Abstract
In the following experiments, we sought to understand the triggering mechanism which propels galectin-3 to be secreted into the extracellular compartment from its intracellular stores in breast carcinoma cells. We also wanted to analyze in greater details, the role of galectin-3 in cellular adhesion and spreading. To do this, we made use of two pairs of breast carcinoma cell lines where one of the pair has high expression of galectin-3 and the other low expression of the lectin. We determined that galectin-3 secreted into the conditioned medium of sub confluent and spread cells in culture was quite low, almost negligible. However, once the cells were detached and rounded up, a mechano-sensing mechanism triggered the rapid secretion of galectin-3 into the conditioned medium. The secretion was constitutive as long as the cells remained detached. Galectin-3 was shown to be actively taken up from the conditioned medium by spreading cells. The cells which express and secrete high levels of galectin-3 adhered and spread much faster on plastic than those with reduced expression. The uptake of galectin-3 according to our data was important in cell spreading because if this process was compromised significantly, cells failed to spread. The data suggested that galectin-3 uptake modulates the adhesion plaques in that cells which express high levels of galectin-3 have thin-dot like plaques that may be suited for rapid adhesion and spreading while cells in which galectin-3 expression is reduced or knocked-down, have thick and elongated plaques which may be suited for a firmer adhesion to the substratum. Recombinant galectin-3 added exogenously reduced the thickness of the adhesion plaques of tumor cells with reduced galectin-3 expression. Taken together, the present data suggest that galectin-3 once externalized, is a powerful modulator of cellular adhesion and spreading in breast carcinoma cells.
Keywords: Galectin-3, secretion, uptake, extra cellular, adhesion plaques, mechano- transduction, integrin, breast, carcinoma, growth factor
Introduction
Galectin-3 is a chimera type member of the galectin family of animal lectins, with an amino terminal domain, a collagen like domain and a carboxyl terminal domain [1]. Galectin-3 like other members of the family, lacks signal peptide and is largely located in the cytosolic compartment of the cell [2]. However, the protein is unique in that it has been located in the nucleus [3], mitochondria [4] and other intracellular organelles [5]. A nuclear localization signal in the molecule has been identified [6]. Despite its lack of the classical signal peptide for secretion, galectin-3 can be transported into the extracellular milieu, via a non-classical pathway [2,7]. Recently we demonstrated the ability of galectin-3 to cross the lipid bilayer of large unilamellar vesicles [8], suggesting that the lectin has yet an unknown novel sequence that enables it to traverse lipid membranes.
A number of studies have suggested that galectin-3 is preferentially secreted via the exosomal transport mechanism [5,9]. Exosomes are membrane vesicles that are secreted into the conditioned medium of cells upon the exocytic fusion of multivesicular bodies with the cell surface. However, the triggering event that externalizes galectin-3 in this pathway has been elusive [9]. Once in the extracellular milieu, galectin-3 being a sugar binding protein interacts with a myriad of partners including the extracellular matrix proteins laminin and fibronectin [10]. Galectin-3 also interacts with a number of cell surface proteins that are rich in polylactosamine residues, the preferred receptors of the lectin [10,11]. One of the key physiological functions that has been repeatedly attributed to galectin-3 is cellular adhesion [10,12]. Currently, the prevailing opinion is that the interaction of galectin-3 and other members of the galectin family with the well established players of cellular adhesion such as integrins is responsible for the adhesive properties [13–16]. In other words, galectin-3 is more likely to play regulatory roles in the adhesive process rather than a direct role. Initial studies suggested that the interaction of galectin-3 with integrins on the cell surface reduced their adhesion to their extracellular matrix ligands [15]. Other studies further suggested that galectin-3 modulates the endocytic uptake of β1 integrins [17] in a manner similar to its role in the endocytosis of advanced glycation end products [18]. The endocytic uptake of the integrins would in theory down-regulate the avidity of the integrins.
In the present studies, we have demonstrated that galectin-3 is secreted and taken up by the cells using a mechanotransduction mechanism. Detached and spherical cells secrete galectin-3 in a constitutive manner while attached and spreading cells take up galectin-3 from the conditioned medium. Our data suggest that the secreted galectin-3 remodels the adhesion plaques such that the plaques formed in cells which express high levels of galectin-3 are thinner and dot like in appearance compared to the thick and elongated adhesion plaques in breast carcinoma cells with reduced expression of galectin-3.
Materials and Method
Materials
The anti-galectin-3 mAb producing hybridoma TIB 166 was purchased from ATCC (Manassas, VA). The rabbit polyclonal antibodies against galectin-3 were kindly provided by Dr. Avraham Raz of Karmanos Cancer Research Institute, Detroit, MI. Recombinant galectin-3 was kindly donated by Dr. Richard Cummings through the Consortium for Functional Glycomics. Fetuin-A was purchased from Calbiochem (San Diego, CA). All the other reagents used were purchased from Sigma (St. Louis, MO) unless otherwise stated.
Cell Culture
Two sets of breast carcinoma cell lines kindly provided to us by Dr. Avraham Raz of Karmanos Cancer Research Institute, Detroit, MI., were used in the study. The first set comprised of two sub-lines of the breast carcinoma MDA-MB-435 transfected with either galectin-3 in the antisense orientation [19] resulting in MDA-MB-435-Gal3As (435 AS) or with vector only (435 V) as control. The other set was obtained by introducing galectin-3 into the null-expressing non-tumorigenic BT-549 cells resulting in a sub-line named 549 Gal-3. As control, BT5–549 was transfected with vector only to give the sub-line 549-PCN [20]. The cell lines were routinely maintained in complete medium (CM) consisting of DMEM/F12 supplemented with essential and non-essential amino acids, 100 μg/ml penicillin-streptomycin, 2.5 μg/ml Fungizone, 20 ng/ml epidermal growth factor, 10 μg/ml insulin, 0.5 μg/ml hydrocortisone, 98 ng/ml cholera toxin, and 10% heat inactivated Fetal bovine serum. Most of the experiments were done in serum free medium (SFM) either without or supplemented with the growth factor cocktail (hydrocortisone, cholera toxin, insulin, and epidermal growth factor). The LNCaP cells (purchased from ATCC) were maintained in RPMI-1640 medium with 10% serum. The cells adhere much more slowly to the substratum and do not spread well.
Secretion of galectin-3 by detached cells
The galectin-3 expressing 549-Gal-3 carcinoma cells were grown until approximately 70% confluent. The cells were then detached and plated in 12-well microtiter plates at 5 x 105cells/well and allowed to grow for 2 days at 37°C in the humidified incubator. After 48 h, the complete medium was replaced with 200 μl/well of serum free medium (SFM) without (control) or with 2.5 mM EDTA for 5, 10, 30, and 60 min. At the indicated time points, 100 μl of the conditioned medium from each well was centrifuged to pellet any suspended cell and the supernatant assayed for galectin-3 by Western blot as previously described [21]. At the end of the experiment, the wells were washed twice and the adherent cells fixed and stained with crystal violet. The dye was released from the cells with acetic acid and O.D. 570 nm determined as described [22]. The cells were also allowed to adhere in the 12-well microtiter plate (2.5 x 105 cells/well) in 400 μl of SFM in the presence or absence of 2.5 mM EDTA. After 6 h incubation, the conditioned medium (100 μl) was centrifuged to pellet any suspended cells and the supernatant assayed for galectin-3. The experiment was repeated with the 435 V/435 AS pair.
We were also interested in determining how much galectin-3 is secreted over a period of 3 h relative to the cytoplasmic galectin-3. To do this, 435 V cells were allowed to adhere and spread in complete medium in 12-well microtiter plate (2.5 x 105 cells/ml; 200 μl/well) in the absence or presence of 2.5 mM EDTA for 3 h. At the end of the incubation period, the conditioned medium was centrifuged to pellet floating cells and the relative concentration of galectin-3 in the conditioned medium determined by Western blot as described above. The same number of cells, 2.5 x 105 cells/tube were lysed in 200 μl of RIPA (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1% Triton X-100; 1% Sodium deoxycholate; 0.1% SDS; 1 mM PMSF; 1 mM EDTA; 5 μg/ml aprotinin; 5 μg/ml leupeptin) buffer and the relative concentration of galectin-3 determined.
Speed of adhesion and spreading as a function of galectin-3 expression
The speed of adhesion and spreading was investigated using BT-549 and its galectin-3 transfected sub-line 549 Gal-3 as well as the vector control 549-PCN. The cells were detached and plated on 96 well microtiter plates at 4 x 104 cells/well. After 10 min, the non-adherent cells were washed off and the adherent cells fixed in 5% glutaraldehyde in PBS, stained with crystal violet, washed with water and photographed after drying the wells. The stain was then removed from the cells and optical density at 570 nm determined as described [22]. The BT-549, 549-PCN and 549 Gal-3 cells were also incubated in eppendorf tubes (1 x 106 cells/tube) in triplicates for 2 h. At the end of the incubation period, the cells were centrifuged and the supernatant assayed for galectin-3 expression by Western blot. The speed of adhesion and spreading experiment was repeated with the 435 V/435 AS pair.
Galectin-3 uptake by tumor cells
We hypothesize that the ability of cells to spread is driven by the rapid uptake of galectin-3 from the extracellular milieu. To directly demonstrate this process, two approaches were undertaken. In the first approach, we made use of human prostate carcinoma LNCaP cell line. It has been reported that this cell line does not synthesize endogenous galectin-3 [23]. The LNCaP cells in chambered glass slides were incubated with recombinant galectin-3 (5 μM) in SFM for 30 min. The cells were briefly washed in PBS, and then permeabilized and fixed by incubating with cold methanol for 5 min. Control LNCaP cells (incubated with only SFM) were also permeabilized and fixed. Another control slide of LNCaP cells was fixed with 3% paraformaldehyde (non-permeabilized) after incubating with 5 μM recombinant galectin-3. The slides were then incubated with rat monoclonal antibodies against galectin-3 (TIB-166), followed by FITC labeled anti-rat secondary antibodies. After extensive washing with PBS, the chambers were carefully removed and a drop of 50% glycerol saturated with n-propylgallate (anti-fade) added to the slides, cover slipped and examined by fluorescence microscopy (Olympus BX41). In the second approach, recombinant galectin-3 was labeled with rhodamine isothiocynate as previously described [17]. Breast tumor 549 Gal-3 cells were then allowed to adhere to chambered glass slides in the presence of recombinant galectin-3 in the presence of either 50 mM sucrose or 50 mM lactose. After incubation for 1 h at 37°C, the cells were washed in PBS and fixed for 30 min in a solution of 3% paraformaldehyde in PBS. The chambers were then carefully removed and the slides prepared as above, examined and images captured digitally.
Modulation of galectin-3 expression in the conditioned medium by growth factors
The galectin-3 expressing 549 Gal-3 cells were detached, washed and suspended in SFM without growth factors for 10 min at room temperature. The cells were divided into 7 eppendorf tubes (5 x 105 cells/tube) and centrifuged. SFM was aspirated and replaced with 500 μl/tube of SFM without (control # 1), and containing cholera toxin (98 ng/ml), EGF (20 ng/ml), fetuin-A (0.25% w/v), hydrocortisone (0.5 μg/ml), and insulin (10 μg/ml). Each of the five experimental tubes contained only the indicated growth factor in SFM. The last tube (control # 2) contained all the five growth factors in SFM. At the end of the experiments, the tubes were centrifuged at 1,000 x g to pellet the cells and the supernatant assayed for galectin-3 by western blot as described above. The cells were also detached and washed in SFM without insulin, hydrocortisone, insulin and EGF. They were then allowed to adhere to 96-well microtiter plates (4 x 104 cells/well) in complete medium; SFM containing insulin, hydrocortisone, insulin and EGF; and in SFM without growth factors.
The role of hydroxy propyl-betacyclodextrin (HPBCD) in the uptake of galectin-3 by breast carcinoma cells
Hydroxy propyl-betacyclodextrin has the capacity to disrupt transport mechanism mediated by the cholesterol rich lipid rafts [24]. However, HPBCD has no effect on the secretion of exosomes [25]. The galectin-3 expressing cells (549 Gal-3) were detached and allowed to adhere to the wells of 96 well microtiter plate (4 x 104 cells/well) in SFM containing all the five growth factors or complete medium (CM) and graded doses of HPBCD (0–14 mM). After 2 h of incubation, the non-adherent cells were washed off and adherent cells fixed and stained as described above, and photographed. The stain was removed and optical density determined [22]. The cells were also allowed to adhere to 12-well microtiter plate (2.5 x 105 cells/well; 400 μl/well) in SFM without and with 7 mM of HPBCD for 2 h. At the end of the incubation, 100 μl of conditioned medium from each well was centrifuged and the supernatant assayed for galectin-3.
Remodeling of adhesion plaques and cytoskeleton by galectin-3
In majority of cases, cells that express galectin 3 tend to be more spread in any substratum relative to the galectin-3 null cells which are normally spindle shaped [26]. We therefore investigated the disposition of adhesion plaques in our two pairs of high and low galectin-3 expressing breast carcinoma cells. The cells were plated in 8-chamber glass slides such that after 24 hours of incubation, they were approximately 60% confluent. The medium was removed from the wells and the cells fixed for exactly 2 minutes in a solution of PBS containing 3% para formaldehyde and 0.5% v/v Triton X-100. After 2 min, the buffer was removed and the chambers washed once with 3% Para formaldehyde in PBS and fixed in this buffer for another 30 min. The chambers were washed once more and then a monoclonal mouse anti-human vinculin (Sigma, St Lous, MO) at a dilution of 1:500 added to the wells and incubated for 40 min. The wells were washed thoroughly followed by incubation with rabbit anti-mouse secondary antibody (Cy3). After incubation with secondary antibodies, the wells were washed 5x with PBS, the chambers removed, and the slides cover-slipped. The slides were examined by an Olympus BX41 epifluorescence microscope and the images captured digitally. The cells (435 AS) were also grown in chamber slides as above and then incubated without (control) or with 5 μM of recombinant galectin-3 for 10 min and then fixed as above. The adhesion plaques (vinculin staining) in the cells incubated with excess galectin-3 was determined as above.
Growth of galectin-3 expressing and gelectin-3 knockdown cells in matrigel
Whereas the growth rates of galectin-3 expressing and cells in which the lectin is knocked down are not significantly different on plastic, we questioned whether growth on matrigel, an extracellular matrix that normally favor the rapid growth of tumor cells would distinguish the growth potential between galectin-3 expressing 435 V and 435 AS. Matrigel at 4°C was added to the wells of a 96-well microtiter plate (100 μl/well) and allowed to gel at 37°C for 30 min. The cells 2 x 104 cells/well were added on top of the matrigel and allowed to grow for 7 days. The cells were then photographed.
Results
The signal which triggers the secretion or release of galectin-3 from the intracellular compartments has been elusive. We have observed in many experiments, that negligible quantities of galectin-3 are detected in conditioned media of adherent galectin-3 expressing cells such as 549 Gal-3 and 435 V that are sub-confluent. In confluent and overgrown cultures of the tumor cells consisting of a number of floating cells on the other hand, galectin-3 could be easily detected in the conditioned medium. We therefore hypothesized that overgrowth and loose attachment of the cells on the substrata could signal the rapid secretion of galectin-3. Indeed when EDTA was added to the wells of a 6-well plate in which the cells were approximately ~70% confluent, galectin-3 was detectable in the conditioned medium within 5 min of incubation (Fig. 1A). In EDTA containing wells, cells began to detach as early as 2 min after the switch to SFM containing EDTA. However, in the absence of EDTA, galectin-3 was not detected in the conditioned medium up to 1 h after the switch to SFM (Fig. 1A). The cells remained attached and well spread in SFM for the duration of the experiment (Fig. 1B). We then questioned what would happen to galectin-3 in the conditioned medium if the detached cells are allowed to adhere and spread in the absence of EDTA. We therefore detached 549 Gal-3 and 435 V cells with EDTA, washed twice in SFM and allowed the cells (2.5 x 105 cells/well; 400 μl/well) to adhere and spread in 12-well plates in SFM in the presence and absence of EDTA. In the presence of EDTA, the cells did not attach nor spread and galectin-3 could be detected in the conditioned medium after 6 hours of incubation (Fig. 1C, lanes 1 and 2). The cells however remained viable (viable for up to 10 hours in SFM containing EDTA) as determined by trypan blue dye exclusion. In the absence of EDTA on the other hand, the cells attached and spread within 1 h and galectin-3 could hardly be detected in the conditioned medium after 6 hours of incubation (Fig. 1C, lanes 3 and 4).
Fig. 1. Secretion of galectin-3 by detaching and uptake by spreading 549-Gal-3 cells.
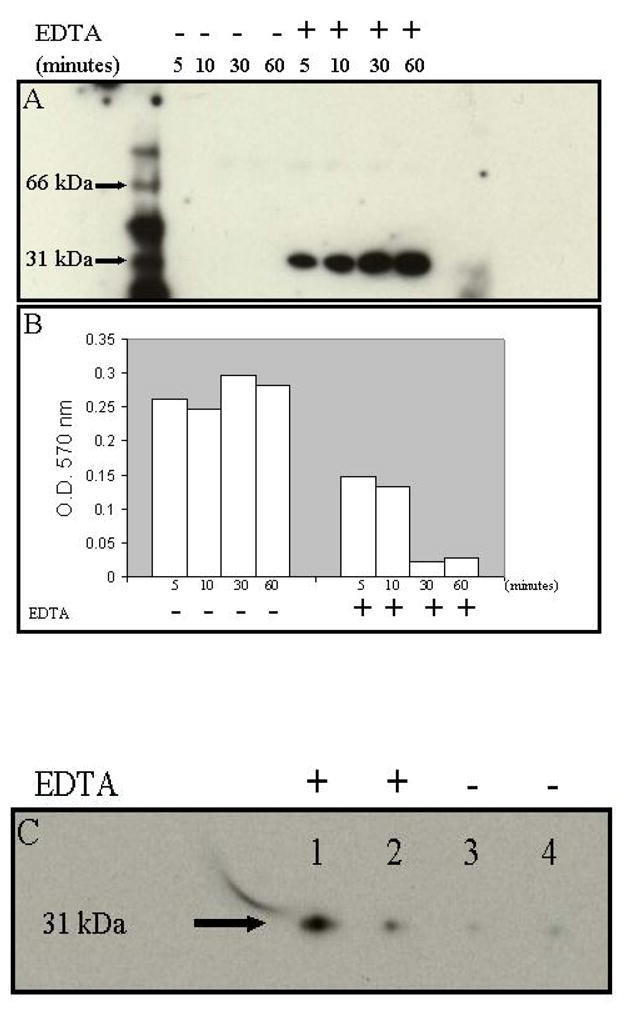
In panel A, the carcinoma cells were plated in 12-well microtiter plates at 5 x 105 cells/well and allowed to grow for 2 days in the cell incubator. After 48 h, the growth medium was replaced with 200 μl of SFM without and with 2.5 mM EDTA for 5, 10, 30, and 60 min. At the indicated time points, 100 μl of the conditioned medium from each well was assayed for galectin-3 by Western blot analysis, and the cells adhered in the wells fixed, stained with crystal violet and washed with water. The dye was released from the cells and absorbance at 570 nm determined as described in Materials and Methods (panel B). In panel C, the cells were also allowed to adhere in the 12-well microtiter plate (2.5 x 105 cells/well) in 400 μl of SFM in the presence (lanes 1 and 2) and absence (lanes 3 and 4) of 2.5 mM EDTA. After 6 h of incubation, the conditioned medium was assayed for galectin-3.
To rule out the possibility that EDTA was directly responsible for the secretion of galectin-3, we detached the cells by mechanical scraping and trypsinization and determined that galectin-3 was expressed in the conditioned medium of the detached but not adhered cells. Galectin-3 was also detected in the “conditioned medium” if the detached cells were washed and incubated in 12-well microtiter plate (5 x 105 cells/well) in Ca2+/Mg2+ free PBS (data not shown). The experiments were repeated with the 435 V/435 AS pair. Similar to the data shown for 549 Gal-3, there is considerably less galectin-3 in the conditioned medium of adhered and spread cells in comparison to rounded non-adherent cells (Fig. 2A). The 435 V cells are known to express very high levels of galectin-3 in comparison to other breast carcinoma cells. Therefore as the data suggest, the uptake of galectin-3 by spreading cells may not be as complete as in 549 Gal-3 cells and therefore galectin-3 can still be detected in the conditioned medium of adhered and spread cells. As expected a similar number of adhered and spread 435 AS express even lesser concentration of galectin-3 in their conditioned medium compared to non-adherent rounded cells (Fig. 2B). We compared the proportion of galectin-3 in the conditioned medium of adhered and spread, rounded and non-adherent as well as intracellular galectin-3 from the same number of lysed 435 AS cells. Whereas there was a decrease in the level of galectin-3 in the conditioned medium of adhered and spread cells (Fig 2C, lane 1), the concentration of galectin-3 secreted by rounded and non-adherent cells (Fig. 2C, lane 2) was almost similar to the concentration of intracellular galectin-3 (Fig. 2C, lane 3). Our data show that the relative concentration of galectin-3 secreted to the extracellular milieu over a 3 h period almost approaches that of galectin-3 in the intracellular milieu of the same number of tumor cells.
Fig. 2. Galectin-3 in the conditioned medium of adherent and non-adherent rounded MDA-MB-435 cells.

In panel A, MDA-MB-435 (435V) cells were plated in 12-well microtiter plates at 5 x 105 cells/well and allowed to grow for 2 days in the cell incubator. The complete medium was replaced with serum free medium (200 μl/well) in the absence or presence of 2 mM EDTA. After 30 min of incubation, galectin-3 in the conditioned media was measured by Western blot. In panel B, the same experiment as panel A was repeated with 435 AS. In panel C, MDA-MB-435 (435V) cells were allowed to adhere and spread in complete medium in 12-well microtiter plates (2.5 x 105 cells/well; 200 μl/well) in the absence (lane 1) or presence of 2.5 mM EDTA (lane 2) for 3h. At the end of the incubation period, the conditioned medium was centrifuged to pellet cells and the relative concentration of galectin-3 determined as above. The same number of cells (2.5 x 105 cells/tube) was lysed in 200 μl of RIPA lysis buffer containing protease inhibitors and the relative concentration of galectin-3 determined by Western blot as above (lane 3).
Galectin-3 expressing tumor cells adhere and spread faster than galectin-3 null cells
We next questioned the physiological significance of galectin-3 that is secreted following detachment of cells from the substrata. We previously reported that spreading of cells could be slowed down by lactose or thiodigalactoside in the medium [21], implying a role for extracellular galectin-3 in the modulation of cellular adhesion and spreading. We postulated that galectin-3 secreted by detached cells was responsible for the speed of adhesion and spreading on ECM proteins. We made use of three cell lines to address this problem: 1) the parental BT-549 that are known to express negligible quantities of galectin-3, 2) galectin-3 expressing 549 Gal-3 and 3) 549-PCN. When the cells (4 x 104 cells/well) were plated in 96 well plates and allowed to adhere for 10 min, only the 549 Gal-3 cells adhered and spread sufficiently within this time frame (Figs. 3B and 3C). The parental BT-549 as well as 549-PCN adhered poorly without spreading within 10 min (Figs. 3A and 3C). However if the cells were allowed to adhere for at least 7 h, the BT-549 and 549-PCN achieved similar levels of adhesion to plastic (data not shown). When the cells were incubated in eppendorf tubes for 2 h, 549 gal-3 cells secreted substantial quantities of galectin-3 (Fig. 3D, lanes 1–3). The parental BT-549 (Fig. 3D, lane 5) and 549-PCN clones (Fig. 3D, lanes 6 and 7) secreted minimal quantities of galectin-3 into the conditioned medium within 2 h. Similar adhesion and spreading data was obtained with the 435 V/435 AS pair (Fig. 4). The 435 V which express high levels of galectin-3 adhered and spread rapidly within the 10 min period in comparison to the 435 AS (Fig. 4A). Interestingly, after 7 hours of incubation both cell lines adhered and spread to the same extent (Fig. 4B).
Fig. 3. Speed of adhesion and spreading as a function of galectin-3 expression.

The cells (BT-549, 549-PCN and 549 Gal-3) were plated in 96-well microtiter plate at 4 x 104 cells/well. In panels A and B, after 10 min, the non-adherent cells were washed off and the adherent cells fixed and stained with crystal violet and photographed. In panel C, the stain was removed and the used to determine the relative numbers of cells adhered as described in Materials and Methods. In panel D, the BT-549 cells and its sub-clones were incubated in eppendorf tubes (1 x 106 cells/tube) for 2 h. At the end of the incubation, the tubes were centrifuged and galectin-3 in the conditioned medium determined by Western blot. The lane assignments were 549 Gal-3 (lanes 1–3), BT-549 (lane 5) and 549-PCN (lanes 6 and 7).
Fig. 4. The speed of adhesion and spreading of 435 V/435 AS cells.

The cells 435 AS and 435 V were plated in 96-well microtiter plates at 4 x 104 cells/well in quadriplicates. After 10 min of incubation at 37°C in panel A, the non-adherent cells were washed off and the adherent cells fixed, and stained with crystal violet. The stain was subsequently dissolved in 10% acetic acid and O.D. at 570 nm determined. In panel B, the same experiment as panel A was repeated with the exception that the cells were allowed to adhere for 7 hours before fixing and assaying for adherent cells.
Uptake of Galectin-3 and Cell Spreading
In order to show that recombinant galectin-3 can be endocytosed by tumor cells, we made use of the LNCaP prostate cancer cells that do not synthesize endogenous galectin-3 [23]. When the cells were incubated with recombinant galectin-3 and then permeabilized and incubated with rat monoclonal antibodies against galectin-3, there was clear evidence that these cells uptake galectin-3 (Fig. 5, pane A). The plasma membrane of the cells was intact because when the cells were incubated with galectin-3 and then fixed with paraformaldehyde (non-permeabilized), the antibodies could not reach the internalized galectin-3 (Fig. 5, panel A). Control cells (not incubated with recombinant galectin-3) confirmed that indeed these cells do not express endogenous galectin-3 (Fig. 5, panel A). Lastly, incubation of tumor cells with recombinant galectin-3 in the presence of sucrose suggested that under these conditions galectin-3 is internalized by the cells via structures that resemble adhesion plaques (Fig. 5 panel B, arrow). Interestingly, as the cells internalize galectin-3 in the presence of sucrose, they spread more and appear larger while in the presence of lactose the internalization of galectin-3 is slowed considerably and the cells are more rounded with less galectin-3 staining (Fig. 5, panel B).
Fig. 5. Uptake of Galectin-3 by LNCaP and 549 Gal3 cells tumor cells.
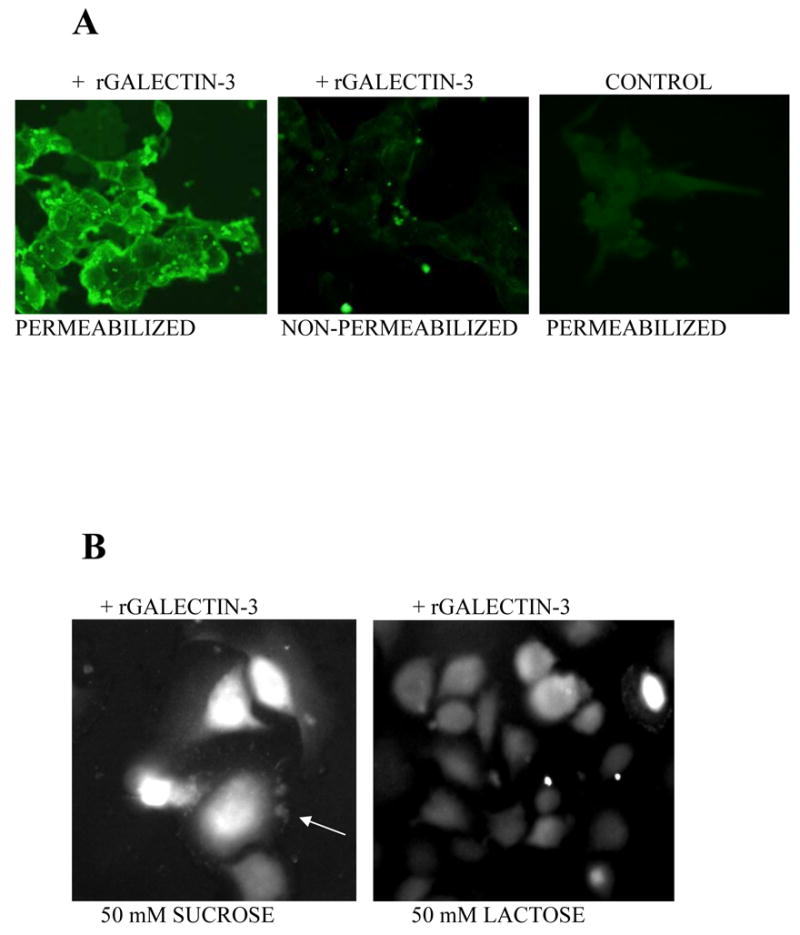
The LNCaP prostate cancer cells were plated in fibronectin coated chambered glass slides and allowed to adhere overnight in complete RPMI 1640 medium. The medium was replaced with serum free medium without (control) or with 5 μM galectin-3 and incubated for 30 min at 37°C. The cells were then fixed with cold methanol (permeabilized) or 3% paraformaldehyde (non-permeabilized) and then incubated with rat monoclonal antibodies against galectin-3 and finally with FITC-labeled anti-rat secondary antibodies. In panel B, 549 Gal3 tumor cells were allowed to adhere and spread in chambered glass slides in the presence of recombinant galectin-3 labeled with rhodamine isothiocynate (5 μM) in the presence of 50 mM sucrose or 50 mM lactose. After 1 h of incubation, the cells were examined by a fluorescence microscope and images captured digitally.
Modulation of galectin-3 expression in the conditioned medium by growth factors, and their likely roles in cellular adhesion and spreading on plastic
To achieve optimal growth of breast carcinoma cells either in serum free medium (SFM) or complete medium, supplementation with cholera toxin, EGF, hydrocortisone and insulin has been shown to be essential [27]. It appears that in the absence of any of the growth factors including fetuin-A, galectin-3 concentration in the conditioned medium of detached cells is high (Fig. 6, panels A and B). If only cholera toxin is added, the concentration of galectin-3 goes even higher (Fig. 6, panels A and B). However, the presence of either fetuin-A (0.25% w/v), hydrocortisone, or insulin, reduced the levels of galectin-3 in the conditioned medium considerably (Fig. 6, panels A and B). The presence of epidermal growth factor alone in SFM did not appreciably alter the concentration of galectin-3 in the conditioned medium. Similar data was recorded in at least 3 separate experiments. The data suggest that these supplements have a role in regulating the concentration of galectin-3 in the conditioned medium such that optimal adhesion and spreading is achieved. We previously demonstrated that incubation of cells following detachment from the flask, in SFM containing the growth factors and 0.25% w/v fetuin-A, increased the secretion of galectin-3 into the conditioned medium [21].
Fig. 6. Modulation of galectin-3 expression in the conditioned medium by growth factors.

In panel A, the 549 Gal-3 cells were detached, washed and suspended in SFM without growth factors for 10 min at RT. The cells were divided into 7 eppendorf tubes (5 x 105 cells/tube; 500μl/tube), pelleted, SFM aspirated and replaced with 200 μl/tube of fresh SFM without growth factors (control # 1) and with: cholera toxin; EGF; fetuin; hydrocortisone; insulin; and all the five growth factors (control # 2). Panel B is the densitometric scans of the gel in panel A. In panel C, the cells were detached and washed in SFM without growth factors and allowed to adhere to micro-titer wells in: complete medium; SFM containing cholera toxin, EGF, hydrocortisone, and insulin; and in SFM without growth factors.
Finally, the cells were detached in SFM without growth supplements, and allowed to adhere to 96-well micro-titer plates (4 x 104 cells/well) in complete medium, SFM containing insulin, cholera toxin, epidermal growth factors and hydrocortisone and SFM without any growth supplement (Fig. 6, panel C). It was evident that in complete medium as well as SFM with growth factors, cellular adhesion and spreading was enhanced. Cells in SFM devoid of growth supplement adhered poorly and lacked the capacity to spread, underscoring the significance of the growth factors for optimal adhesion and spreading of the cells on plastic. These experiments have been repeated at least 4 times with similar results. We have also demonstrated that when cells are allowed to adhere to plastic in SFM without growth supplements, in addition to poor adhesion and spreading, there is an accumulation of galectin-3 in the conditioned medium (data not shown).
Modulation of galectin-3 uptake by hydroxyl propyl betacyclodextrin (HPBCD)
Assuming the exosomal mechanism for secretion of galectin-3 and uptake via lipid raft domains in the cell membrane, we reasoned that the use of a non-toxic form of beta-cyclodextrin (HPBCD) which has been shown to inhibit internalization of HIV via raft domains [24], could also interfere with the uptake of galectin-3. We allowed 549 Gal-3 cells to adhere and spread on plastic for at least 24 h in SFM containing growth factors and in complete medium that contained graded doses of HPBCD (0–14 mM). Our data showed that in complete medium, adhesion and cell spreading was not affected by HPBCD (Fig. 7A). In SFM containing HPBCD on the other hand, the adhesion was poor and there was no spreading at concentrations above 3.5 mM (Fig. 7, panels A and B). In Fig. 7C, as expected, there was hardly any galectin-3 in the conditioned medium of adherent and spread cells (control). However, in the presence of 7 mM HPBCD, there was galectin-3 accumulation in the wells and the cells failed to adhere and spread (Fig. 7C).
Fig. 7. Inhibition of cellular adhesion and spreading and galectin-3 uptake by HPBCD in SFM but not complete medium.

In panel A, the 549 Gal-3 cells were allowed to adhere to the wells of 96-well microtiter plate (4 x 104 cells/well) in SFM containing insulin, EGF, hydrocortisone and cholera toxin or complete medium in the absence and presence of graded doses of HPBCD (0–14 mM). After 2 h incubation the relative numbers of adherent cells were determined as described in Materials and Methods. In panel B, the cells adhered to wells in SFM without and with 7 mM HPBCD were fixed and stained as described and photographed. In panel C, the cells we allowed to adhere to 12-well plates (2.5 x 105 cells/well; 400 μl/well) in SFM without and with 7 mM of HBCD for 2 h and the conditioned medium assayed for galectin-3.
Remodeling of adhesion plaques by galectin-3
Adhesion plaques are specialized domains of the plasma membrane which are in closest contact with the substrata. On the cytosolic side of the plaques are proteins such as vinculin, paxillin, actininin and talin [28]. On the extracellular side of the plaques are extracellular domains of integrins. Adhesion plaques are well organized and prominent in fibroblasts which are the prototypic cells used for studies pertaining to the plaques. In order to begin to understand how galectin-3 modulates cell spreading in breast carcinoma cells, we sought to analyze the organization of adhesion plaques in these cells using the indirect immunofluorescence approach. We used the galectin-3 null parental BT-549 and its galectin-3 transfected sub line, 549 Gal-3 as well as the parental MDA-MB-435 and its subclone transfected with galectin-3 antisense plasmid [19]. In all the studies we have done, adhesion plaques in BT-549 and 435 AS (visualized using antibodies to vinculin) are thick and elongated (Fig. 8, panels B and D) compared to those in 549 Gal-3 and 435 V which form thin punctate pattern evenly distributed around the cell (Fig. 8, panels A and C). Interestingly, the distribution of the adhesion plaques in 549 Gal-3 cells was similar to the punctuate pattern of galectin-3 expression observed in non-permeabilized cells [20]. To directly determine how galectin-3 modulates the expression of adhesion plaques, the medium in the chambers with 435 AS cells that were well adhered and expressing adhesion plaques (3 h after plating), was replaced with fresh serum free medium without (Fig. 8, panel E) or with 5 μM recombinant galectin-3 and incubated for 10 min at 37°C (Fig. 8, panel F). This treatment alone drastically reduced the thickness of the adhesion plaques (arrows) as expected within 10 min, confirming the direct role of galectin-3 in the modulation of adhesion plaque density.
Fig. 8. Remodeling of adhesion plaques and cytoskeleton by galectin-3.

The galectin-3 expressing breast carcinoma cell lines; 549 Gal-3 (panel A) and 435 V (panel C) as well as null cell lines; BT-549 (panel B) and 435 AS (panel D), were plated in 8 -chamber glass slides. At approximately 60% confluence, the cells were fixed briefly in a solution of PBS containing 3% para formaldehyde and 0.5% Triton X-100. They were then incubated with a mouse monoclonal antibody against human vinculin followed by secondary antibodies labeled with Cy3. The slides were processed and visualized under a fluorescence microscope. The adhered and spread 435 AS cells were also incubated without (panel E) and with 5 μM galectin-3 in SFM (panel F) for 10 min. They were then fixed and expression of adhesion plaques (vinculin) examined as above and images captured digitally.
Galectin-3 modulates the growth of the breast tumor cells in matrigel
Both MDA-MB-435 and the control cell line transfected with vector alone (435V) grew rapidly and formed stellate outgrowth in matrigel while the sub line transfected with anti-sense galectin-3 gene (435 AS) formed only small colonies consisting of spherical cells that lacked the capacity to spread and grow into the gel (Fig 9). The stellate outgrowth in matrigel is typical of aggressive and invasive tumor cells [29]. Likewise the growth of 435 AS in matrigel is similar to the growth pattern of benign and non-metastatic cells in this extra cellular matrix. The 549 Gal-3/BT-549 pair also had similar growth pattern in matrigel (data not shown).
Fig. 9. Galectin-3 modulates the growth of breast carcinoma cells in Matrigel.

The galectin-3 expressing 435 V (panel A) and its subclone 435 AS (panel B) in which galectin-3 expression is knocked down (2 x 104 cells/well), were allowed to grow on matrigel in 96-well microtiter plate for 7 days. The cells were then photographed.
Discussion
The present studies demonstrate that galectin-3 is secreted rapidly from breast carcinoma as they detach from the substratum by a mechano-sensing signaling pathway. The galectin-3 is most likely secreted from membrane bound vesicles (exosomes) that accumulate below the plasma membrane and are pinched off before being secreted into the conditioned medium [7]. The data suggest that this secretion or release of galectin-3 from the cells is sustained as long as the cells are viable and remain detached with a spherical morphology. Interestingly, as the cells adhere and spread on the substratum, they take up galectin-3 from the conditioned medium.
Mechano-transduction is a well known phenomenon in cellular adhesion and spreading [30]. Engagement of integrins to their extracellular ligands such as fibronectin, mediate adhesive and stretching forces, which are transmitted by one set of mechano-sensors to the intracellular organelles via cytoskeleton proteins, resulting in phosphorylation of proteins such as FAK and activation of PI3 kinase/Akt signaling involved in growth related mechanisms [31]. Disengagement of integrins by chelating the divalent ions in the medium on the other hand is relayed by the same or another set of mechano-sensors, resulting in the rapid and sustained secretion of galectin-3 from the cells.
The constitutive secretion of galectin-3 by the detached and rounded cells brings into sharp focus the role of the lectin in the extracellular milieu in subsequent adhesion and spreading of the cells. One of the adhesive roles that has been consistently suggested for galectin-3 expression is cell spreading [20,26,32]. Based on the present data, galectin-3 expression controls the speed at which breast carcinoma cells adhere and spread on the substrata. Most breast carcinoma cells with reduced expression of galectin-3 are spindle shaped similar to macrophages with reduced galectin-3 expression [26] in culture while cells with optimum galectin-3 expression are well spread on plastic, assuming the cobblestone morphology (data not shown).
How galectin-3 modulates cell spreading in the breast tumor cells by all accounts is a complex process. Whereas galectin-3 can interact with other intracellular proteins such as synexin [4], by far their most important binding partners are in the extracellular milieu. Upon cellular detachment from the substrata, the race is on for galectin-3 to be secreted into the extracellular space where it interacts with its receptors on the cell surface to mediate its extracellular functions [10]. The ability of breast carcinoma cells to adhere and spread on different kinds of substrata obviously depends on many factors. For example the cells adhere and spread poorly on plastic in SFM containing divalent ions but lacking growth factors. However, if the plastic is coated with collagen, laminin or fibronectin and then cells added in the minimal SFM, they adhere and spread rapidly particularly if they express galectin-3 (data not shown). Therefore, if there is a need to culture breast carcinoma cells on plastic in SFM, growth factor cocktail that includes cholera toxin, epidermal growth factor, insulin and hydrocortisone should be included [27]. The present data suggest that some of these factors enhance the secretion while others the uptake of galectin-3 by the cells. Both the secretion and uptake of galectin-3 are important for cellular division that is accompanied by cellular detachment, adhesion and spreading. Conservatively, we can hypothesize that insulin and EGF both of which are known to activate the PI3/Akt pathway [33,34], stimulate the uptake of galectin-3 which then modulates adhesion and spreading while cholera toxin speeds the secretion of galectin-3 in non-adherent cells to prepare them for subsequent adhesion and spreading. We have shown that fetuin-A which also facilitates the secretion and uptake of galectin-3, and is a major adhesion protein in serum [35], activates the PI3 kinase/Akt in tumor cells [36]. Galectin-3 may also signal the cells to synthesize and secrete collagen to which integrins binds, resulting in mechano-sensing and uptake of more galectin-3 [37].
Regarding the transport of galectin-3 through the membrane, the data suggest that secretion is mainly dependent on cell shape. As cells round up, there is galectin-3 build up in the conditioned medium. The rounded cells obviously have reduced adhesion to the substratum. The uptake of galectin-3 bound on the cell surface is most likely via lipid raft domains that can be compromised by HPBCD, particularly under serum free conditions. In complete medium (10% serum), it may require much higher concentrations of HPBCD to inhibit internalization via the lipid rafts. Our data demonstrate that galectin-3 is readily endocytosed by tumor cells including those that do not synthesize it such as LNCaP cells. The uptake of galectin-3 is facilitated by the carbohydrate recognition domain (CRD) because lactose which binds to the CRD significantly reduces uptake while sucrose that does not bind to CRD has no effect. Interestingly, galectin-3 uptake is accompanied by cell spreading, a process that can be slowed down by lactose. We previously demonstrated the ability of breast carcinoma cells to uptake galectin-3 from the extracellular compartment [8,17]. Furthermore, treatment of the breast carcinoma cells with filipin which binds to cholesterol in lipid rafts on the cell surface resulted in the rounding of the cells, accumulation of galectin-3 in the conditioned medium, and reduced adhesion to plastic [17]. Whereas we cannot conclude that the interruption of galectin-3 entry into the cells by these agents is the reason for lack of adhesion, it is safe to say that the endocytic recycling and ordering of the cell surface complex (focal adhesion) to which galectin-3 binds on the cell surface is important for adhesion and spreading of tumor cells to the substrata [38]. We hypothesize, based on the present data, that galectin-3 is one of the key regulators of membrane order of focal adhesions. We have determined that drugs such as chloroquine and methylamine which increase the pH of endosomal vesicles can also abrogate the adhesion and spreading of the breast carcinoma cells to various substrata, resulting in a build up of galectin-3 in the conditioned medium of the cells (unpublished information).
The clear difference between galectin-3 expressing cells and those that have reduced expression is the size and shape of their adhesion plaques, visualized by vinculin immunofluorescence staining. Formation of thick elongated adhesion plaques reminiscent of fibroblastic cells [39] in galectin-3 deficient breast carcinoma cells was significant. It is well established in literature that aggressive tumor cells adhere less to the extracellular matrix proteins in comparison to benign non-invasive cells [40]. The breast carcinoma cells with reduced expression of galectin-3 are less aggressive relative to those with high expression of the lectin [19]. We propose a model system where galectin-3 once secreted from the cells, binds to integrins via their polylactosamine residues, mediates their endocytosis, and finally re-distributes them evenly in adhesion plaques around the cell. The shape, size and pattern of the resulting plaques depend on the concentration of galectin-3 on the cell surface. If galectin-3 is limiting as in galectin-3 null cells, the plaques are thick and resemble those seen on fibroblasts. The present data (Fig. 8F) demonstrate that high levels of exogenously added galectin-3 can readily remodel the thick adhesion plaques on cells with reduced galectin-3 expression (435 AS). The plaques in cells which express high levels of galectin-3, are suited for rapid cell spreading but looser adhesion to the substratum. These plaques may also be more suited for growth in vivo where the cells encounter substrata such as collagen, laminin, fibronectin and matrigel [29]. Although unrelated, a similar phenomenon occurs in the clustering of T-cell receptors. In the presence of galectin-3, the receptors in Mgat5+/+ cells are well distributed on the cell surface. If lactose is added, thus competing for galectin-3 binding, the receptors cluster. In wild type Mgat5+/+ T cells, galectin-3 interact with the polylactosamine residues on the receptors to re-distribute them on the cell surface [41].
Transformation of cells is normally accompanied by elevated expression of β1,6GlcNAc-branched N-glycans, a product of Golgi β1,6-acetylglucosaminyltransferase V (Mgat5) and a ligand for galectin-3. A recent report by Lagana et al [42], demonstrated that fibronectin fibrillogenesis and fibronectin dependent cell spreading are deficient in Mgat5−/− mammary epithelial tumor cells. These processes are inhibited in Mgat5+/+ cells by swansonine or competitive inhibition of galectin-3 binding [42]. They further demonstrated that at an optimum dosage, exogenous galectin-3 added to Mgat5+/+ breast tumor cells activates focal adhesion kinase (FAK) and phosphatidyl inositol kinase (PI3K) and recruits conformationally active α5β1-integrin to fibrillar adhesion, and increases F-actin turnover in these cells. These studies reinforce a role for externalized galectin-3 in the remodeling of adhesion plaques and cell spreading in transformed breast cells.
In summary, our studies suggest that once cellular detachment from the substrata is initiated, mechano-sensors trigger the rapid secretion or release of galectin-3 into the conditioned medium. Eventually when conditions are ripe for adhesion, the extracellular galectin-3 interacts with integrins and other appropriate glycans on the cell surface to mediate the remodeling of adhesion plaques and cytoskeleton during cellular adhesion and spreading. Cells which express and secrete high levels of galectin-3 adhere and spread faster than those with reduced galectin-3 expression. However, due to the prominent adhesion plaques in cells with reduced galectin-3 expression, they are expected to adhere more firmly to the substrata.
Acknowledgments
The work was supported by IU54 CA091408 (J.O.); and grant GM62116 from the National Institutes of Health to the Consortium for Functional Glycomics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–20810. [PubMed] [Google Scholar]
- 2.Hughes RC. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta. 1999;1473:172–185. doi: 10.1016/s0304-4165(99)00177-4. [DOI] [PubMed] [Google Scholar]
- 3.Dagher SF, Wang JL, Patterson RJ. Identification of galectin-3 as a factor in pre-mRNA splicing. Proc Natl Acad Sci U S A. 1995;92:1213–1217. doi: 10.1073/pnas.92.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu F, Finley RL, Jr, Raz A, Kim HR. Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria. A role for synexin in galectin-3 translocation. J Biol Chem. 2002;277:15819–15827. doi: 10.1074/jbc.M200154200. [DOI] [PubMed] [Google Scholar]
- 5.Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 6.Li SY, Davidson PJ, Lin NY, Patterson RJ, Wang JL, Arnoys EJ. Transport of Galectin-3 between the Nucleus and Cytoplasm. II. Identification of the Signal for Nuclear Export. Glycobiology. 2006 doi: 10.1093/glycob/cwj089. [DOI] [PubMed] [Google Scholar]
- 7.Nickel W. The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. Eur J Biochem. 2003;270:2109–2119. doi: 10.1046/j.1432-1033.2003.03577.x. [DOI] [PubMed] [Google Scholar]
- 8.Lukyanov P, Furtak V, Ochieng J. Galectin-3 interacts with membrane lipids and penetrates the lipid bilayer. Biochem Biophys Res Commun. 2005;338:1031–1036. doi: 10.1016/j.bbrc.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 9.Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J. 2002;19:527–535. doi: 10.1023/B:GLYC.0000014082.99675.2f. [DOI] [PubMed] [Google Scholar]
- 11.Ochieng J, Warfield P, Green-Jarvis B, Fentie I. Galectin-3 regulates the adhesive interaction between breast carcinoma cells and elastin. J Cell Biochem. 1999;75:505–514. doi: 10.1002/(sici)1097-4644(19991201)75:3<505::aid-jcb14>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Hughes RC. Galectins as modulators of cell adhesion. Biochimie. 2001;83:667–676. doi: 10.1016/s0300-9084(01)01289-5. [DOI] [PubMed] [Google Scholar]
- 13.Dong S, Hughes RC. Macrophage surface glycoproteins binding to galectin-3 (Mac-2-antigen) Glycoconj J. 1997;14:267–274. doi: 10.1023/a:1018554124545. [DOI] [PubMed] [Google Scholar]
- 14.Gu M, Wang W, Song WK, Cooper DN, Kaufman SJ. Selective modulation of the interaction of alpha 7 beta 1 integrin with fibronectin and laminin by L-14 lectin during skeletal muscle differentiation. J Cell Sci. 1994;107(Pt 1):175–181. doi: 10.1242/jcs.107.1.175. [DOI] [PubMed] [Google Scholar]
- 15.Ochieng J, Leite-Browning ML, Warfield P. Regulation of cellular adhesion to extracellular matrix proteins by galectin-3. Biochem Biophys Res Commun. 1998;246:788–791. doi: 10.1006/bbrc.1998.8708. [DOI] [PubMed] [Google Scholar]
- 16.Zick Y, Eisenstein M, Goren RA, Hadari YR, Levy Y, Ronen D. Role of galectin-8 as a modulator of cell adhesion and cell growth. Glycoconj J. 2004;19:517–526. doi: 10.1023/B:GLYC.0000014081.55445.af. [DOI] [PubMed] [Google Scholar]
- 17.Furtak V, Hatcher F, Ochieng J. Galectin-3 mediates the endocytosis of beta-1 integrins by breast carcinoma cells. Biochem Biophys Res Commun. 2001;289:845–850. doi: 10.1006/bbrc.2001.6064. [DOI] [PubMed] [Google Scholar]
- 18.Zhu W, Sano H, Nagai R, Fukuhara K, Miyazaki A, Horiuchi S. The role of galectin-3 in endocytosis of advanced glycation end products and modified low density lipoproteins. Biochem Biophys Res Commun. 2001;280:1183–1188. doi: 10.1006/bbrc.2001.4256. [DOI] [PubMed] [Google Scholar]
- 19.Honjo Y, Nangia-Makker P, Inohara H, Raz A. Down-regulation of galectin-3 suppresses tumorigenicity of human breast carcinoma cells. Clin Cancer Res. 2001;7:661–668. [PubMed] [Google Scholar]
- 20.Warfield PR, Makker PN, Raz A, Ochieng J. Adhesion of human breast carcinoma to extracellular matrix proteins is modulated by galectin-3. Invasion Metastasis. 1997;17:101–112. [PubMed] [Google Scholar]
- 21.Zhu WQ, Ochieng J. Rapid release of intracellular galectin-3 from breast carcinoma cells by fetuin. Cancer Res. 2001;61:1869–1873. [PubMed] [Google Scholar]
- 22.Kundranda MN, Ray S, Saria M, Friedman D, Matrisian LM, Lukyanov P, Ochieng J. Annexins expressed on the cell surface serve as receptors for adhesion to immobilized fetuin-A. Biochim Biophys Acta. 2004;1693:111–123. doi: 10.1016/j.bbamcr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Ellerhorst J, Nguyen T, Cooper DN, Lotan D, Lotan R. Differential expression of endogenous galectin-1 and galectin-3 in human prostate cancer cell lines and effects of overexpressing galectin-1 on cell phenotype. Int J Oncol. 1999;14:217–224. [PubMed] [Google Scholar]
- 24.Graham DR, Chertova E, Hilburn JM, Arthur LO, Hildreth JE. Cholesterol depletion of human immunodeficiency virus type 1 and simian immunodeficiency virus with beta-cyclodextrin inactivates and permeabilizes the virions: evidence for virion-associated lipid rafts. J Virol. 2003;77:8237–8248. doi: 10.1128/JVI.77.15.8237-8248.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem. 2005;280:23349–23355. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- 26.Hsu DK, Yang RY, Pan Z, Yu L, Salomon DR, Fung-Leung WP, Liu FT. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol. 2000;156:1073–1083. doi: 10.1016/S0002-9440(10)64975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salomon DS, Liotta LA, Kidwell WR. Differential response to growth factor by rat mammary epithelium plated on different collagen substrata in serum-free medium. Proc Natl Acad Sci U S A. 1981;78:382–386. doi: 10.1073/pnas.78.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jockusch BM, Bubeck P, Giehl K, Kroemker M, Moschner J, Rothkegel M, Rudiger M, Schluter K, Stanke G, Winkler J. The molecular architecture of focal adhesions. Annu Rev Cell Dev Biol. 1995;11:379–416. doi: 10.1146/annurev.cb.11.110195.002115. [DOI] [PubMed] [Google Scholar]
- 29.Sharp JA, Waltham M, Williams ED, Henderson MA, Thompson EW. Transfection of MDA-MB-231 human breast carcinoma cells with bone sialoprotein (BSP) stimulates migration and invasion in vitro and growth of primary and secondary tumors in nude mice. Clin Exp Metastasis. 2004;21:19–29. doi: 10.1023/b:clin.0000017167.17065.61. [DOI] [PubMed] [Google Scholar]
- 30.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. Faseb J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 31.Lee BH, Ruoslahti E. alpha5beta1 integrin stimulates Bcl-2 expression and cell survival through Akt, focal adhesion kinase, and Ca2+/calmodulin-dependent protein kinase IV. J Cell Biochem. 2005;95:1214–1223. doi: 10.1002/jcb.20488. [DOI] [PubMed] [Google Scholar]
- 32.Matarrese P, Fusco O, Tinari N, Natoli C, Liu FT, Semeraro ML, Malorni W, Iacobelli S. Galectin-3 overexpression protects from apoptosis by improving cell adhesion properties. Int J Cancer. 2000;85:545–554. [PubMed] [Google Scholar]
- 33.Chang L, Chiang SH, Saltiel AR. Insulin signaling and the regulation of glucose transport. Mol Med. 2004;10:65–71. doi: 10.2119/2005-00029.Saltiel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu Q, Yang M, Tsang BK, Gruslin A. Both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signalling are required in epidermal growth factor-induced human trophoblast migration. Mol Hum Reprod. 2004;10:677–684. doi: 10.1093/molehr/gah088. [DOI] [PubMed] [Google Scholar]
- 35.Brown WM, Saunders NR, Mollgard K, Dziegielewska KM. Fetuin--an old friend revisited. Bioessays. 1992;14:749–755. doi: 10.1002/bies.950141105. [DOI] [PubMed] [Google Scholar]
- 36.Kundranda MN, Henderson M, Carter KJ, Gorden L, Binhazim A, Ray S, Baptiste T, Shokrani M, Leite-Browning ML, Jahnen-Dechent W, Matrisian LM, Ochieng J. The serum glycoprotein fetuin-A promotes Lewis lung carcinoma tumorigenesis via adhesive-dependent and adhesive-independent mechanisms. Cancer Res. 2005;65:499–506. [PubMed] [Google Scholar]
- 37.Sasaki S, Bao Q, Hughes RC. Galectin-3 modulates rat mesangial cell proliferation and matrix synthesis during experimental glomerulonephritis induced by anti-Thy1.1 antibodies. J Pathol. 1999;187:481–489. doi: 10.1002/(SICI)1096-9896(199903)187:4<481::AID-PATH263>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 38.Gaus K, Le Lay S, Balasubramanian N, Schwartz MA. Integrin-mediated adhesion regulates membrane order. J Cell Biol. 2006;174:725–734. doi: 10.1083/jcb.200603034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volberg T, Romer L, Zamir E, Geiger B. pp60(c-src) and related tyrosine kinases: a role in the assembly and reorganization of matrix adhesions. J Cell Sci. 2001;114:2279–2289. doi: 10.1242/jcs.114.12.2279. [DOI] [PubMed] [Google Scholar]
- 40.Nair KS, Naidoo R, Chetty R. Expression of cell adhesion molecules in oesophageal carcinoma and its prognostic value. J Clin Pathol. 2005;58:343–351. doi: 10.1136/jcp.2004.018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- 42.Lagana A, Goetz JG, Cheung P, Raz A, Dennis JW, Nabi IR. Galectin binding to Mgat5-modified N-glycans regulates fibronectin matrix remodeling in tumor cells. Mol Cell Biol. 2006;26:3181–3193. doi: 10.1128/MCB.26.8.3181-3193.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


