Abstract
Humans and nonhuman animals make use of sensory hierarchies in “selecting” strategies for solving many cognitive and behavioral tasks. Often, if a preferred type of sensory information is unavailable or is not useful for solving a given task, the animal can switch to a lower-priority strategy, making use of a different class of sensory information. In the case of rats performing a classic reach-to-grasp-food task, however, prior studies indicate that the reaching maneuver may be a fixed action pattern that is guided exclusively by the food’s odor plume until the point of contact with the food morsel [1–3]. We sought to confirm and extend these findings in several ways. In Experiment 1, using a GO/NO-GO variant of the classic task, we demonstrated that rats used the GO target’s odor both to trigger and guide their reaches. In Experiment 2, we showed that rats deprived of (a) vision, (b) object-recognizing rostral whiskers and forearm sinus hairs, or (c) both, displayed no deficits in triggering and guiding their reaches. Finally, in a third experiment in which the GO target’s location varied randomly across trials and only olfactory cues were available, we demonstrated that rats could determine the spatial endpoint of their reach without any loss of accuracy. Combined with results from a prior study in which bulbectomized rats never developed a new, successful reaching strategy despite extensive post-operative training [1], these results indicate that rats do not have a sensory hierarchy for solving the reach-to-grasp-food task, but rather, are guided by olfaction alone until their paw contacts the food morsel.
Keywords: rats, food handling, olfaction, vibrissae, vision, modularity, evolution
Introduction
In 1983, the philosopher of mind Jerry Fodor published a book called The Modularity of Mind [4] that had a wide impact on researchers studying human and nonhuman animal perception, cognition and movement. One of this book’s most significant claims was that certain mental processes, termed “modules,” are “informationally encapsulated” in that they can only process highly restricted types of information. Thus, even when another cognitive or brain system detects information that could disambiguate processing within the encapsulated module, its encapsulation prevents it from making use of that information. At the time, one example of an apparently encapsulated cognitive process concerned rats’ reorientation process after becoming disoriented in their environment, as measured by their ability to find a piece of hidden food whose location they had known prior to the disorientation procedure. Cheng [5] found that the rats reoriented themselves using the macroscopic, three-dimensional shape of the environment even when symmetries in the environmental shape indicated (incorrectly) that multiple locations were correct, and failed to use disambiguating markings such as distinctive scents or visual cues that they clearly used for many non-reorientation tasks. Accordingly, Hermer and Spelke [6,7] found that young children appear to rely on a similarly encapsulated system for reorientation.
More recent reports, however, have demonstrated that very few cognitive processes are entirely modular and encapsulated. Rather, it appears that humans and nonhuman animals will solve a task in a given manner depending on the precise demands of that task. In the case of spatial reorientation, the degree to which the task is aversive [8], the task’s difficulty [9], and the exact size and shape of the environment [10–12] all play a large role in determining which cues will be used to solve the task. Similarly, despite the common notion that “rats are blind” [13], rats preferentially use visual cues for navigation while foraging away from the home site, followed by olfactory cues and then self-motion cues [14]. Furthermore, they can flexibly switch between different cue classes depending on their availability and utility in a given situation [14,15]. Results such as these demonstrate that for many tasks, animals have a cue or solution hierarchy rather than a set of encapsulated modules.
One type of task that has long resisted being associated with a sensory hierarchy, however, is rats’ reaching to grasp food pellets. For example, Metz and Whishaw found that despite the food pellet’s diameter, the animals do not change their grip size as they move to grasp the pellet [2]. Furthermore, Whishaw and Tomie found that rats with their olfactory bulbs ablated “blindly” reached for pellets located on a shelf, even though the pellets were fully visible, and even after 21 days of post-operation training [1]. Indeed, in this latter study, rats’ performance did not decline when they were blindfolded. Combined, these two studies suggest that in rats, reaching to grasp food pellets is a modular process that is guided by olfaction alone, even when visual or other disambiguating information that the rats can in fact perceive is available.
We sought to confirm and extend these findings both out of a long-standing interest in modularity [6,7,16,17], and to provide a greater behavioral understanding of a very similar task [18] that is now commonly used in neurophysiological studies [19–25] including studies in our own laboratory. For example, one of our ongoing projects is to determine how information flows through olfactory, prefrontal (decision-making) and motor circuits. For logical electrode placement and computational modeling of the physiological results, we needed to know whether senses other than olfaction were used to trigger and guide rats’ reaches before contact with the food target. In Experiment 1, we used a GO/NO-GO variant of the classic reach-to-grasp-food task [18] to determine whether, on the basis of olfactory cues alone, GO odorants would trigger and guide rats’ reaches whereas NO-GO odorants would cue rats not to lift their reaching paw. In Experiment 2, we tested whether vision, rats’ vibrissal sense (both myostacial and forearm), or both would play a role in triggering or guiding their reaches. Finally, because the prior two experiments made use of a constant pellet location across trials, in Experiment 3 we tested whether rats could use olfaction alone to determine the food pellet’s location, which randomly varied across trials. This last experiment controlled for the possibility that rats succeeded in Experiments 1 and 2 by using a single, memorized reaching trajectory rather than using the target’s odor as a guide. The results of all three experiments indicated that rats could perform at high, baseline accuracy levels exclusively by using olfactory cues.
Methods
Baseline training and tasks
All experimental methods were approved by the SUNY Health Science Center Institutional Animal Care and Use Committee. Seven adult female Long-Evans rats, food-restricted to 90% of their ad libitum weight, were screened against anosmia and high anxiety using a task in which rats had to locate, dig for, and eat a piece of sweetened cereal that had been buried under 2″ of woodchips in a 3′ × 3′ arena. Rats were given 10 trials in this task and all but two of them quickly began exploring the center of the arena and finding and digging for the cereal (by the second trial, those five rats found the buried food in less than three minutes on nearly all attempts). These five were further trained and tested in all conditions of the experiments reported here (i.e. the same rats were used for all three experiments). The order of the experiments was the same for all rats as well, because two of the experiments required the rats’ whiskers to be shaved (see below), and these whiskers take considerable time to regrow to full length. The rats were food-restricted to ~90% of their ad libitum body weight before the start of the reaching experiments. All trials were videorecorded and coded at 17 ms resolution (Lafayette Instruments, Lafayette, IN), and the experiments were performed in regular lighting near the end of the light portion of the rats’ days.
Rats first learned two different baseline conditions. The “constant pellet location baseline” was essentially the classic reach-to-grasp-food task developed by Whishaw and colleagues [18]. In this task, the animals learned to reach through a narrow plastic slot to grasp a 45 mg food pellet from one of two wells – the well contralateral to the reaching paw – located 13 mm beyond the slot on a plastic shelf at roughly the height of the rat’s snout. The slot’s width was such that the rat could place its nose directly behind the pellet in the well, thereby gaining information on the pellet’s odor quality and location, but still could not retrieve it with its tongue. Rats were trained on approximately 20 trials per day until they reached an asymptotic performance level, which was defined as the rat’s percentage of successful reaches not differing from one another by more than 5% across 3 testing days. For the purposes of our study, a reach was counted as accurate or successful if the rat grasped the pellet on its first try. Rats were shaped not to double-reach when they missed the pellet on the first try. Once rats had asymptoted, they sniffed the target for 1–3 respiratory cycles before withdrawing their snout, lifting their reaching paw off the ground and maneuvering it through the slot to a position just beyond where their snout had been, allowing them to grasp the pellet and bring it to their mouth. The pellets were either banana- or chocolate-flavored and served as the reward when rats successfully retrieved them. Approximately twenty such trials (range 18–25) were run the day following the rats’ third asymptotic day. These trials plus the prior two asymptotic days’ data were used as three data points per rat in the constant-location baseline task. The trials were identical to the GO trials run in Experiment 1, in preparation for the subsequent tests of the role of odors in triggering and guiding the rats’ reaches.
The second baseline condition resembled the first, except that randomly across trials, the food pellet could be located at any of three positions 13 mm away from the animal’s nose, with a 33% probability for each. These locations were the two pellet wells contralateral or ipsilateral to the reaching paw, plus a third well located midway between and slightly behind the left and right wells, so that it too was 13 mm from the slot. The rats received ~20 training trials in this condition, and the next day, each rat was tested in this “variable pellet location baseline” task.
Experiment 1
Following training in the two baseline tasks, rats were run in a GO/NO-GO variant of the reach-to-grasp task. This task tested (a) whether rats used the GO odors to trigger their reaches, as measured by the percentage of trials on which the rats lifted their paws, and (b) whether the GO odors played a role in guiding their reaches, as measured by percentage of accurate grasps on GO trials. Rats were run in this task for three days, with approximately 20 trials/day (range 17–24). On approximately 50% of trials, a real food pellet (chocolate or banana) served as the target, whereas randomly, on the remaining 50% of trials, a visually identical plastic bead (Fireside Stitchery, Frazer, PA), coated with non-food odors on some trials and uncoated with any additional scent on others (see below), served as the target. On approximately 18% of trials, the beige, plastic-only bead was used as the target; on another 16% of trials the bead was coated in pure vanilla extract, and finally, on the remaining 16% of trials, the bead was coated in acetone. The target’s location was constant across trials in this experiment (contralateral to the rat’s reaching paw), and the control condition for this experiment was the baseline condition with the constant pellet location. Although the rats’ ability to detect the plastic odor was not tested, a sample of humans was able to detect the odor of the plastic alone at an above-chance rate.
Experiment 2
This experiment tested which sensory modalities rats used to trigger and guide their reaches, as measured by percent of reaches attempted and percent of reaches that were accurate (i.e. on which the rat grasped the food pellet). A real chocolate or banana food pellet and a constant target location contralateral to the rat’s reaching paw were used throughout this experiment, and the baseline condition with the constant target location was used as the control condition. In the first experimental condition, the rats’ rostral whiskers, which are used for detecting and recognizing objects [26], were shaved off. The caudal whiskers, which are used primarily for head-centered proprioceptive control [26], were left intact so that the rats could detect when they were positioned correctly relative to the slot. Additionally, the sinus hairs on the underside of rats’ forearms were clipped off. The animals were then run in 3 days of trials (approximately 20 trials/day) with no vibrissae that could project through the slot, as confirmed by a field-by-field video analysis.
In the second experimental condition of this experiment, rats were acclimated over a two-week period to wearing a blindfold similar to the one used with rats by Whishaw and colleagues [14,27]. Once the rats had become comfortable enough to eat pellets off the cage floor with their blindfolds on, their rostral whiskers and forearm sinus hairs were again removed and their ability to initiate reaches and guide them to the correct location, without vision or object-detecting vibrissae, was tested.
Experiment 3
In the previous two experiments, rats may have succeeded across trials because the pellet was always located in the well contralateral to the reaching paw; thus, they could have proprioceptively memorized a single reaching trajectory rather than using odor cues to determine the endpoint of their reaches. To examine this possibility, we ran a control experiment in which we varied the pellet’s position randomly across trials, with three pellet wells, each 13 mm beyond the slot (as was the single well we used before). We ran this experiment with the rats blindfolded and with their rostral and forearm whiskers shaved, so that the only sensory modality useful for locating the randomly positioned pellet was olfaction. The control condition for this experiment was the second baseline task, in which rats with normal visual, haptic and olfactory abilities performed the reaching task with three different, evenly distributed target positions. The experimental condition involved the same rats with blindfolds on and vibrissae removed. As before, animals were run for 3 days in this task, with approximately 20 trials/day.
Data analysis
We used analyses of variance (ANOVAs) to compare data from the different baseline and experimental conditions. For all three experiments, percentage of reaches triggered, and percentage of trials on which the target was successfully grasped, served as the dependent variable. For Experiment 1, the percentage of reaches triggered, and the percentage of reaches leading to a successful grasp of the pellet on the first try, were compared across the baseline task with a constant pellet location, on GO (chocolate or banana) trials, and on NO-GO (plastic-only, vanilla or acetone) trials. For each experimental condition, the percentages of reaches triggered or successful from each of the three testing days were used, and similarly, for the first baseline condition, the data from the three days of asymptotic performance were used. For the second baseline condition in which the target position varied across trials, the one day’s data of approximately 20 trials were divided into three portions (e.g. if there were 21 baseline trials, the first portion would consist of the average across the first 7 trials) to facilitate within-subjects analyses across conditions. All analyses started with rat and condition as the independent variables, with condition being a within-subjects factor. In Experiment 3, in addition to the ANOVAs, we performed single-sample t-tests to determine whether reaching accuracy in the variable-location baseline condition and the variable-location, no whiskers/no vision condition was above the chance level of 33.3%. Also, as determined to be appropriate given an ANOVA’s results, post hoc tests were carried out using a Bonferroni correction method.
Results
Baseline task acquisition
In the first baseline task with the constant pellet location, all rats achieved asymptotic performance (with percentage of accurate grasps of the food pellet varying by 5% across three contiguous days) within 12 days (range 8–12 days). They varied considerably in asymptotic accuracy, however: the “best” rat averaged 88% correct whereas the “worst” rat averaged 58%. On average, shown in the bold dark blue line in Figure 1a, the sample asymptoted on days 8–10 with a 69% accuracy rate, an accuracy rate very similar to that reported in the original skilled reaching paper [18] published in the early 1990s by Whishaw and colleagues. Figure 1b shows the timeline of different phases of the reach across asymptotic days. After protruding their noses through the slot, not all rats took a definable first sniff (“Sniff1”), but virtually all rats took a Sniff2 and a Final Sniff before lifting the paw. From the Final Sniff to Contact with the food pellet, the movements were performed with relatively little variance in timing, as observed previously [25].
Figure 1.
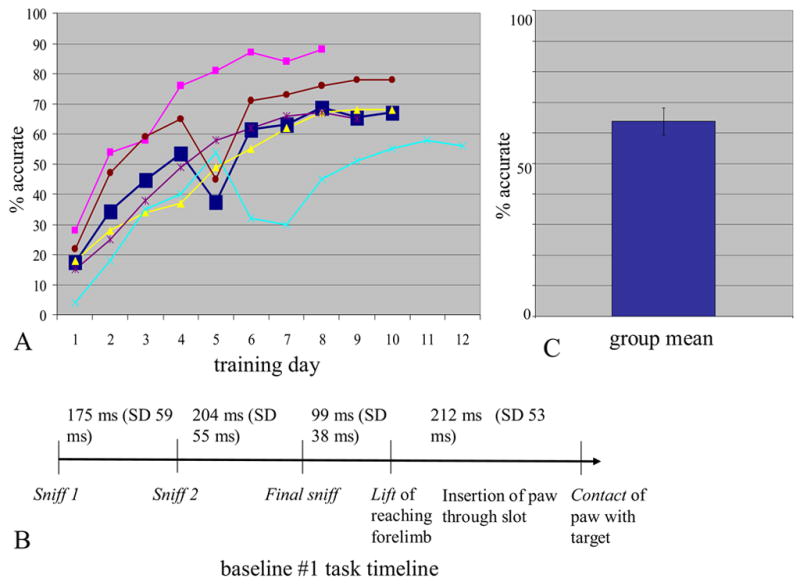
Performance in the two baseline tasks. (A) Learning curve for constant-location baseline, in which a real food pellet was used and the pellet was always located contralateral to the rat’s reaching paw. Rats (n=5) received approximately 20 trials on each training day. “Percent accurate” refers to the percent of trials on which the paw successfully grasped the food pellet. The dark blue, bold line shows the average performance of the group, while the other 5 lines show the performance of the individual rats. (B) Average timeline, derived from videocoding, for the events in each trial of the constant-location baseline task once rats had learned the task well. (C) Performance of rats in the variable-location baseline task.
Rats received less training on the second baseline task, in which the pellet’s location varied randomly across trials, but still performed at an average of 64% correct on the testing day for that task (Figure 1c). This rate was statistically equivalent to the rats’ performance in the constant-location baseline (proportion test, p=.871).
Experiment 1
We first examined the percentage of reaches triggered by the different GO and NO-GO odors in this experiment. An initial comparison across conditions (5: Baseline #1, Real targets in the GO/NO task, and plastic-only, vanilla-scented, and acetone-scented NO-GO targets) yielded a large main effect of condition (F(4,50)=1484.1, p<.000001), with rats reaching for GO targets on more than 90% of trials, and reaching for NO-GO targets on less than 10% of trials (Figure 2a). This pattern held across all rats in the sample, as there was neither a main effect of rat (F(4,50)=.903, p=.469) nor an interaction between rat and condition (F(16,50)=.660, p=.817). Rats rapidly learned to confine their reaching to banana- and chocolate-scented targets, as shown by a post hoc test of reaching on GO versus NO-GO trials (F(1,73)=5387, Bonferroni-corrected p<.00001). However, on NO-GO trials they reached more often for vanilla-scented beads than acetone-coated or uncoated ones (F(2,42)=5.67, Bonferroni-corrected p=.009).
Figure 2.
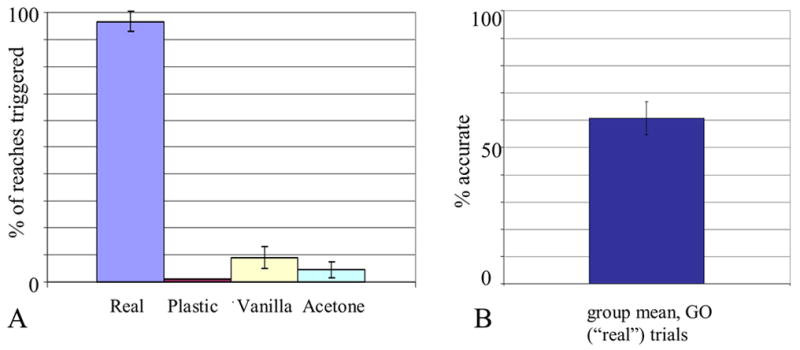
Performance of the same 5 rats in the GO/NO-GO variant of the reach-to-grasp task, in which the decision to reach on a particular trial had to be guided by olfaction alone (GO and NO-GO targets were visually and haptically identical). (A) Percent of reaches triggered by the GO odors (banana and chocolate trials are combined under the heading “Real”) versus the NO-GO odors (vanilla, acetone or plastic-only), judging from a video analysis of whether the rat lifted its paw off the ground on each trial. (B) Accuracy on GO (“Real” food pellets) trials.
We also examined whether rats’ accuracy was similar across the two GO conditions, i.e. the constant-location baseline task versus the GO trials of the GO/NO-GO task (Fig. 1a versus Fig. 2b). Their performance on these two tasks did not differ (proportion test, p=.771). In contrast, as in the Baseline #1 task, the rats differed greatly in their accuracy overall (F(4,20)=38.66, p<.000001). Some rats also showed a greater tendency than others toward lower accuracy in the Real condition of the GO/NO-GO experiment (F(4,20)=2.74, p=.057).
Experiment 2
This experiment was designed to test whether visual or tactile abilities played a role in triggering or guiding rats’ reaches. Compared to the constant-location baseline task, rats in the no-whiskers (i.e. no rostral whiskers or forearm sinus hairs) condition, and the no-whiskers and no-vision conditions reached for the food pellets equally often (Figure 3a; F(2,30)=.069, p=.933). Remarkably, there were no detectable differences across rats in the percentage of reaches triggered (F(4,30)=.384, p=.817).
Figure 3.
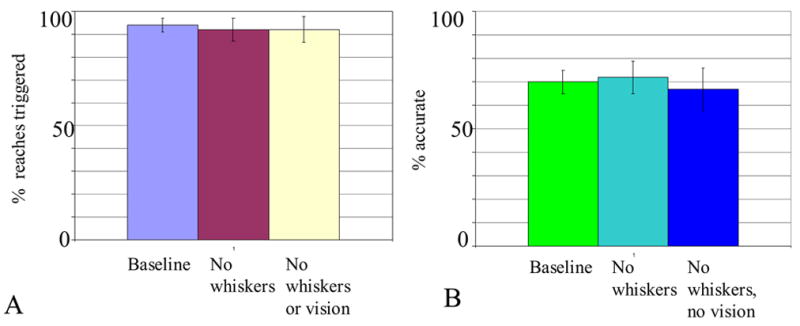
Role of olfactory versus somatosensory and visual information in triggering and guiding rats’ reaches (again, rats were the same 5 used before). (A) Percent of reaches triggered in the constant-location baseline relative to conditions in which rats were deprived of somatosensory input or both visual and somatosensory input. (B) Reaching accuracy in the same conditions.
Also strikingly, there were no major effects of condition on accuracy in this task (F((2,30)=.860, p=.433; Figure 3b). Although rats that had asymptoted on a low percentage correct in the first baseline task continued to perform more poorly than the other rats in the sample, as reflected in a large main effect of rat (F(4,30)=10.39, p=.00002). Once again, however, rats differed considerably in their accuracy on this task. This difference in skill was displayed similarly by rats across the baseline and the two sensory-loss conditions. Thus, there was no effect of condition (F(2,30)=.860, p=.433), nor an interaction between rat and condition (F(8,30)=1.68, p=.144).
Experiment 3
The first two experiments appeared to establish that rats can perform the standard reach-to-grasp-food task with olfactory information alone. However, because of the constant pellet location across trials, they may have been solving the task proprioceptively, as a procedural “motor memory” task rather than as an olfactory-guided task. Thus we tested the accuracy of a control group of rats in the variable-location baseline task, and later ran the same group of rats in this variable-location task when they could not rely on vibrissae or vision. In this latter case, rats would have to solve the task with different movements across trials, using olfaction alone as a guide.
As a comparison between Figure 1c and Figure 1a shows, rats performed the variable-location baseline task with nonsignificantly less accuracy than the constant-location baseline task (approximately 69% correct for the constant-location baseline versus approximately 63% correct for the variable-location baseline). However, rats in the experimental condition of Experiment 3, who were blindfolded and had their rostral whiskers and forearm vibrissae removed, performed nonsignificantly better than when they were tested with all their senses intact in the variable-location baseline (condition F(1,20)=.294, p=.593; Figure 4). Single-sample t-tests confirmed that rats in both conditions performed far above the chance level of 33.3% (variable-location baseline, t(14)=10.91, p<.000001; variable-location with no whiskers or vision, t(14)=11.88, p<.000001). Rats in both conditions also attempted reaching at similar, high rates (> 90% in both cases; data not shown). As in the earlier experiments, though, rats differed from one another in the accuracy of their reaching and grasping (rat F(4,20)=17.20, p=.00001), with the group of inaccurately performing rats similar across conditions (condition × rat F(4,20)=1.57, p=.219).
Figure 4.
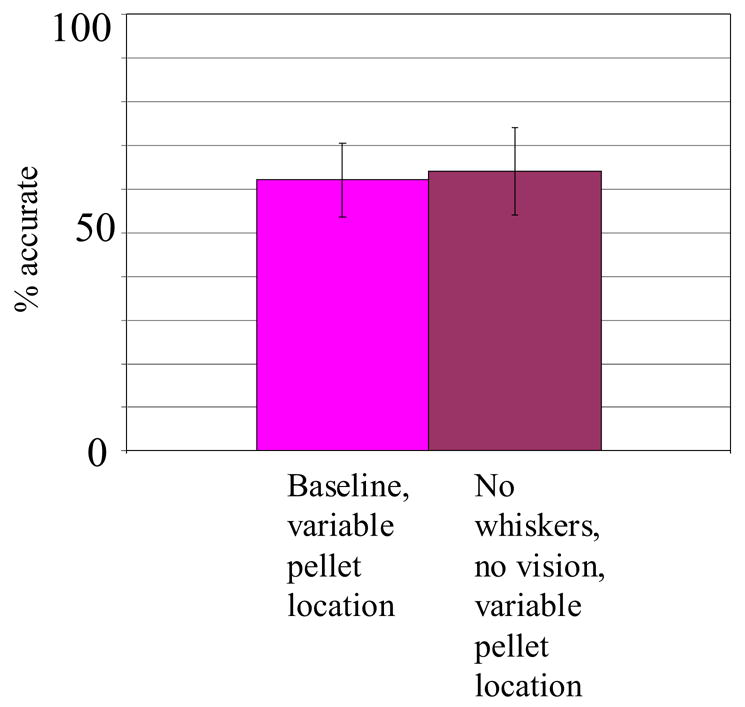
Performance of the same 5 rats in the variable-location baseline task and the sensory deprivation (same groups as in Figure 3)/variable-location tasks. In these conditions, rats could not rely on a unitary procedural memory of how to execute the reaching maneuver.
Discussion
The results of these experiments suggest that rats’ reaches for food targets are triggered and guided solely by olfaction. In Experiment 1, on over 90% of trials the sample confined their reaching to the GO targets with a chocolate or banana scent, reaching for the NO-GO vanilla, acetone, or plastic scented beads on less than 10% of trials. Moreover, their grasping accuracy on GO trials remained high. In Experiment 2, the loss of vibrissae, vision, or both did not affect the triggering or accuracy of reaching in any detectable way. Finally, in Experiment 3, in which the pellet location varied randomly across trials and olfaction was the only available cue to determine these randomly presented locations, the rats’ reaches were no less accurate than in the baseline condition.
We cannot rule out the possibility that when other sensory modalities are intact, rats may partly rely on them. However, after Whishaw and Tomie [1] bulbectomized the rats in their experiment (which used a similar reaching task), the rats did not gain the ability to rely on vision or another sensory modality to improve their post-operative performance even after 21 days of post-operative training. Taken together, our findings and theirs suggest that, first, normal rats rely exclusively on olfaction to trigger and guide their reaches in this type of reaching task, and second, olfactory-impaired rats lack the neural plasticity to develop a reliance on a non-olfactory sensory domain, even when they easily rely on those other domains to solve other behavioral tasks (e.g. [14]). They are also consistent with recent experimental results showing that when first learning the reaching task, rats align their noses opposite the food target (as constrained by the slot), then align their paw opposite the pellet as they reach to where the end of their nose was, again underscoring the importance of the pellet’s odor and position in determining the reaching trajectory [28]. These results support the overall description by Whishaw and colleagues that rats’ reaching is guided by olfaction until the reaching paw’s contact with the food pellet, at which point it is driven by hapsis [3,29].
More broadly, these findings contribute to a more general conceptual framework for mammalian brain evolution. Aboitiz and colleagues have suggested that the development of 6-layered isocortex was initially driven by heavy olfactory input to the hippocampus and amygdala, followed by increasing visual projections to the hippocampus in animals who evolved to become more diurnal [30,31]. Fossil endocasts of early mammals indicate that they may have relied on olfaction for most behavioral tasks. In contrast, most primates have a far greater number of visual cortical regions and rely primarily on vision for many more behavioral tasks, including reaching and navigation. Rats appear to occupy an intermediate place on this continuum, in that, early in their development for instance, they rely mainly on olfactory cues for navigation, but through learning to associate olfactory cues with visual cues, as adolescents and adults they come to rely mainly on vision [14,32] (with some limitations, however [33]). Also, to develop a cognitive map, rats have to locomote across the parts of the environment that they will come to represent [34–36]. In contrast, human and nonhuman primates can represent an environment by viewing it, without having to locomote over each portion to be represented [37–41]. Thus, unlike primates, rats’ sensory world is often tied to the region just beyond their noses, whiskers and paws.
Similarly, when reaching for a food target, olfaction plays a primary role even though one would think that rats’ visual acuity should allow them to rely on vision as well. Rats first place their nose as close to the food as possible, sniff several times and move their snout so that, through stereoscopic olfaction [3,42,43], the target is just beyond their nose and in line with their head. After this calibration process, they move their upper body out of the way so they can reach to the spot just beyond the snout [3]. Whishaw has suggested that in primates, the greater development of visual areas, and the uncoupling of somatosensory and motor forelimb cortex (which overlap in the rat caudal forelimb motor cortex, but not in primates), allowed them much greater behavioral flexibility [3]. Obviously, we are not suggesting that there was a simple, hierarchical progression in evolution from rodents to primates; the two orders shared a common insectivore ancestor approximately 50–60 million years ago [44] and have been evolving separately since then. However, we are suggesting that evolutionary pressures have selected for a similar functional switch from olfaction to vision in species that have become partly or wholly diurnal. Thus, while adult rats make ample use of visual as well as olfactory and self-motion cues for navigation [14], they still appear to rely exclusively on olfaction for reaching. Rats’ reaching process does seem to be modular, therefore, but it will likely come to incorporate other sensory cues as the species continues to evolve.
Acknowledgments
We thank three anonymous reviewers and David Root for helpful comments on this manuscript. This research was supported by DARPA-ONR grants 02SCNSF1015 and N000149911097 to J.K.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whishaw IQ, Tomie JA. Olfaction directs skilled forelimb reaching in the rat. Behav Brain Res. 1989;32(1):11–21. doi: 10.1016/s0166-4328(89)80067-1. [DOI] [PubMed] [Google Scholar]
- 2.Metz GA, Whishaw IQ. Skilled reaching an action pattern: stability in rat (Rattus norvegicus) grasping movements as a function of changing food pellet size. Behav Brain Res. 2000;116(2):111–122. doi: 10.1016/s0166-4328(00)00245-x. [DOI] [PubMed] [Google Scholar]
- 3.Whishaw IQ. Did a change in sensory control of skilled movements stimulate the evolution of the primate frontal cortex? Behav Brain Res. 2003;146(1–2):31–41. doi: 10.1016/j.bbr.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 4.Fodor JA. The modularity of mind: an essay on faculty psychology. MIT Press; Cambridge, Mass: 1983. [Google Scholar]
- 5.Cheng K. A purely geometric module in the rat’s spatial representation. Cognition. 1986;23(2):149–178. doi: 10.1016/0010-0277(86)90041-7. [DOI] [PubMed] [Google Scholar]
- 6.Hermer L, Spelke ES. A geometric process for spatial reorientation in young children. Nature. 1994;370(6484):57–59. doi: 10.1038/370057a0. [DOI] [PubMed] [Google Scholar]
- 7.Hermer L, Spelke E. Modularity and development: the case of spatial reorientation. Cognition. 1996;61(3):195–232. doi: 10.1016/s0010-0277(96)00714-7. [DOI] [PubMed] [Google Scholar]
- 8.Dudchenko PA, Goodridge JP, Seiterle DA, Taube JS. Effects of repeated disorientation on the acquisition of spatial tasks in rats: dissociation between the appetitive radial arm maze and aversive water maze. J Exp Psychol Anim Behav Process. 1997;23(2):194–210. doi: 10.1037//0097-7403.23.2.194. [DOI] [PubMed] [Google Scholar]
- 9.Gibson BM, Shettleworth SJ, McDonald RJ. Finding a goal on dry land and in the water: differential effects of disorientation on spatial learning. Behav Brain Res. 2001;123(1):103–111. doi: 10.1016/s0166-4328(01)00196-6. [DOI] [PubMed] [Google Scholar]
- 10.Learmonth AE, Newcombe NS, Huttenlocher J. Toddlers’ use of metric information and landmarks to reorient. J Exp Child Psychol. 2001;80(3):225–244. doi: 10.1006/jecp.2001.2635. [DOI] [PubMed] [Google Scholar]
- 11.Learmonth AE, Nadel L, Newcombe NS. Children’s use of landmarks: implications for modularity theory. Psychol Sci. 2002;13(4):337–341. doi: 10.1111/j.0956-7976.2002.00461.x. [DOI] [PubMed] [Google Scholar]
- 12.McGregor A, Jones PM, Good MA, Pearce JM. Further evidence that rats rely on local rather than global spatial information to locate a hidden goal: reply to Cheng and Gallistel (2005) J Exp Psychol Anim Behav Process. 2006;32(3):314–321. doi: 10.1037/0097-7403.32.3.314. [DOI] [PubMed] [Google Scholar]
- 13.Keller J, Strasburger H, Cerutti DT, Sabel BA. Assessing spatial vision - automated measurement of the contrast-sensitivity function in the hooded rat. J Neurosci Methods. 2000;97(2):103–110. doi: 10.1016/s0165-0270(00)00173-4. [DOI] [PubMed] [Google Scholar]
- 14.Maaswinkel H, Whishaw IQ. Homing with locale, taxon, and dead reckoning strategies by foraging rats: sensory hierarchy in spatial navigation. Behav Brain Res. 1999;99(2):143–152. doi: 10.1016/s0166-4328(98)00100-4. [DOI] [PubMed] [Google Scholar]
- 15.Shettleworth SJ, Sutton JE. Multiple systems for spatial learning: dead reckoning and beacon homing in rats. J Exp Psychol Anim Behav Process. 2005;31(2):125–141. doi: 10.1037/0097-7403.31.2.125. [DOI] [PubMed] [Google Scholar]
- 16.Hermer-Vazquez L, Spelke ES, Katsnelson AS. Sources of flexibility in human cognition: dual-task studies of space and language. Cognit Psychol. 1999;39(1):3–36. doi: 10.1006/cogp.1998.0713. [DOI] [PubMed] [Google Scholar]
- 17.Hermer-Vazquez L, Moffet A, Munkholm P. Language, space, and the development of cognitive flexibility in humans: the case of two spatial memory tasks. Cognition. 2001;79(3):263–299. doi: 10.1016/s0010-0277(00)00120-7. [DOI] [PubMed] [Google Scholar]
- 18.Whishaw IQ, Pellis SM. The structure of skilled forelimb reaching in the rat: a proximally driven movement with a single distal rotatory component. Behav Brain Res. 1990;41(1):49–59. doi: 10.1016/0166-4328(90)90053-h. [DOI] [PubMed] [Google Scholar]
- 19.Hyland B. Neural activity related to reaching and grasping in rostral and caudal regions of rat motor cortex. Behav Brain Res. 1998;94(2):255–269. doi: 10.1016/s0166-4328(97)00157-5. [DOI] [PubMed] [Google Scholar]
- 20.Hyland BI, Jordan VM. Muscle activity during forelimb reaching movements in rats. Behav Brain Res. 1997;85(2):175–186. doi: 10.1016/s0166-4328(97)87582-1. [DOI] [PubMed] [Google Scholar]
- 21.Jarratt H, Hyland B. Neuronal activity in rat red nucleus during forelimb reach-to-grasp movements. Neuroscience. 1999;88(2):629–642. doi: 10.1016/s0306-4522(98)00227-9. [DOI] [PubMed] [Google Scholar]
- 22.Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci. 2004;24(3):628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80(6):3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- 24.Kleim JA, Barbay S, Cooper NR, et al. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002;77(1):63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- 25.Hermer-Vazquez L, Hermer-Vazquez R, Moxon KA, et al. Distinct temporal activity patterns in the rat M1 and red nucleus during skilled versus unskilled limb movement. Behav Brain Res. 2004;150(1–2):93–107. doi: 10.1016/S0166-4328(03)00226-2. [DOI] [PubMed] [Google Scholar]
- 26.Brecht M, Preilowski B, Merzenich MM. Functional architecture of the mystacial vibrissae. Behav Brain Res. 1997;84(1–2):81–97. doi: 10.1016/s0166-4328(97)83328-1. [DOI] [PubMed] [Google Scholar]
- 27.Whishaw IQ, Maaswinkel H. Rats with fimbria-fornix lesions are impaired in path integration: a role for the hippocampus in “sense of direction”. J Neurosci. 1998;18(8):3050–3058. doi: 10.1523/JNEUROSCI.18-08-03050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gharbawie OA, Whishaw IQ. Parallel stages of learning and recovery of skilled reaching after motor cortex stroke: “Oppositions” organize normal and compensatory movements. Behav Brain Res. 2006 doi: 10.1016/j.bbr.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 29.Ballermann M, McKenna J, Whishaw IQ. A grasp-related deficit in tactile discrimination following dorsal column lesion in the rat. Brain Res Bull. 2001;54(2):237–242. doi: 10.1016/s0361-9230(01)00431-2. [DOI] [PubMed] [Google Scholar]
- 30.Aboitiz F, Morales D, Montiel J. The evolutionary origin of the mammalian isocortex: towards an integrated developmental and functional approach. Behav Brain Sci. 2003;26(5):535–552. doi: 10.1017/s0140525x03000128. discussion 552–585. [DOI] [PubMed] [Google Scholar]
- 31.Aboitiz F, Montiel J, Morales D, Concha M. Evolutionary divergence of the reptilian and the mammalian brains: considerations on connectivity and development. Brain Res Brain Res Rev. 2002;39(2–3):141–153. doi: 10.1016/s0165-0173(02)00180-7. [DOI] [PubMed] [Google Scholar]
- 32.Rossier J, Schenk F. Olfactory and/or visual cues for spatial navigation through ontogeny: olfactory cues enable the use of visual cues. Behav Neurosci. 2003;117(3):412–425. doi: 10.1037/0735-7044.117.3.412. [DOI] [PubMed] [Google Scholar]
- 33.Knierim JJ, Kudrimoti HS, McNaughton BL. Interactions between idiothetic cues and external landmarks in the control of place cells and head direction cells. J Neurophysiol. 1998;80(1):425–446. doi: 10.1152/jn.1998.80.1.425. [DOI] [PubMed] [Google Scholar]
- 34.Loewen I, Wallace DG, Whishaw IQ. The development of spatial capacity in piloting and dead reckoning by infant rats: use of the huddle as a home base for spatial navigation. Dev Psychobiol. 2005;46(4):350–361. doi: 10.1002/dev.20063. [DOI] [PubMed] [Google Scholar]
- 35.Whishaw IQ, Brooks BL. Calibrating space: exploration is important for allothetic and idiothetic navigation. Hippocampus. 1999;9(6):659–667. doi: 10.1002/(SICI)1098-1063(1999)9:6<659::AID-HIPO7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 36.Muir GM, Bilkey DK. Theta- and movement velocity-related firing of hippocampal neurons is disrupted by lesions centered on the perirhinal cortex. Hippocampus. 2003;13(1):93–108. doi: 10.1002/hipo.10052. [DOI] [PubMed] [Google Scholar]
- 37.O’Keefe J, Burgess N, Donnett JG, Jeffery KJ, Maguire EA. Place cells, navigational accuracy, and the human hippocampus. Philos Trans R Soc Lond B Biol Sci. 1998;353(1373):1333–1340. doi: 10.1098/rstb.1998.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O’Keefe J. Knowing where and getting there: a human navigation network. Science. 1998;280(5365):921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- 39.Washburn DA, Astur RS. Exploration of virtual mazes by rhesus monkeys (Macaca mulatta) Anim Cogn. 2003;6(3):161–168. doi: 10.1007/s10071-003-0173-z. [DOI] [PubMed] [Google Scholar]
- 40.Sato N, Sakata H, Tanaka Y, Taira M. Navigation in virtual environment by the macaque monkey. Behav Brain Res. 2004;153(1):287–291. doi: 10.1016/j.bbr.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 41.Hori E, Nishio Y, Kazui K, et al. Place-related neural responses in the monkey hippocampal formation in a virtual space. Hippocampus. 2005;15(8):991–996. doi: 10.1002/hipo.20108. [DOI] [PubMed] [Google Scholar]
- 42.Wilson DA. Binaral interactions in the rat piriform cortex. J Neurophysiol. 1997;78(1):160–169. doi: 10.1152/jn.1997.78.1.160. [DOI] [PubMed] [Google Scholar]
- 43.Rajan R, Clement JP, Bhalla US. Rats smell in stereo. Science. 2006;311(5761):666–670. doi: 10.1126/science.1122096. [DOI] [PubMed] [Google Scholar]
- 44.Matsubara K, Ishikawa A, Kuroiwa A, et al. Comparative FISH mapping of human cDNA clones to chromosomes of the musk shrew (Suncus murinus, Insectivora) Cytogenet Cell Genet. 2001;93(3–4):258–262. doi: 10.1159/000056994. [DOI] [PubMed] [Google Scholar]


