Abstract
The HIV-1 (HIV) transgenic (Tg) rat develops several immune abnormalities in association with clinical impairments that are similar to what are seen with HIV infection in humans. In HIV infection, retinoids and opioids can have separate and potentially combined effects on the clinical course of HIV disease. In these studies, the effects of a vitamin A deficient diet on T cell proinflammatory cytokine and mu opioid receptor (MOR) expression were examined in the Tg and in wild-type (WT) rats. The effects of the diet on HIV gene expression were also analyzed in the Tg rats. Phytohemagglutinin-stimulated T cells from WT rats on the vitamin A diet and from Tg rats on either diet were more likely to either produce increased percentages of T cells expressing intracytoplasmic IFN-γ, secrete higher levels of TNF-α, and express higher levels of MOR mRNA and surface MOR. Mitogen stimulation also increased Tg rat HIV env, tat, and nef mRNA expression with even higher env and nef mRNA produced in association with the vitamin A deficient diet. All together, these data suggest that a vitamin A deficient diet can result in cellular effects that increase T cell proinflammatory responses and HIV expression, which may alter the course of disease in the HIV Tg rat model.
Keywords: HIV-1, vitamin A, retinoids, transgenic rat, opioid receptor, cytokines, proinflammatory
Introduction
Vitamin A deficiency has been associated with the development of abnormal T cell responses to exogenous and host antigens. Among children and adults with HIV-1 (HIV) infection, deficiency has been linked to an increased risk of infection-related morbidity and mortality (Humphrey et al. 2006; Fawzi 2003). These clinical effects of vitamin A deficiency may be enhanced by the use of illicit drugs (Semba et al. 1993a) and, in opioid users, may be mediated by activation of surface mu opioid receptor (Peterson et al. 1990). While among individuals with HIV infection such complications generally occur in the setting of progressive immunosuppression, vitamin A deficiency also results in enhanced T cell proinflammatory responses, which have been shown to underlie immune-mediated tissue damage that occurs in multiple sclerosis and other natural and experimental autoimmune disorders (Driscoll et al. 1996; Kinoshita et al. 2003; Cantorna et al. 1994; Cantorna et al. 1995; Gershwin et al. 1984; Royal, III et al. 2002; Vladutiu and Cringulescu 1968; Warren 1982; Brinckerhoff et al. 1980; Cantorna and Hayes 1996; Comstock et al. 1997). In studies of mice rendered vitamin A deficient, T lymphocyte precursors from purified lymph node cell cultures were observed to take on a Th1 (proinflammatory) phenotype and, with activation, to secrete increased levels of interferon (IFN)-γ (Cantorna et al. 1994). Deficiency also induced decreased numbers of Th2 (anti-inflammatory) precursors and decreased secretion of interleukin (IL)-4.
The HIV Tg rat model incorporates a non-infectious viral genome that is under similar regulatory control mechanisms in vivo that exist with natural infection (Reid et al. 2001). Over time, the rats develop immune abnormalities and clinical manifestations in the presence of the transgene that are similar to what occurs with infection in humans (Reid et al. 2004; Reid et al. 2001). In humans, retinoids have been demonstrated to either enhance or suppress replication of HIV in infected cultures (Yamaguchi et al. 1994; Lee et al. 1994; Kitano et al. 1990; Semmel et al. 1994; Poli et al. 1992; Towers et al. 1995; Maciaszek et al. 1998). The cellular effects of retinoids are mediated by retinoid binding to retinoid receptors, of which there are two classes, retinoic acid receptor (RAR) and retinoid X receptor (RXR). The combined effects of vitamin A deficiency on proinflammatory cytokine responses and HIV gene expression have not been previously studied. Therefore, in this report we describe studies in which we examined IFN-γ, tumor necrosis factor (TNF)-α, and HIV gene expression by PHA-stimulated T cells from HIV-1 transgenic (Tg) rats on vitamin A deficient or normal diet and compared these data to results obtained from wild-type (WT) rats on the same diets. In addition, to identify possible factors that may regulate effects of opioids in the context of vitamin A deficiency, we also measured mu opioid receptor expression by these cells. The studies demonstrate that these responses are increased in the presence of vitamin A deficiency with the changes in proinflammatory cytokine and MOR production being most prominent in the Tg rats.
Materials and methods
HIV-1 Tg Rat
The details on the construction of the HIV-1 Tg rat have been previously described (Reid et al. 2001). All experiments were performed using whole blood samples from 3-6 month old specific pathogen free Tg and age-matched WT Fisher 344/NHsd control rats. The rats Tg and WT rats were administered a diet previously used to induce vitamin A deficiency in mice (Carman and Hayes 1991), except that the rats were fed the Bio-Serv AIN-93M rodent maintenance diet (Bio-Serv; Frenchtown, NJ), which contains 400,000 IU/kg of retinyl palmitate, the major dietary form of vitamin A, or the same diet mix formulated minus retinyl palmitate. Female rats maintained on the normal maintenance diet were mated then randomly divided into two groups at 2 wks gestation. One group of pregnant females was subsequently fed a vitamin A deficient diet and the other was fed the vitamin A-sufficient diet. Weanlings were maintained on the same diets as their dams. For collection of blood, the rats were anesthetized using a combination of 60mg/kg Ketamine and 7.5 mg/kg xylazine and blood was removed by capillary stick from the cavernous sinus. All studies were approved by the University of Maryland Biotechnology Institute of the University of Maryland, Baltimore Animal Care and Use Committee. .
Polymerase chain reaction assays
Samples were analyzed by PCR for TNF-α and IFN-γ, for HIV tat, nef, and vif and, as an internal control, for 18s gene expression as previously described (Nakagawa et al. 2001; Bae et al. 2005; Hudson et al. 2000; Arrigo et al. 1989; Arrigo et al. 1990; Jacque et al. 2002; Royal, III et al. 2005). For these studies RNA from PHA-stimulated and unstimulated samples from the Tg and control rats was isolated and DNase treated using RNeasy Mini Columns with Qiashredder column inserts and RNase-free DNase I (Qiagen, Valencia, CA) according to the product instructions. RT-PCR and subsequent PCR amplification was performed in an iCycler (Bio-Rad, Hercules, CA). Amplification was initiated by enzyme activation for 5 minutes at 94 oC followed by 40 cycles of denaturation at 94 oC for 30 seconds, annealing at 56 oC for 30 seconds, and extension at 72 oC for 30 seconds. The following primers were used: TNF-α forward: TACTGAACTTCGGGGTGATTGGTCC, TNF-α reverse: CAGCCTTGTCCCTTGAAGAGAACC (product length 295 bp); IFN-γ forward: GGCCATCAGCAACAACATAA, IFN-γ reverse: GACTCCTTTTCCGCTTCCTT (product length 206 bp); vif forward: ATTGTGTGGCAAGTAGACAGGATGA, vif reverse: CTAGTGGGATGTGTACTTCTGAACT (product length 154 bp); tat forward: GCGCGCACAGCAAGAGGCGA, tat reverse: GCAATGAAAGCAACACTTTTTACAATA (product length 181 bp); nef forward: GACAGGGCTTGGAAAGG, nef reverse: TTAGCAGTTCTGAAGTACTC (product length 640 bp); and env forward: GCG CGC ACA GCA AGA GGC GA, env reverse: CCACAAGTGCTGATACTTCTCC (product length 311 bp); the 18s primers were purchased from Ambion (Austin, TX) with PCR performed as recommended by the manufacturer. The PCR products were run on 1% agarose gels and the
Flow cytometry
T cell intracytoplasmic antigen detection
For intracytoplasmic detection of cytokine, whole blood samples were collected as described above and 0.5 ml of the samples was incubated in 1.5 ml of medium consisting of RPMI 1640 (BioWhittaker, Frederick, MD) supplemented with 10% fetal bovine serum (BioWhittaker), 50 units/ml of penicillin, 50 μg/ml of streptomycin, and 2 mM L-Glutamine (ICN Biomedical, Costa Mesa, CA). The samples were then incubated in medium alone or medium plus 12.5 μg/ml PHA for 4 hours in the presence of GolgiStop (brefeldin A; BD Biosciences), added to the cultures at of final concentration of 60 μM. The cells were then washed and surface labeling for T cell subtypes was performed with CD3-APC, CD4-PC5, and CD8-FITC antibodies (BD Biosciences) with subsequent lysis of the red blood cells in the cell suspension with FACS Lysing Solution (BD Biosciences) as recommended by the manufacturer. The samples were then permeabilized using the Cytofix/Cytoperm Kit with GolgiPlug (BD Biosciences), which contains the protein transport inhibitor brefeldin A and Perm/Wash buffer, and stained for intracytoplasmic cytokine expression by incubation with TNF-α-PE or IFN-γ-PE antibodies (BD Biosciences), also as recommended by the manufacturer. After a final wash, the samples were resuspended in 1% paraformaldehyde in PBS and four color flow cytometry was performed using a FACSCalibur flow cytometer equipped with CellQuest Software (BD Biosciences; San Jose, CA) and calibrated using Calibrite Beads (BD Biosciences). Mononuclear cell populations were identified on dot plots of forward versus side scatter gated for CD3+ cells, and the CD4+ and CD8+ cells within this gate were subsequently examined by quadrant analysis of dot plots for positive staining for either for IFN-γ or TNF-α.
Surface T cell MOR Expression
Surface MOR expression was examined as previously described (Royal, III et al. 2005). Briefly, the whole blood samples were incubated for 30 minutes with a 1:500 dilution of rabbit anti-human MOR antibody, generated against a recombinant protein corresponding to amino acids 1-80 of the amino terminus of human MOR-1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), then washed twice in phosphate buffered saline pH 7.4 (PBS) and incubated for 30 minutes with a 1:1000 dilution of Alexa Fluor 488-conjugated mouse anti-rabbit polyclonal antibodies (Molecular Probes; Eugene, OR). After a final wash, the stained samples were analyzed by flow cytometry as described above using unstained samples and samples incubated with secondary antibody alone as negative controls.
Cytokine Enzyme-linked Immunosorbent Assays
TNF-α and IFN-γ secretion by PBMCs from the Tg and WT rats were analyzed by antigen-specific ELISA. Briefly, PBMCs were purified on Ficoll gradients and 2x105 cells were incubated in 200 μl of RPMI 1640 supplemented with 10% fetal bovine serum, 50 units/ml of penicillin, 50 μg/ml of streptomycin, and 2 mM L-glutamine alone or medium containing 10 μg/ml PHA in 5% CO2 at 37 degrees Celsius for 72 hours. Culture supernatants were then analyzed for TNF-α and IFN-γ secretion in antigen capture ELISA using, respectively, using the rat TNF-α and IFN-γ BD OptEIA ELISA Set (BD Biosciences Pharmingen) according to the product directions.
Statistical Analyses
T cell percentages for the Tg and WT rats were analyzed using the Mann Whitney-U test and mean levels of cytokine secretion were compared for these groups using the student’s t-test.
Results
IFN-γ and TNF-α gene expression by PHA-stimulated and unstimulated whole blood samples
Whole blood samples from Tg and WT rats on either a normal or vitamin A deficient diet were examined for proinflammatory cytokine gene expression by PCR (figure 1). Expression levels were quantitated by measuring the intensity of the band produced by the PCR product on the agarose gel then calculating a ratio of the cytokine and 18s band intensities. These studies showed low levels of IFN-γ mRNA for non-stimulated samples. Following PHA stimulation, these was a slight increase in IFN-γ gene expression by cells from the WT rat on the control diet, whereas larger increases were observed for the samples from the WT rat on the vitamin A deficient diet and from Tg rats on either diet. In contrast, analysis of TNFα mRNA expression showed prominent relative baseline levels for rats from all groups which increased slightly with PHA stimulation for all except the sample from the WT animal on the normal diet, for which expression was decreased.
Figure 1.
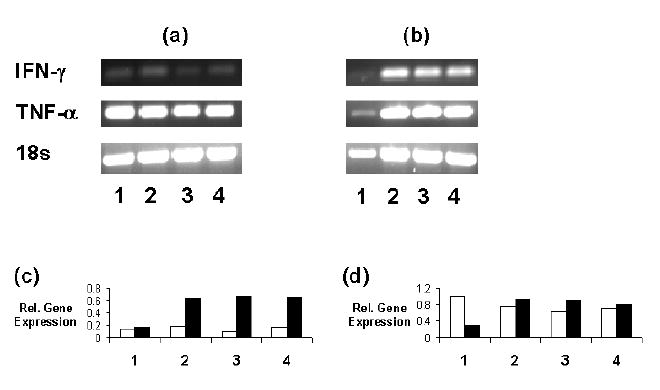
PCR analysis of IFN-γ and TNF-α mRNA expression by cells in (a) non-stimulated and (b) PHA-stimulated whole blood samples from rats in the following groups: (1) WT on the normal diet; (2) Tg on the normal diet; (3) WT on the vitamin A deficient; and (4) Tg on the vitamin A deficient. The figure is representative of results from 3 experiments. The graphs depict the relative gene expression of (c) IFN-γ and (d) TNF-α mRNA versus the 18s internal control gene for the PHA- (open bars) and PHA+ (filled bars) samples.
Intracytoplasmic IFN-γ and TNF-α expression by activated T cells
For these studies, the effects of mitogen stimulation on intracytoplasmic IFN-γ and TNF-α expression by CD4+ and CD8+ T cells in whole blood was analyzed. These studies showed that, relative to unstimulated samples from WT rats on the normal diet, PHA stimulation increased percentages of CD4+IFN-γ+ T cells detected in samples from WT rats on the deficient diet and from Tg rats on either diet (figure 2). In addition, percentages of IFN-γ+ CD4+ T cells were higher for PHA stimulated samples from WT rats on the vitamin A deficient diet than for unstimulated samples from these rats whereas no such difference was observed for Tg rats on the deficient diet. Analysis of CD8+ T cells also showed that percentages of IFN-γ+ cells were higher for PHA stimulated samples from WT rats on the vitamin A deficient diet as compared to non-stimulated samples from these animals. In addition, INF-γ+ percentages were also higher for CD8+ T cells from Tg rats on the normal diet.
Figure 2.
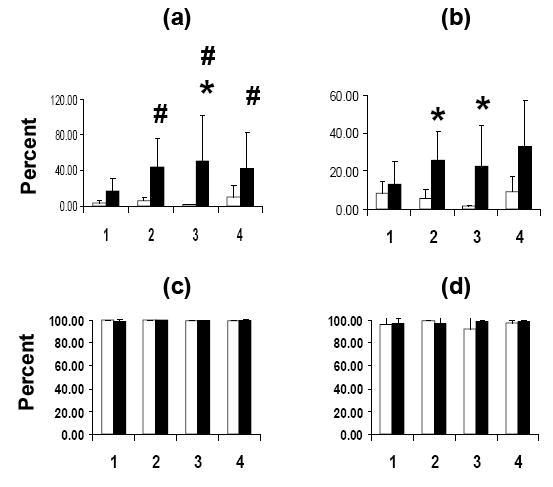
Flow cytometry of CD4+ (a, c) and CD8+ (b, d) T cells stained for intracytoplasmic IFN-γ (a, b) and TNF-α (c, d). Open bars: PHA-; filled bars: PHA+. See figure 2 legend for descriptions of the animal groups (n = 4 per group). * = p < 0.05 (PHA- vs. PHA+); # = p < 0.05 (for comparisons vs. Group 1 PHA-)
In contrast to what was observed for intracytoplasmic IFN-γ+ expression, high percentages of TNF-α+ CD4+ and TNF-α+CD8+ T cells were observed both in non-stimulated and PHA-stimulated cultures from rats in all groups (figure 2).
Secretion of IFN-γ and TNF-α by Tg and WT rat T cells
PBMCs from the rats were examined by ELISA for levels of IFN-γ and TNF-α secretion. These studies showed that, for all cultures, there was no detectable secretion of IFN-γ in the absence of PHA stimulation (figure 3). For PHA-stimulated cultures, high levels of IFN-γ were detected but levels were similar for Tg and WT rats on either diet. In contrast, detectable TNF-α was secreted in the absence of mitogen and at levels that were similar for the various animal groups. With PHA stimulation, secreted TNF-α levels were significantly higher for PBMCs from WT rats on a deficient diet and from Tg rats on either a normal or a vitamin A deficient diet relative to levels measured for non-stimulated samples from WT rats fed a normal diet. In addition, PHA stimulation ncreased TNF-α secretion by cells from Tg rats on either a normal diet or on a vitamin A deficient diet as compared non-stimulated cells from these rats.
Figure 3.
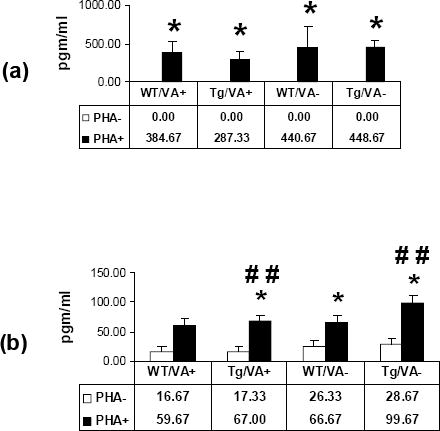
Measurement of (a) IFN-γ and (b) TNF-α secretion by PBMCs from Tg (Tg) and WT rats on either a normal (VA+) or a vitamin A deficient (VA-) diet by ELISA. The mean levels of secreted cytokine are shown in the table below the graph. Open bars: PHA-; filled bars: PHA+. Mean values in pgm/ml are listed in the tables below the graphs (n = 2 per group). * = p < 0.05 (PHA- vs. PHA+); ## = p < 0.01 (comparison vs. Group 1 PHA-).
MOR gene expression by cells in whole blood from Tg and WT rats
MOR gene expression in whole blood was also examined by PCR (figure 4). Again, samples were obtained from Tg and WT rats on vitamin A deficient and normal diets and analyzed unstimulated or following stimulation with PHA. These studies showed that the sample from the Tg rat on the vitamin A deficient diet had the lowest level of expression at baseline. With PHA stimulation there were increases in expression for rats in all groups with the highest levels of expression noted for the Tg and WT rats on the vitamin A deficient diet.
Figure 4.
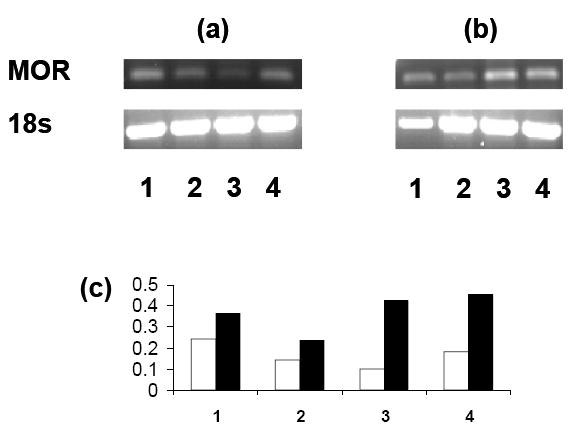
PCR analysis of MOR gene expression in (a) unstimulated and (b) PHA-stimulated whole blood samples from Tg and WT rats on either a vitamin A deficient or normal diet (representative of results from 3 experiments; see figure 2 for group descriptions). The graph depicts the relative gene expression of MOR mRNA versus the 18s internal control gene for the PHA- (open bars) and PHA+ (filled bars) samples.
T cell expression of surface MOR
Surface MOR expression by T cells in whole blood was examined by indirect immunofluorescence staining and analysis by flow cytometry (figure 5). These studies showed, relative to non-stimulated samples from WT rats, increased MOR expression by CD4+ and CD8+ T cells in stimulated samples from Tg rats on the vitamin A-deficient diet. In addition, percentages of CD4+MOR+ T cells in stimulated samples from vitamin A-deficient Tg rats were also higher than for samples from non-stimulated samples from these rats.
Figure 5.

Flow cytometry of CD4+ (a) and CD8+ (b) T cells surface stained for MOR. Open bars: PHA-; filled bars: PHA+. See figure 2 legend for descriptions of the animal groups (n = 4 per group). * = p < 0.05 (PHA- vs. PHA+); # = p < 0.05 (for comparisons vs. Group 1 PHA-).
HIV-1 gene expression by cells in whole blood from Tg and WT rats
In these studies, we examined relative expression of env, tat, nef, and vif in whole blood from Tg and, as a negative control, WT rats on normal and vitamin A-deficient diets following incubation with either medium alone or PHA (figure 6). These studies, as expected, showed no detection of HIV-specific mRNA in the samples from the WT rats. For the non-stimulated HIV Tg rat sample, there was no detectible env or tat gene expression; expression was higher for vif than for nef. Stimulation of the Tg rat samples with PHA resulted in an increase in expression env, tat, and nef mRNA with relative levels of tat and vif expression noted to be similar for the rats on the vitamin A deficient and normal diets. In contrast, mitogen stimulation of cells from the rat on the vitamin A deficient diet resulted in higher relative env and nef expression than what was observed for stimulated sample from the Tg rat on the normal diet.
Figure 6.

PCR analysis of HIV (env, tat, nef, and vif) gene expression in a sample of (a) unstimulated and (b) PHA-stimulated whole blood samples from Tg and WT rats on either a vitamin A deficient or normal diet (representative result from 3 experiments; see figure 2 for group descriptions). The graph depicts the relative gene expression versus the 18s internal control gene for rats fed (c) the normal diet or (d) the vitamin A deficient diet. No env or tat expression was noted for the PHA- sample. Open bars: PHA-; filled bars: PHA+.
Discussion
In humans with HIV infection, treatment with retinoids has been shown to have potentially beneficial effects. For example, 13-cis retinoic acid induced regression of oral leukoplakia (Beenken et al. 1994), and AIDS-related Kaposi’s sarcoma lesions have been shown to respond to treatment with topical preparations of either 9-cis RA or the synthetic retinoid bexarotene (Walmsley et al. 1999; Duvic et al. 2000). The effects on these retinoids on HIV replication and clinical consequences of the infection appear to be mediated by retinoid binding to receptors which then interact with retinoid receptor response elements in the viral long terminal repeat (LTR) and in the core promoter (Lee et al. 1994; Towers et al. 1995; Maciaszek et al. 1998). The two classes of retinoid receptors, RAR and RXR, exist in cells as RAR-RXR heterodimers and as RXR homodimers. RAR is bound by all-trans retinoic acid, 9-cis retinoic acid, and 13 cis-retinoic acid; RXR is also bound by 9-cis retinoic acid as well as by bexarotene. Indeed, the response elements for both RAR and RXR are present in the HIV promoter (Xu et al. 1996; Lee et al. 1994; Ladias 1994; Desai-Yajnik and Samuels 1993; Orchard et al. 1993).
Earlier stages of HIV infection are characteristically associated with enhanced humeral and cellular immune responses, with the latter primarily reflecting the activity of virus-specific cytotoxic T cells (Letvin and Walker 2003). With acute HIV infection there is often an initial fall in CD4+ T cell numbers followed by a return to normal or near-normal levels. Subsequently, individuals with established HIV infection typically show a fall in numbers of CD4+ T cells and lower IFN-γ expression levels which continue to decrease with progression to AIDS (Klein et al. 1997). Vitamin A deficiency has been independently associated with decreased CD4+ T cell percentages in children (Semba et al. 1993b) and with lower splenic CD4+ T cell numbers in rats (Zhao and Ross 1995). In our studies, we did not observe any difference in total whole blood CD4+ T cell percentages for the animals in the various groups (data not shown). The effects of advanced HIV disease on proinflammatory responses have been previously examined in samples from phorbol ester-stimulated T cells from 12-15 month-old Tg rats, and these studies showed that levels of IFN-γ secretion were lower than those for age-matched WT rats (Reid et al. 2004). In our studies of younger rats, PHA-induced increases in proinflammatory cytokine gene expression were most consistently seen for samples from Tg rats on either diet and from WT rats on the vitamin A deficient diet. Notably, no difference in percentages of TNF-α+ T cell percentages and secreted IFN-γ levels was seen between groups. This suggests that rat T cells store preformed TNF-α which is released following exposure of the cell to the appropriate stimulus. It is also possible that significant levels of TNF-α are may be secreted by monocyte/macrophages present in the samples, which could also account for the differences noted in these measures. Similarly, IFN-γ is produced by natural killer cells as well by T cells, explaining, at least in part, the similar levels of this cytokine measured in supernatants from the stimulated cells.
The data presented in this report also show that MOR gene expression was increased by PHA stimulation with the largest increases noted for the cells from vitamin A deficient rats. Of note is the fact that significant mitogen-induced elevations of surface MOR were noted only for cells from Tg rats on the vitamin A deficient diet. The mechanisms that underlie these effects are yet to be elucidated. However, activation of NF-kappa B is required for expression of TNF-α, and it has been demonstrated that, in vitamin A deficient mice, NK-kappa B activity is increased (Austenaa et al. 2004). In murine macrophages, nanomolar concentrations of morphine have been demonstrated to activate NF-kappa B and to stimulate TNF-α production (Roy et al. 1998; Wang et al. 2003). In immune cells, binding of TNF-α to tumor necrosis factor receptor type 2 results in increased production of NF-kappa B, which binds to a responsive element in the MOR gene promoter to stimulate MOR expression (Kraus et al. 2001). Therefore, the enhanced expression of MOR that we observed in the vitamin A deficient rats likely represents unsuppressed complementary interactions between the mechanisms that control expression of TNF-α and MOR.
Increased expression of HIV genes has been demonstrated for monocytes isolated from the HIV Tg rat (Mazzucchelli et al. 2004). Similarly, HIV gp120, tat, and nef has been demonstrated immunohistochemically in spleen from and gp120 has been detected in serum from the Tg animals and on Western blots of lysates of T cell, B cells, and monocytes (Reid et al. 2001). It is notable in our studies that PHA stimulation also increased HIV env and nef gene expression with expression following stimulation being more prominent in samples from animals on the diet deficient in vitamin A. In contrast, no increase was noted for tat and vif transcripts; in fact, a decrease was observed in vif gene expression. Gp120, nef, and tat have been demonstrated to induce cytotoxicity to neighboring cells, and it is possible that this may be increased in the setting of retinoid deficiency. In addition, morphine binding to MOR can enhance HIV infectivity and cellular gp120 binding by increasing the expression of CCR5, the HIV co-receptor, and by decreasing expression of alpha- and beta- chemokines, which can serve to block HIV and gp120 binding (Mahajan et al. 2002; Mahajan et al. 2005; Peterson et al. 1994; Peterson et al. 1990; Li et al. 2002).
In summary, the studies presented in this paper demonstrate that dietary depletion of vitamin A in the HIV Tg rat results in altered generation of T cell phenotypes and effects of immune responses that may significantly alter the course of disease induced by the presence of the transgene. For human populations who are at risk for HIV infection, these data suggest mechanisms by which vitamin A deficiency may affect the course of the human disease, and notably so for those individuals who are opioid users. These conditions remain highly prevalent in a number of areas of the world, and, therefore, future studies are will likely have a significant impact on the health of the affected public.
Footnotes
Supported by: R01 DA15311 (WR) and the Veterans Affairs Medical System Multiple Sclerosis Center of Excellence – East, Baltimore Veterans Administration Medical Center, 10 North Greene Street, Baltimore, MD 21201
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Arrigo SJ, Weitsman S, Rosenblatt JD, Chen IS. Analysis of rev gene function on human immunodeficiency virus type 1 replication in lymphoid cells by using a quantitative polymerase chain reaction method. J Virol. 1989;63:4875–4881. doi: 10.1128/jvi.63.11.4875-4881.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo SJ, Weitsman S, Zack JA, Chen IS. Characterization and expression of novel singly spliced RNA species of human immunodeficiency virus type 1. J Virol. 1990;64:4585–4588. doi: 10.1128/jvi.64.9.4585-4588.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austenaa LM, Carlsen H, Ertesvag A, Alexander G, Blomhoff HK, Blomhoff R. Vitamin A status significantly alters nuclear factor-kappaB activity assessed by in vivo imaging. FASEB J. 2004;18:1255–1257. doi: 10.1096/fj.03-1098fje. [DOI] [PubMed] [Google Scholar]
- Bae JS, Ahn SJ, Yim H, Jang KH, Jin HK. Prevention of intraperitoneal adhesions and abscesses by polysaccharides isolated from Phellinus spp in a rat peritonitis model. Ann Surg. 2005;241:534–540. doi: 10.1097/01.sla.0000154281.79639.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenken SW, Huang P, Sellers M, Peters G, Listinsky C, Stockard C, Hubbard W, Wheeler R, Grizzle W. Retinoid modulation of biomarkers in oral leukoplakia/dysplasia. J Cell Biochem. 1994;19(Suppl):270–277. [PubMed] [Google Scholar]
- Brinckerhoff CE, McMillan RM, Dayer JM, Harris ED., Jr Inhibition by retinoic acid of collagenase production in rheumatoid synovial cells. N Engl J Med. 1980;303:432–436. doi: 10.1056/NEJM198008213030805. [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Hayes CE. Vitamin A deficiency exacerbates murine Lyme arthritis. J Infect Dis. 1996;174:747–751. doi: 10.1093/infdis/174.4.747. [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Nashold FE, Hayes CE. In vitamin A deficiency multiple mechanisms establish a regulatory T helper cell imbalance with excess Th1 and insufficient Th2 function. J Immunol. 1994;152:1515–1522. [PubMed] [Google Scholar]
- Cantorna MT, Nashold FE, Hayes CE. Vitamin A deficiency results in a priming environment conducive for Th1 cell development. Eu J Immunol. 1995;25:1673–1679. doi: 10.1002/eji.1830250629. [DOI] [PubMed] [Google Scholar]
- Carman JA, Hayes CE. Abnormal regulation of IFN-gamma secretion in vitamin A deficiency. J Immunol. 1991;147:1247–1252. [PubMed] [Google Scholar]
- Comstock GW, Burke AE, Hoffman SC, Helzlsouer KJ, Bendich A, Masi AT, Norkus EP, Malamet RL, Gershwin ME. Serum concentrations of alpha tocopherol, beta carotene, and retinol preceding the diagnosis of rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis. 1997;56:323–325. doi: 10.1136/ard.56.5.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai-Yajnik V, Samuels HH. The NF-kappa B and Sp1 motifs of the human immunodeficiency virus type 1 long terminal repeat function as novel thyroid hormone response elements. Mol Cell Biol. 1993;13:5057–5069. doi: 10.1128/mcb.13.8.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll HK, Chertow BS, Jelic TM, Baltaro RJ, Chandor SB, Walker EM, Dadgari JM, Pofahl AB. Vitamin A status affects the development of diabetes and insulitis in BB rats. Metabolism. 1996;45:248–253. doi: 10.1016/s0026-0495(96)90062-1. [DOI] [PubMed] [Google Scholar]
- Duvic M, Friedman-Kien AE, Looney DJ, Miles SA, Myskowski PL, Scadden DT, Von Roenn J, Galpin JE, Groopman J, Loewen G, Stevens V, Truglia JA, Yocum RC. Topical treatment of cutaneous lesions of acquired immunodeficiency syndrome-related Kaposi sarcoma using alitretinoin gel: results of phase 1 and 2 trials. Arch Dermatol. 2000;136:1461–1469. doi: 10.1001/archderm.136.12.1461. [DOI] [PubMed] [Google Scholar]
- Fawzi W. Micronutrients and human immunodeficiency virus type 1 disease progression among adults and children. Clin Infect Dis. 2003;37(Suppl 2):S112–S116. doi: 10.1086/375882. [DOI] [PubMed] [Google Scholar]
- Gershwin ME, Lentz DR, Beach RD, Hurley LS. Nutritional factors and autoimmunity. Dietary vitamin A deprivation induces a selective increase in IgM auroantibodies and hypergammaglobulinemia in New Zealand black mice. J Immunol. 1984;133:222–226. [PubMed] [Google Scholar]
- Hudson L, Liu J, Nath A, Jones M, Raghavan R, Narayan O, Male D, Everall I. Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J Neurovirol. 2000;6:145–155. doi: 10.3109/13550280009013158. [DOI] [PubMed] [Google Scholar]
- Humphrey JH, Iliff PJ, Marinda ET, Mutasa K, Moulton LH, Chidawanyika H, Ward BJ, Nathoo KJ, Malaba LC, Zijenah LS, Zvandasara P, Ntozini R, Mzengeza F, Mahomva AI, Ruff AJ, Mbizvo MT, Zunguza CD. Effects of a single large dose of vitamin A, given during the postpartum period to HIV-positive women and their infants, on child HIV infection, HIV-free survival, and mortality. J Infect Dis. 2006;193:860–871. doi: 10.1086/500366. [DOI] [PubMed] [Google Scholar]
- Jacque JM, Triques K, Stevenson M. Modulation of HIV-1 replication by RNA interference. Nature. 2002;418:435–438. doi: 10.1038/nature00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita K, Yoo BS, Nozaki Y, Sugiyama M, Ikoma S, Ohno M, Funauchi M, Kanamaru A. Retinoic acid reduces autoimmune renal injury and increases survival in NZB/W F1 mice. J Immunol. 2003;170:5793–5798. doi: 10.4049/jimmunol.170.11.5793. [DOI] [PubMed] [Google Scholar]
- Kitano K, Baldwin GC, Raines MA, Golde DW. Differentiating agents facilitate infection of myeloid leukemia cell lines by monocytotropic HIV-1 strains. Blood. 1990;76:1980–1988. [PubMed] [Google Scholar]
- Klein SA, Dobmeyer JM, Dobmeyer TS, Pape M, Ottmann OG, Helm EB, Hoelzer D, Rossol R. Demonstration of the Th1 to Th2 cytokine shift during the course of HIV-1 infection using cytoplasmic cytokine detection on single cell level by flow cytometry. AIDS. 1997;11:1111–1118. doi: 10.1097/00002030-199709000-00005. [DOI] [PubMed] [Google Scholar]
- Kraus J, Borner C, Giannini E, Hickfang K, Braun H, Mayer P, Hoehe MR, Ambrosch A, Konig W, Hollt V. Regulation of mu-opioid receptor gene transcription by interleukin-4 and influence of an allelic variation within a STAT6 transcription factor binding site. J Biol Chem. 2001;276:43901–43908. doi: 10.1074/jbc.M107543200. [DOI] [PubMed] [Google Scholar]
- Ladias JA. Convergence of multiple nuclear receptor signaling pathways onto the long terminal repeat of human immunodeficiency virus-1. J Biol Chem. 1994;269:5944–5951. [PubMed] [Google Scholar]
- Lee MO, Hobbs PD, Zhang XK, Dawson MI, Pfahl M. A synthetic retinoid antagonist inhibits the human immunodeficiency virus type 1 promoter. Proc Natl Acad Sci U S A. 1994;91:5632–5636. doi: 10.1073/pnas.91.12.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letvin NL, Walker BD. Immunopathogenesis and immunotherapy in AIDS virus infections. Nat Med. 2003;9:861–866. doi: 10.1038/nm0703-861. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang X, Tian S, Guo CJ, Douglas SD, Ho WZ. Methadone enhances human immunodeficiency virus infection of human immune cells. J Infect Dis. 2002;185:118–122. doi: 10.1086/338011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciaszek JW, Coniglio SJ, Talmage DA, Viglianti GA. Retinoid-induced repression of human immunodeficiency virus type 1 core promoter activity inhibits virus replication. J Virol. 1998;72:5862–5869. doi: 10.1128/jvi.72.7.5862-5869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan SD, Aalinkeel R, Reynolds JL, Nair BB, Fernandez SF, Schwartz SA, Nair MP. Morphine exacerbates HIV-1 viral protein gp120 induced modulation of chemokine gene expression in U373 astrocytoma cells. Curr HIV Res. 2005;3:277–288. doi: 10.2174/1570162054368048. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Schwartz SA, Shanahan TC, Chawda RP, Nair MP. Morphine regulates gene expression of alpha- and beta-chemokines and their receptors on astroglial cells via the opioid mu receptor. J Immunol. 2002;169:3589–3599. doi: 10.4049/jimmunol.169.7.3589. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli R, Amadio M, Curreli S, Denaro F, Bemis K, Reid W, Bryant J, Riva A, Galli M, Zella D. Establishment of an ex vivo model of monocytes-derived macrophages differentiated from peripheral blood mononuclear cells (PBMCs) from HIV-1 transgenic rats. Mol Immunol. 2004;41:979–984. doi: 10.1016/j.molimm.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Oono H, Nishio A. Enhanced production of IL-1beta and IL-6 following endotoxin challenge in rats with dietary magnesium deficiency. J Vet Med Sci. 2001;63:467–469. doi: 10.1292/jvms.63.467. [DOI] [PubMed] [Google Scholar]
- Orchard K, Lang G, Harris J, Collins M, Latchman D. A palindromic element in the human immunodeficiency virus long terminal repeat binds retinoic acid receptors and can confer retinoic acid responsiveness on a heterologous promoter. J Acquir Immune Defic Syndr. 1993;6:440–445. [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Anderson WR, Kravitz F, Portoghese PS, Balfour HH, Jr, Chao CC. Morphine amplifies HIV-1 expression in chronically infected promonocytes cocultured with human brain cells. J Neuroimmunol. 1994;50:167–175. doi: 10.1016/0165-5728(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Sharp BM, Gekker G, Portoghese PS, Sannerud K, Balfour HH., Jr Morphine promotes the growth of HIV-1 in human peripheral blood mononuclear cell cocultures. AIDS. 1990;4:869–873. doi: 10.1097/00002030-199009000-00006. [DOI] [PubMed] [Google Scholar]
- Poli G, Kinter AL, Justement JS, Bressler P, Kehrl JH, Fauci AS. Retinoic acid mimics transforming growth factor beta in the regulation of human immunodeficiency virus expression in monocytic cells. Proc Natl Acad Sci U S A. 1992;89:2689–2693. doi: 10.1073/pnas.89.7.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid W, Abdelwahab S, Sadowska M, Huso D, Neal A, Ahearn A, Bryant J, Gallo RC, Lewis GK, Reitz M. HIV-1 transgenic rats develop T cell abnormalities. Virology. 2004;321:111–119. doi: 10.1016/j.virol.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Jr, Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O'Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Cain KJ, Chapin RB, Charboneau RG, Barke RA. Morphine modulates NF kappa B activation in macrophages. Biochem Biophys Res Commun. 1998;245:392–396. doi: 10.1006/bbrc.1998.8415. [DOI] [PubMed] [Google Scholar]
- Royal W, III, Gartner S, Gajewski CD. Retinol measurements and retinoid receptor gene expression in patients with multiple sclerosis. Mult Scler. 2002;8:452–458. doi: 10.1191/1352458502ms858oa. [DOI] [PubMed] [Google Scholar]
- Royal W, III, Leander MV, Bissonnette R. Retinoid-induced mu opioid receptor expression by phytohemagglutinin-stimulated U937 cells. J Neurovirol. 2005;11:157–165. doi: 10.1080/13550280590922766. [DOI] [PubMed] [Google Scholar]
- Semba RD, Graham NM, Caiaffa WT, Margolick JB, Clement L, Vlahov D. Increased mortality associated with vitamin A deficiency during human immunodeficiency virus type 1 infection. Arch Int Med. 1993a;153:2149–2154. [PubMed] [Google Scholar]
- Semba RD, Muhilal, Ward BJ, Griffin DE, Scott AL, Natadisastra G, West JKP, Sommer A. Abnormal T-cell subset proportions in vitamin-A-deficient children. Lancet. 1993b;341:5–8. doi: 10.1016/0140-6736(93)92478-c. [DOI] [PubMed] [Google Scholar]
- Semmel M, Macho A, Coulaud D, Alileche A, Plaisance S, Aguilar J, Jasmin C. Effect of retinoic acid on HL-60 cells infected with human immunodeficiency virus type 1. Blood. 1994;84:2480–2488. [PubMed] [Google Scholar]
- Towers G, Harris J, Lang G, Collins MK, Latchman DS. Retinoic acid inhibits both the basal activity and phorbol ester- mediated activation of the HIV long terminal repeat promoter. AIDS. 1995;9:129–136. [PubMed] [Google Scholar]
- Vladutiu A, Cringulescu N. Suppression of experimental allergic encephalomyelitis by vitamin A. Experimentia. 1968;24:718–719. doi: 10.1007/BF02138337. [DOI] [PubMed] [Google Scholar]
- Walmsley S, Northfelt DW, Melosky B, Conant M, Friedman-Kien AE, Wagner B. Treatment of AIDS-related cutaneous Kaposi's sarcoma with topical alitretinoin (9-cis-retinoic acid) gel. Panretin Gel North American Study Group. J Acquir Immune Defic Syndr. 1999;22:235–246. doi: 10.1097/00126334-199911010-00004. [DOI] [PubMed] [Google Scholar]
- Wang J, Barke RA, Charboneau R, Loh HH, Roy S. Morphine negatively regulates interferon-gamma promoter activity in activated murine T cells through two distinct cyclic AMP-dependent pathways. J Biol Chem. 2003;278:37622–37631. doi: 10.1074/jbc.M301224200. [DOI] [PubMed] [Google Scholar]
- Warren TR. Multiple sclerosis and infants fed on diets deficient in vitamin A or in selenium and vitamin E. Medical Hypotheses. 1982;8:443–454. doi: 10.1016/0306-9877(82)90003-2. [DOI] [PubMed] [Google Scholar]
- Xu J, Luznik L, Wong-Staal F, Gill GN. Hormone Receptor Regulation of the Human Immunodeficiency Virus Type 1 and Type 2 Long Terminal Repeats. J Biomed Sci. 1996;3:323–331. doi: 10.1007/BF02257962. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Groopman JE, Byrn RA. The regulation of HIV by retinoic acid correlates with cellular expression of the retinoic acid receptors. AIDS. 1994;8:1675–1682. doi: 10.1097/00002030-199412000-00006. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Ross AC. Retinoic acid repletion restores the number of leukocytes and their subsets and stimulates natural cytotoxicity in vitamin A-deficient rats. J Nutr. 1995;125:2064–2073. doi: 10.1093/jn/125.8.2064. [DOI] [PubMed] [Google Scholar]


