Abstract
Gemtuzumab ozogamicin (GO) contains an anti-CD33 antibody to facilitate uptake of a toxic calicheamicin-γ1 derivative. While recent in vitro data demonstrated a quantitative relationship between CD33 expression and GO cytotoxicity, previous correlative studies failed to identify a significant association between CD33 expression and clinical outcome. Studying patients undergoing GO monotherapy for relapsed acute myeloid leukemia (AML), we now find that AML blasts of responders have a significantly higher mean CD33 level and lower P-glycoprotein (Pgp) activity compared with nonresponders. CD33 expression and Pgp activity are inversely correlated. While both variables are associated with outcome, Pgp remains significantly associated with outcome even after adjusting for CD33, whereas CD33 does not show such an association after adjusting for Pgp. The inverse relationship between CD33 and Pgp suggests a maturation-stage–dependent expression of both proteins, and offers the rationale for using cell differentiation–promoting agents to enhance GO-induced cytotoxicity.
Introduction
Due to its restricted, maturation stage–dependent expression on normal myeloid cells and its presence on tumor cells of 85% to 90% of adult and pediatric patients with acute myeloid leukemia (AML), CD33 has been exploited as a target for antibody-based AML therapies.1–4 The endocytic property of CD33 led to the development of gemtuzumab ozogamicin (GO; Mylotarg; Wyeth Pharmaceuticals, Collegeville, PA), an immunoconjugate consisting of a humanized IgG4 anti-CD33 monoclonal antibody (hP67.6) joined to a toxic calicheamicin-γ1 derivative.4,5 Encouraging results on 277 patients treated in phase 2 trials revealed that GO monotherapy induces a complete remission (CR) or CR with incomplete platelet recovery (CRp) in 26% of adults with relapsed CD33+ AML.6,7
hP67.6 itself is largely nontoxic and primarily facilitates uptake of the calicheamicin-γ1 derivative, which is then cleaved and eventually induces DNA damage and cell death,4 implying a critical role of the intracellular calicheamicin-γ1 accumulation for GO-induced cytotoxicity. Indeed, drug efflux mediated by P-glycoprotein (Pgp) results in resistance to GO and predicts for adverse outcome after GO monotherapy; conversely, inhibition of Pgp function effectively increases GO-induced cytotoxicity in vitro.8–12 Furthermore, recent in vitro studies revealed a quantitative relationship between CD33 expression and GO-induced cytotoxicity in human CD33+ AML cell lines,13 and higher CD33 expression levels on circulating AML blasts were associated with clearance of AML blasts from peripheral blood after one dose of GO.14 In contrast, an analysis of the first 142 patients treated in the phase 2 trials failed to show a significant impact of CD33 expression levels of bone marrow AML blasts in response to GO.6 Additional smaller studies similarly did not find a predictive role of CD33 expression.15,16 To resolve these apparent discrepancies, we re-examined the role of CD33 expression and Pgp function for clinical response to GO, using the complete phase 2 trial patient cohort for analysis.
Patients, materials, and methods
Patients and treatment
Details on the 3 multicenter, open-label, single-arm, phase 2 GO protocols have been reported previously.6–8 Briefly, adult patients with primary AML in untreated first relapse were eligible if they had CD33+ AML with CD33 at a fluorescence intensity more than or equal to 4 times the fluorescence intensity of an isotype-matched control antibody on at least 80% of the myeloid blasts. Patients were scheduled to receive 2 doses of GO 9 mg/m2 as a 2-hour single-agent intravenous infusion with 14 to 28 days between doses. CR was defined as (1) absence of circulating leukemic blasts; (2) 5% or fewer leukemic blasts in the bone marrow by morphology; (3) peripheral blood counts with hemoglobin level more than or equal to 90 g/L, absolute neutrophil count more than or equal to 1.5 × 109/L, and platelets more than or equal to 100 × 109/L; and (4) RBC/platelet transfusion independence. CRp defined patients who fulfilled CR criteria except for failure to recover a platelet count of more than or equal to 100 × 109/L. All patients provided written informed consent before treatment, in accordance with the Declaration of Helsinki, and all studies were approved by the participating centers' institutional review boards.
Assessment of CD33 expression and Pgp function
Pretreatment bone marrow samples were used to assess CD33 expression and Pgp function. Cell surface expression of CD33 on the myeloid blast population was determined by flow cytometry using hP67.6 as the primary antibody, and expressed as a ratio between the mean CD33 fluorescence of the CD33+ blast subfraction and the mean fluorescence of an isotype control antibody.14 Pgp function was cytofluorometrically assessed by efflux of the fluorescent Pgp substrate, DiOC2, and expressed as mean DiOC2 fluorescence intensity after dye loading divided by DiOC2 intensity after dye efflux.8
Statistical analysis
Mean values between responders and nonresponders were compared using the 2-sample t test with nonequal variances. Additional analyses were conducted by dividing CD33 and Pgp into quartiles and using logistic regression to perform a trend test on the association between proportion of responders and quartile rank. The correlation between CD33 and Pgp was estimated using the Pearson correlation coefficient. The impact of Pgp on the probability of response after adjusting for CD33 (and vice versa) was assessed using logistic regression and the likelihood ratio test.
Results and discussion
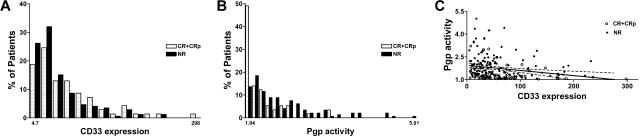
There were 276 adult patients enrolled on the phase 2 trials (median age, 61 years; range, 20-87 years) who were available for analysis. Seventy-one (25.4%) patients achieved either a CR (n = 35) or a CRp (n = 36). Among patients who achieved a CR or CRp, the mean CD33 expression level and CD33 expression to blast percentage ratio were each statistically significantly higher, whereas the percentage of AML blasts in the bone marrow was statistically significantly lower than that among non-responders, that is, patients with a smaller amount of AML blasts but higher blast CD33 levels were more likely to respond to GO (Figure 1A and Table 1). Twenty-seven patients died within 28 days of therapy initiation, and a separate analysis was done after exclusion of these patients. Qualitatively, the same results were seen as those among all patients (Table 1). Among patients in the first to fourth quartiles of mean CD33 expression, response rates were 23%, 23%, 27%, and 41%, respectively, after exclusion of patients who experienced early death (P < .03, trend test). Previous reports failed to identify a significant association between CD33 expression level and outcome.6,15,16 This discrepancy may be explained by differences in the method of quantifying CD33 expression, the larger sample size yielding increased statistical power compared with previous reports, and/or differences in the manner in which associations were assessed.
Figure 1.
CD33 expression and Pgp activity in responders and nonresponders. (A) CD33 expression on AML blasts of responders (CR+CRp) and nonresponders (NR) who survived beyond day 28 of study initiation, determined by flow cytometry after staining with the anti-CD33 antibody hP67.6. (B) Pgp activity of AML blasts of responders and nonresponders who survived beyond day 28 of study initiation, determined by flow cytometry using the fluorescent Pgp substrate DiOC2. (C) Correlation between CD33 expression and Pgp activity. Dot plot graph showing individual data on CD33 expression levels and Pgp activity in responders (CR+CRp; ○) as well as nonresponders (NR; ●) who survived beyond day 28 of study initiation; n = 208. The same magnitude of correlation was seen when the patients who died before day 28 were not excluded from the analysis (r = −0.23; n = 230; P < .001).
Table 1.
Association of parameters with response among all patients and among those who survived beyond day 28 of therapy initiation
| All nonresponders |
Nonresponders surviving beyond day 28* |
Responders† |
P‡ | ||||
|---|---|---|---|---|---|---|---|
| n | Mean (95% CIs) | n | Mean (95% CIs) | n | Mean (95% CIs) | ||
| CD33 expression | 197 | 54.8 (48.6-61.0) | 172 | 53.9 (47.2-60.7) | 69 | 71.2 (57.2-85.2) | .04; .03 |
| Pgp efflux | 170 | 1.84 (1.73-1.96) | 148 | 1.86 (1.74-1.99) | 60 | 1.44 (1.31-1.57) | <.001; <.001 |
| CD33 expression-blast % ratio | 197 | 1.52 (1.26-1.78) | 172 | 1.56 (1.27-1.85) | 69 | 3.31 (2.11-4.53) | .005; .006 |
| % AML blasts | 197 | 55.9 (51.8-60.0) | 172 | 54.7 (50.3-59.2) | 69 | 44.7 (37.7-51.8) | .008; .02 |
n indicates the number of patients with available data; 95% CIs, 95% confidence intervals.
This group excludes patients who died within 28 days of therapy initiation.
CR + CRp.
First P value among all patients; second value excludes patients who died within 28 days of therapy initiation.
Previous analysis of a subset of the phase 2 trial patients has demonstrated that Pgp activity was associated with a poorer response.6,8 Consistent with these earlier findings, we found that patients who achieved a CR or CRp had statistically significantly lower mean Pgp efflux, even after excluding patients who died before day 28 (Figure 1B and Table 1). This association was also apparent when the relationship between Pgp activity and clinical outcome was analyzed by quartiles of Pgp, with response rates of 54%, 27%, 23%, and 12% among patients in the first to fourth quartiles, respectively, after exclusion of patients who experienced early death (P < .001, trend test). This observation for a GO-based therapy is similar to anthracycline-based conventional anti-AML chemotherapies.17,18
Finally, since increasing evidence suggests that Pgp function is related to the maturation stage of the AML blast, with higher activity in more immature cells19–21 and CD33 being expressed in a maturation-stage–dependent manner in myeloid cells,1 we estimated the correlation between these 2 parameters. We found a statistically significant inverse relationship (P < .001) between CD33 expression and Pgp efflux, although the magnitude of the correlation was only moderate (r = −0.23, Figure 1C). Given this correlation, it was of interest to examine the association of one parameter with outcome after adjusting for the other. The addition of Pgp statistically significantly improved a logistic regression model containing only CD33 (P < .001), whereas the addition of CD33 did not lead to such an improvement relative to the model containing only Pgp (P = .14).
In conclusion, our data indicate that CD33 expression levels and Pgp efflux are each associated with clinical outcome of patients treated with GO monotherapy, and Pgp remains statistically significantly associated with outcome even after adjusting for CD33 whereas CD33 does not show such an association after adjusting for Pgp function. Importantly, these variables may not necessarily be independent of other prognostic factors, or in all situations. The inverse relationship between CD33 abundance and Pgp activity is consistent with the notion of maturation-stage–dependent expression of these proteins. This offers the rationale for using drug efflux inhibitors and/or cell differentiation–promoting agents (eg, cytokines or growth factors) to enhance GO-induced cytotoxicity by increasing CD33 expression/drug uptake and/or reducing drug efflux,16,22–24 thereby possibly improving clinical outcome of patients undergoing GO-containing AML therapy.
Acknowledgments
We thank Jeroen G. te Marvelde and Patricia G. Hoogeveen for excellent technical assistance.
This work was supported in part by research funding from the Leukemia & Lymphoma Society (Specialized Center of Research [SCOR] grant no. 7040) and the National Institutes of Health/National Cancer Institute (grant no. CA091 316). R.B.W. is the recipient of an American Society of Hematology Clinical/Translational Research Fellow Scholar Award and a Leukemia & Lymphoma Society Special Fellow Award (no. 3588-07). I.D.B. is the recipient of an American Cancer Society (ACS) Clinical Research Professorship (no. CRP-95-129-11).
Footnotes
Presented in part at the 48th Annual Meeting of the American Society of Hematology, December 9 to 12, 2006, Orlando, FL.25
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.B.W. designed research, analyzed data, and wrote the paper; T.A.G. analyzed data and wrote the paper; V.H.J.v.d.V. and J.J.M.v.D. collected data and wrote the paper; M.R.L. and D.A.F. collected data; and I.D.B. and F.R.A. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: R. B. Walter, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D2-373, Seattle, WA 98109-1024; e-mail: rwalter@fhcrc.org.
References
- 1.Varki A, Angata T. Siglecs—the major subfamily of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum FR. Antibody-targeted therapy for myeloid leukemia. Semin Hematol. 1999;36:2–8. [PubMed] [Google Scholar]
- 3.Ravandi F, Kantarjian H, Giles F, Cortes J. New agents in acute myeloid leukemia and other myeloid disorders. Cancer. 2004;100:441–454. doi: 10.1002/cncr.11935. [DOI] [PubMed] [Google Scholar]
- 4.Linenberger ML. CD33-directed therapy with gemtuzumab ozogamicin in acute myeloid leukemia: progress in understanding cytotoxicity and potential mechanisms of drug resistance. Leukemia. 2005;19:176–182. doi: 10.1038/sj.leu.2403598. [DOI] [PubMed] [Google Scholar]
- 5.Giles F, Estey E, O'Brien S. Gemtuzumab ozogamicin in the treatment of acute myeloid leukemia. Cancer. 2003;98:2095–2104. doi: 10.1002/cncr.11791. [DOI] [PubMed] [Google Scholar]
- 6.Sievers EL, Larson RA, Stadtmauer EA, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19:3244–3254. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- 7.Larson RA, Sievers EL, Stadtmauer EA, et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer. 2005;104:1442–1452. doi: 10.1002/cncr.21326. [DOI] [PubMed] [Google Scholar]
- 8.Linenberger ML, Hong T, Flowers D, et al. Multidrug-resistance phenotype and clinical responses to gemtuzumab ozogamicin. Blood. 2001;98:988–994. doi: 10.1182/blood.v98.4.988. [DOI] [PubMed] [Google Scholar]
- 9.Walter RB, Raden BW, Hong TC, Flowers DA, Bernstein ID, Linenberger ML. Multidrug resistance protein attenuates gemtuzumab ozogamicin-induced cytotoxicity in acute myeloid leukemia cells. Blood. 2003;102:1466–1473. doi: 10.1182/blood-2003-02-0396. [DOI] [PubMed] [Google Scholar]
- 10.Walter RB, Raden BW, Cronk MR, Bernstein ID, Appelbaum FR, Banker DE. The peripheral benzodiazepine receptor ligand PK11195 overcomes different resistance mechanisms to sensitize AML cells to gemtuzumab ozogamicin. Blood. 2004;103:4276–4284. doi: 10.1182/blood-2003-11-3825. [DOI] [PubMed] [Google Scholar]
- 11.Naito K, Takeshita A, Shigeno K, et al. Calicheamicin-conjugated humanized anti-CD33 monoclonal antibody (gemtuzumab ozogamicin, CMA-676) shows cytocidal effect on CD33-positive leukemia cell lines, but is inactive on P-glycoprotein-expressing sublines. Leukemia. 2000;14:1436–1443. doi: 10.1038/sj.leu.2401851. [DOI] [PubMed] [Google Scholar]
- 12.Matsui H, Takeshita A, Naito K, et al. Reduced effect of gemtuzumab ozogamicin (CMA-676) on P-glycoprotein and/or CD34-positive leukemia cells and its restoration by multidrug resistance modifiers. Leukemia. 2002;16:813–819. doi: 10.1038/sj.leu.2402459. [DOI] [PubMed] [Google Scholar]
- 13.Walter RB, Raden BW, Kamikura DM, Cooper JA, Bernstein ID. Influence of CD33 expression levels and ITIM-dependent internalization on gemtuzumab ozogamicin-induced cytotoxicity. Blood. 2005;105:1295–1302. doi: 10.1182/blood-2004-07-2784. [DOI] [PubMed] [Google Scholar]
- 14.van der Velden VHJ, te Marvelde JG, Hoogeveen PG, et al. Targeting of the CD33-calicheamicin immunoconjugate Mylotarg (CMA-676) in acute myeloid leukemia: in vivo and in vitro saturation and internalization by leukemic and normal myeloid cells. Blood. 2001;97:3197–3204. doi: 10.1182/blood.v97.10.3197. [DOI] [PubMed] [Google Scholar]
- 15.Jilani I, Estey E, Huh Y, et al. Differences in CD33 intensity between various myeloid neoplasms. Am J Clin Pathol. 2002;118:560–566. doi: 10.1309/1WMW-CMXX-4WN4-T55U. [DOI] [PubMed] [Google Scholar]
- 16.Jedema I, Barge RM, van der Velden VH, et al. Internalization and cell cycle-dependent killing of leukemic cells by gemtuzumab ozogamicin: rationale for efficacy in CD33-negative malignancies with endocytic capacity. Leukemia. 2004;18:316–325. doi: 10.1038/sj.leu.2403205. [DOI] [PubMed] [Google Scholar]
- 17.Leith CP, Kopecky KJ, Godwin J, et al. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood. 1997;89:3323–3329. [PubMed] [Google Scholar]
- 18.Leith CP, Kopecky KJ, Chen I-M, et al. Frequency and clinical significance of the expression of the multidrug resistance protein MDR1/p-glycoprotein, MRP1, and LRP in acute myeloid leukemia. A Southwest Oncology Group study. Blood. 1999;94:1086–1099. [PubMed] [Google Scholar]
- 19.Wuchter C, Karawajew L, Ruppert V, et al. Clinical significance of CD95, Bcl-2 and Bax expression and CD95 function in adult de novo acute myeloid leukemia in context of P-glycoprotein function, maturation stage, and cytogenetics. Leukemia. 1999;13:1943–1953. doi: 10.1038/sj.leu.2401605. [DOI] [PubMed] [Google Scholar]
- 20.van der Kolk DM, de Vries EG, Noordhoek L, et al. Activity and expression of the multidrug resistance proteins P-glycoprotein, MRP1, MRP2, MRP3 and MRP5 in de novo and relapsed acute myeloid leukemia. Leukemia. 2001;15:1544–1553. doi: 10.1038/sj.leu.2402236. [DOI] [PubMed] [Google Scholar]
- 21.Suarez L, Vidriales MB, Moreno MJ, et al. Differences in anti-apoptotic and multidrug resistance phenotypes in elderly and young acute myeloid leukemia patients are related to the maturation of blast cells. Haematologica. 2005;90:54–59. [PubMed] [Google Scholar]
- 22.Sivaraman S, Manjali J, Gregory SA, Manson S, Raza A, Venugopal P. G-CSF and other cytokines modulated expression of CD33 antigen on the surface of myeloid cells [abstract]. Blood. 2002;100:554a. [Google Scholar]
- 23.Leone G, Rutella S, Voso MT, Fianchi L, Scardocci A, Pagano L. In vivo priming with granulocyte colony-stimulating factor possibly enhances the effect of gemtuzumab-ozogamicin in acute myeloid leukemia: results of a pilot study. Haematologica. 2004;89:634–636. [PubMed] [Google Scholar]
- 24.Rutella S, Bonanno G, Procoli A, et al. Granulocyte colony-stimulating factor enhances the in vitro cytotoxicity of gemtuzumab ozogamicin against acute myeloid leukemia cell lines and primary blast cells. Exp Hematol. 2006;34:54–65. doi: 10.1016/j.exphem.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Walter RB, Gooley TH, van der Velden VHJ, et al. Relationship between CD33 expression, P-glycoprotein-mediated drug efflux, and clinical outcome in patients treated in phase II trials with gemtuzumab ozogamicin monotherapy. Blood. 2006;108:658a. doi: 10.1182/blood-2006-09-047399. [DOI] [PMC free article] [PubMed] [Google Scholar]