Abstract
We compared cellular and humoral immunity to vaccinia virus (VV) in individuals exposed to 3 different orthopoxviruses: 154 individuals previously vaccinated with VV, 7 individuals with a history of monkeypox virus infection, and 8 individuals with a history of variola virus infection. Among individuals vaccinated >20 years prior, 9 (14%) of 66 individuals demonstrated VV-specific interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) assay responses; 21 (50%) of 42 had lymphoproliferative (LP) responses, and 29 (97%) of 30 had VV-specific neutralizing antibodies. One year after monkeypox virus infection, 6 of 7 individuals had IFN-γ ELISPOT responses, all had VV-specific LP responses, and 3 of 7 had VV-specific neutralizing antibodies. Of 8 individuals with a history of variola virus infection, 1 had a VV-specific IFN-γ ELISPOT response, 4 had LP responses against whole VV, 7 had LP responses against heat-denatured vaccinia antigen, and 7 had VV-specific neutralizing antibodies. Survivors of variola virus infection demonstrated VV-specific CD4 memory cell responses and neutralizing antibodies >40 years after infection.
The last known case of variola virus infection in the United States occurred in 1949 in Texas [1]. The discontinuation of routine immunization against variola virus in the United States in 1972 and the worldwide eradication of natural infection with variola virus in 1979 provide a unique opportunity for the study of long-term immunological memory to a virus without circulating homologous antigen exposure. The potential use of remaining variola virus stocks as a bioterrorism agent has prompted investigators to measure immunological memory in previously vaccinated individuals. These prior studies revealed the presence of vaccinia virus (VV)-specific memory T and B cell responses [2-4], although the level of protection against natural variola virus infection that this may confer remains unclear, and comparison to individuals infected with variola virus has not been reported previously.
Protective immunity conferred after surviving natural infection with variola virus is thought to be lifelong [5], although correlates of that protection have not been examined with contemporary immunological tools. Improved understanding of human immune responses to poxviruses, including variola virus, could have important implications for the development of safer VV-based vaccines. We describe here the human memory immune responses after vaccination with VV and also after natural infection with monkeypox or variola virus.
METHODS
Study Population
We studied 4 cohorts of individuals from whom blood samples were collected between April 2002 and October 2005. Volunteers were recruited through the Veterans Affairs New York Harbor Healthcare System (Institutional Review Board-approved protocol 00385) and the University of Massachusetts Medical School (UMMS; protocol H-10849 or H-3348).
History of VV vaccination
All 154 individuals had written documentation of the date of VV vaccination. For individuals who had had ≥2 vaccinations, the date of the last vaccination served as the reference date for the determination of time since last vaccination. Eighty-six individuals were vaccinated ≤2 years prior, 2 were vaccinated 3-20 years prior, and 66 were vaccinated >20 years prior.
History of monkeypox virus infection
A year after a monkeypox outbreak in Wisconsin in 2003 [6], 7 individuals with a history of monkeypox virus infection were enrolled. Five individuals with laboratory-confirmed monkeypox virus infection were identified through their physician. Two additional individuals were identified through these 5 individuals: one individual was the wife of a confirmed case patient who had had contact with an ill prairie dog and who had symptoms of monkeypox virus infection but did not have laboratory confirmation. The second individual was a veterinary-clinic employee who was exposed to an ill prairie dog during the out-break period and who developed a typical pox rash and headache within 21 days after contact but did not have laboratory confirmation. Using a standardized questionnaire, we collected information on clinical symptoms, history of VV vaccination, exposure history, and results of diagnostic laboratory tests. Diagnosis of monkeypox was categorized as confirmed, probable, or suspect, as defined by the Centers for Disease Control and Prevention [7].
History of variola virus infection
We identified 8 individuals with a history of variola virus infection through advertisements in Indian-American newspapers. Volunteers were questioned in a face-to-face interview about the nature of the variola virus infection and their history of VV vaccination and were surveyed for the presence of facial pockmarks and VV-vaccination scars. The facial scar survey for evidence of prior variola virus infection was conducted as described elsewhere [8]. A probable case of variola virus infection was defined as having occurred in an individual with a history of variola illness before 1975 and the presence of ≥5 facial scars [8]. The main confounder in the facial scar survey is a history of severe chickenpox, which also can leave residual scars. However, a facial scar survey conducted for 12 months in Bangladesh, after the eradication of variola virus infection in 1975, indicated that chickenpox rarely results in ≥5 facial pockmarks [8]. Loss of scars over time has been reported almost exclusively when variola virus infection occurred at 6-12 months of age and does not appear to increase with time [8]. Thus, 1 volunteer who reported having had variola virus infection at <1 year of age (she and several members of her family had variola virus infection) was categorized as having a ‘probable’ history of variola virus infection, since facial scarring was absent. Because 3 subjects had a history of both variola virus infection and VV vaccination and because these events occurred within 1-2 years of one another, we used the year of variola virus infection as the reference year.
Poxvirus-naive individuals
We enrolled 15 healthy individuals living in the United States, 18-33 years of age, who did not have a history of VV vaccination. These individuals also had the pertinent negative military and travel history, and pock lesions and vaccine scars were absent.
Laboratory Assays
Blood specimens obtained for serum and peripheral blood mononuclear cells (PBMCs) were harvested from sodium citrate cell-separator tubes (Becton Dickinson). Samples were processed within 24 h of blood donation. Separated PBMCs were counted and resuspended with 10% serum from blood-group AB donors in RPMI 1640 medium, at a concentration of 2 × 106 cells/mL, for assays using fresh or cryopreserved samples as described elsewhere [3]. The New York City Board of Health strain of VV, derived from the Dryvax vaccine, was used in all assays. Enzyme-linked immunospot (ELISPOT) and lymphoproliferation assays were conducted at New York University (NYU) and UMMS; cytotoxic T lymphocyte (CTL) and neutralizing-antibody assays were conducted at UMMS.
Interferon (IFN)-γ ELISPOT assay
We quantified VV-specific IFN-γ-producing T cells by using the IFN-γ ELISPOT assay on both fresh and cryopreserved PBMCs. The results of the ELISPOT assays did not differ significantly between the fresh and frozen specimens (data not shown). The assays were conducted as described by Kennedy et al. [3] and Borkowsky et al. [9], with the following modifications. VV was added at 2 μL/well at an MOI of 2 virions/cell. Phytohemagglutinin (PHA [Sigma]; 1:100 dilution and a final concentration of 10 μg/mL) at 10 μL/well served as a positive control well. Results represent the mean value of triplicate wells, expressed as IFN-γ spot-forming units per 106 PBMCs. On the basis of results from 15 poxvirus-naive individuals, a positive cutoff of >15 sfu/106 PBMCs was used.
Lymphoproliferation assay
The lymphoproliferation assay was a modification of the methods described by Valentine et al. [10]. Fresh PBMCs were isolated by the Ficoll-Hypaque method and then were washed and suspended in RPMI 1640 medium. Cells were counted and adjusted to a concentration of 106 cells/mL, and then 0.1 mL of the cell suspension was added to a 96-well (U-bottom) plate containing quadruplicate wells of a 1:5000 dilution of VV, cytomegalovirus, PHA, and a control medium prepared at twice the final concentration in RPMI 1640-20% heat-inactivated serum from blood-group AB donors, 2% penicillin, and 2% streptomycin. The VV dilutions were prepared from a stock solution of 108 pfu/mL (the same stock that was used to prepare the VV dilutions for the ELI-SPOT assays). This virus preparation did not stimulate lymphocytes from unvaccinated healthy individuals.
In assays using vaccinia antigen (VacAg), the VacAg was prepared by heat inactivation and was tested for residual live virus by means of a plaque assay. A single lot of virus-free antigen was used at dilutions of 1:40 and 1:80, for comparison. Stimulation of 2 × 105 cells for 5 days was done as described else-where for VV [11, 12].
The stimulation index (SI) was calculated by dividing the median counts per minute from 4 wells containing cells exposed to antigen by the median counts per minute for cells incubated with medium alone. A positive cutoff was defined as an SI of >3.
VV-specific CTL assays
Blood specimens were received within 16 h of donation, and separated PBMCs were cryopreserved within 24 h of blood donation. B lymphoblastoid cell lines were prepared from PBMCs from each donor by transformation with Epstein-Barr virus [3, 11]. Cryopreserved donor PBMCs were thawed, washed, and suspended in 5 mL of RPMI 1640 with 10% heat-inactivated fetal bovine serum and then were counted and prepared in accordance with well-defined protocols [3, 11]. In brief, CTL assays were performed by the infection of 0.2-1 million target cells (autologous BLCL) with VV 1 day before labeling for 60 min with 0.25-mCi 51Cr (New England Nuclear). Target cells were washed, counted, and resuspended to 15,000 cells/mL, and 0.1 mL of target cells was added per well in 96-well (U-bottom) plates. Effector cells were counted and washed and then were added to each well at effector-to-target cell (E:T) ratios of 90, 30, and 10, in triplicate. Target cells with 0.1 mL of medium served as minimum lysis controls. Plates were incubated at 37 °C for 4.5 h. Well supernatants were harvested by use of the Skatron supernatant collection system and were counted in a Packard gamma counter. Target cells lysed with 0.1 mL of RENEX (detergent) served as maximum lysis controls [4]. At each E:T ratio, a percent-specific lysis of VV-infected target cells was calculated as the difference between the percent-specific lysis of VV-infected target cells and the percent-specific lysis of uninfected target cells. Lytic units (LU) per 106 PBMCs were determined from the percent-specific lysis at each E:T ratio, by use of the exponential fit method based on software provided by Proteins International [13]. A normal LU value was between 1 and 1000, and the LU value represents a semiquantitative measure of the cell-mediated cytotoxicity observed in 106 PBMCs. In prior clinical studies, a positive CTL response was defined as >5 LU, and 5 LU indicates an ∼5-fold increase above the background lysis level [11].
Neutralizing-antibody assay
Humoral immune responses were assessed by serial plaque-reduction neutralization titer (PRNT) assays [12]. Samples obtained at blood-cell harvest were tested for the presence of VV-specific neutralizing antibodies, by means of 2-fold serial dilutions of heat-inactivated serum. VV at a concentration of 1 × 103 pfu/mL was added to multiple dilutions of serum (1:10 through 1:3840) and to positive (New York City Board of Health strain) and negative control samples. The resulting mixtures were incubated for 1 h and then were plated on Vero 76 cells. Plaques were stained with neutral red and counted at 72 h, and the reduction in plaques was plotted against the dilution factor. Antibody titers (the reciprocal of the dilution) resulting in plaque neutralization of 50% (i.e., PRNT50) were calculated from the plot. A positive cutoff was defined as a titer ≥1:20.
RESULTS
Immune responses after VV vaccination
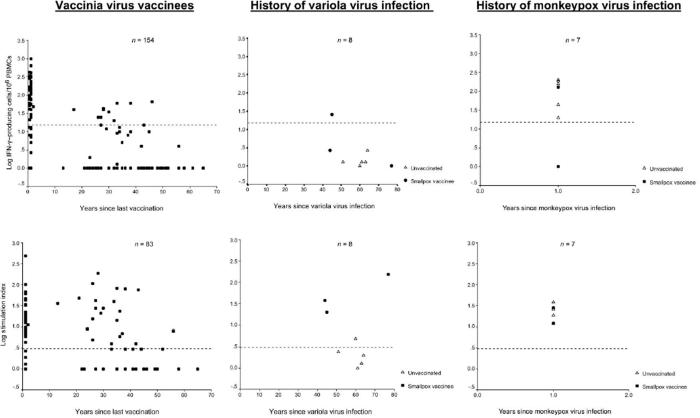
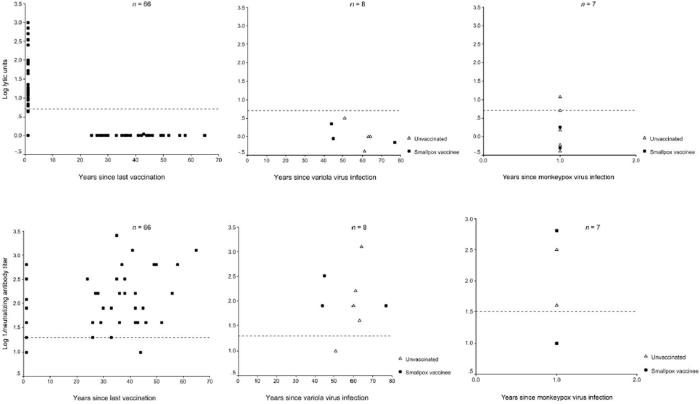
VV-specific IFN-γ ELISPOT assays were positive for 64 (42%) of the 154 vaccinees, and lymphoproliferative (LP) responses were positive for 56 (67%) of 83 vaccinees tested (figure 1). Of the 83 vaccinees tested by both the ELISPOT and lymphoproliferation assays, 30 (36%) had both VV-specific IFN-γ-producing lymphocytes and proliferative memory responses; 26 (31%) demonstrated proliferative memory responses without the detection of IFN-γ-producing cells; and the remaining 27 (33%) demonstrated neither IFN-γ-producing lymphocytes nor proliferative responses.
Figure 1.
Distribution of laboratory measures of cellular and humoral immunity in individuals with a history of vaccinia virus vaccination, variola virus infection, or monkeypox virus infection. IFN, interferon; PBMCs, peripheral blood mononuclear cells.
CTL responses were detected in 27 (41%) of 66 vaccinees tested. The median number of years since last vaccination was 1 for those with a CTL response, compared with a median of 35 years (range, 1-65 years) for those without a CTL response. All 27 vaccinees with CTL responses also had VV-specific proliferative responses, and 21 also had VV-specific IFN-γ-producing lymphocytes.
The proportion of VV vaccinees with a detectable VV-specific memory T cell immune response decreased over time (table 1). At >20 years after vaccination, 9 (14%) of 66 vaccinees tested had detectable IFN-γ-producing lymphocytes, and 21 (50%) of 42 vaccinees had VV-specific LP responses. CTL responses were not detected in any subjects, but 29 (97%) of 30 vaccinees had a neutralizing-antibody titer ≥1:20. VV-specific IFN-γ-producing lymphocytes were detected in 1 individual 46 years after this person’s last VV vaccination, and LP responses to virus were detected in another individual 56 years after vaccination.
Table 1.
Cell-mediated and humoral immune responses among individuals vaccinated with vaccinia virus (VV), by type of immunological test and interval since last vaccination.
| Years since last VV vaccination | IFN-γ ELISPOT assay | Lymphoproliferation assay | Cytotoxic T cell lysis | Neutralizing antibody |
|---|---|---|---|---|
| ≤2 | 54/86 (63) | 34/40 (85) | 27/36 (75) | 31/36 (86) |
| 3-20 | 1/2 (50) | 1/1 (100) | ND | ND |
| >20 | 9/66 (14) | 21/42 (50) | 0/30 (0) | 29/30 (97) |
NOTE. Data are no. of positive individuals/total no. tested (%). IFN, interferon; ELISPOT,enzyme-linked immunospot; ND, not determined.
Overall, 60 (91%) of 66 vaccinees had detectable neutralizing-antibody levels (titer ≥1:20); strong neutralizing-antibody responses (titer ≥1:1280) were detected in 3 individuals ≥35 years after their last VV vaccination.
Immune responses after monkeypox virus infection
Seven individuals with suspected or confirmed monkeypox virus infection were identified in Wisconsin (4 women and 3 men; median age, 29 years; range, 20-43 years). Two of 7 individuals also had a history of VV vaccination. All donors resided in southeastern Wisconsin; 4 individuals worked in veterinary clinics, 1 worked in a pet store, 1 was an animal distributor, and 1 was the wife of the animal distributor. All reported exposure to ill prairie dogs in May 2003 and subsequent onset of illness within 21 days. Types of exposure included scratches or bites by an ill prairie dog, occupying the same room as an ill prairie dog in a veterinary clinic, and contact with or urination on intact skin by an ill prairie dog. Clinical signs and symptoms included skin lesions, headache, fever, chills, and lymphadenopathy. The number of skin lesions ranged between 1 and >100, and the number of days of illness ranged between 3 and 21 (table 2).
Table 2.
Characteristics and assay results for 7 individuals with a history of monkeypox virus infection and with cell-mediated and humoral immunity 1 year after infection, United States, 2004-2005.
| Clinical symptoma |
III days, no. | Assay result |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Individual | VV vaccination | Typical lesions, no. | Headache | Fever | Chills | LAD | ELISPOT, sfu/106PBMCs | CTL, LU | Lymphoproliferation, SI | nAb titer | |
| 1 | Yes (>43 years ago) | 7 | + | - | + | - | 3 | 0 | .5 | 28b | <1:10 |
| 2 | No | 24 | + | + | + | + | 20 | 159b | 11.8b | 12b | <1:10 |
| 3 | No | 20 | + | + | + | + | 21 | 20b | 5b | 26b | <1:10 |
| 4 | No | 1 | + | + | + | + | 7 | 203b | .4 | 19b | <1:10 |
| 5 | No | >100 | + | + | + | + | 14 | 188b | .6 | 28b | 1:320b |
| 6 | Yes (year unknown) | 12 | + | + | + | + | 10 | 127b | 1.8 | 12b | 1:640b |
| 7 | No | 50 | + | + | + | + | 15 | 44b | 1.5 | 39b | 1:40b |
NOTE. CTL, cytolytic T lymphocyte; ELISPOT, enzyme-linked immunospot; LAD, lymphadenopathy; LU, lytic unit; nAb, neutralizing antibody; PBMCs, peripheral blood mononuclear cells; SI, stimulation index; VV, vaccinia virus.
A plus sign (+) indicates presence of a symptom, and a minus sign (-) indicates absence of a symptom.
Value exceeds positive cutoff level for assay.
One year after monkeypox virus infection, all 7 subjects had VV-specific LP responses; 6 subjects had detectable IFN-γ ELI-SPOT responses, and 2 subjects had CTL responses (figure 1). Three (43%) of 7 subjects had a VV-specific neutralizing-antibody titer ≥1:20 (table 2). The 2 individuals who had a positive response for all 3 cellular immune-response assays did not have detectable serum neutralizing antibody to VV. The absence of neutralizing-antibody titers was confirmed with a second assay, described by Kennedy et al. [3].
Immune responses after variola virus infection
We identified 8 individuals with a history of variola virus infection before 1975 (table 3). The median age was 63 years (range, 50-78 years), and 5 (63%) were men. The variola virus infections occurred in India (7 individuals) and Bangladesh (1 individual) between 1928 and 1961. The median number of years since variola virus infection was 57 (range, 44-77 years), and the median age at the time of variola virus infection was 6 years (range, <1-16 years). Three individuals reported having received a VV vaccination and had a vaccination scar; 5 individuals denied a history of VV vaccination and did not have a vaccination scar. Six individuals reported that other household members also had had variola virus infection.
Table 3.
Characteristics and assay results for 8 individuals with a history of variola virus infection.
| Assay result |
||||||||
|---|---|---|---|---|---|---|---|---|
| Individual (age in years, sex) | Years since variola virus infection | Facial scars, no. | VV vaccinationa | ELISPOT, sfu/106 PBMCs | CTL, LU | Lymphoproliferation, SI |
nAb titer | |
| Whole VV | Heat-inactivated VacAg | |||||||
| 1 (62, male) | 60 | ∼50 | - | 0 | NA | 5b | 5b | 1:80b |
| 2 (55, male) | 51 | 25 | - | 1.3 | 3.2 | 2.4 | 2 | 1:10 |
| 3 (69, male) | 63 | 25 | - | 1.3 | 0 | 1.3 | 16b | 1:40b |
| 4 (71, female) | 64 | 20 | - | 2.7 | 0 | 2 | 24b | 1:1280b |
| 5 (64, male) | 61 | ∼100 | - | 1.3 | .4 | 1 | 29b | 1:160b |
| 6 (78, female) | 77 | 0 | + | 0 | .7 | 153b | 131b | 1:80b |
| 7 (50, male) | 44 | ∼50 | + | 2.7 | 2.2 | 38b | 86b | 1:80b |
| 8 (61, female) | 45 | ∼50 | + | 25.3b | .9 | 20b | 8b | 1:320b |
NOTE. CTL, cytotoxic T lymphocyte; ELISPOT, enzyme-linked immunospot; LU, lytic unit; NA, not available; nAb, neutralizing antibody; PBMC, peripheral blood mononuclear cell; SI, stimulation index; VacAg, vaccinia antigen; VV, vaccinia virus.
A plus sign (+) indicates presence of a symptom, and a minus sign (-) indicates absence of a symptom.
Value exceeds positive cutoff level for assay.
Three individuals had facial scars only, and 4 had scars to the face and extremities. The number of facial scars was between 25 and >100 pockmarks. Figure 2 shows the typical facial scars of 2 survivors of variola virus infection who were included in this study.
Figure 2.
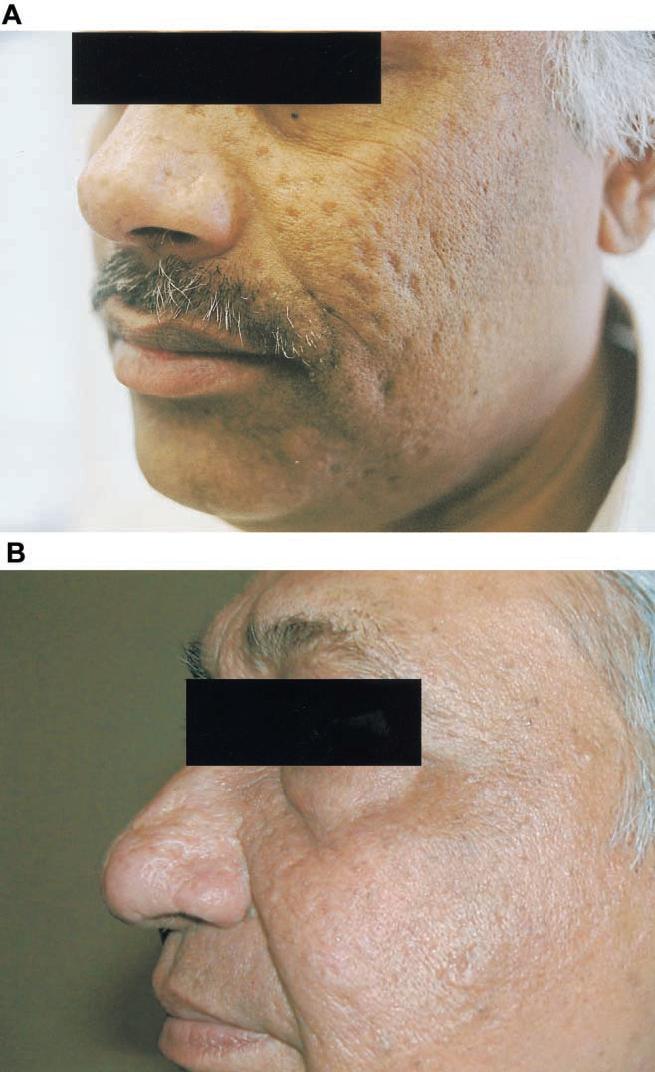
Facial scar survey of individuals with a history of variola virus infection. A, 50-year-old Bangladeshi man who had variola virus infection 44 years ago and 2 vaccinia virus (VV) vaccinations. Assay results were as follows: interferon (IFN)-γ enzyme-linked immunospot (ELISPOT), 2.7 sfu/106 peripheral blood mononuclear cells (PBMCs); lymphoproliferation, stimulation index (SI) of 38; cytotoxic T lymphocyte (CTL), 2.2 lytic units (LU); and VV-specific neutralizing-antibody titer, 1:80. B, 62-year-old Indian man who had variola virus infection 60 years ago and no history of VV vaccination. IFN-γ ELISPOT, 0 sfu/106 PBMCs; lymphoproliferation, SI of 5; CTL, not available; and VV-specific neutralizing-antibody titer, 1:80.
Of the 5 individuals without a history of VV vaccination, 4 had neutralizing-antibody titers, and 1 had a VV-specific LP response; positive IFN-γ ELISPOT and CTL responses were not detected. All 3 individuals with a history of both variola virus infection and VV vaccination had neutralizing-antibody titers ≥1:20 and LP responses; 1 individual had an IFN-γ ELISPOT response, and none had detectable CTL activity (table 3).
For all survivors of variola virus infection, lymphoproliferation assays were repeated with VacAg (instead of whole VV), to determine whether the use of VacAg would increase VV-specific CD4 T cell-dependent proliferation. Seven of 8 individuals showed a positive response, including 3 of 4 individuals who did not have a proliferative response to whole VV (table 3). By means of flow cytometry, this response to VacAg was characterized as IFN-γ-producing CD4 cells (CD8 cells were not detected) and was not detected in 6 VV vaccinees, who were vaccinated a median of 40 years prior, or in 3 pox-naive individuals (data not shown). These results suggest that the T lymphocytes proliferating in response to VacAg were long-lasting CD4 memory cells and that survivors of variola virus infection maintained a low but extant population of VV-specific CD4 memory cells, despite the lack of ongoing antigen exposure to variola virus or VV.
DISCUSSION
By using contemporary assays, we provide the first description of residual cell-mediated and humoral immunity in individuals who survived variola virus infection, compared with individuals with a history of monkeypox virus infection or VV vaccination. Individuals who survived an episode of variola virus infection were thought to have lifelong protection against reinfection [5], although a reinfection rate of 1 in 1000 cases has been reported in India, with an average interval of ∼15-20 years between attacks [14, 15]. We found high titers of neutralizing antibody against VV in 4 of 5 unvaccinated survivors of variola virus infection who had had the infection >40 years ago, suggesting that the antibody response after natural variola virus infection is long lasting. In contrast, none of the 5 unvaccinated individuals with a history of variola virus infection had IFN-γ production detected by ELISPOT assay, and only 1 had an LP response to VV. Stimulation with heat-denatured VacAg led to a more robust expansion of VV-specific CD4 cells; such cells were undetectable when whole VV was used. This discrepancy may reflect differences in concentration and types of epitopes present in VacAg and whole VV preparations; alternatively, it may reflect differences in processing and presentation of antigen in cultured cells.
One year after monkeypox virus infection, VV-specific IFN-γ-producing T cells were present at a level comparable to levels in those who had received a VV vaccination 1-2 years prior, which is within the time frame of maximal vaccine-induced protection against variola virus. T cell LP responses to VV also were robust in these survivors of monkeypox virus infection. The presence of VV-specific IFN-γ-producing T cells and LP responses in individuals with monkeypox virus infection who had never received VV vaccination reflects cross-protective immunity between VV and monkeypox virus [16]. Only 3 of the 7 survivors of monkeypox virus infection demonstrated VV-specific neutralizing-antibody titers ≥1:20, including 2 without a history of VV vaccination. This finding may be explained by the serotype-specific nature of neutralizing-antibody responses, compared with the more cross-reactive T cell responses to epitopes in orthopoxviruses [17, 18]. In contrast to our findings, other studies have found higher antibody responses in patients with previous monkeypox virus infection, by use of ELISA [16, 19]. This inconsistency likely reflects the higher specificity and lower sensitivity of PRNT assays, relative to ELISAs; however, neutralizing antibodies are more likely to correlate with protective immunity against reinfection.
Several recent studies have demonstrated immunological longevity of memory T cells and antibody titers from previous VV vaccination [2, 4, 20, 21]. One study reported that 50% of volunteers had detectable VV-specific IFN-γ-producing CD8 lymphocytes 20-30 years after VV vaccination, whereas 100% had IFN-γ-producing CD4 lymphocytes; similarly, 50% of volunteers had CD4 or CD8 memory lymphocytes >50 years after VV vaccination [2]. The same study also found an earlier preferential loss of IFN-γ-producing CD8 cells, relative to CD4 cells. In contrast, our data demonstrate that only 14% of vaccinated individuals retained IFN-γ-producing lymphocytes >20 years after VV vaccination, and none (0/8) retained IFN-γ-producing lymphocytes after 50 years, which is consistent with the findings of other recent studies [22, 23]. The discrepancy between these study results likely reflects individual differences in the generation and maintenance of long-term immunological memory rather than differences in the assays. All these studies are limited by the number of volunteers tested, but the cumulative data suggest that important questions about why some individuals do not appear to maintain detectable long-lasting memory T cells to orthopoxviruses need to be answered.
Our study has several limitations. First, in the assays, VV was used as the surrogate antigen for monkeypox and variola viruses. The cellular or humoral response to VV may not accurately reflect the immunological responses that survivors of monkeypox and variola virus infection would have if they were to be rechallenged with monkeypox or variola virus. Second, although there is highly conserved genetic homology between orthopoxviruses, particularly between VV and variola virus, the use of an immune-response marker to VV may not fully disclose the total repertoire of variola virus-specific T cell and B cell immunity that could explain lifelong protective immunological memory after infection with variola virus. However, immune responses induced by VV clearly do protect against clinical infection with variola virus, and it is important to report any insights gained regarding the phenotype and durability of immune responses induced against VV. Second, because we analyzed only IFN-γ production by ELISPOT assay, a negative response does not rule out the possibility that other cytokines were produced by memory T lymphocytes, as is suggested by the presence of interleukin-2-dependent proliferative responses. Third, the small number of survivors of monkeypox and variola virus infection limits statistical comparisons of the immune responses in these groups and provides only observational data. However, this study describes, for the first time, memory cell-mediated and humoral immunity after variola virus infection, as compared with that seen after monkeypox infection or VV vaccination.
In summary, survivors of variola virus infection display sustained vaccinia-specific neutralizing-antibody responses; in our study, 7 of 8 individuals had VV-specific proliferative responses to heat-denatured VacAg, indicating the presence of low-level circulating VV-specific CD4 memory cells >40 years after infection. Survivors of monkeypox virus infection had strong cell-mediated responses 1 year after infection, although the cytolytic T cell response was more limited. We also found that only 14% of individuals had IFN-γ-producing lymphocytes 20 years after the last VV vaccination, although 50% had detectable VV-specific proliferative responses; this contrast suggests the longer persistence of CD4 memory cells after natural infection or vaccination with orthopoxviruses.
Future studies could address the issue of lifelong immunity induced by variola virus infection by challenging survivors of variola virus infection with Dryvax vaccine or with experimental vaccines, to assess in vitro immune responses. These studies could help delineate the components of postchallenge antibody and T cell responses that are specific for both variola virus and VV protein epitopes. Such a study of survivors of variola virus infection would require comparison to vaccinated individuals and would significantly improve our understanding of the role of prior infection in protecting against or attenuating the response to subsequent exposure to a homologous virus.
Acknowledgments
We thank the volunteers for offering their time and enthusiastic participation in this study. We also acknowledge the following people for their invaluable assistance: Maura Laverty, for recruitment of volunteers with monkeypox and variola virus infection; Mary Graham, in Wisconsin; Tiina Ilmet, for laboratory assistance; John Cruz, for help with the cell-mediated immune-function assays; and Deborah Hirsch-Temple, Joanne Flannery, Ruth Abrams, Howard Leaf, Jose Farrer, Noreen Haren, Patty Masterson, Elizabeth Maccario, Sadma Sathe, Merigene White, Melanie Maslow, and Michael Simberkoff with the Veterans Affairs New York Harbor Healthcare System and Karen Longtine and Melissa O’Neil at the University of Massachusetts, for patient recruitment and interviews.
Footnotes
Potential conflicts of interest: none reported.
Financial support: New York University School of Medicine (National Institutes of Health [NIH] grants M01 RR00096 and AI027742); University of Massachusetts (New York State Department of Health contract C018439 and NIH grants AI057319 and AI49320).
Presented in part: 44th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 30 October-2 November 2004 (abstract G-535).
References
- 1.Irons JV, Sullivan TD, Cook EB, Cox GW, Hale RA. Outbreak of smallpox in the lower Rio Grande Valley of Texas in 1949. Am J Public Health. 1953;43:25–9. doi: 10.2105/ajph.43.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammarlund E, Lewis MW, Hansen SG, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–7. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy JS, Frey SE, Yan L, et al. Induction of human T cell-mediated immune responses after primary and secondary smallpox vaccination. J Infect Dis. 2004;190:1286–94. doi: 10.1086/423848. [DOI] [PubMed] [Google Scholar]
- 4.Demkowicz WE, Jr, Littaua RA, Wang J, Ennis FA. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J Virol. 1996;70:2627–31. doi: 10.1128/jvi.70.4.2627-2631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breman JG, Henderson DA. Diagnosis and management of smallpox. N Engl J Med. 2002;346:1300–8. doi: 10.1056/NEJMra020025. [DOI] [PubMed] [Google Scholar]
- 6.Reed KD, Melski JW, Graham MB, et al. The detection of monkeypox in humans in the Western hemisphere. N Engl J Med. 2004;350:342–50. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Updated interim case definition for human monkeypox. 2004 January; Available at: http://www.cdc.gov/ncidod/monkeypox/casedefinition.htm. Accessed 4 April 2006.
- 8.Hughes K, Foster SO, Tarantola D, Mehta H, Tulloch JL, Joarder AK. Smallpox surveillance in Bangladesh. II. Smallpox facial scar survey assessment of surveillance effectiveness. Int J Epidemiol. 1980;9:335–40. doi: 10.1093/ije/9.4.335. [DOI] [PubMed] [Google Scholar]
- 9.Borkowsky W, Zhan M-X, Chen S-H, et al. Correlation between HIV-specific CD8 cell production of interferon-γ and plasma levels of HIV RNA in perinatally infected pediatric populations. J Infect Dis. 2004;190:722–6. doi: 10.1086/422757. [DOI] [PubMed] [Google Scholar]
- 10.Valentine FT, Kundu S, Haslett PA, et al. A randomized, placebo-controlled study of the immunogenicity of human immunodeficiency virus (HIV) rgp160 vaccine in HIV-infected subjects with ≥400/mm3 CD4 T lymphocytes. AIDS Clinical Trials Group Protocol 137. J Infect Dis. 1996;173:1336–46. doi: 10.1093/infdis/173.6.1336. [DOI] [PubMed] [Google Scholar]
- 11.Frey SE, Newman FK, Cruz J, et al. Dose-related effects of smallpox vaccine. N Engl J Med. 2002;346:1275–80. doi: 10.1056/NEJMoa013431. [DOI] [PubMed] [Google Scholar]
- 12.Weltzin R, Liu J, Pugachev KV, et al. Clonal vaccinia virus grown in cell culture as a new smallpox vaccine. Nat Med. 2003;9:1125–30. doi: 10.1038/nm916. [DOI] [PubMed] [Google Scholar]
- 13.Pross HF, Baines MG, Rubin P, Shragge P, Patterson MS. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. IX. The quantitation of natural killer cell activity. J Clin Immunol. 1981;1:51–63. doi: 10.1007/BF00915477. [DOI] [PubMed] [Google Scholar]
- 14.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication. World Health Organization; Geneva: 1988. [Google Scholar]
- 15.Rao A. Smallpox. Kothari Book Depot; Bombay: 1972. [Google Scholar]
- 16.Hammarlund E, Lewis MW, Carter SV, et al. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat Med. 2005;11:1005–11. doi: 10.1038/nm1273. [DOI] [PubMed] [Google Scholar]
- 17.Terajima M, Cruz J, Leporati AM, Demkowicz WE, Jr, Kennedy JS, Ennis FA. Identification of vaccinia CD8+ T-cell epitopes conserved among vaccinia and variola viruses restricted by common MHC class I molecules, HLA-A2 or HLA-B7. Hum Immunol. 2006;67:512–20. doi: 10.1016/j.humimm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Terajima M, Cruz J, Raines G, et al. Quantitation of CD8+ T cell responses to newly identified HLA-A*0201-restricted T cell epitopes conserved among vaccinia and variola (smallpox) viruses. J Exp Med. 2003;197:927–32. doi: 10.1084/jem.20022222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karem KL, Reynolds M, Olson V, Li Y, Damon IK. Monkeypox out-break diagnostics and implications for vaccine protective effect. Nat Med. 2006;12:495–6. doi: 10.1038/nm0506-495. [DOI] [PubMed] [Google Scholar]
- 20.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–73. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 21.el-Ad B, Roth Y, Winder A, et al. The persistence of neutralizing antibodies after revaccination against smallpox. J Infect Dis. 1990;161:446–8. doi: 10.1093/infdis/161.3.446. [DOI] [PubMed] [Google Scholar]
- 22.Combadiere B, Boissonnas A, Carcelain G, et al. Distinct time effects of vaccination on long-term proliferative and IFN-γ-producing T cell memory to smallpox in humans. J Exp Med. 2004;199:1585–93. doi: 10.1084/jem.20032083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsieh SM, Pan SC, Chen SY, Huang PF, Chang SC. Age distribution for T cell reactivity to vaccinia virus in a healthy population. Clin Infect Dis. 2004;38:86–9. doi: 10.1086/380460. [DOI] [PubMed] [Google Scholar]