Abstract
The purpose of the present experiments was to evaluate the contribution of the glutamate-glutamine cycle in retinal glial (Müller) cells to photoreceptor cell synaptic transmission. Dark-adapted isolated rat retinas were superfused with oxygenated bicarbonate-buffered media. Recordings were made of the b-wave of the electroretinogram as a measure of light-induced photoreceptor to ON-bipolar neuron transmission. L-methionine sulfoximine (1–10 mM) was added to superfusion media to inhibit glutamine synthetase, a Müller cell specific enzyme, by more than 99% within 5–10 min, thereby disrupting the conversion of glutamate to glutamine in the Müller cells. Threo-hydroxyaspartic acid and D-aspartate were used to block glutamate transporters. The amplitude of the b-wave was well maintained for 1–2 h provided 0.25 mM glutamate or 0.25 mM glutamine was included in the media. Without exogenous glutamate or glutamine the amplitude of the b-wave declined by about 70% within 1 h. Inhibition of glutamate transporters led to a rapid (2–5 min) reversible loss of the b-wave in the presence and absence of the amino acids. In contrast, inhibition of glutamine synthetase did not alter significantly either the amplitude of the b-wave in the presence of glutamate or glutamine or the rate of decline of the b-wave found in the absence of these amino acids. Excellent recovery of the b-wave was found when 0.25 mM glutamate was resupplied to L-methionine sulfoximine–treated retinas. The results suggest that in the isolated rat retina uptake of released glutamate into photoreceptors plays a more important role in transmitter recycling than does uptake of glutamate into Müller cells and its subsequent conversion to glutamine.
Keywords: Electroretinogram, b-wave, Glutamate-glutamine cycle, Müller cells, Glutamine synthetase, Glutamate transporters
Introduction
It is now well recognized that glutamate is the transmitter released in darkness by photoreceptors and that absorption of light by these cells slows this release (Copenhagen & Jahr, 1988; Massey, 1991; Maple et al., 1994). The released glutamate acts at several different types of glutamate receptors on bipolar cells and horizontal cells, and the interaction between glutamate and these receptors shapes their specific and varied responses to light. Given the high release in the dark, for continued normal function of excitatory glutamatergic synapses in the retina, it is important that the extracellular concentration of glutamate be controlled so that the receptors are not saturated. This is usually accomplished by uptake of the released glutamate into cells within the localized region bordering the synaptic region, rather than by extracellular enzymatic degradation (Hertz, 1979; Brew & Attwell, 1987; Barbour et al., 1991; Kanai et al., 1993; Eliasoff & Werblin, 1993; Rauen & Kanner, 1994; Derouiche & Rauen, 1995; Sontheimer, 1995; Yang & Wu, 1997). The cells which could participate in the uptake of synaptically released glutamate include the photoreceptor cells themselves, the horizontal cells, the bipolar cells, and the glial (Müller) cells. It was proposed many years ago (Hertz, 1979; Shank & Aprison, 1981) that at brain glutamatergic synapses glial cells take up glutamate, convert this glutamate to glutamine which diffuses out of the glia and into neurons, where glutamine is converted into glutamate, thereby replenishing transmitter content in the appropriate presynaptic neurons; this cycle is called the glutamate-glutamine cycle.
Autoradiographic studies have clearly shown that incubation of mammalian and nonmammalian vertebrate retinas with micromolar concentrations of labeled glutamate leads to its rapid uptake into Müller cells and photoreceptor cells (Ehinger & Falck, 1971; Ehinger, 1977; Marc & Lam, 1981; Pow & Robinson, 1994), which is consistent with the postulated roles of these two cell types in controlling the synaptic concentration of glutamate. Furthermore, the recycling of the glutamate through Müller cells requires that the glutamate which is taken up be enzymatically converted to glutamine within these cells by glutamine synthetase, which has been localized to Müller cells in the retina (Riepe & Norenberg, 1977, 1978; Linser & Moscona, 1979). Thus, the high-affinity uptake system, the presence of glutamine synthetase in Müller cells, and the close morphological association of Müller cell processes with photoreceptor synapses (Sarantis & Mobbs, 1992; Pow & Robinson, 1994; Derouiche & Rauen, 1995; but see Lasansky, 1973) are all consistent with a role for the Müller cells in the recycling of glutamate.
In the present study, we have evaluated the role of the Müller cells and the glutamate-glutamine cycle in regulating synaptic transmission between rat rod photoreceptors and ON-bipolar cells, as measured by the low intensity b-wave (Granit’s PII) of the electroretinogram (ERG). This wave has recently been suggested to reflect bipolar cell currents (Stockton & Slaughter, 1989; Robson & Frishman, 1995; Tian & Slaughter, 1995). Since the primary function of the Müller cells in the recycling scheme is the synthesis of glutamine, we have disrupted this cycle by incubating isolated rat retinas with L-methionine sulfoximine, which inhibits glutamine synthetase irreversibly by binding to the active site of the enzyme as methionine sulfoximine phosphate (Rowe & Meister, 1970; Karasawa & Nakata, 1988), in conjunction with inhibition of glutamate uptake, and examined the effects of these inhibitors on the generation of the b-wave. Our results show that inhibition of glutamine synthetase has little or no effect on b-wave generation over many tens of minutes. This leads us to suggest that Müller cells need not be primarily responsible for replenishment of glutamate in photoreceptor terminals. Rather, glutamate transporters in photoreceptors can function as the major system for controlling the level of glutamate in the synaptic regions in the outer plexiform layer.
Methods
Isolation of retina and superfusion chamber
Albino rats (Sprague-Dawley) weighing 200–250 g were dark adapted for 12–24 h. Animals were anesthetized with sodium pentobarbital (i.p., 30 mg/kg), then decapitated, and both eyes removed under infrared light using night vision devices (NAV-3, Intervac, Palo Alto, CA). The eyecup was obtained following cutting of the globe just posterior to the ora serrata and was kept in oxygenated (95% O2/5% CO2) Ringer solution at room temperature. The retina was subsequently detached from the retinal pigment epithelium (RPE) and, just prior to mounting in the superfusion chamber, was cut into 3 × 3 mm pieces. A piece of retina was mounted receptor side up on a Millipore filter, and both the retina and filter paper were positioned horizontally between two larger pieces of mesh in a specialized chamber. The fluid entered the chamber from above, flowing both over the receptor side of the tissue and through the tissue, the fluid exiting the chamber from the compartment under the retina. Total volume of fluid in the chamber was approximately 0.5 ml. The outflow was collected in a reservoir and, when necessary, could be recirculated through the perfusion system. Temperature was maintained inside the chamber at 36–37°C, as measured by a small thermistor probe.
Solution composition
The control superfusion solution contained in mmoles/l: NaCl, 110; KCl, 5; Na2HPO4, 0.8; NaH2PO4, 0.1; NaHCO3, 30; MgSO4, 1.0; CaCl2, 1.8; and Glucose, 22. In addition, L-glutamate was typically included in the control solution (see later) at 0.25 mmoles/l. All media were saturated and continuously bubbled in the main storage reservoir with 95% O2/5% CO2, pH was 7.4–7.5 and osmolarity was about 310 milliosmoles. The flow rate was maintained at 3 ml/min, thereby providing for rapid (less than 1 min) exchanges of different test media. Test compounds were directly dissolved into the media at the appropriate concentrations.
Electrophysiology
Transretinal recordings of ERG potentials were made between an Ag-AgCl macroelectrode in the bottom of the chamber and a saline-filled glass capillary microelectrode in contact with the distal tips of the rod outer segments. The tip diameter of the microelectrode was between 1.5–5 μm and tip resistance was 5–25 MΩ. Potentials were amplified with a high-gain amplifier (CyberAmp 320, Axon Instruments, San Francisco, CA) with a band width of 0–400 Hz. Responses were digitized and stored using an on-line computer data acquisition system (LabView, National Instruments, Austin, TX).
Light stimulation
Light stimuli were projected onto the dark-adapted retina through a Zeiss operating microscope. Stimuli were monochromatic (500 nm) and were produced from a 40-ms shuttered flash. The shutter was placed in front of a 150-W xenon arc lamp. The intensity of the stimuli was varied by placing calibrated neutral density filters in the stimulus beam. The timing of the flash was controlled by a computer. Data were collected over a series of intensities, though the emphasis in the present experiments was on the b-wave in response to relatively dim stimuli, 1–3 log units above threshold (a 10-μV response). Stimuli were spaced in time to ensure full dark adaptation between successive light flashes. The peak amplitude of the b-wave (and other ERG components) was measured by fitting second-order polynomials to segments of the record containing the peak of interest. Typically, the b-wave (in response to dim stimuli) was measured from the baseline to the peak, while with brighter stimuli which elicited an a-wave, the b-wave amplitude was measured from the peak of the a-wave to the peak of the b-wave.
Glutamine synthetase assay
Enzyme activity was measured in cytosolic extracts of rat retinas using a modification of procedures described previously (Chader, 1971; Thorndike & Reif-Lehrer, 1971; Darrow et al., 1997). One isolated rat retina was first rinsed with ice-cold saline and then was homogenized in 1 ml of a buffer mixture containing 10 mM HEPES, 137 mM NaCl, 4.6 mM KCl, 1.1 mM KH2PO4, 0.6 mM MgSO4, 1.1 mM EDTA, and a cocktail of protease inhibitors (PMSF, Pepstatin A, and leupeptin). The homogenate was centrifuged at 15,000g for 10 min, and the supernatant kept on ice until just before the start of the assay. The assay mixture (330 μl) contained 180 mM glutamine, 48 mM NH2OH (pH = 6.3), 51 mM Na Citrate (pH = 6.3), 0.6 mM MnCl2, and 0.09 mM NaADP to which either 10-, 25-, or 70-μl aliquots of the retinal supernatant (with appropriate amounts of d-H2O) was added to bring the final reaction volume to 400 μl. The assay tubes (with and without supernatant and with and without ADP) were incubated for 10 min at 37°C. Then 400 μl of Stop Reagent (equal volumes of 7% FeCl3, 15% TCA, and 0.1 M HCl) was added. Absorbencies were measured at 500 nm with a Bausch and Lomb spectrophotometer. Standards (10, 25, and 70 μl) of L-glutamic monohydroxamate were run to generate standard curves for each experiment. One additional point is that inclusion of the protease inhibitors was absolutely essential for a successful assay, because glutamine synthetase is a very labile enzyme which rapidly loses its activity when homogenates and supernatants are prepared in the absence of such inhibitors.
Results
In the first set of experiments, intact retinas were incubated for varying times with 1 and 5 mM L-methionine sulfoximine and the extent of inhibition of glutamine synthetase was subsequently examined in cytosolic extracts of retina, which were prepared after the exposure of the intact tissue to the drug. In this way, we obtained information on the time course of inhibition of the enzyme in the presence of the inhibitor during the ongoing incubation of the intact retina. The activity of glutamine synthetase in control (uninhibited) rat retinas was 7.6 μmoles/min/mg protein (n = 12), a value similar to that reported previously for the specific activity of this enzyme in retina (Reif-Lehrer & Coglin, 1973; Darrow et al., 1997). As shown in Fig. 1, nearly complete inhibition of glutamine synthetase was observed when rat retinas (n = 8) were incubated for 10 min with either 1 or 5 mM L-methionine sulfoximine. This shows that the drug readily entered the Müller cells, the only retinal cell containing this enzyme, and rapidly inactivated glutamine synthetase. Glutamine synthetase was not inhibited when intact retinas (or extracts) were incubated with methionine sulfoxide, an inactive analog of methionine sulfoximine. The rapid (within minutes), complete (more than 99%) and irreversible inhibition (Rowe & Meister, 1970) of glutamine synthetase by 1 mM or higher concentrations (2–10 mM) of methionine sulfoximine was clearly a prerequisite for examining the role of this enzyme and Müller cells in glutamate cycling in the subsequent electrophysiological experiments.
Fig. 1.
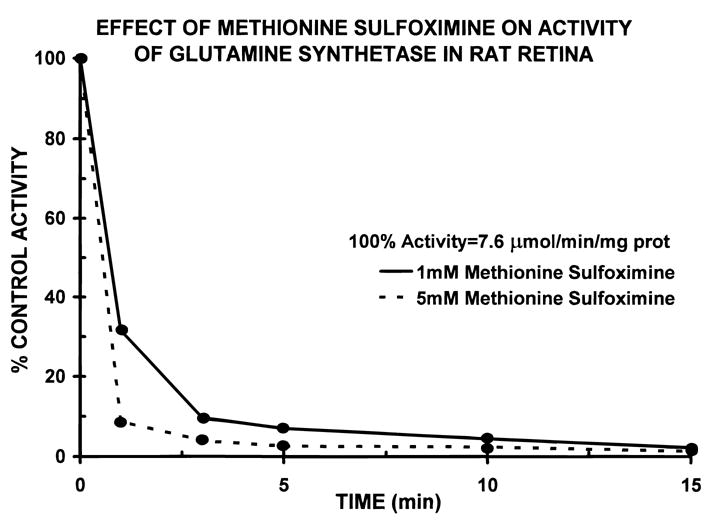
Time course of the decline in activity of glutamine synthetase during incubation of isolated rat retinas with 1 mM (solid line) and 5 mM (dashed line) L-methionine sulfoximine. Though not shown, 10 mM MSO yielded an inhibition curve which was similar to the rate of loss of enzyme activity with 5 mM MSO. Each point is the average of eight individual retinas. Standard errors of the mean were smaller than the size of the symbols.
In our experiences, when a piece of rat retina (see Methods) was superfused with the control medium lacking any added glutamate or glutamine, the amplitude of the b-wave declined over the first hour of incubation (Fig. 2, left tracings). This was a consistent finding in recordings from more than 50 individual retinas. However, the amplitude of the b-wave was maintained, i.e. no significant decay, for several hours when either 0.25 mM glutamine (Fig. 2, middle tracings) or 0.25 mM glutamate (Fig. 2, right tracings) was added to the control solution. Since it was clearly important for the “control” b-wave to be stable over many tens of minutes, 0.25–0.50 mM glutamate (or 0.25–0.50 mM glutamine) was routinely added to the superfusion solutions.
Fig. 2.
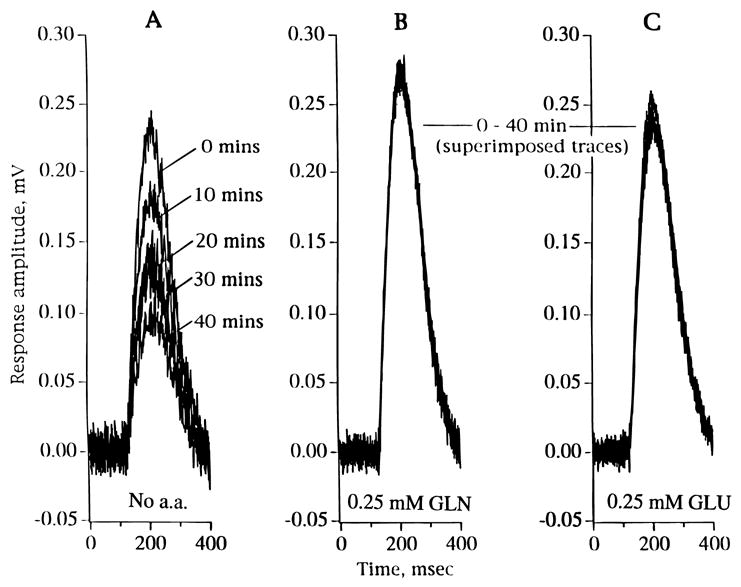
The response waveforms of ERG b-waves during 40-min incubations of isolated rat retinas under the following conditions: A: Control medium with no added amino acids (no a.a.). B: Control medium with 0.25 mM glutamine (GLN). C: Control medium with 0.25 mM glutamate (GLU). Note decline in b-wave amplitude over time in A, but not in B and C.
Fig. 3 shows the effects of adding 2 or 10 mM L-methionine sulfoximine to media containing 0.25 mM glutamate on the amplitude of the b-wave. The retina was initially incubated for 30 min in control media to establish stable baseline recordings (open circles). Then media containing L-methionine sulfoximine was introduced (t = 0) and recordings of the b-wave were carried out over the next 50 min. It can be seen that the amplitude of the b-wave remained within 85% of its starting amplitude after 50 min, despite the fact that glutamine synthetase was inhibited by more than 99% after the initial 10 min of the incubation (see Fig. 1). In two additional experiments, effects of L-methionine sulfoximine were followed for up to 100 min and the b-wave amplitude also remained at greater than 80% of its initial amplitude.
Fig. 3.
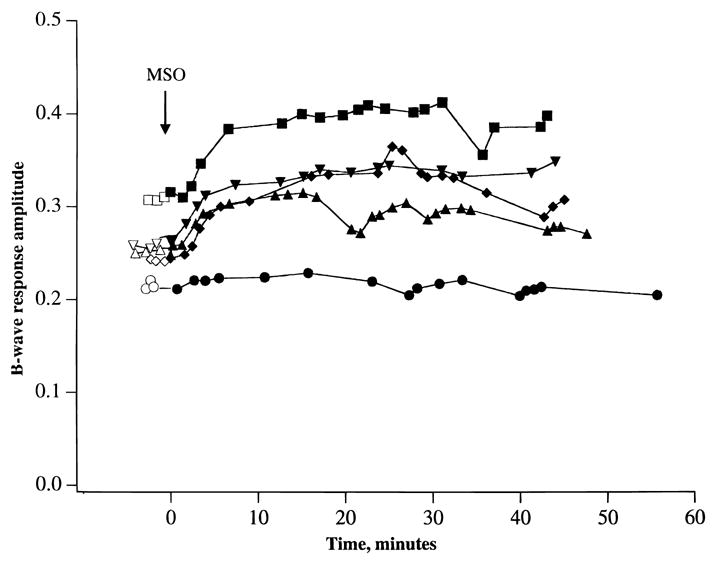
A graph of the effects of adding 2–10 mM L-methionine sulfoximine (MSO) on the amplitude of the ERG b-wave. 0.25 mM glutamate was present in both the control and MSO-containing media. Preincubation period in control media (open symbols) was for 30 min. Then media containing MSO were introduced (t = 0) and recordings were made over the next 50 min (closed circles). Each curve represents an individual experiment. Lowest curve is with 2 mM MSO, while other four curves are with 10 mM MSO.
Fig. 4 provides an additional test of the effects of 1 mM L-methionine sulfoximine on the amplitude of the b-wave, but in this series of experiments the superfusion media lacked exogenous glutamate. In this case, the declines in b-wave amplitude were very similar with only a suggestion that b-wave decline was slightly faster and more pronounced in the presence of L-methionine sulfoximine (closed circles, n = 5) than in its absence (open circles, n = 5). However, after 40 min b-wave amplitudes in the presence and absence of L-methionine sulfoximine were decreased by more than 50% in comparison to the starting values. Thus, inhibition of glutamine synthetase did not appear to substantially alter the generation of the b-wave in the presence and absence of exogenous glutamate. This view receives support from the experiments shown in Fig. 5, which shows excellent recovery of the b-wave when retinas (n = 3) were returned to control media containing 0.25 mM glutamate following a 45-min incubation with media containing 1 mM L-methionine sulfoximine (no added glutamate). Thus, despite the irreversible loss in glutamine synthetase activity, re-addition of glutamate was sufficient to restore the b-wave to a high amplitude that was maintained for another 60 min. Recovery of the b-wave under this condition amounted to 85% in comparison to the control, starting value, e.g. 210 μV (n = 10). As shown in Fig. 6, strong recovery of the b-wave was also obtained when 0.25 mM glutamine was added to control media following a 45-min incubation with L-methionine sulfoximine. A similar recovery of the b-wave was also observed when glutamate (n = 3) or glutamine (n = 3) was added to the incubation media in the absence of L-methionine sulfoximine (data not shown).
Fig. 4.
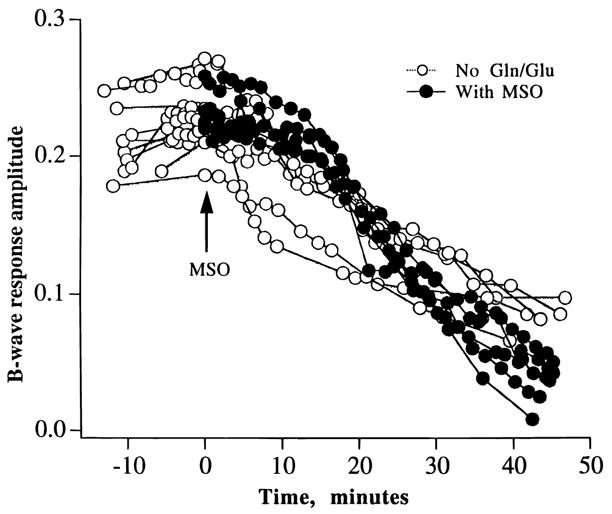
A graph of the effects of adding 1 mM L-methionine sulfoximine (MSO) on the amplitude of the ERG b-wave. Control (open circles) and MSO-containing (closed circles) media did not contain glutamate or glutamine. Note similar declines in b-wave amplitudes in the presence and absence of MSO.
Fig. 5.
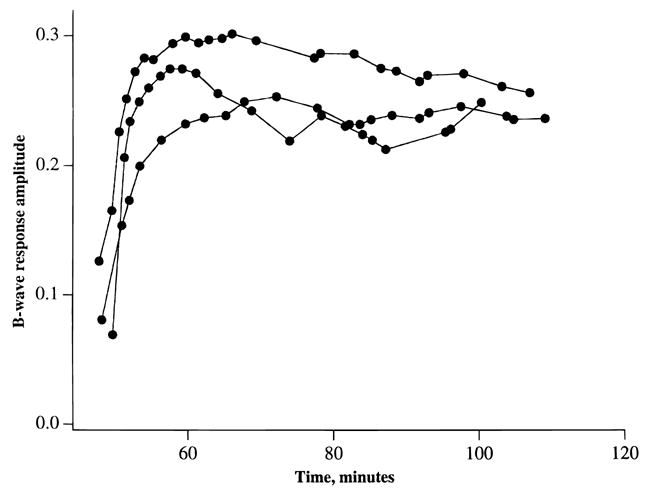
Recovery of b-waves following addition of 0.25 mM glutamate to media containing 1 mM L-methionine sulfoximine. In effect, these data represent a continuation of Fig. 4, where it was shown that the amplitude of the b-wave declined when rat retinas were superfused with medium lacking glutamate. L-methionine sulfoximine was present throughout the experiment. Each curve represents data from one retina.
Fig. 6.
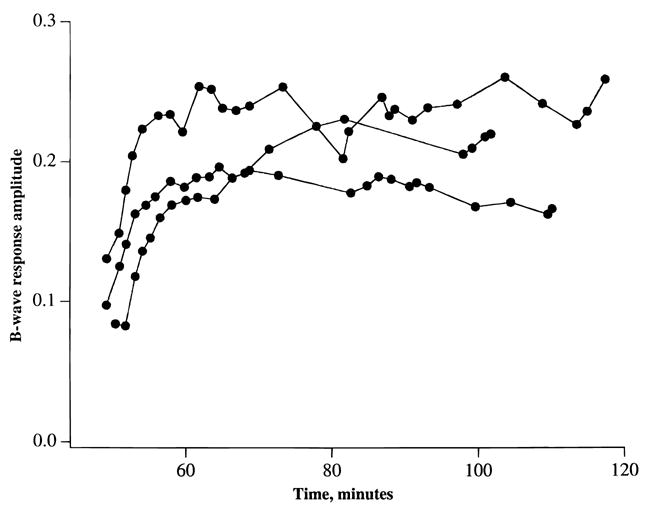
An identical experiment to that described in Fig. 5, except that recovery of the b-wave was elicited by adding 0.25 mM glutamine to MSO-containing medium.
We next studied the effects of inhibiting glutamate uptake transporters on the generation of the b-wave. Fig. 7 shows that in the presence of 0.25 mM glutamate, incubation of retinas with 2 mM threo-hydroxyaspartic acid (HA), a glutamate transporter substrate, led to a rapid decline and virtual loss of the b-wave in less than 5 min. Removal of HA led to a partial recovery of the b-wave. Essentially the same result, that is, suppression of the b-wave, was seen when 2–4 mM D-aspartate, another glutamate transporter substrate, was added to the media containing 0.25 mM glutamate. Fig. 8 shows that HA (or D-aspartate, data not shown) also suppressed the b-wave reversibly when effects of the inhibitor was examined in the presence of 0.50 mM glutamine. Fig. 9 shows ERG tracings in response to high-intensity light flashes showing that HA did not inhibit the leading edge of the a-wave, an indicator of light-induced photoreceptor activity. Rather, the HA-induced loss of the b-wave was accompanied by an unmasking of the full extent of the a-wave and the appearance of the PIII component of the ERG.
Fig. 7.
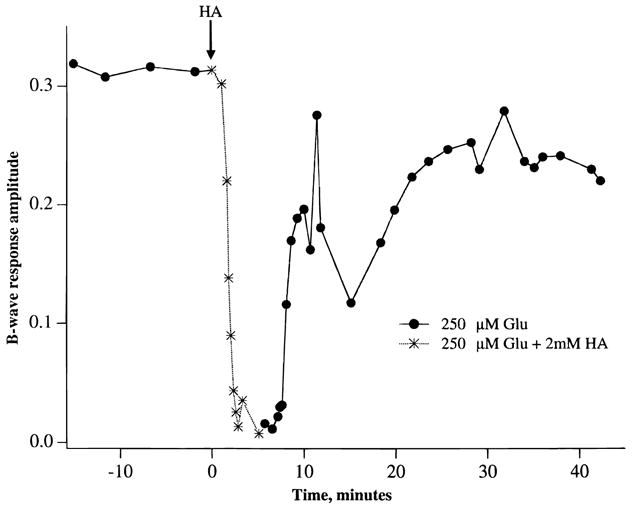
A graph of the changes in b-wave amplitude elicited by 2 mM threo-hydroxyaspartic acid (HA) and by returning to the control condition. 0.25 mM glutamate was present in all media. Note rapid decline in the amplitude of the b-wave following superfusion of retinas with HA. Each point is the average of two individual experiments.
Fig. 8.
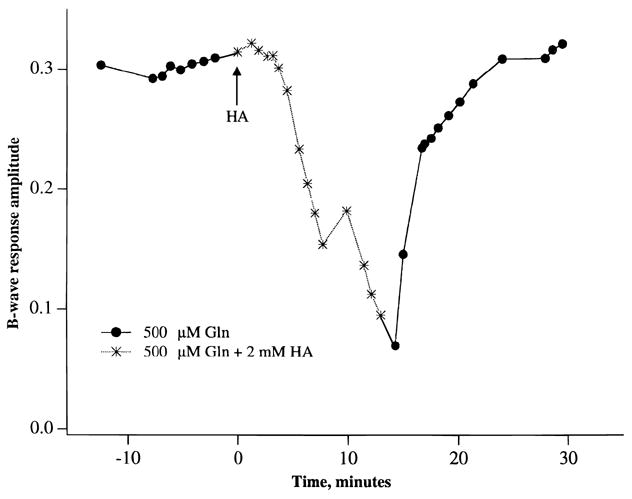
Similar protocol as described in Fig. 7 except that 0.5 mM glutamine was present throughout the experiment, that is, before, during, and after superfusion with 2 mM threo-hydroxyaspartic acid (HA). Each point is the average of two experiments.
Fig. 9.
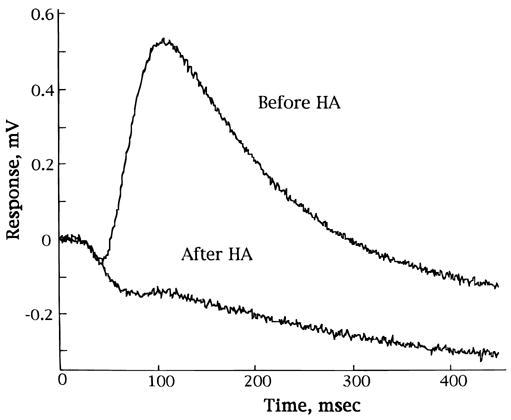
ERG tracings in response to high intensity light flashes, which elicit a- and b-waves, before and 5 min after the addition of 2 mM HA from a representative experiment. The “after HA” trace shows loss of b-wave, but a-wave and PIII component are not affected by this glutamate uptake blocker.
Discussion
In this paper, we have shown that inhibition of glutamine synthetase with L-methionine sulfoximine has virtually no affect on the b-wave of the ERG of the isolated rat retina over many tens of minutes. Use of this inhibitor was intended to disrupt the conversion of glutamate to glutamine specifically in retinal Müller cells, which is the cell type in the retina which contains glutamine synthetase (Riepe & Norenberg, 1977, 1978; Linser & Moscona, 1979), and to impair the recycling scheme (glutamate-glutamine cycle) “in which Müller cells take up released glutamate, convert it to glutamine, and release glutamine as a precursor for neuronal glutamate synthesis into the extracellular space” (Sontheimer, 1995, pg. 123). A previous study (Pow & Robinson, 1994) showed that incubation of rabbit eyecups with 2 mM D,L methionine D,L sulfoximine for 90 min resulted in the virtual absence of glutamate immunoreactivity, that is, depletion of glutamate reserves, in bipolar and ganglion cells, a buildup in glutamate reactivity in Müller cells, and a slight reduction in glutamate immunoreactivity in photo-receptor cells. In that paper, Pow and Robinson suggested that the total lack of glutamate immunoreactivity in the bipolar and ganglion cells following inhibition of glutamine synthetase resulted from the inhibition of Müller cell–derived de novo synthesis of glutamate. They were less certain about the extent to which Müller cells supplied glutamate to the photoreceptor cells, since glutamate labeling was still detectable in these cells despite the absence of glutamine synthetase activity. Indeed, in a subsequent paper, Pow and Crook (1996) concluded that the photoreceptor cells in the rabbit retina were not “heavily reliant upon uptake of glutamine for maintenance of their content of neurotransmitter glutamate” (p. 301). The results in the present paper show clearly that the activity of glutamine synthetase is rapidly and completely inhibited following incubation of isolated rat retinas with 1–10 mM L-methionine sulfoximine. These concentrations of the inhibitor are not known to inhibit glutamate uptake transporters or the uptake, distribution, or metabolism of other putative neurotransmitters, including GABA and glycine (Balcar & Johnston, 1972; Pow & Robinson, 1994). Thus, in the present study, the effects of L-methionine sulfoximine are likely to be confined to inhibition of glutamine synthetase.
Our findings showing that the b-wave is unaffected by the absence of glutamine synthetase activity raise concerns about whether Müller cells are primarily responsible for glutamate removal in the outer plexiform layer and for resupply of this neurotransmitter to the photoreceptor cells. We have used the amplitude of the light-evoked b-wave as a functional test of synaptic transmission between photoreceptor cells and ON-bipolar cells and as a qualitative measure of ambient levels of extracellular glutamate in the synapse in dark and light. We believe that the b-wave is a useful functional tool in this context, since its complete blockage by APB (Stockton & Slaughter, 1989; Gurevich & Slaughter, 1993; Robson & Frishman, 1995; Tian & Slaughter, 1995) shows it is secondary to ON-bipolar cell activity. Recent evidence (Gurevich & Slaughter, 1993; Tian & Slaughter, 1995) showing that the b-wave waveform closely resembles the waveform of the ON-bipolar cell suggests that “the b-wave results directly from the light response of ON bipolar neurons” (Tian & Slaughter, 1995, p. 1364). On the basis of combined electrophysiological and electron-microscopic (EM) measurements of synaptic interactions between photoreceptor, horizontal, and bipolar cells in retinas from larval tiger salamanders (Ambystoma tigrinum), Yang and Wu (1997) concluded that Müller cells are not primarily responsible for glutamate removal in the outer plexiform layer. Rather, they suggested (p. 837) that “glutamate transporters in photoreceptors are efficient enough to adjust the extracellular glutamate concentrations in the synaptic clefts under various physiological conditions,” in agreement with the work from an earlier study of Eliasoff and Werblin (1993). The present results (Figs. 7 and 8) showing the rapid loss in the b-wave following incubation of the rat retina with inhibitors of glutamate uptake, that is, D-asparate and threo-hydroxyaspartic acid, are consistent with this view, and they show how important these transporters are in controlling the extracellular concentration of glutamate at retinal synapses. Inhibition of glutamate transporters rapidly and profoundly adversely affects synaptic transmission between photoreceptors and their postsynaptic neurons. In this regard, the rate of the decline in the amplitude of the b-wave following inhibition of glutamate uptake in the isolated rat retina is similar to the decline observed when synaptic transmission is blocked by raising the extracellular concentration of glutamate to 5 mM (data not shown).
A final point for consideration concerns that fact that stable b-wave amplitudes were obtained only when the superfusion medium contained 0.25 mM glutamate or 0.25 mM glutamine. When these amino acids were not present, the amplitude of the b-wave declined by 70% in less than 1 h after the start of an experiment. In earlier publications on ERG (a-wave, b-wave, and wavelets or oscillatory potentials) recordings from the superfused, isolated rat retina, Winkler (1972, 1973) added 2–5% horse serum to the media to improve the long-term survival of the b-wave and wavelets. At that time, it was unclear exactly what in the horse serum was responsible for its beneficial effects. In view of the present experiments, it is entirely reasonable that the critical ingredients supplied by horse serum were glutamate and glutamine, since, for example, the glutamate concentration in rat plasma is close to 0.25 mM (Reddy, 1967) and the glutamine concentration in human plasma is approximately 0.6 mM (Dickinson et al., 1968). Normal b-waves were recorded (see Fig. 1) over a long incubation period in the presence of glutamate and glutamine, and for this reason we consider this modification in the perfusate to be both beneficial and valuable. To follow the effects of inhibiting glutamine synthetase on the amplitude of the b-wave over a prolonged period, the control b-wave had to be maintained. The inclusion of 0.25 mM glutamate (or glutamine) in the media fulfilled this need. Since the amplitude of the b-wave was maintained in the rat retinas incubated in media containing 0.25 mM glutamate, we believe that the level of glutamate in the synaptic regions in the outer plexiform layer is at a physiological concentration, perhaps in the range of 1–10 μM (Brew & Attwell, 1987; Barbour et al., 1991), though its concentration at this site has not been measured in this tissue. An unphysiologically high level of glutamate in the synaptic zones between photoreceptor cells and ON-bipolars would be expected to saturate the glutamate receptors on the bipolar cells in darkness and in light, thereby resulting in a decline in the amplitude of the b-wave, as is observed when the bath concentration of glutamate is raised to 2–5 mM (data not shown).
It is tempting to suggest reasons why exogenous glutamate or glutamine is needed to keep up the amplitude of the b-wave, but at this point the possibilities are only speculative. An obvious starting point is that because of washout of synaptically released glutamate under the conditions of our superfusion system with no amino acids in the media, the ambient, steady-state level of glutamate (and glutamine) in the extracellular spaces in the outer plexiform layer is less than in the in vivo situation. This possibility is also a potential concern regarding our interpretation of the effects of interfering with the glutamate-glutamine cycle. For example, wash-out of glutamine, in particular, could make it difficult to observe a role for Müller cells, while exogenously added glutamate or glutamine could be supplying the photoreceptors with enough amino acid to render the glutamate-glutamine cycle unnecessary. The fact that exogenously added glutamine (see Figs. 1 and 6) is as effective as glutamate in maintaining the b-wave is consistent with the notion that glutamine readily enters photoreceptors where it is converted (via glutaminase) to glutamate, which is available for synaptically mediated release. Clearly, this result is also consistent with the postulated recycling role of Müller cells as a supplier of glutamine to neuronal cells. However, Fig. 4 shows a similar decline in the amplitude of the b-wave in the presence and absence of L-methionine sulfoximine and Fig. 5 shows that exogenous glutamate (0.25 mM) restores the b-wave in L-methionine sulfoximine-treated retinas, that is, tissues lacking activity of glutamine synthetase. If our experimental conditions accurately reflect glutamate and glutamine concentrations in vivo, then these latter two sets of results suggest that in the isolated rat retina uptake of released glutamate by photoreceptors plays a more important role than does uptake of glutamate into Müller cells and its subsequent conversion to glutamine in the overall scheme of transmitter recovery and maintenance of synaptic transmission in photoreceptor cells.
Acknowledgments
This work was support by NEI Grants EY RO1-10015 (B.S.W.) and EY RO1-00379 (D.G.G.) and by Core Grants for Vision Research EY P30-05230 (Eye Research Institute, Oakland University) and EY P30-07003 (Department of Ophthalmology, University of Michigan). The authors also acknowledge the insightful comments and constructive suggestions of the reviewers.
References
- Balcar VJ, Johnston GAR. The structural specificity of the high affinity uptake of L-glutamate and L-aspartate by rat brain slices. Journal of Neurochemistry. 1972;19:2657–2666. doi: 10.1111/j.1471-4159.1972.tb01325.x. [DOI] [PubMed] [Google Scholar]
- Barbour B, Brew H, Attwell D. Electrogenic uptake of glutamate and aspartate into glial cells isolated from the salamander (Ambystoma) retina. Journal of Physiology. 1991;436:169–193. doi: 10.1113/jphysiol.1991.sp018545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew H, Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axototl retinal glial cells. Nature. 1987;327:707–709. doi: 10.1038/327707a0. [DOI] [PubMed] [Google Scholar]
- Chader GJ. Hormonal effects on the neural retina: 1: glutamine synthetase development in the retina and liver of the normal and triiodothyronine-treated rat. Archives of Biochemistry and Biophysics. 1971;144:657–662. doi: 10.1016/0003-9861(71)90372-9. [DOI] [PubMed] [Google Scholar]
- Copenhagen DR, Jahr CE. Release of endogenous excitatory amino acids from turtle photoreceptors. Nature. 1988;341:536–539. doi: 10.1038/341536a0. [DOI] [PubMed] [Google Scholar]
- Darrow RA, Darrow RM, Organisciak DT. Biochemical characterization of cell specific enzymes in light-exposed rat retinas: Oxidative loss of all-trans retinol dehydrogenase activity. Current Eye Research. 1997;16:144–151. doi: 10.1076/ceyr.16.2.144.5092. [DOI] [PubMed] [Google Scholar]
- Derouiche A, Rauen T. Coincidence of L-glutamate/L-aspartate transporter (GLAST) and glutamine synthetase (GS) immunoreactions in retinal glia: Evidence for coupling of GLAST and GS in transmitter clearance. Journal of Neuroscience Research. 1995;42:131–143. doi: 10.1002/jnr.490420115. [DOI] [PubMed] [Google Scholar]
- Dickinson JC, Durham DG, Hamilton PB. Ion exchange chromatography of free amino acids in aqueous fluid and lens of the human eye. Investigative Ophthalmology. 1968;7:551–563. [PubMed] [Google Scholar]
- Ehinger B. Glial and neuronal uptake of GABA, glutamic acid, glutamine and glutathione in the rabbit retina. Experimental Eye Research. 1977;25:221–234. doi: 10.1016/0014-4835(77)90089-6. [DOI] [PubMed] [Google Scholar]
- Ehinger B, Falck B. Autoradiography of some suspected neurotransmitter substances: GABA, glycine, glutamic acid, histamine, dopamine, L-dopa. Brain Research. 1971;33:157–172. doi: 10.1016/0006-8993(71)90314-3. [DOI] [PubMed] [Google Scholar]
- Eliasoff S, Werblin F. Characterization of the glutamate transporter in retinal cones of the tiger salamander. Journal of Neuroscience. 1993;13:402–411. doi: 10.1523/JNEUROSCI.13-01-00402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich L, Slaughter MM. Comparison of the waveforms of the ON bipolar neuron and the b-wave of the electroretinogram. Vision Research. 1993;33:2431–2435. doi: 10.1016/0042-6989(93)90122-d. [DOI] [PubMed] [Google Scholar]
- Hertz L. Functional interactions between neurons and astrocytes, I. Turnover and metabolism of putative amino acid transmitters. Progress in Neurobiology. 1979;13:277–323. doi: 10.1016/0301-0082(79)90018-2. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Smith CP, Hediger MA. The elusive transporters with a high affinity for glutamate. Trends in Neuroscience. 1993;16:365–370. doi: 10.1016/0166-2236(93)90094-3. [DOI] [PubMed] [Google Scholar]
- Karasawa Y, Nakata C. Effects of varying doses of methionine sulfoximine on liver glutamine synthetase activity and time course of blood and urinary nitrogenous compounds in the chicken. Comparative Biochemistry and Physiology. 1988;91B:789–792. doi: 10.1016/0305-0491(88)90209-x. [DOI] [PubMed] [Google Scholar]
- Lasansky A. Organization of the outer synaptic layer in the retina of the larval tiger salamander. Philosophical Transactions of the Royal Society B (London) 1973;265:471–489. doi: 10.1098/rstb.1973.0033. [DOI] [PubMed] [Google Scholar]
- Linser P, Moscona AA. Induction of glutamine synthetase in embryonic neural retina: Localization in Müller fibers and dependence on cell interactions. Proceedings of the National Academy of Sciences of the U.S.A. 1979;76:6476–6480. doi: 10.1073/pnas.76.12.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maple BR, Werblin FS, Wu SM. Miniature excitatory postsynaptic currents in bipolar cells of the tiger salamander retina. Vision Research. 1994;34:2357–2362. doi: 10.1016/0042-6989(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Marc RE, Lam DMK. Proceedings of the National Academy of Sciences of the U.S.A. Vol. 78. 1981. Uptake of aspartic acid and glutamic acid by photoreceptors in goldfish retina; pp. 7185–7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey SC. Cell types using glutamate as a neurotransmitter in the vertebrate retina. In: Osborne N, Chader GJ, editors. Progress in Retinal Research. New York, New York: Pergamon Press; 1991. pp. 399–425. [Google Scholar]
- Pow DV, Crook DK. Direct immunocytochemical evidence for the transfer of glutamine from glial cells to neurons: Use of specific antibodies directed against the D-stereoisomers of glutamate and glutamine. Neuroscience. 1996;70:295–302. doi: 10.1016/0306-4522(95)00363-n. [DOI] [PubMed] [Google Scholar]
- Pow DV, Robinson SR. Glutamate in some retinal neurons is derived solely from glia. Neuroscience. 1994;60:355–366. doi: 10.1016/0306-4522(94)90249-6. [DOI] [PubMed] [Google Scholar]
- Rauen T, Kanner BI. Localization of the glutamate transporter GLT-1 in rat and macaque monkey retinae. Neuroscience Letters. 1994;169:137–140. doi: 10.1016/0304-3940(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Reddy DVN. Distribution of free amino acids and related compounds in ocular fluids, lens and plasma of various mammalian species. Investigative Ophthalmology. 1967;6:478–483. [PubMed] [Google Scholar]
- Reif-Lehrer L, Coglin J. Conversion of glutamic acid to glutamine by retinal glutamine synthetase. Experimental Eye Research. 1973;17:321–328. doi: 10.1016/0014-4835(73)90241-8. [DOI] [PubMed] [Google Scholar]
- Riepe RE, Norenberg MD. Müller cell localization of glutamine synthetase in rat retina. Nature. 1977;268:654–655. doi: 10.1038/268654a0. [DOI] [PubMed] [Google Scholar]
- Riepe RE, Norenberg MD. Glutamine synthetase in the developing rat retina: An immunohistochemical study. Experimental Eye Research. 1978;27:435–444. doi: 10.1016/0014-4835(78)90022-2. [DOI] [PubMed] [Google Scholar]
- Robson JG, Frishman LJ. Response linearity and kinetics of the cat retina: The bipolar cell component of the dark-adapted electroretinogram. Visual Neuroscience. 1995;12:837–850. doi: 10.1017/s0952523800009408. [DOI] [PubMed] [Google Scholar]
- Rowe WB, Meister A. Identification of L-methionine-S-sulfoximine as the convulsant isomer of methionine sulfoximine. Proceedings of the National Academy of Sciences of the U.S.A. 1970;66:500–506. doi: 10.1073/pnas.66.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantis M, Mobbs P. The spatial relationships between Müller cell processes and the photoreceptor output synapse. Brain Research. 1992;584:299–304. doi: 10.1016/0006-8993(92)90909-s. [DOI] [PubMed] [Google Scholar]
- Shank RP, Aprison MH. Present status and significance of the glutamine cycle in neural tissues. Life Sciences. 1981;28:837–842. doi: 10.1016/0024-3205(81)90044-8. [DOI] [PubMed] [Google Scholar]
- Sontheimer H. Glial influences on neuronal signaling. The Neuroscientist. 1995;1:123–126. [Google Scholar]
- Stockton RA, Slaughter MM. B-wave of the electroretinogram: A reflection of ON bipolar cell activity. Journal of General Physiology. 1989;93:101–122. doi: 10.1085/jgp.93.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike J, Reif-Lehrer L. A sensitive assay for glutamyl transferase. Enzyme. 1971;12:235–241. doi: 10.1159/000459536. [DOI] [PubMed] [Google Scholar]
- Tian N, Slaughter MM. Correlation of dynamic responses in the ON bipolar neuron and the b-wave of the electroretinogram. Vision Research. 1995;35:1359–1364. doi: 10.1016/0042-6989(95)98715-l. [DOI] [PubMed] [Google Scholar]
- Winkler BS. The electroretinogram of the isolated rat retina. Vision Research. 1972;12:1183–1198. doi: 10.1016/0042-6989(72)90106-x. [DOI] [PubMed] [Google Scholar]
- Winkler BS. Dependence of fast components of the electroretinogram of the isolated rat retina on the ionic environment. Vision Research. 1973;13:457–463. doi: 10.1016/0042-6989(73)90123-5. [DOI] [PubMed] [Google Scholar]
- Yang JH, Wu SM. Characterization of glutamate transporter function in the tiger salamander retina. Vision Research. 1997;37:827–838. doi: 10.1016/s0042-6989(96)00231-3. [DOI] [PubMed] [Google Scholar]


