Abstract
One factor limiting the therapeutic efficacy of cord blood (CB) hematopoietic progenitor cell (HPC) transplantation is the low cell dose of the graft. This is associated with an increased incidence of delayed or failed engraftment. Cell dose can be increased and the efficacy of CB transplantation potentially improved, by ex vivo CB expansion before transplantation. Two ex vivo CB expansion techniques were compared: (1) CD133+ selection followed by ex vivo liquid culture and (2) co-culture of unmanipulated CB with bone-marrow-derived mesenchymal stem cells (MSCs). Ex vivo culture was performed in medium supplemented with granulocyte colony-stimulating factor, stemcell factor and either thrombopoietin or megakaryocyte growth and differentiation factor. Expansion was followed by measuring total nucleated cell (TNC), CD133+ and CD34+ cell, colony-forming unit and cobblestone area-forming cell output. When compared to liquid culture, CB-MSC co-culture (i) required less cell manipulation resulting in less initial HPC loss and (ii) markedly improved TNC and HPC output. CB-MSC co-culture therefore holds promise for improving engraftment kinetics in CB transplant recipients.
Keywords: cord blood, ex vivo expansion, mesenchymal stem cell, CD34+, CD133+, cobblestone area-forming cell assay
Introduction
Umbilical cord blood (CB) is becoming an important source of hematopoietic progenitor cell (HPC) support following myeloablative and non-myeloablative therapies. 1–6 A major limitation to the therapeutic efficacy of CB is the low cell dose available for transplantation, which can lead to delayed engraftment and higher rates of graft failure.1,3,5,7–9 Two approaches being investigated to increase the total number of CB cells include the transplantation of two CB units10–13 and the ex vivo expansion of CB before transplantation.
There is evidence of functional and phenotypic heterogeneity within the HPC compartment.14–16 One concern associated with ex vivo expansion is that short-term reconstituting, lower-‘quality’ HPC will be expanded at the expense of long-term reconstituting, higher-‘quality’ HPC, thereby significantly impacting on the hematopoietic reserve of the graft.17 Evidence, primarily in animal models, suggests that this may occur under certain conditions. Compromised long-term repopulating activity following ex vivo expansion has been reported in fetal sheep,18–20 non-human primate,21 feline22 and mouse models.23–25 Clinically, while the absence of durable engraftment from ex vivo-expanded CD34+ cells has once been reported,26 durable engraftment has been reported in patients who received expanded autologous peripheral blood progenitor cells as the sole source of hematopoietic support following high-dose therapy.19 This observation, together with other evidence, suggests that the primitive HPC compartment,27–32 HPC homing after transplantation33 and basic biological and genetic characteristics of HPC,34 are preserved following ex vivo expansion.
Currently, several approaches exist for ex vivo HPC expansion. The majority of static liquid culture ex vivo expansion systems first require the isolation of CD34+ or CD133+ HPC from fresh or frozen hematopoietic tissue.35–37 Positively selected CD34+ or CD133+ cells are subsequently incubated in culture medium supplemented with granulocyte colony-stimulating factor (G-CSF), stem cell factor (SCF) and thrombopoietin (TPO).38 Clinically, liquid ex vivo expansion of CD34+ CB cells was reported to increase total nucleated cells (TNC) by 56-fold and the total number of CD34+ cells by fourfold.38 A two-step, 14-day ex vivo CB expansion protocol was subsequently developed and yields >400-fold increase in TNC and >20-fold increase in CD34+ cells.39
Ex vivo liquid CB expansion removes the primitive HPC from the hematopoietic microenvironment and relies on the addition of exogenous growth factors to prevent apoptosis and stimulate proliferation, potentially driving differentiation at the expense of self-renewal. An alternative approach for ex vivo expansion is the co-culture of CB cells with components of the hematopoietic microenvironment. The hematopoietic microenvironment contains the putative stem cell ‘niche’ and is composed of hematopoietic and non-hematopoietic (cellular and extracellular) components, 40,41 thought to provide the complex molecular cues that direct HPC self-renewal and proliferation and regulate the differentiation and maturation of hematopoietic progeny.42–47 This would be consistent with the observation that ex vivo contact with stromal components of the hematopoietic microenvironment preserves hematopoietic stem cell activity.48–53 One component of the hematopoietic microenvironment, mesenchymal stem cells (MSCs), can be isolated as plastic adherent cells from a variety of fetal and adult tissues.54–57 In addition to providing an alternative (co-culture) strategy for CB expansion, allogeneic MSC co-administration has been shown to promote engraftment of human CD34+ cells in NOD/SCID mice and fetal sheep,57–61 and MSCs have been shown to have clinical immunomodulatory activity that may impact on graft-versus-host disease in transplant patients.59,62–69
CB-MSC co-culture does not require the isolation of CD34+ or CD133+ cells before expansion, minimizing manipulation and loss of HPC. McNiece et al.56 have demonstrated a 10- to 20-fold increase in TNC, a seven- to 18-fold increase in committed progenitor cells (colony-forming cells), a two- to five-fold increase in primitive progenitor cells (high proliferative potential colony-forming cells) and a 16- to 37-fold increase in CD34+ cells, using an MSC co-culture expansion technique. In our current clinical CB expansion protocols, CD133+ cells are isolated before culture.38 Despite the >50-fold expansion in TNC, the upfront loss of CD34+ and CD133+ cells owing to the positive selection procedure is significant, reducing the final cell dose available for transplant. Thus, we sought to develop a CB expansion procedure, which would maximize the expanded cell dose available for transplantation. Here, we compared the efficacy of an ex vivo liquid culture expansion protocol that required CD133+ isolation before expansion, with a CB-MSC co-culture expansion protocol that did not require CB manipulation before expansion.
Materials and methods
CB units
Pre- and post-ex vivo liquid expansion, TNC, CD133+, CD34+ and colony-forming unit (CFU) data were obtained from 11 frozen CB units processed for either validation studies or clinical use as described below. An additional three frozen CB units were cultured using the same liquid culture method to evaluate the yield of the more primitive cobblestone area (CA)-forming cells (CAFC). CB units were thawed and at least 10% of the mononuclear cell (MNC) fraction co-cultured with MSC as described below. Data from these partial CB units were each normalized to provide an estimate of the numbers of TNC, CD133+, CD34+, CFU and CAFC following ex vivo CB-MSC co-culture expansion and compared to the liquid culture results.
Ex vivo liquid expansion of CD133+-selected CB cells
After signing informed consent, eight patients were enrolled on University of Texas MD Anderson Cancer Center Protocol 02-407 and had one entire frozen CB unit expanded using a liquid culture expansion technique. Cells were infused following myeloablative therapy. An additional three CB units were also used in initial validation studies (total 11 CB units). For each CB unit, CD133+ cells were selected using the CliniMACS device and AC133 Reagent (Miltenyi Biotec Inc., Auburn, CA, USA) according to the manufacturer’s instructions. The CD133+selected cells were incubated in a 100 ml Teflon-coated culture bag (American Fluoroseal Corp., Gaithersburg, MD, USA) at 37°C in a total volume of 50 ml of ex vivo expansion medium: αMEM (HyClone, Logan, UT, USA) supplemented with 50 μg/ml gentamicin sulfate (Irvine Scientific, Santa Ana, CA, USA), 2mM L-glutamine (HyClone, Logan, UT, USA) and 10% (v/v) irradiated fetal bovine serum (FBS) (HyClone, Logan, UT, USA) and containing 100 ng/ml each of G-CSF (Amgen, Thousand Oaks, CA, USA), SCF (Amgen) and either megakaryocyte growth and differentiation factor (Kirin Brewery Co., Ltd, Tokyo, Japan) or TPO (R&D Systems, Minneapolis, MN, USA).
After 7 days of culture, the 50 ml cell suspension was added to 750 ml of ex vivo expansion medium in a 1 l Teflon-coated culture bag (American Fluoroseal Corp.) and culture continued for an additional 7 days (14 days total) at which time they were harvested and assayed for TNC, CFU, CD133+ and CD34+.
As detailed previously, specifically to investigate the proportion of CAFCwk2–6 present in pre- and post-CD133+ selection samples and post-ex vivo liquid expansion culture, part (approximately 70%) of an additional three frozen CB units were subjected to CD133+ selection using Mid-iMACS apparatus and AC133 Reagent (Miltenyi Biotec Inc., Auburn, CA, USA) and cultured in 10 ml of ex vivo expansion medium in 25 cm2 flasks. After 7 days of culture, a 2 ml aliquot of the cell suspension was added to 30 ml ex vivo expansion medium and cultured for an additional 7 days (14 days total). At day 14, cells were harvested and assayed for CAFC.
MSC preparation
MSCs were isolated from fresh bone marrow from healthy donors obtained under University of Texas MD Anderson Cancer Center Institutional Review Board (IRB) Protocol Lab02-630. Briefly, bone marrow MNCs were collected from the interface generated following Histopaque (Sigma Chemical Co., St Louis, MO, USA) density separation and washed once in MSC culture medium: αMEM supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin sulfate, 2mM L-glutamine and 20% (v/v) FBS. The MNC from a 10ml bone marrow aspirate were cultured in a 75 cm2 culture flask at a concentration of 1–5 × 106 MNC/ml at 37°C. After 2–3 days, non-adherent (hematopoietic) cells were removed and adherent cell culture continued in MSC culture medium until cells achieved approximately 70% confluence. Adherent cells were released by Trypsin digestion (1 × Trypsin–EDTA; Gibco Invitrogen Corp., Grand Island, NY, USA) and passaged into new 75 cm2 culture flasks. The MSCs were passaged at least three times before freezing to ensure removal of residual hematopoiesis and maintained as confluent monolayers in 175 cm2 tissue culture flasks.
Ex vivo expansion of non-selected CB cells with MSC co-culture
CB units were diluted in ex vivo expansion medium containing 20% (v/v) FBS (HyClone) and 100 ng/ml each of G-CSF (Amgen), SCF (R&D Systems, Minneapolis, MN, USA) and TPO (R&D Systems), and plated over the pre-established confluent MSC monolayers in 175 cm2 culture flasks at 10% of a CB unit per flask. Co-culture was performed for 7 days at 37°C. After 7 days, the 50 ml of culture medium containing non-adherent cells were removed and replaced with 50 ml of fresh expansion medium and culture continued for an additional 7 days (14 days total). Two milliliter aliquots of the non-adherent cells were cultured with 30 ml of fresh expansion medium in 75 cm2 flasks and culture continued for an additional 7 days (14 days total), as previously described for ex vivo liquid expansion. At day 14, non-adherent cells from both cultures were harvested and assayed to yield TNC, CFU, CD133+, CD34+ and CAFC data. Also, at day 14 adherent cells from the CB-MSC co-culture flasks were removed by trypsinization and assayed for the presence of CD133+ and CD34+ cells by flow cytometry to provide a measure of hematopoietic progenitors remaining associated with the MSC monolayer.
Flow cytometric analysis
Flow cytometric analyses were performed to (1) determine the proportion of CD133+ and CD34+ cells in pre- and post-CD133+-selected positive samples of CB and following the ex vivo expansion processes, and (2) confirm the phenotype of bone marrow-derived serially passaged and frozen and thawed MSC.
Pre- and post-CD133+-selected positive fractions of CB cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD45 (BD Biosciences Pharmingen, San Jose, CA, USA), phycoerythrin (PE)-conjugated anti- CD133/2 (AC141, Miltenyi Biotec Inc., Auburn, CA, USA) and allophycocyanin-conjugated anti-CD34 (8G12) (BD Biosciences Pharmingen, San Jose, CA, USA) according to the manufacturer’s instructions. For ex vivo-expanded hematopoietic cells, samples were stained with anti-CD45-FITC- and PE-conjugated anti-CD133/2, or PE-conjugated anti-CD34 (8G12) (BD Biosciences Pharmingen, San Jose, CA, USA) owing to FL3 and FL4 autofluorescence. The proportion of CD133+ and CD34+ cells was expressed both as a percentage of TNC (used subsequently to calculate CD133+ and CD34+ numbers) and as a percentage of a ‘lymphocyte gate’, which specifically contained the stem cell activity.
MSCs were assayed for CD31, CD34, CD45, CD73, CD80, CD90, CD105, CD166, HLA-ABC (I) and HLA-DR (II) expression according to the manufacturer’s instructions. With the exception of anti-CD105-FITC (Serotec, Kidlington, Oxford, UK), all antibodies were purchased from BD Biosciences (San Jose, CA, USA).
CFU assay
For each sample assayed, duplicate CFU assays were performed. Briefly, CFU assays were performed in semisolid methylcellulose culture medium (MethoCult, Stem- Cell Technologies, Vancouver, BC, Canada) supplemented with 50 ng/ml SCF, 20 ng/ml granulocyte–macrophage colony-stimulating factor (GM-CSF), 20 ng/ml interleukin (IL)-3, 20 ng/ml IL-6, 20 ng/ml G-CSF, 3 U/ml EPO and containing 30% (v/v) FBS (MethoCult GF+ H4435, Stem-Cell Technologies), as per the manufacturer’s instructions. CFU assays were cultured for 21 days in a fully humidified, 37°C, 5% CO2 in air atmosphere. Colonies were defined as clusters consisting of ≥40 cells and were enumerated using a dissecting microscope and dark field illumination. CFU frequencies and total CFU numbers per CB unit were determined.
CAFC assay
The frequency of CAFC in samples was determined by limiting dilution analysis (LDA), as described originally. 70,71 Cultures were plated and refed weekly with CAFC medium: Iscove’s modified Dulbecco’s medium (Gibco Invitrogen Corp., Grand Island, NY, USA) supplemented with 10% (v/v) each of FBS (HyClone, Logan, UT, USA) and horse serum (HS, Gibco Invitrogen Corp., Grand Island, NY, USA), 100 U/ml penicillin and 100 μg/ml streptomycin sulfate (Gibco Invitrogen Corp., Grand Island, NY, USA), 2mM L-glutamine (HyClone, Logan, UT, USA), 10−5 M hydrocortisone-21-hemisuccinate (Sigma Chemical Co., St Louis, MO, USA), and containing 10 ng/ml IL-3 (R&D Systems, Minneapolis, MN, USA) and 20 ng/ml G-CSF (Neupogen Filgrastim, Amgen). CAFC microwell cultures were scored ‘positive’ or ‘negative’ at weekly intervals for the presence of CAs using an inverted microscope. Assay of CA present in culture at 2 weeks of culture (CAFCwk2) is considered to provide a measure of relatively mature HPC, whereas assay of CA persisting in culture for 6 weeks (CAFCwk6) is considered to provide a measure of relatively primitive HPC.72,73 The proportion of ‘negative’ wells at each cell concentration was used in a Poisson-based LDA calculation to determine CAFC frequency and the numbers of CAFC (CAFCwk2 and CAFCwk6) per CB unit were calculated.
Statistical analysis
Means were compared using the Student’s two-sample t-test (Microsoft Excel, Microsoft Corp.) and significance was assumed at P ≤ 0.05.
Results
CB units
The cellular composition of the thawed CB units (n = 11) is shown in Table 1. After thawing and washing, CB units yielded >109 TNC and contained approximately 5 × 106 CD133+ cells, 14 × 106 CD34+ cells, 1 × 106 CFU, 1.5 × 106 CAFCwk2 and 9.9 × 106 CAFCwk6. Following CD133+ selection, >63% of CD133+ cells were recovered and the percentage of TNC that were CD133+ was increased from 0.3 ± 0.1 to 23.5 ± 3.6%. When a ‘lymphocyte gate’ specifically containing the stem cell activity was applied to the TNC population, the CD133+ selection process increased the proportion of CD133+ cells from 0.5 ± 0.1 to 75.7 ± 5.2%.
Table 1.
Comparison of the TNC and HPC expansion following ex vivo CB-MSC co-culture or ex vivo liquid culture techniques
| TNC or HPC | CB unit ( × 106) | Post-selection ( × 106) | Post-expansion ( × 106) | Fold expansion | |
|---|---|---|---|---|---|
| TNC | 1770±450 | Liquid
Co-culture |
17.5±8.1
N/A |
810±160a
10980±2510b,c |
46*
6 |
| CD133+ | 4.9±1.8 | Liquid
Co-culture |
3.1±1.1
N/A |
19.4±10.5
151.8±34.9b,c |
6*
31 |
| CD34+ | 14.0±6.6 | Liquid
Co-culture |
3.3±0.6
N/A |
7.6±3.6
112.0±32.6b,c |
2*
8 |
| CFU | 1.0±0.2 | Liquid
Co-culture |
0.4±0.1
N/A |
7.9±2.7a
201.5±42.9b,c |
20*
202 |
| CAFCwk2 | 1.5±0.7 | Liquid
Co-culture |
0.01±0.01
N/A |
0.3±0.2
75.1±22.1b,c |
30*
50 |
| CAFCwk6 | 9.9±5.2 | Liquid
Co-culture |
0.04±0.02
N/A |
0.01±0.01
0.5±0.2b,c |
0.3*
0.05 |
N/A = not applicable, no selection was performed for co-culture.
Pre- and post-CD133+ selection data and fold expansion data are provided (*fold expansion for liquid culture calculated as post-expansion divided by postselection). Data as mean ± s.e.m. (liquid culture, n = 11 and CB-MSC co-culture, n = 6). Significant difference (P ≤ 0.05):
Post-selection vs post-expansion.
Cord unit vs post-expansion.
Post-expansion: liquid vs co-culture.
The CD133+ selection process also recovered approximately 20% of CD34+ cells and increased the proportion of the TNC that were CD34+ from 0.3 ± 0.1% pre-selection to 24.9 ± 4.1% post-selection. When a ‘lymphocyte gate’ was applied to the TNC population, the proportion of CD34+ cells was increased from 0.6 ± 0.1% pre-selection to 77.6 ± 4.5% post-selection. Approximately 40% of CFU and <1% of CAFC activity was recovered from the CB units following the CD133+ selection process, further demonstrating the marked loss of HPC that is associated with the selection process. These data underscore the need to develop CB expansion strategies that do not require positive selection before culture.
Expansion of CD133+-selected CB cells
Fourteen days of ex vivo liquid culture of the CD133+-selected CB cells generated >800 × 106 TNC, a significant (>40-fold, P = 0.00002) increase from the <20 × 106 TNC input into the culture system. (Table 1) The number of CFU generated during the 14-day ex vivo liquid culture was significantly increased (20-fold, P = 0.013) to >7 × 106. This was significantly greater (eightfold, P = 0.025) than the numbers of CFU present in the thawed, washed CB units (approximately 1 × 106).
Expansion of non-selected CB cells byMSC co-culture MSC products
Phenotypically, MSC expressed CD73, CD90, CD105, CD166 and HLA-ABC (I) and did not express CD31, CD34, CD45, CD80 and HLA-DR (II). This phenotype was conserved through repeated cell passage and following freezing and thawing (data not shown).
CB-MSC co-culture
Interactions between MSC and primitive hematopoietic progenitors contained in the thawed, washed CB sample were observed by the presence of CAs (areas of phase-distinct cells beneath the MSC monolayer) and foci of hematopoiesis on the surface of the MSC (Figure 1). A significant (sixfold, P = 0.002) increase over the initially input TNC was observed after 14 days of CB-MSC co-culture (Table 1). This expansion data is consistent with that obtained from a limited number (n = 2) of clinical-scale CB-MSC co-culture experiments (unpublished data), where approximately four- and ninefold increases in TNC were observed. Thus, approximately 1010 TNC could be generated from thawed, washed CB units initially containing 1–2 × 109 TNC. Flow cytometry also revealed a significant (31-fold, P = 0.003) increase in CD133+ cell from an initial 5 × 106 in thawed, washed CB units to approximately 150 × 106 after 14 days of CB-MSC co-culture. Similarly, flow cytometry revealed a significant (eightfold, P = 0.003) increase in CD34+ cells from an initial 14 × 106 in thawed, washed CB units to approximately 112 × 106 after 14 days of CB-MSC co-culture. Further, CFU assay revealed a significant (>200-fold, P = 0.001) increase in CFU from approximately 1 × 106 in thawed, washed CB units to >200 × 106 after 14 days of CB-MSC co-culture. CAFC assay revealed a significant (>50-fold, P = 0.007) increase in CAFCwk2 from an initial 1.5 × 106 to approximately 75 × 106 after 14 days of CB-MSC co-culture, although a significant (95%) reduction (P = 0.001) in CAFCwk6 from an initial 10 × 106 to approximately 0.5 × 106 was observed. These data suggest that during the 14-day CB-MSC co-culture, the significant increase in TNC number and the numbers of CD133+, CD34+, CFU and CAFCwk2 was, at least in part, at the expense of the CAFCwk6.
Figure 1.
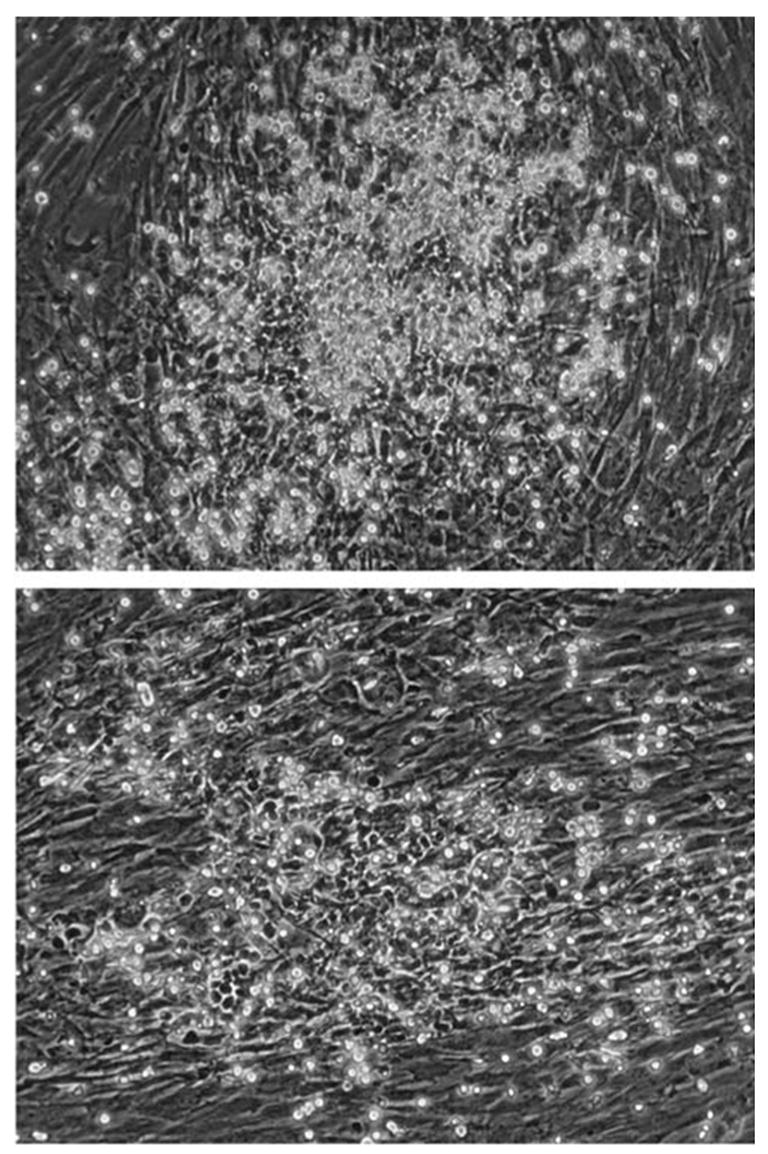
Evidence of cellular interaction between CB and MSC. Coculture of unmanipulated CB cells with MSC revealed foci of hematopoiesis and CAs. This provides evidence for the active participation of MSC in the culture and demonstrates that MSCs are not simply an inert ‘feeder’ layer. A confluent monolayer of MSC fills the field. Phase bright hematopoietic cells are floating or attached to the MSC. Phase dark cells are hematopoietic cells beneath the MSC layer (CA) (× 100 magnification).
Adherent cells
Trypsinization of the adherent layer remaining after 14 days of CB-MSC co-culture and flow cytometric analysis revealed that an estimated 130.5 ± 30.7 × 106 hematopoietic cells (approximately 1% of the total TNC output from the CB-MSC co-culture) remained associated with the adherent MSC, of which an estimated 4.0 ± 0.8 × 106 (approximately 3%) were CD133+ cells and 8.6 ± 0.8 × 106 (approximately 7%) were CD34+ cells.
Discussion
Preliminary clinical trials using CD34+- or CD133+-selected CB cells expanded in a liquid culture system have resulted in time to neutrophil engraftment of 25–35 days.38 In recipients of unmanipulated CB products, there is growing clinical evidence suggesting that patients who receive higher doses of TNC3 and CD34+ cells3,74 experience faster engraftment than do patients who receive lower TNC and CD34+ CB doses. This stimulated our interest in strategies that might improve the expansion capability of CB above that generated with liquid cultures, with the goal of achieving neutrophil engraftment in 15 days, or less, as typically occurs in unrelated bone marrow or peripheral blood HPC transplant recipients. It can be postulated that MSC co-culture with CB cells without the need for an initial positive selection step (and the associated loss of HPC) might produce a CB graft with better engraftment potential. One report where fresh CB cells were co-cultured with MSC56 further stimulated our interest in this strategy.
Data presented here demonstrate that the ex vivo coculture of non-selected CB cells with MSC generated superior TNC and HPC expansion than did the ex vivo liquid culture of CD133+-selected CB cells. The improved TNC and HPC expansion observed during the CB-MSC co-culture would be consistent with the observation that ex vivo contact with stromal components of the hematopoietic microenvironment preserves hematopoietic stem cell activity.48–53 MSC co-culture with CB units (containing approximately 109 TNC) resulted in estimated CB TNC numbers that were >10-fold higher (approximately 1010 TNC, P = 0.0005) than liquid ex vivo expansion of CD133+-selected CB cells (<109 TNC) (Figure 2). CB-MSC co-culture also generated a significantly greater output of CFU (>25-fold, P = 0.0002), CD133+ cells (>7-fold, P = 0.01), CD34+ cells (>14-fold, P = 0.003), CAFCwk2 (>200-fold, P = 0.0002) and CAFCwk6 (>44-fold, P = 0.009) than did ex vivo liquid culture of CD133+-selected CB. These data demonstrate that the expansion of TNC and HPC appears superior using CB-MSC co-culture (without CD133+ selection) when compared to the use of ex vivo liquid culture of CD133+-selected CB cells and the considerable initial loss of HPC associated with positive selection processes.
Figure 2.
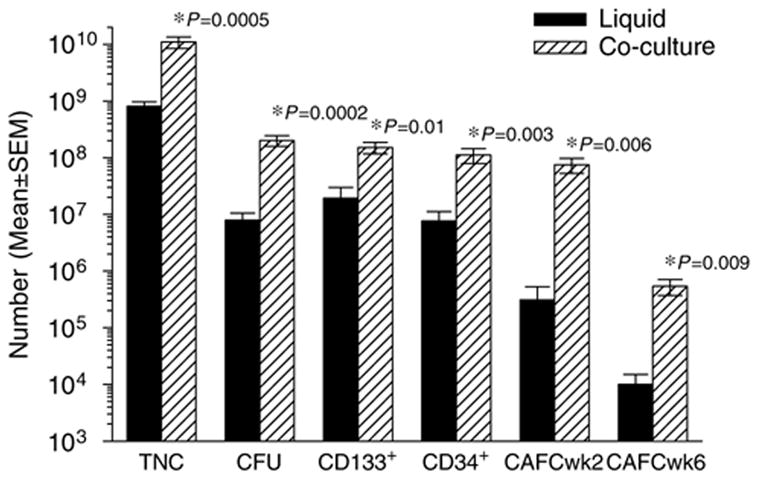
Comparison of TNC and HPC output after 14-day ex vivo liquid culture of CD133+-selected CB cells (solid bar) and 14-day ex vivo CB-MSC co-culture (shaded bar). TNC and HPC (CFU, CD133+, CD34+ and CAFCs assayed at week (wk) 2 and wk 6 of culture). Data are shown as mean ± s.e.m. (ex vivo liquid culture, n = 11 and ex vivo CB-MSC co-culture, n = 6). Ex vivo CB-MSC co-culture generated TNC numbers that were >13-fold (P = 0.0005), CFU numbers that were >25-fold (P = 0.0002), CD133+ cell numbers that were >7-fold (P = 0.01), CD34+ cell numbers that were >14-fold (P = 0.003), CAFCwk2 numbers that were >200-fold (P = 0.006) and CAFCwk6 numbers that were >44-fold (P = 0.009) those obtained following ex vivo liquid culture. These data demonstrate the superior TNC and HPC expansion obtained following ex vivo CB-MSC co-culture compared with ex vivo liquid culture of CD133+-selected CB cells.
Several studies report that adult recipients of CB experience faster engraftment, less engraftment failure and, in selected reports, better survival, if they receive >107 TNC/kg (and >105 CD34+/kg).75 The typical CB unit in current CB bank inventories contains approximately 109 TNC; thus, the majority of patients who weigh >75 kg will rarely have access to CB units with cell doses sufficient to provide >1 × 107 TNC/kg. However, our data suggest that MSC co-culture of a CB unit will yield approximately 1010 TNC (and approximately 108 CD34+ cells). This yield would be sufficient to provide a transplant dose of approximately 108 TNC/kg (and 106 CD34+/kg) for a >75 kg recipient. This procedure would therefore potentially permit the transplantation of patients >75 kg who are currently precluded from receiving CB units because single CB units with acceptable cell doses are not available. Further, the potentially elevated TNC and CD34+ cell doses achieved following CB-MSC co-culture may also reduce engraftment time.38 The possibility of using multiple cords, one of which is transplanted unmanipulated, the other of which is co-cultured with MSC before transplant, may yield a combined graft that contains both HPC responsible for long-term repopulation (largely derived from the unmanipulated CB unit) and HPC responsible for short-term repopulation (derived from the ex vivo CB-MSC co-cultured CB unit). Such a combination of donor material may potentially provide the recipient with more rapid (short-term) and durable (long-term) engraftment.
In neither ex vivo culture system (liquid or co-culture) was there expansion of more primitive CAFCwk6. In both cases, the expansion of the more mature hematopoietic HPC appeared to be, at least in part, at the expense of the more primitive CAFCwk6 population. However, during 14-day ex vivo CB-MSC co-culture, a greater proportion (5%) of the initial CB CAFCwk6 population was maintained than was observed during 14-day ex vivo liquid culture (0.1%). The CAFCwk6 population may represent higher-quality HPC within the expanded CB sample and may be the cells most responsible for long-term, sustained hematopoiesis following transplantation. The fact that their numbers were not completely exhausted during the expansion process would be consistent with previous reports.27–29 A clinical trial to assess the safety and efficacy of CB expansion using an MSC co-culture strategy will be conducted.
Acknowledgments
We gratefully acknowledge the helpful advice of Michael Thomas, Nirmali Ponweera and Dr Sean O’Connor, Department of Blood and Marrow Transplantation, University of Texas MD Anderson Cancer Center and Dr WE Fibbe, Laboratory of Experimental Hematology, Department of Hematology, Leiden University Medical Center, Leiden, The Netherlands, in the isolation and propagation of MSC. This research was supported by NCI 5R01CA061508-13 (EJS).
References
- 1.Gluckman E, Rocha V, Chevret S. Results of unrelated umbilical cord blood hematopoietic stem cell transplantation. Rev Clin Exp Hematol. 2001;5:87–99. doi: 10.1046/j.1468-0734.2001.00034.x. [DOI] [PubMed] [Google Scholar]
- 2.Broxmeyer HE, Hangoc G, Cooper S, Ribeiro RC, Graves V, Yoder M, et al. Growth characteristics and expansion of human umbilical cord blood and estimation of its potential for transplantation in adults. Proc Natl Acad Sci USA. 1992;89:4109–4113. doi: 10.1073/pnas.89.9.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gluckman E, Rocha V, Arcese W, Michel G, Sanz G, Chan KW, et al. Factors associated with outcomes of unrelated cord blood transplant: guidelines for donor choice. Exp Hematol. 2004;32:397–407. doi: 10.1016/j.exphem.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Kurtzberg J, Laughlin M, Graham ML, Smith C, Olson JF, Halperin EC, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 6.Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA. 1989;86:3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laughlin MJ, Barker J, Bambach B, Koc ON, Rizzieri DA, Wagner JE, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344:1815–1822. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 8.Migliaccio AR, Adamson JW, Stevens CE, Dobrila NL, Carrier CM, Rubinstein P. Cell dose and speed of engraftment in placental/umbilical cord blood transplantation: graft progenitor cell content is a better predictor than nucleated cell quantity. Blood. 2000;96:2717–2722. [PubMed] [Google Scholar]
- 9.Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 10.de Lima M, St John LS, Wieder ED, Lee MS, McMannis J, Karandish S, et al. Double-chimaerism after transplantation of two human leucocyte antigen mismatched, unrelated cord blood units. Br J Haematol. 2002;119:773–776. doi: 10.1046/j.1365-2141.2002.03893.x. [DOI] [PubMed] [Google Scholar]
- 11.Barker JN, Weisdorf DJ, Wagner JE. Creation of a double chimera after the transplantation of umbilical-cord blood from two partially matched unrelated donors. N Engl J Med. 2001;344:1870–1871. doi: 10.1056/NEJM200106143442417. [DOI] [PubMed] [Google Scholar]
- 12.Barker JN, Weisdorf DJ, Defor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102:1915–1919. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 13.Barker JN, Weisdorf DJ, Defor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 14.Guenechea G, Gan OI, Dorrell C, Dick JE. Distinct classes of human stem cells that differ in proliferative and self-renewal potential. Nat Immunol. 2001;2:75–82. doi: 10.1038/83199. [DOI] [PubMed] [Google Scholar]
- 15.Lemischka IR, Jordan CT. The return of clonal marking sheds new light on human hematopoietic stem cells. Nat Immunol. 2001;2:11–12. doi: 10.1038/83115. [DOI] [PubMed] [Google Scholar]
- 16.Hogan CJ, Shpall EJ, Keller G. Differential long-term and multilineage engraftment potential from subfractions of human CD34+ cord blood cells transplanted into NOD/SCID mice. Proc Natl Acad Sci USA. 2002;99:413–418. doi: 10.1073/pnas.012336799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams DA. Ex vivo expansion of hematopoietic stem and progenitor cells – robbing Peter to pay Paul? Blood. 1993;81:3169–3172. [PubMed] [Google Scholar]
- 18.McNiece IK, Almeida-Porada G, Shpall EJ, Zanjani E. Ex vivo expanded cord blood cells provide rapid engraftment in fetal sheep but lack long-term engrafting potential. Exp Hematol. 2002;30:612–616. doi: 10.1016/s0301-472x(02)00805-6. [DOI] [PubMed] [Google Scholar]
- 19.McNiece I, Jones R, Bearman SI, Cagnoni P, Nieto Y, Franklin W, et al. Ex vivo expanded peripheral blood progenitor cells provide rapid neutrophil recovery after high-dose chemotherapy in patients with breast cancer. Blood. 2000;96:3001–3007. [PubMed] [Google Scholar]
- 20.Shimizu Y, Ogawa M, Kobayashi M, Almeida-Porada G, Zanjani ED. Engraftment of cultured human hematopoietic cells in sheep. Blood. 1998;91:3688–3692. [PubMed] [Google Scholar]
- 21.Tisdale JF, Hanazono Y, Sellers SE, Agricola BA, Metzger ME, Donahue RE, et al. Ex vivo expansion of genetically marked rhesus peripheral blood progenitor cells results in diminished long-term repopulating ability. Blood. 1998;92:1131–1141. [PubMed] [Google Scholar]
- 22.Abkowitz JL, Taboada MR, Sabo KM, Shelton GH. The ex vivo expansion of feline marrow cells leads to increased numbers of BFU-E and CFU-GM but a loss of reconstituting ability. Stem Cells. 1998;16:288–293. doi: 10.1002/stem.160288. [DOI] [PubMed] [Google Scholar]
- 23.Von Drygalski A, Alespeiti G, Ren L, Adamson JW. Murine bone marrow cells cultured ex vivo in the presence of multiple cytokine combinations lose radioprotective and long-term engraftment potential. Stem Cells Dev. 2004;13:101–111. doi: 10.1089/154732804773099308. [DOI] [PubMed] [Google Scholar]
- 24.Peters SO, Kittler EL, Ramshaw HS, Quesenberry PJ. Ex vivo expansion of murine marrow cells with interleukin-3 (IL-3), IL-6, IL-11, and stem cell factor leads to impaired engraftment in irradiated hosts. Blood. 1996;87:30–37. [PubMed] [Google Scholar]
- 25.Peters SO, Kittler EL, Ramshaw HS, Quesenberry PJ. Murine marrow cells expanded in culture with IL-3, IL-6, IL-11, and SCF acquire an engraftment defect in normal hosts. Exp Hematol. 1995;23:461–469. [PubMed] [Google Scholar]
- 26.Holyoake TL, Alcorn MJ, Richmond L, Farrell E, Pearson C, Green R, et al. CD34 positive PBPC expanded ex vivo may not provide durable engraftment following myeloablative chemoradiotherapy regimens. Bone Marrow Transplant. 1997;19:1095–1101. doi: 10.1038/sj.bmt.1700799. [DOI] [PubMed] [Google Scholar]
- 27.Piacibello W, Sanavio F, Severino A, Dane A, Gammaitoni L, Fagioli F, et al. Engraftment in nonobese diabetic severe combined immunodeficient mice of human CD34(+) cord blood cells after ex vivo expansion: evidence for the amplification and self-renewal of repopulating stem cells. Blood. 1999;93:3736–3749. [PubMed] [Google Scholar]
- 28.Lewis ID, Almeida-Porada G, Du J, Lemischka IR, Moore KA, Zanjani ED, et al. Umbilical cord blood cells capable of engrafting in primary, secondary, and tertiary xenogeneic hosts are preserved after ex vivo culture in a noncontact system. Blood. 2001;97:3441–3449. doi: 10.1182/blood.v97.11.3441. [DOI] [PubMed] [Google Scholar]
- 29.Guenechea G, Segovia JC, Albella B, Lamana M, Ramirez M, Regidor C, et al. Delayed engraftment of nonobese diabetic/severe combined immunodeficient mice transplanted with ex vivo-expanded human CD34(+) cord blood cells. Blood. 1999;93:1097–1105. [PubMed] [Google Scholar]
- 30.Traycoff CM, Cornetta K, Yoder MC, Davidson A, Srour EF. Ex vivo expansion of murine hematopoietic progenitor cells generates classes of expanded cells possessing different levels of bone marrow repopulating potential. Exp Hematol. 1996;24:299–306. [PubMed] [Google Scholar]
- 31.Muench MO, Moore MA. Accelerated recovery of peripheral blood cell counts in mice transplanted with in vitro cytokine-expanded hematopoietic progenitors. Exp Hematol. 1992;20:611–618. [PubMed] [Google Scholar]
- 32.Holyoake TL, Freshney MG, McNair L, Parker AN, McKay PJ, Steward WP, et al. Ex vivo expansion with stem cell factor and interleukin-11 augments both short-term recovery post-transplant and the ability to serially transplant marrow. Blood. 1996;87:4589–4595. [PubMed] [Google Scholar]
- 33.Zhai QL, Qiu LG, Li Q, Meng HX, Han JL, Herzig RH, et al. Short-term ex vivo expansion sustains the homing-related properties of umbilical cord blood hematopoietic stem and progenitor cells. Haematologica. 2004;89:265–273. [PubMed] [Google Scholar]
- 34.Tian H, Huang S, Gong F, Tian L, Chen Z. Karyotyping, immunophenotyping, and apoptosis analyses on human hematopoietic precursor cells derived from umbilical cord blood following long-term ex vivo expansion. Cancer Genet Cytogenet. 2005;157:33–36. doi: 10.1016/j.cancergencyto.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Purdy MH, Hogan CJ, Hami L, McNiece I, Franklin W, Jones RB, et al. Large volume ex vivo expansion of CD34-positive hematopoietic progenitor cells for transplantation. J Hematother. 1995;4:515–525. doi: 10.1089/scd.1.1995.4.515. [DOI] [PubMed] [Google Scholar]
- 36.Briddell RA, Kern BP, Zilm KL, Stoney GB, McNiece IK. Purification of CD34+ cells is essential for optimal ex vivo expansion of umbilical cord blood cells. J Hematother. 1997;6:145–150. doi: 10.1089/scd.1.1997.6.145. [DOI] [PubMed] [Google Scholar]
- 37.McNiece IK, Stoney GB, Kern BP, Briddell RA. CD34+ cell selection from frozen cord blood products using the Isolex 300i and CliniMACS CD34 selection devices. J Hematother. 1998;7:457–461. doi: 10.1089/scd.1.1998.7.457. [DOI] [PubMed] [Google Scholar]
- 38.Shpall EJ, Quinones R, Giller R, Zeng C, Baron AE, Jones RB, et al. Transplantation of ex vivo expanded cord blood. Biol Blood Marrow Transplant. 2002;8:368–376. doi: 10.1053/bbmt.2002.v8.pm12171483. [DOI] [PubMed] [Google Scholar]
- 39.McNiece I, Kubegov D, Kerzic P, Shpall EJ, Gross S. Increased expansion and differentiation of cord blood products using a two-step expansion culture. Exp Hematol. 2000;28:1181–1186. doi: 10.1016/s0301-472x(00)00520-8. [DOI] [PubMed] [Google Scholar]
- 40.Lemischka IR, Moore KA. Stem cells: interactive niches. Nature. 2003;425:778–779. doi: 10.1038/425778a. [DOI] [PubMed] [Google Scholar]
- 41.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 42.Hackney JA, Charbord P, Brunk BP, Stoeckert CJ, Lemischka IR, Moore KA. A molecular profile of a hematopoietic stem cell niche. Proc Natl Acad Sci USA. 2002;99:13061–13066. doi: 10.1073/pnas.192124499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Etheridge SL, Spencer GJ, Heath DJ, Genever PG. Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells. Stem Cells. 2004;22:849–860. doi: 10.1634/stemcells.22-5-849. [DOI] [PubMed] [Google Scholar]
- 44.Kadereit S, Deeds LS, Haynesworth SE, Koc ON, Kozik MM, Szekely E, et al. Expansion of LTC-ICs and maintenance of p21 and BCL-2 expression in cord blood CD34(+)/CD38(−) early progenitors cultured over human MSCs as a feeder layer. Stem Cells. 2002;20:573–582. doi: 10.1634/stemcells.20-6-573. [DOI] [PubMed] [Google Scholar]
- 45.Rattis FM, Voermans C, Reya T. Wnt signaling in the stem cell niche. Curr Opin Hematol. 2004;11:88–94. doi: 10.1097/01.moh.0000133649.61121.ec. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 47.Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 48.Yildirim S, Boehmler AM, Kanz L, Mohle R. Expansion of cord blood CD34+ hematopoietic progenitor cells in coculture with autologous umbilical vein endothelial cells (HUVEC) is superior to cytokine-supplemented liquid culture. Bone Marrow Transplant. 2005;36:71–79. doi: 10.1038/sj.bmt.1705001. [DOI] [PubMed] [Google Scholar]
- 49.Breems DA, Blokland EA, Siebel KE, Mayen AE, Engels LJ, Ploemacher RE. Stroma-contact prevents loss of hematopoietic stem cell quality during ex vivo expansion of CD34+ mobilized peripheral blood stem cells. Blood. 1998;91:111–117. [PubMed] [Google Scholar]
- 50.Brandt JE, Galy AH, Luens KM, Travis M, Young J, Tong J, et al. Bone marrow repopulation by human marrow stem cells after long-term expansion culture on a porcine endothelial cell line. Exp Hematol. 1998;26:950–961. [PubMed] [Google Scholar]
- 51.Rafii S, Shapiro F, Pettengell R, Ferris B, Nachman RL, Moore MA, et al. Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood. 1995;86:3353–3363. [PubMed] [Google Scholar]
- 52.Chute JP, Saini AA, Chute DJ, Wells MR, Clark WB, Harlan DM, et al. Ex vivo culture with human brain endothelial cells increases the SCID-repopulating capacity of adult human bone marrow. Blood. 2002;100:4433–4439. doi: 10.1182/blood-2002-04-1238. [DOI] [PubMed] [Google Scholar]
- 53.Davis TA, Robinson DH, Lee KP, Kessler SW. Porcine brain microvascular endothelial cells support the in vitro expansion of human primitive hematopoietic bone marrow progenitor cells with a high replating potential: requirement for cell-to-cell interactions and colony-stimulating factors. Blood. 1995;85:1751–1761. [PubMed] [Google Scholar]
- 54.Zhang Y, Li C, Jiang X, Zhang S, Wu Y, Liu B, et al. Human placenta-derived mesenchymal progenitor cells support culture expansion of long-term culture-initiating cells from cord blood CD34+ cells. Exp Hematol. 2004;32:657–664. doi: 10.1016/j.exphem.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Kanai M, Hirayama F, Yamaguchi M, Ohkawara J, Sato N, Fukazawa K, et al. Stromal cell-dependent ex vivo expansion of human cord blood progenitors and augmentation of transplantable stem cell activity. Bone Marrow Transplant. 2000;26:837–844. doi: 10.1038/sj.bmt.1702634. [DOI] [PubMed] [Google Scholar]
- 56.McNiece I, Harrington J, Turney J, Kellner J, Shpall EJ. Ex vivo expansion of cord blood mononuclear cells on mesenchymal stem cells. Cytotherapy. 2004;6:311–317. doi: 10.1080/14653240410004871. [DOI] [PubMed] [Google Scholar]
- 57.in’t Anker PS, Noort WA, Kruisselbrink AB, Scherjon SA, Beekhuizen W, Willemze R, et al. Nonexpanded primary lung and bone marrow-derived mesenchymal cells promote the engraftment of umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2003;31:881–889. doi: 10.1016/s0301-472x(03)00202-9. [DOI] [PubMed] [Google Scholar]
- 58.Noort WA, Kruisselbrink AB, in’t Anker PS, Kruger M, van Bezooijen RL, de Paus RA, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2002;30:870–878. doi: 10.1016/s0301-472x(02)00820-2. [DOI] [PubMed] [Google Scholar]
- 59.Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE, et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 60.Almeida-Porada G, Porada CD, Tran N, Zanjani ED. Cotransplantation of human stromal cell progenitors into preimmune fetal sheep results in early appearance of human donor cells in circulation and boosts cell levels in bone marrow at later time points after transplantation. Blood. 2000;95:3620–3627. [PubMed] [Google Scholar]
- 61.Angelopoulou M, Novelli E, Grove JE, Rinder HM, Civin C, Cheng L, et al. Cotransplantation of human mesenchymal stem cells enhances human myelopoiesis and megakaryocytopoiesis in NOD/SCID mice. Exp Hematol. 2003;31:413–420. doi: 10.1016/s0301-472x(03)00042-0. [DOI] [PubMed] [Google Scholar]
- 62.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus- host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 63.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485–489. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- 64.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 65.Gotherstrom C, Ringden O, Westgren M, Tammik C, Le Blanc K. Immunomodulatory effects of human foetal liver-derived mesenchymal stem cells. Bone Marrow Transplant. 2003;32:265–272. doi: 10.1038/sj.bmt.1704111. [DOI] [PubMed] [Google Scholar]
- 66.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 67.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 68.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 69.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 70.Neben S, Anklesaria P, Greenberger J, Mauch P. Quantitation of murine hematopoietic stem cells in vitro by limiting dilution analysis of cobblestone area formation on a clonal stromal cell line. Exp Hematol. 1993;21:438–443. [PubMed] [Google Scholar]
- 71.Ploemacher RE, van der Sluijs JP, Voerman JS, Brons NH. An in vitro limiting-dilution assay of long-term repopulating hematopoietic stem cells in the mouse. Blood. 1989;74:2755–2763. [PubMed] [Google Scholar]
- 72.Kusadasi N, van Soest PL, Mayen AE, Koevoet JL, Ploemacher RE. Successful short-term ex vivo expansion of NOD/SCID repopulating ability and CAFC week 6 from umbilical cord blood. Leukemia. 2000;14:1944–1953. doi: 10.1038/sj.leu.2401917. [DOI] [PubMed] [Google Scholar]
- 73.Breems DA, Blokland EA, Neben S, Ploemacher RE. Frequency analysis of human primitive haematopoietic stem cell subsets using a cobblestone area forming cell assay. Leukemia. 1994;8:1095–1104. [PubMed] [Google Scholar]
- 74.Wagner JE, Barker JN, Defor TE, Baker KS, Blazar BR, Eide C, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 75.Barker JN, Wagner JE. Umbilical cord blood transplantation: current practice and future innovations. Crit Rev Oncol Hematol. 2003;48:35–43. doi: 10.1016/s1040-8428(03)00092-1. [DOI] [PubMed] [Google Scholar]


