Living organisms go to extreme lengths to maintain a constant internal environment. A conspicuous example of this is the level of circulating metabolites, most critically glucose, which in normal individuals is maintained within a restricted range. Accomplishing this feat is not trivial, as considerable variability in food intake and the need for the generation of energy leads to wide swings in the input and consumption of substrates, respectively. One of the major regulatory mechanisms controlling blood glucose is provided by the endocrine system, most notably the beta cells of the pancreas, which secrete insulin in response to an increase in circulating nutrients. Insulin suppresses production of glucose by the liver as well as accelerating sugar uptake into muscle and adipose tissue, the consequence of which is to maintain blood glucose in a healthy range. However, a group of diseases that share a defect in the ability of insulin to elicit its appropriate biological actions can further stress the beta cell; this increased demand compels the endocrine pancreas to increase the production of insulin in an effort to overcome its end organ resistance (1, 2). The beta cells, like most endocrine organs, accomplish this in two ways: increasing the absolute secretion of insulin per cell as well as expanding the total beta cell mass (3). In this issue of PNAS, Okada et al. (4) provide an indication of one of the critical signaling pathways that is necessary for compensatory beta cell hyperplasia.
The major cell extrinsic determinants of cell and organ growth are growth factors and nutrients. The peptides that control beta cell mass have been a subject of considerable interest with much of the recent focus on insulin itself and its close relative, insulin-like growth factor 1 (IGF-1). Although it is clear that the insulin and IGF-1 signaling are operative in cell culture models of pancreatic beta cells, the consequences of activation of each of these pathways in vivo has generated controversy (5). Most recent data, particularly of the genetic type reported by Okada et al. (4), support a positive role for these pathways in maintaining or stimulating beta cell growth and/or function (6–9). An indication that there are important differences between these signaling pathways is that deletion of the insulin receptor exclusively in the mouse beta cell (BIRKO) results in a progressive loss of beta cell mass and insulin content, whereas the beta cell-specific IGF-1 receptor knockout mice (BIGFRKO) display an insulin secretory defect (6–9). Combined deletion of these receptors in the beta cell results in a further deterioration of function and mass suggesting some degree of overlap or compensation between the two receptor signaling cascades (10).
In the current report, Okada et al. (4) again delete the insulin receptor in beta cells but now induce insulin resistance by two different means. The first of these models is the liver-specific insulin receptor knockout (LIRKO) mouse, which exhibits severe insulin resistance but does not develop overt hyperglycemia (11). This is most likely due to a dramatic increase in pancreatic beta cell mass. To determine whether this compensatory response requires the insulin receptor, Okada et al. generated mice with both liver and beta cell insulin receptor deficiency (BIRKO/LIRKO). In contrast to the LIRKO mice, which maintain normal blood sugar until 4 weeks of age, the BIRKO/LIRKO mice develop hyperglycemia by 2–3 weeks. However, ablation of insulin receptor in the liver also results in a functional decrease in IGF-1 action throughout the organism. This confuses the question of whether insulin receptor is required in the beta cell during compensatory hyperplasia when there is a functional IGF-1 response also present. To resolve this issue, the other model of insulin resistance Okada et al. use is diet-induced obesity, which is not known to alter IGF-1 signaling and is thought to more closely mimic the common cause of insulin resistance in humans (12). In agreement with the BIRKO/LIRKO mice, removal of the insulin receptor from the beta cells prevented the increase in beta cell mass in response to high-fat diet. In contrast, mice with the IGF-1 receptor deleted in the beta cells expanded their beta cell mass in a manner similar to wild-type mice.
One conclusion from these studies is the striking disparity between the lack of need for insulin or IGF-1 receptor during development of the endocrine pancreas and the requirement for insulin receptor in compensatory hyperplasia. These data provide a strong argument against a model in which provoked proliferation of adult beta cells is preceded by “dedifferentiation” to a less mature phenotype followed by a recapitulation of normal developmental expansion. What then is the role of the insulin receptor in the stressed adult beta cell? Okada et al. (4) observe a correlation between elevated insulin levels and beta cell proliferation and suggest that the insulin receptor itself is the primary recipient of the message to divide. In support of this, they point out that LIRKO mice eventually develop hypoglycemia but that increased insulin and beta cell mass persist (11). However, there remain a number of difficulties with the model that autocrine production of insulin provides the primary regulatory signal for beta cell expansion. First, it is difficult to conceive of how a cell secreting insulin into its immediate environs could be geared to sense the level of the hormone in blood (5). Second, and more problematic, are the well established consequences of altering blood insulin levels independent of resistance. For example, hyperinsulinemia, whether by administration or insulinomas, results in decreased beta cell mass (14, 15). In addition, ablation of the genes that encode mouse insulin leads to the expected diabetes but with a marked islet hyperplasia (15, 16). Thus, it is difficult to imagine how simply raising insulin levels during beta cell stress could activate compensatory hyperplasia unless there is also a strong concomitant signal.
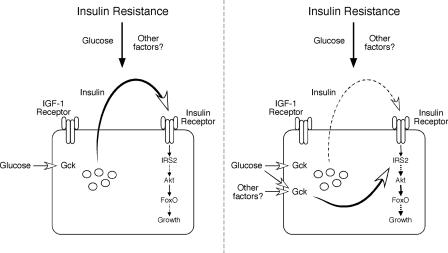
Nonetheless, in view of the data from Okada et al. (4), one might postulate an alternative requirement for insulin receptor, not as the primary determinate of compensatory hyperplasia but in the maintenance of an essential, permissive pathway (Fig. 1). The notion that insulin might provide a basal activity for certain signaling intermediates to allow proliferation is neither implausible nor, to our knowledge, refuted by existing data. For example, despite the textbook assertion of lack-of-insulin effects in the unfed animal, the true absence or even a severe reduction in insulin signaling during fasting has profound consequences for the organism, most notably fasting hyperglycemia, the major diagnostic criterion for diabetes mellitus. In other words, if some insulin signaling is required for metabolic homeostasis apparently under all conditions, might the same also be true for adult beta cell proliferation? Perhaps this provides a “failsafe” mechanism, such that if persistent beta cell proliferation causes a reduction in function, then the falling insulin levels would curb expansion and allow the beta cells to recover.
Fig. 1.
Insulin resistance provokes expansion of the adult beta cell mass, probably by stimulating proliferation of mature beta cells. In their report, Okada et al. (4) show that such compensatory hyperplasia requires the presence of insulin receptors but not insulin-like growth factor 1 (IGF-1) receptors. They describe two models to explain this process. In the first (Left), insulin resistance leads to an increase in circulating glucose, which, through the carbohydrate sensor glucokinase (Gck), augments insulin secretion from the beta cell. The insulin then acts in an autocrine/paracrine manner to activate a well described signaling cascade through IRS2 and Akt to initiate cell growth. This process is independent of the IGF-1 receptor. In an alternative mechanism (Right), insulin resistance also leads to an increase in glucose as well as other unknown factors that act on the beta cell. The critical difference in this model, however, is that insulin is not the primary initiating signal for growth but provides a permissive input to allow response to glucose or other factors by another mechanism, possibly also dependent on Gck. Note that in both cases, the hyperplastic response is absolutely dependent on cellular insulin but not IGF-1 receptors, as clearly shown by Okada et al.
If insulin is not the primary determinant of beta cell growth, then what does report the existence of insulin resistance to the beta cell? Considerable evidence supports a dominant role for glucose in this process (15). For example, Terauchi et al. (17) induced insulin resistance by placing mice on a high-fat diet but used mice heterozygous for a null mutation in glucokinase, the canonical glucose sensor of the beta cell. As expected, beta cells from these mice demonstrated a blunted insulin secretory response to glucose but also were impaired in their ability to proliferate in response to insulin resistance. The authors posit that the signal through glucokinase works, at least in part, by increasing the level of insulin receptor substrate 2 (IRS2) and thus amplifying the insulin signaling cascade. If this model is correct, it would stand to reason that some signaling through the insulin receptor would be required to allow IRS2 to exert its effect.
In spite of the logic of this model, there remains considerable resistance to the idea that glucose is the primary signal for beta cell growth. The rationale is that because during insulin resistance beta cell hyperplasia often precedes measurable hyperglycemia, increased serum glucose cannot be the signal for growth. However, this argument relies on the physician investigator being the equal of the beta cell in perceiving modest alterations in blood glucose. More likely, intermittent sampling and current instruments fail to replicate the sensitivity of the endocrine pancreas, and metabolic abnormalities exist long before we can detect them. This is not to say that other adult regulators of beta cell proliferation do not exist; however, proof of this awaits identification of such factors.
Finally, why should we care about how the pancreatic beta cell responds to the stress of insulin resistance? As alluded to above, a group of diseases characterized by insulin resistance is increasing in prevalence in both Western and developing societies at an alarming rate, largely because of the marked changes in lifestyle and diet of the last 50 years (18). In particular, modern conveniences and the decline of manual labor have reduced our energy expenditure such that obesity is reaching epidemic proportions. Although the precise molecular mechanisms are still hotly debated, increased adipose load invariably leads to insulin resistance and enhanced demands on the beta cell (1). How the endocrine pancreas responds to such stress determines whether disease ensues and, if so, the nature of the illness. Should hormone secretion not keep pace with worsening insulin resistance, the result is diabetes mellitus. In fact, a prevalent view asserts that the critical genetic determinant of progression from obesity through insulin resistance to diabetes mellitus is the capacity of the beta cell to maintain enhanced insulin secretion in the face of circulating toxins such as carbohydrate and lipids (1). Thus, the clear demonstration of a critical role for the insulin receptor in supporting compensatory beta cell hyperplasia is an important step toward understanding this process and ultimately designing therapeutics to enhance it.
Footnotes
The authors declare no conflict of interest.
See companion article on page 8977.
References
- 1.Kahn SE, Hull RL, Utzschneider KM. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 2.Lingohr MK, Buettner R, Rhodes CJ. Trends Mol Med. 2002;8:375–384. doi: 10.1016/s1471-4914(02)02377-8. [DOI] [PubMed] [Google Scholar]
- 3.Bouwens L, Rooman I. Physiol Rev. 2005;85:1255–1270. doi: 10.1152/physrev.00025.2004. [DOI] [PubMed] [Google Scholar]
- 4.Okada T, Liew CW, Hu J, Hinault C, Michael MD, Krutzfeldt J, Yin C, Holzenberger M, Stoffel M, Kulkarni RN. Proc Natl Acad Sci USA. 2007;104:8977–8982. doi: 10.1073/pnas.0608703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leibiger IB, Leibiger B, Berggren P-O. FEBS Lett. 2002;532:1–6. doi: 10.1016/s0014-5793(02)03627-x. [DOI] [PubMed] [Google Scholar]
- 6.Mauvais-Jarvis F, Kulkarni RN, Kahn CR. Clin Endocrinol. 2002;57:1–9. doi: 10.1046/j.1365-2265.2002.01563.x. [DOI] [PubMed] [Google Scholar]
- 7.Lu Y, Herrera PL, Guo Y, Sun D, Tang Z, LeRoith D, Liu J-L. Diabetes. 2004;53:3131–3141. doi: 10.2337/diabetes.53.12.3131. [DOI] [PubMed] [Google Scholar]
- 8.Kulkarni RN, Holzenberger M, Shih DQ, Ozcan U, Stoffel M, Magnuson MA, Kahn CR. Nat Genet. 2002;31:111–115. doi: 10.1038/ng872. [DOI] [PubMed] [Google Scholar]
- 9.Xuan S, Kitamura T, Nakae J, Politi K, Kido Y, Fisher PE, Morroni M, Cinti S, White MF, Herrera PL, et al. J Clin Invest. 2002;110:1011–1019. doi: 10.1172/JCI15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueki K, Okada T, Hu J, Liew CW, Assmann A, Dahlgren GM, Peters JL, Shackman JG, Zhang M, Artner I, et al. Nat Genet. 2006;38:583–588. doi: 10.1038/ng1787. [DOI] [PubMed] [Google Scholar]
- 11.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 12.West DB, Boozer CN, Moody DL, Atkinson RL. Am J Physiol. 1992;262:R1025–R1032. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- 13.Charge SBP, Rudnicki MA. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 14.Blume N, Skouv J, Larsson LI, Holst JJ, Madsen OD. J Clin Invest. 1995;96:2227–2235. doi: 10.1172/JCI118278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weir GC, Bonner-Weir S. J Clin Invest. 2007;117:81–83. doi: 10.1172/JCI30862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duvillie B, Currie C, Chrones T, Bucchini D, Jami J, Joshi RL, Hill DJ. Endocrinology. 2002;143:1530–1537. doi: 10.1210/endo.143.4.8753. [DOI] [PubMed] [Google Scholar]
- 17.Terauchi Y, Takamoto I, Kubota N, Matsui J, Suzuki R, Komeda K, Hara A, Toyoda Y, Miwa I, Aizawa S, et al. J Clin Invest. 2007;117:246–257. doi: 10.1172/JCI17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmet P, Alberti KG, Shaw J. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]