2, 15a1 and 15a3, respectively. Analysis of their deduced sequences using a signal predicting program (http://www.cbs.dtu.dk/services/SignalP/) indicated the presence of a signal peptide in the three major chorion proteins. The first 19, 16, and 13 N-terminal residues were indeed not observed in the peptide maps 15a1 (Table 1), 15a2 (Table 2) and 15a3 (Table 3), respectively, suggesting that their signal peptide was proteolytically processed during choriogenesis.
Table 1.
Identification of chorion protein c-15 as 15a1.
| Fragment masses that matched 15a1 peptides | ||||
|---|---|---|---|---|
| Observed mass | Deduced mass | Start | End | Sequences a |
| 1055.22 | 1055.21 | 17 | 25 | MADYPKPAY |
| 2266.35 | 2266.60 | 21 | 41 | PKPAYHAPPPPPPHHLHAHPA |
| 988.41 | 988.16 | 28 | 36 | PPPPPPHHL |
| 1404.19 | 1404.60 | 29 | 41 | PPPPPHHLHAHPA |
| 1488.33 | 1488.69 | 47 | 60 | HTYPVHAPHAKCGA |
| 921.16 | 921.02 | 48 | 55 | TYPVHAPH |
| 1467.72 | 1467.50 | 54 | 67 | PHAKCGANLLVGCA |
| 702.71 | 702.82 | 64 | 70 | VGCAPSV |
| 892.25 | 892.08 | 71 | 78 | AHVPCVPL |
| 1171.75 | 1171.25 | 79 | 90 | PGHAPAHGYGHA |
| 1461.18 | 1461.51 | 95 | 106 | YRAPESDSFDQF |
|
| ||||
| 1 | MNKFIILAIFALAVGAMADYPKPAYHAPPPPPPHHLHAHPAPAPVVHTYP | 50 | ||
| 51 | VHAPHAKCGANLLVGCAPSVAHVPCVPLPGHAPAHGYGHAPAPHYRAPES | 100 | ||
| 101 | DSFDQFEE | |||
The artificial modifications of amino acid residues, i.e. carboxyamidomethyl cysteine, propionamide cysteine, or methionine sulfoxide, are not shown. The sequence at the bottom of the Table is 15a1 with the identified peptides underlined.
Table 2.
Identification of chorion protein c-13 as 15a2
| Fragment masses that matched 15a2 peptides | ||||
|---|---|---|---|---|
| Observed mass | Deduced mass | Start | End | Sequences a |
| 1435.37 | 1435.64 | 20 | 32 | DYPAPPPPPPKPY |
| 2501.07 | 2500.84 | 20 | 42 | DYPAPPPPPPKPYHAPPPPPYHA |
| 1157.30 | 1157.38 | 22 | 32 | PAPPPPPPKPY |
| 2763.20 | 2762.17 | 22 | 47 | PAPPPPPPKPYHAPPPPPYH APPHHA |
| 1846.26 | 1846.16 | 24 | 40 | PPPPPPKPYHAPPPPPY |
| 1180.28 | 1180.33 | 33 | 43 | HAPPPPPYHAP |
| 1388.28 | 1388.55 | 37 | 49 | PPPYHAPPHHAPA |
| 1246.49 | 1246.45 | 58 | 69 | YPVKAPAAKCGA |
| 846.20 | 846.01 | 65 | 72 | AKCGANLL |
| 702.68 | 702.82 | 73 | 79 | VGCAPSV |
| 892.21 | 892.08 | 80 | 87 | AHVPCVPV |
| 1708.22 | 1709.00 | 80 | 95 | AHVPCVPVHPHPPPPA |
| 1937.26 | 1938.24 | 81 | 97 | HVPCVPVHPHPPPPAHY |
| 1345.21 | 1345.53 | 86 | 97 | PVHPHPPPPAHY |
|
| ||||
| 1 | MNKIIAALVLFTAVIGALADYPAPPPPPPKPYHAPPPPPYHAPPHHAPAP | 50 | ||
| 51 | LHPVVHTYPVKAPAAKCGANLLVGCAPSVAHVPCVPVHPHPPPPAHY | 97 | ||
The artificial modifications of amino acid residues, i.e. carboxyamidomethyl cysteine, propionamide cysteine, or methionine sulfoxide, are not shown. The sequence at the bottom of the Table is 15a2 with the identified peptides underlined.
These three major chorion proteins contained dominant hydrophobic regions rich in proline, alanine, valine or leucine (Tables 1–3). The presence of conserved three cysteine residues and the requirement of strong reducing agents for their solubilization from the dissected chorion suggest that these proteins undergo disulfide bond crosslinking prior to oviposition. In addition, there are several conserved tyrosine residues in these low molecular weight major eggshell proteins, suggesting the possibility of their crosslinking through dityrosine formation during eggshell hardening.
3.5 Distribution of 15a1, 15a2 and 15a3 in endochorion
Under the EM, the chorion is a two-layer structure. To determine the distribution of the three major chorion proteins between the endochorion and exochorion, 20% sodium hypochlorite, a regent that has been used for the separation of exochorion from endochorion in Drosophila and mosquitoes (Monnerat et al., 1999), was used to treat dissected mature eggs, but this reagent destroyed the primary structures of the chorion proteins, even at a much lower concentration (not shown). Subsequently, it was found that treatment of chorion sediments with 1% SDS resulted in the separation of exochorion from endochorion (Fig. 4A and 4B). When protein samples, solubilized from the collected exochorion and endochorion pieces, respectively, were analyzed by SDS-PAGE, the overall protein profiles between solubilized exochorion and endochorion samples were quite different (Fig. 4C). Furthermore, 15a1, 15a2 and 15a3 were present only in solubilized endochorion protein samples (Fig. 4C).
Fig. 4.
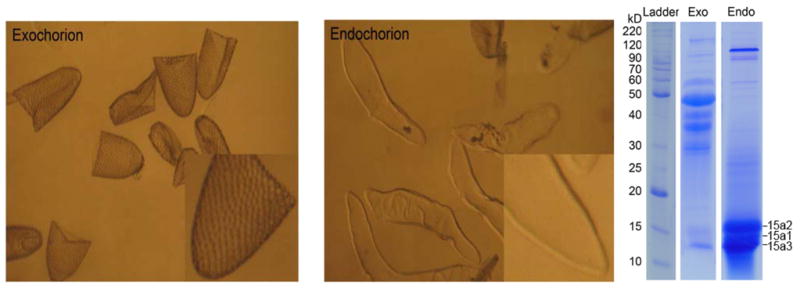
Separated exochorion (left) and endochorion pieces (middle), and solubilized exochorion (Exo) and endochorion proteins (Endo) on a SDS-PAGE gel (right). 15a1, 15a2 and 15a3 are present in the endochorion. The relative amount of endochorion proteins is higher than that of exochorion proteins on an individual egg basis. For electrophoresis, a similar amount of protein from endo- and exochorion protein extractions was applied for SDS-PAGE analysis.
3.6 Insolubilization of major endochorion proteins in relation to CPO and phenoloxidase-catalyzed reactions
CPO catalyzes protein crosslinking via intermolecular dityrosine and trityrosine formation (Kay and Shapiro, 1987; Chapman, 1998). When chorion sediments from dissected mature eggs were hydrolyzed and analyzed by HPLC with fluorescent detection, a trace amount of dityrosine was detected in the hydrolysate (Fig. 5B). The concentrations of dityrosine and trityrosine increased sharply in the chorion hydrolysates from oviposited eggs that were collected at 30 min and 2 hours after oviposition (Fig. 5C & 5D). Dityrosine was also detected in the chorion hydrolysates from DETC-treated eggs that were collected at 30 min and 3 hours after oviposition (Fig. 5E and 5F), but the amount was much lower than that detected in the hydrolysate of control samples. A similar result was also observed in 10 mM EDTA-treated eggs (Fig. 5G and 5H).
Fig. 5.
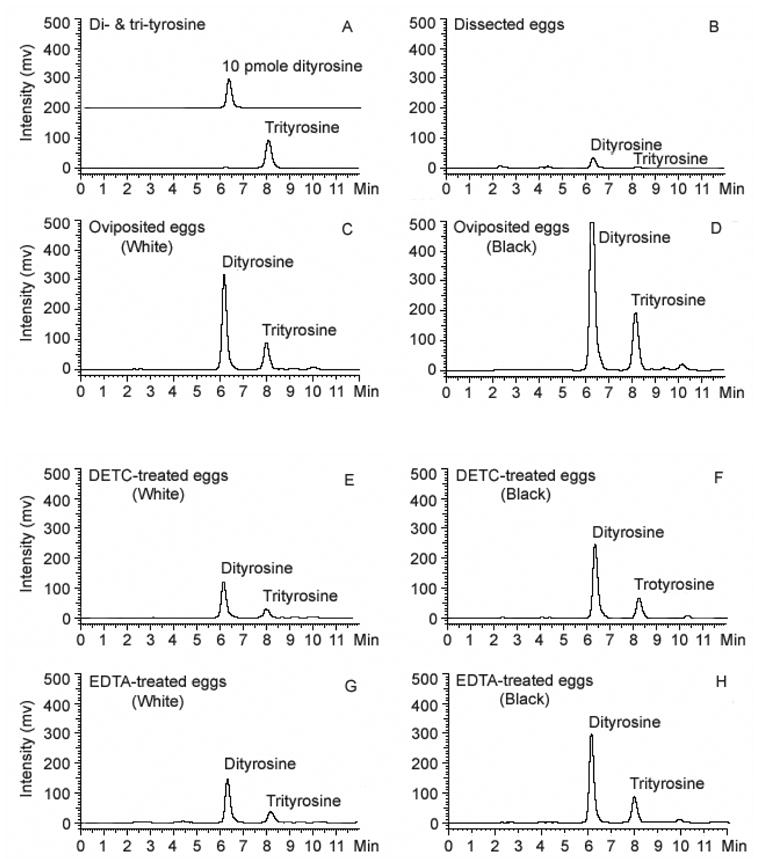
Determination of dityrosine and trityrosine in chorion hydrolysates of A. aegypti eggs using HPLC with fluorescence detection. Dityrosine and trityrosine standards were illustrated in (A). The relative amounts of dityrosine and trityrosine in chorion hydrolysates from dissected mature eggs (B); from oviposited control eggs at 30 min (C) and 2 hours (D) after oviposition; from DETC-treated oviposited eggs at 30 min (E) and 3 hours (F) after oviposition; from EDTA-treated oviposited eggs at 30 min (G) and 3 hours after oviposition. Chorion sediments were obtained from 1000 eggs in each group and gas-phase acid hydrolyzed. Analytical conditions: column, Polaris C18, 5 μm, 150 X 4.6 mm (MetaChem Technologies, Torrance, CA, USA); mobile phase, 7% acetonitrile and 0.1% TFA, 1 ml/min; detection, Ex 280 nm/Em 410 nm. The peaks are collected and applied to ESI/MS for identification: dityrosine, m/z 361.1400 C18H21N2O6; trityrosine, m/z 540.1992, C27H30N3O9.
4. Discussion
In mature A. aegypti eggs (i.e., ready to be oviposited), essentially all chorion proteins, though densely packed into an intact structure, remain soluble in the presence of a strong reducing agent and detergent, which seems quite different from some other model species. For example, a considerable amount of insoluble materials remained when chorion sediments from dissected Drosophila eggs were treated under the same conditions (unpublished data). However, most of the chorion proteins in Ae. aegypti eggs become insoluble after the chorion undergoes a hardening process shortly after oviposition. Our earlier studies indicated that both CPO- and phenoloxidase-catalyzed reactions contribute to the overall chorion hardening in mosquito eggs (Li et al., 1996), but a lack of detailed information or specific examples for CPO and phenoloxidase-catalyzed reactions limited our further understanding of the specific role these two enzymes play in chorion hardening. Consequently, we have no problem or concern proposing the involvement and the importance of these two enzymes in chorion hardening, but have difficulty explaining their role in chorion hardening in a specific or quantitative manner. By monitoring the insolubilization process of the three low molecular weight major endochorion proteins (15a1, 15a2 and 15a3) under different conditions during chorion hardening, we were able to establish a closer relationship between their irreversible insolubilization and the CPO-mediated reactions.
Under the EM, the mature mosquito chorion is a two-layer structure (Monnerat et al., 1991; Soumar and Ndiaye, 2005). The data in this study revealed that 15a1, 15a2 and 15a3 are present in the endochorion layer and that they constitute at least 70% of the total amount of endochorion proteins (based on densitometric analysis). These major endochorion proteins have been termed vitelline envelope proteins (Lin et al., 1993; Edwards et al., 1998). It has generally been considered that a vitelline envelope is produced by oocyte, but in Ae. aegypti, the materials used for the formation of the endochorion are produced by follicle cells (Raikhel and Lea, 1991). Therefore, 15a1, 15a2 and 15a3 should henceforth be termed major endochorion proteins. The three major endochorion proteins share high identity in their primary sequences, so their physical characteristics are likely similar, which may explain in part the homogenous and highly electron-dense nature of the endochorion under the EM (Monnerat et al., 1991; Soumar and Ndiaye, 2005).
Each of the identified 15a1, 15a2, and 15a3 major endochorion proteins contains 3 cysteine residues within a highly conserved region (KCGANLLVGCAPSVAHVPCVP) (Tables 1–3). Therefore, these proteins have the structural basis to crosslink through disulfide bond formation. The requirement of a strong reducing agent for their solubilization suggests that 15a1, 15a2, and 15a3 are crosslinked among themselves or with other chorion proteins through disulfide bridges prior to oviposition. After chorion hardening in the environment, all three major endochorion proteins are not solubilized through the same SDS and DTT treatment. Evidently, in addition to possible disulfide bond crosslinking, they must have undergone other structural modifications, leading to their irreversible insolubilization.
Both CPO- and chorion phenoloxidase-mediated chorion hardening reactions are initiated after oviposition. Peroxidase catalyzes one-electron oxidation of tyrosine or tyrosine residues on proteins using hydrogen peroxide as an electron acceptor, leading to the formation of tyrosine radicals and the interaction of tyrosine radicals results in the formation of dityrosine and trityrosine, which is a nonenzymatic process (Fig. 6). In contrast, phenoloxidase, catalyzing two-electron oxidation using molecular oxygen as an electron acceptor, cannot mediate dityrosine or trityrosine formation (Fig. 6). Dityrosine is not produced in the chorion prior to egg oviposition, but can easily be detected in the eggs just a few minutes after oviposition (not shown), suggesting that CPO-mediated chorion hardening reactions initiate rapidly following oviposition. Accompanied with the production of dityrosine and trityrosine in the chorion, 15a1, 15a2 and 15a3 become progressively insoluble. For example, no more than 20% of the three major endochorion proteins remained in monomer form at 30 min after egg oviposition (Fig. 2, lane 1 in panel B). The correlation between dityrosine/trityrosine formation and the insolubilization of the three major endochorion proteins provides some basis to suggest that CPO is involved in their irreversible insolubilization.
Figure 6.
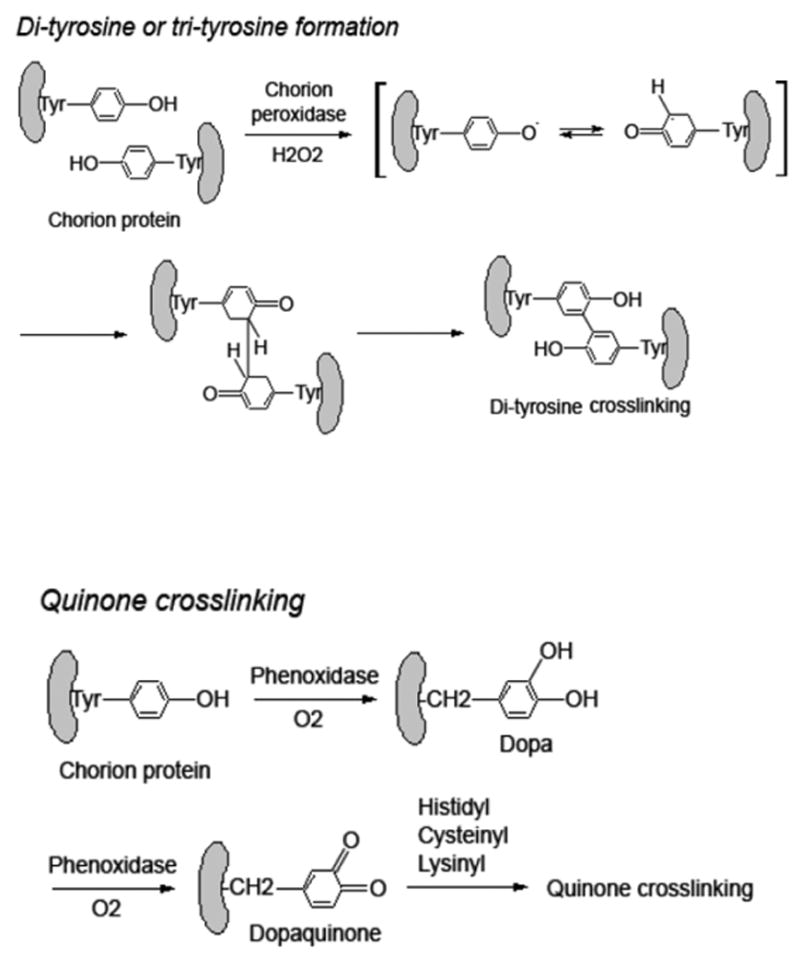
Peroxidase catalyzed chorion protein crosslinking through dityrosine formation in comparison with possible phenoloxidase-catalyzed chorion protein crosslinking reactions.
Unlike CPO-mediated chorion hardening reactions that can be monitored based on the relative quantity of dityrosine and trityrosine formed in the chorion, it is difficult to evaluate phenoloxidase-catalyzed chorion melanization in a quantitative manner. Based on our observation, no noticeable color change of the egg chorion was observed during the first 50 min after oviposition. The chorion becomes slightly grayish around 60 min, and turns completely black about 1.5 hours after oviposition (Fig. 1). The darkening process of mosquito egg chorion does not accurately reflect the progress of phenoloxidase-catalyzed chorion hardening reactions, but in the absence of other indicators, it provides a rough estimate when phenoloxidase-catalyzed reaction likely reaches its peak level after oviposition.
It has been established that phenoloxidase in insects is present in an inactive form and is activated through limited proteolytic processing (Ashida and Brey, 1998; Kanost et al., 2004). Activation of chorion proPO may be initiated soon after egg oviposition, but the process of proPO activation seems to be time consuming. Once proPO molecules are activated, chorion melanization appears to be a rather rapid process (For example, it takes 20–30 min for the chorion to turn from barely noticeable blackish to completely black). These considerations were supported by rapid chorion melanization of the dissected mature eggs upon chemical proPO activation (Kim et al., 2005). When dissected or newly oviposited eggs were treated briefly with some organic solvent, such as ethanol, some areas of the chorion turned black in just a few minutes. The rapid chorion melanization in organic solvent treated eggs suggests that proPO activation is a limiting step during chorion melanization and once the chorion proPO molecules are activated, chorion melanization advances rapidly. Although phenoloxidase-catalyzed reactions do not seem to operate at any efficient level during the early stages of chorion hardening reactions, insolubilization of the three major endochorion proteins takes place promptly following oviposition. These results provide additional support for a primary role of CPO in the irreversible insolubilization of the three major chorion proteins.
In summary, in this study we determined that 15a1, 15a2 and 15a3 (Lin et al., 1993; Edwards et al, 1998) were the most abundant endochorion proteins. By following their insolubilization under different conditions during chorion hardening, we established a close relationship between their insolubilization and CPO catalyzed reactions. Our data in this study provide specific evidence that better attributes CPO-catalyzed reactions to mosquito chorion hardening. However, the determination of 15a1, 15a2 and 15a3 as major endochorion proteins and the close relationship between their insolubilization and CPO-catalyzed reactions bring up a number of new questions. For example, the apparent difference in protein profiles between the endochorion and exochorion and the localization of 15a1, 15a2 and 15a3 in endochorion (Fig. 5) raise a critical question of whether the hardening reactions between the endochorion and exochorion are sufficiently different or if CPO-mediated hardening reactions occur primarily in the endochorion and chorion melanization occurs mostly in the exochorion or vise versa. This question raises another logical question regarding the distribution of proPO and CPO between the two layers. Although we have identified the enzymes and the biochemical events involved in chorion hardening after oviposition, the specific regulations of the two biochemical events remain to be elucidated. To truly achieve a comprehensive understanding of chorion formation and hardening, it is necessary to first determine the protein profiles for both the endochorion and exochorion, which should provide the fundamental basis towards understanding the assembling of individual chorion proteins and their structural modifications during chorion hardening.
Acknowledgments
This work is supported by a NIH grant AI 37789.
Abbreviation
- CPO
chorion peroxidase
- DETC
diethyldithiocarbamic acid
- proPO
prophenoloxidase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahida M, Brey PT. Recent advances in research on the insect prophenol oxidase cascade. In: Brey PT, Hultmark D, editors. Molecular Mechanisms of Immune Responses in Insects. Chapman & Hall; London: 1998. pp. 135–172. [Google Scholar]
- Belli SI, Wallach MG, Luxford C, Davies MJ, Smith NC. Roles of tyrosine-rich precursor glycoproteins and dityrosine- and 3,4-dihydroxyphenylalanine-mediated protein cross-linking in development of the oocyst wall in the coccidian parasite Eimeria maxima. Eukaryot Cell. 2003;2:456–464. doi: 10.1128/EC.2.3.456-464.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RF. The Insects: structure and function. 4. Cambridge University Press; Cambridge: 1998. pp. 295–342. [Google Scholar]
- Christensen BM, Sutherland DR, Gleason LN. Defense reactions of mosquitoes to filarial worms: comparative studies on the response of three different mosquitoes to inoculated Brugia pahangi and Dirofilaria immitis microfilariae. J Invertebr Pathol. 1984;44:267–274. doi: 10.1016/0022-2011(84)90024-7. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Severson DW, Hagedorn HH. Vitelline envelope genes of the yellow fever mosquito, Aedes aegypti. Insect Biochem Mol Biol. 1998;28:915–25. doi: 10.1016/s0965-1748(98)00083-6. [DOI] [PubMed] [Google Scholar]
- Fu S, Davies MJ, Stocker R, Dean RT. Evidence for roles of radicals in protein oxidation in advanced human atherosclerotic plaque. Biochem J. 1998;333:519–525. doi: 10.1042/bj3330519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieseg SP, Simpson JA, Charlton TS, Duncan MW, Dean RT. Protein-bound 3,4-dihydroxyphenylalanine is a major reductant formed during hydroxyl radical damage to proteins. Biochemistry. 1993;32:4780–4786. doi: 10.1021/bi00069a012. [DOI] [PubMed] [Google Scholar]
- Kanost MR, Jiang H, Yu XQ. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol Rev. 2004;198:97–105. doi: 10.1111/j.0105-2896.2004.0121.x. [DOI] [PubMed] [Google Scholar]
- Kay ES, Shapiro BM. Ovoperoxidase assembly into the sea urchin fertilization envelope and dityrosine crosslinking. Dev Biol. 1987;121:325–334. doi: 10.1016/0012-1606(87)90168-0. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li J, Hodgeman BA, Christensen BM. Involvement of peroxidase in chorion hardening in Aedes aegypti. Insect Biochem Mol Biol. 1996;26:309–317. doi: 10.1016/0965-1748(95)00099-2. [DOI] [PubMed] [Google Scholar]
- Li JS, Kim SR, Li J. Molecular characterization of a novel peroxidase involved in Aedes aegypti chorion protein crosslinking. Insect Biochem Mol Biol. 2004;34:1195–1203. doi: 10.1016/j.ibmb.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Lin Y, Hamblin MT, Edwards MJ, Barillas-Mury C, Kanost MR, Knipple DC, Wolfner MF, Hagedorn HH. Structure, expression, and hormonal control of genes from the mosquito, Aedes aegypti, which encode proteins similar to the vitelline membrane proteins of Drosophila melanogaster. Dev Biol. 1993;155:558–568. doi: 10.1006/dbio.1993.1052. [DOI] [PubMed] [Google Scholar]
- Kim SR, Yao Y, Han Q, Christensen, Li J. Identification and molecular characterization of a prophenoloxidase involved in Aedes aegypti chorion melanization. Insect Mol Biol. 2005;14:185–194. doi: 10.1111/j.1365-2583.2004.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malencik DA, Sprouse JF, Swanson CA, Anderson SR. Malencik Dityrosine: preparation, isolation, and analysis. Anal Biochem. 1996;242:202–213. doi: 10.1006/abio.1996.0454. [DOI] [PubMed] [Google Scholar]
- Monnerat AT, Soares MJ, Lima JBP, Rosa-Freitas MG, Valle D. Anopheles albitarsis eggs: ultrastructural analysis of chorion layers after permeabilization. J Insect Physiol. 1999;45:915–922. doi: 10.1016/s0022-1910(99)00071-2. [DOI] [PubMed] [Google Scholar]
- Powell JR, Hollander AL, Fuchs MS. A preparation method for Aedes aegypti and Aedes atropalpus chorion for protein characterization. Insect Biochem. 1986;16:835–842. [Google Scholar]
- Soumar ML, Ndiaye M. Ultrastructural studies of mosquito ovogenesis. Tissue and Cells. 2005;37:117–124. doi: 10.1016/j.tice.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Lea AO. Control of follicular epithelium development and vitelline envelope formation in the mosquito; role of juvenile hormone and 20-hydroxyecdysone. Tissue & Cell. 1991;23:577–591. doi: 10.1016/0040-8166(91)90015-l. [DOI] [PubMed] [Google Scholar]
- Trougakos IP, Margaritis LH. The formation of the functional chorion structure of Drosophila virilis involves inercalation of the "middle" and "late" major chorion proteins: a general model for chorion assembly in Drosophilidae. J Struct Biol. 1998;123:97–110. doi: 10.1006/jsbi.1998.4999. [DOI] [PubMed] [Google Scholar]
- Valle D, Monneratm AT, Soares MJ, Rosa-Freitas MG, Pelajo-Machado M, Vale BS, Lenzi HL, Galler R, Lima JPB. Mosquito embryos and eggs: polarity and terminology of chorionic layers. J Insect Physiol. 1999;45:701–708. doi: 10.1016/s0022-1910(98)00154-1. [DOI] [PubMed] [Google Scholar]
- Wu CC, MacCoss MJ, Howell KE, Yates JR., III A method for the comprehensive proteomic analysis of membrane proteins. Nat Biotechnol. 2003;21:532–538. doi: 10.1038/nbt819. [DOI] [PubMed] [Google Scholar]


