Abstract
The most complete and best-preserved cranium of a Paleogene anthropoid ever found, that of a small female of the early Oligocene (≈29–30 Ma) stem catarrhine species Aegyptopithecus zeuxis, was recovered from the Jebel Qatrani Formation (Fayum Depression, Egypt) in 2004. The specimen is that of a subadult and, in craniodental dimensions, is the smallest Aegyptopithecus individual known. High-resolution computed tomographic (microCT) scanning of the specimen's well preserved cranial vault confirms that Aegyptopithecus had relatively unexpanded frontal lobes and a brain-to-body mass ratio lower than those of living anthropoids. MicroCT scans of a male cranium recovered in 1966 [Egyptian Geological Museum, Cairo (CGM) 40237] reveal that previous estimates of its endocranial volume were too large. Thus, some amount of encephalization evolved independently in platyrrhine and catarrhine anthropoids, and the relative brain size of the last common ancestor of crown Anthropoidea was probably strepsirrhine-like or smaller. A. zeuxis shows extreme sexual dimorphism in craniodental morphology (apparently to a degree otherwise seen only in some highly dimorphic Miocene catarrhines), and the crania of female Aegyptopithecus lack a number of morphological features seen in larger males that have been accorded phylogenetic significance in catarrhine systematics (e.g., a well developed rostrum, elongate sagittal crest, and frontal trigon). Although a unique pattern of craniofacial sexual dimorphism may have characterized advanced stem and basal crown catarrhines, expression of various allegedly “discrete” craniofacial features may have been intraspecifically variable in early catarrhine species due to high levels of dimorphism and so should be treated with caution in phylogenetic analyses.
Keywords: catarrhine, dimorphism, Fayum, Propliopithecus, brain size
The Jebel Qatrani Formation exposed north of Birket Qarun in Egypt's Fayum Depression has long been an important source of Eocene and Oligocene vertebrates (1). Primates are common in the Formation and are today placed in >26 species, 19 genera, and ≈10 families. These primates include both prosimians and anthropoids; among the latter are the oldest known stem catarrhines (2). The specimen reported here, a female cranium of the propliopithecid catarrhine Aegyptopithecus zeuxis [Egyptian Geological Museum, Cairo (CGM) 85785, Fig. 1], is of early Oligocene age (≈29–30 Ma) (3). CGM 85785 was discovered by Rajeev Patnaik in the northeastern part of Quarry M, at a level ≈250 m above the base of the Jebel Qatrani Formation (4). Sweeping of gravel and desert pavement from Quarry M facilitates wind erosion and subsequent “wind harvesting” of fossils from an area approximately as large as two football fields and has led to the recovery of several other craniofacial fragments of Aegyptopithecus over the last 4 decades (5). The specimen described here is, however, by far the smallest mammalian cranium ever found at that locality.
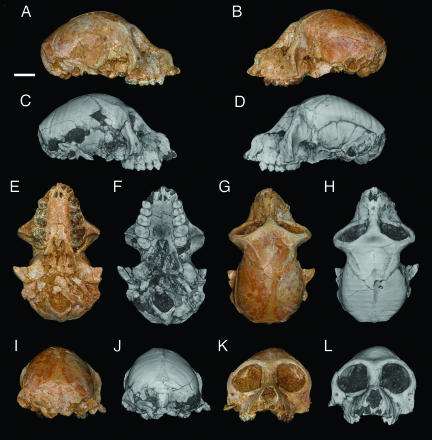
Fig. 1.
Photographs (color) and 3D digital reconstructions (grayscale) of CGM 85785, female cranium of A. zeuxis. Views are right lateral (A and C), left lateral (B and D), ventral (E and F), dorsal (G and H), caudal (I and J), and rostral (K and L). (Scale bar: 1 cm.)
CGM 85785 shows little distortion despite having been encased only in the loose and unconsolidated sands that make up the bulk of sediments at that site. There is some damage to the intracranial region, the anterior margin of the foramen magnum, and the right auditory region, the central portion of both zygomatic arches is broken away, and the six anteriormost teeth have fallen out of their sockets. The specimen is otherwise beautifully preserved, and most of the cranial structures that would be observable in a freshly prepared extant primate cranium can be examined. Observations of external cranial anatomy have been supplemented by high-resolution computed tomographic (microCT) scans that have revealed details of internal cranial morphology.
Craniodental Sexual Dimorphism in Aegyptopithecus.
Previous studies (6, 7) that relied on relatively small sample sizes documented sexual dimorphism in propliopithecid canine, premolar, and mandibular corpus size. The magnitude of craniodental dimorphism within A. zeuxis can now be more accurately gauged thanks to a much larger collection of propliopithecid fossils that has accumulated over the course of the last 25 years of work in the Fayum area. Values for cranial breadth and length of CGM 85785 are intermediate between those of the late Eocene catarrhine Catopithecus browni and those of the best preserved male cranium of A. zeuxis (CGM 40237) [for a comparison of the male and female A. zeuxis specimens, see supporting information (SI) Fig. 5]. In craniofacial dimensions, CGM 85785 is only ≈70% the size of CGM 40237, and the latter is even smaller than some other male A. zeuxis splanchnocrania. Although crushed and broken, another probable female partial cranium of A. zeuxis [Duke Paleontological Collections, Duke Lemur Center (DPC) 5401] is approximately intermediate in facial dimensions between CGM 40237 and CGM 85785, but its braincase is very similar in size. Because CGM 85785 is smaller than all previously known individuals of A. zeuxis in craniodental measurements, the new specimen raises the question, addressed below, of whether all of the propliopithecid specimens from Quarries I and M can be accommodated within two species, Propliopithecus chirobates and A. zeuxis (7).
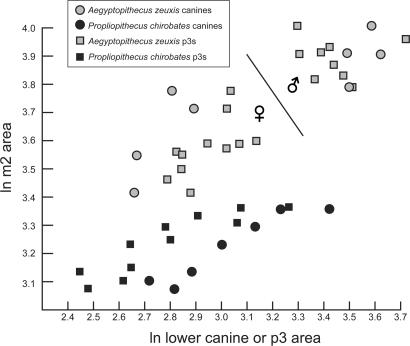
Bivariate plots of lower premolar and molar dimensions (Fig. 2) reveal a relatively cohesive cluster of P. chirobates (restricted to Quarry I) and a more variable, dispersed distribution of Aegyptopithecus, within which coefficients of variation (CVs) for lower molar 1 (m1) and m2 dimensions [m1 length (m1l), 6.1 (n = 28); m1 width (m1w), 8.3 (n = 27); m2 length (m2l), 8.3 (n = 30); m2 width (m2w), 9.3 (n = 30)] exceed those of even the most dimorphic extant hominoids (8). Bivariate plots of the area of the sexually dimorphic canine and third lower premolar versus the area of m2 nevertheless reveal distinct clusters within the Aegyptopithecus sample that are best explained as representing males and females of a single highly dimorphic species (Fig. 2). The pattern resembles that observable in the much younger late Miocene sample of Lufengpithecus from Lufeng, China, which arguably documents a single species characterized by elevated levels of postcanine sexual dimorphism (9). Similarly high postcanine sexual dimorphism has also been identified in other Miocene catarrhines (8, 10), although these cases have long been a matter of debate (11, 12).
Fig. 2.
Bivariate plot of ln canine or p3 area (x axis) versus ln m2 area (y axis) in A. zeuxis and P. chirobates. Larger individuals falling on the right side of the line dividing the Aegyptopithecus sample are presumed to be males, and smaller individuals on the left side are presumed to be females.
For a variety of reasons, we prefer a single species explanation for the distribution of Aegyptopithecus individuals from Quarries I and M. First, the variation evident within each of the two Aegyptopithecus clusters is generally less than that observable within the relatively cohesive P. chirobates assemblage, which exhibits coefficients of variation (CVs) for m1–2 dimensions (m1l, 5.1; m1w, 6.2; m2l, 6.4; m2w, 5.3; all n = 15) that fall well within the limits provided by extant catarrhine samples, despite probable time-averaging. Second, we consider it unlikely that one of two Aegyptopithecus species would have had relatively gracile canines and p3s, and the other relatively large and robust canines and lower premolars 3 (p3s) (note that in Fig. 2, canine area in the smaller cluster is consistently smaller than p3 area, whereas in the larger cluster, canine area is as large or larger than p3 area). Finally, there are no consistent qualitative differences in molar or premolar morphology between the two clusters.
Although the remote possibility exists that two very closely related and morphologically indistinguishable Aegyptopithecus species of similar size coexisted in the same area, and that sampling of both species has led to an inflated estimate of dimorphism, in our opinion it is more parsimonious to conclude that only a single large propliopithecid species, A. zeuxis, has been sampled at the Quarry I/M level of the Jebel Qatrani Formation and that time-averaging might have exaggerated apparent dimorphism and size variation in the species. Quarries I and M are at slightly different stratigraphic levels, and each of the two sites certainly documents a considerable span of time. Nevertheless, there remains no overlap between presumed A. zeuxis males and females in either m1 or m2 area, and thus A. zeuxis appears to provide the earliest evidence for the kind of extreme postcanine dental sexual dimorphism (and, by extrapolation, probably body mass sexual dimorphism) that is otherwise only documented later in the Miocene catarrhine fossil record. The presence of this pattern in an advanced stem catarrhine and among advanced stem or basal crown catarrhines and various Miocene hominoids increases the likelihood that the last common ancestor of crown Catarrhini was similarly highly dimorphic.
Description and Comparisons of CGM 85785.
Orbital, frontal, and jugal region.
The relatively small orbital apertures of CGM 85785 clearly indicate that A. zeuxis was a diurnal primate, as has long been thought. As in other specimens of A. zeuxis, the interorbital region of CGM 85785 is broad, being a little more than approximately half of orbital height. Posteriorly, the interorbital region becomes much thinner; the inner walls of each orbit are damaged, and it is not possible to calculate the area of either optic foramen. It is unlikely that an interorbital fenestra was present. There is no ethmofrontal sinus. The postorbital septa, although fragile, are largely intact, and the portion provided by the jugal bone is expanded and large, providing full postorbital closure. The frontal component of the postorbital septum on both sides is expanded downwards, and although the pterion region is damaged on both sides, the frontal appears to have a small contact with the alisphenoid, as in contemporaneous Parapithecus grangeri (13) and extant catarrhine primates (14). The inferior orbital fissure is small and resembles in outline that of other individuals of Aegyptopithecus. On either side below the orbits there is a zygomaticofacial foramen that is comparatively smaller than that of Parapithecus. Under the right orbit, there is a single infraorbital foramen; two foramina are present under the left orbit.
The angle of orbital convergence in CGM 85785, as measured by the method described by Simons and Rasmussen (15), is ≈130–135°. The angle of convergence is 15–30° lower in the parapithecids Apidium and Parapithecus, and in the stem catarrhine Catopithecus it is between 4° and 15° lower. The degree of frontation of the orbit is also much higher than in Parapithecus. In CGM 85785, the lateral margin of the orbit ascends from the Frankfurt Plane at a little >90°, whereas in Parapithecus (DPC 18651], this angle is closer to 60–62° (13).
Rostral region.
As in P. grangeri and all other early anthropoids, the lacrimal bone and foramen lie within the orbit. Ventrally, the maxilla makes up a portion of the ventral orbital margin between the jugal and lacrimal. As in other A. zeuxis (e.g., DPC 8794) and Parapithecus, the nasal bones are long, but overall the rostrum is not nearly as prominent as in males such as CGM 40237. In CGM 85785, orbital height is ≈90% of the nasal length, which makes the nasals comparatively rather longer than in Parapithecus. Damaged specimens of late Eocene Catopithecus and Proteopithecus suggest that those genera had nasals whose relative length was comparable to that of A. zeuxis. The nasal aperture in CGM 85785 is teardrop shaped in outline, and much deeper than in Parapithecus. The dorsal surface of the rostrum is concave in lateral view.
Premaxilla, maxilla, and palatines.
The premaxillae have very large and broad ascending wings resembling those of other known Aegyptopithecus individuals. Unlike the latter, however, here there is no central foramen in this wing. On the palatal surface, the premaxilla is relatively rostrocaudally elongated when compared with that of P. grangeri, in which this region is abbreviated. The incisive foramina are situated well anterior to a line between the posterior margins of the small canine alveoli. Each is connected by a suture, defining the separation between premaxilla and maxilla, which runs across to the canine alveolus. Incisors and canine alveoli are well preserved, and those of the central incisors are larger than those of the lateral incisors and are closely approximated, as is typical of anthropoid primates. There is, however, less of a discrepancy in the size of the I1-I2 alveoli when compared with those of P. grangeri. Apparently, the adult canines had at least begun to erupt, but, perhaps lacking fully formed roots, fell out postmortem. On both sides of CGM 85785, upper premolar 3 (P3) to upper molar 3 (M3) are present, but the third molars have not erupted as far down as the enamel base on M2. Because of the relative immaturity of the individual, there is almost no wear on the teeth. For dental metrics, see Table 1.
Table 1.
Dental measurements of CGM 85785 in millimeters
| Side | P3 | P4 | M1 | M2 | M3 |
|---|---|---|---|---|---|
| Left | |||||
| Length | 4.8 | 3.3 | 4.9 | 5.2 | 5.0 |
| Width | 5.1 | 6.4 | 6.7 | 8.1 | 7.7 |
| Right | |||||
| Length | 4.6 | 3.4 | 5.1 | 5.2 | 5.0 |
| Width | 5.1 | 6.3 | 6.6 | 7.8 | 7.8 |
The maxilla of CGM 85785 is less tall (dorsoventrally) than in A. zeuxis specimens such as DPC 8794 or CGM 40237. The choanae are situated rostral to the back of the erupting third molars, and the palate is slightly arched and bears a distinct postpalatine spine as in other A. zeuxis. When compared with Parapithecus, the palatine foramina are not as large and well defined, and the pyramidal processes are relatively laterally placed. The palatal surface is ventrally deflected with respect to the external plane of the basioccipital-basisphenoid (i.e., the face is slightly klinorhynch); calculation of the angle between the two surfaces is difficult because the basisphenoid is damaged, but it is probably between 150° and 160°.
Posterior dorsal braincase.
This part of the braincase is preserved with minimal distortion. The temporal lines run posteriorly, and at the middle of the parietals are closely approximated but do not contact at the midline; therefore, there is no frontal trigon and elongate sagittal crest as in previously described individuals of A. zeuxis and the Miocene catarrhines Victoriapithecus and Afropithecus (16). Further back, the temporal lines diverge laterally and meet the middle of the nuchal crests, which are poorly developed when compared with those of CGM 40237. Less extensive development of the temporal and nuchal musculature might be expected given that the individual is subadult, but another, more mature female individual (DPC 5401) also has very weakly developed temporal lines and no frontal trigon or sagittal crest.
Endocranium and brain.
The internal cranial cavity is generally well-preserved, although there is some distortion from breakage in the temporals and damage to the orbital walls. There is a large subarcuate fossa for the parafloccular lobe of the cerebellum and no evidence for an ossified tentorium cerebelli, as occurs in platyrrhine anthropoids (17). There is plastic deformation to the right orbital margin and frontal bone, and this distortion has caused some slight problems with the reconstruction of the endocranial volume, particularly in the area of the temporal and frontal lobes.
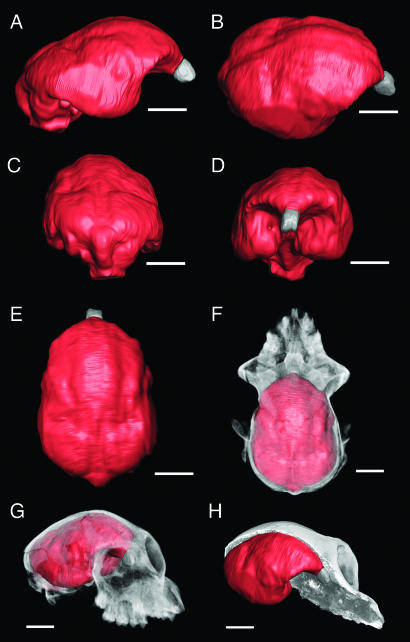
In most respects, the structure of the endocast (Fig. 3) is similar to that of the earlier Aegyptopithecus zeuxis reconstructions (18, 19). When calculated without correction, the endocranial volume of CGM 85785 is ≈14.63 cm3, and the olfactory bulb volume is 0.088 cm3. The plastic deformation evident externally on the cranium is also clearly evident in the three-dimensional reconstruction. This distortion has relatively little impact on the estimated endocranial volume, however. To test the effects of distortion on the volume measurements, the less distorted left half of the reconstruction was extracted along the midline. The volume of this half was 7.28 cm3, resulting in a full, corrected volume of 14.56 cm3. Using the same technique to estimate the olfactory bulb volume yields a somewhat higher estimate of 0.102 cm3. In the case of the endocranial volume, the difference is <0.5%, whereas the difference for the olfactory bulbs is 16%. In our opinion, values of 14.6 cm3 for the endocranial volume and 0.102 cm3 for the olfactory bulb volume represent accurate approximations on the basis of the specimen preservation.
Fig. 3.
Digitally extracted endocast of CGM 85785. (A) Right lateral view. (B) Right dorsolateral view. (C) Dorsocaudal view. (D) Rostral view. (E) Dorsal view. (F) Cranium in dorsal view with bone rendered as translucent and endocast as solid (olfactory bulbs rendered as gray), demonstrating the relationship of endocast to surrounding bones. (G) As in F, rostrolateral view. (H) Cranium sagittally bisected, demonstrating the relationship of endocast to surrounding structures. (Scale bar: 1 cm.)
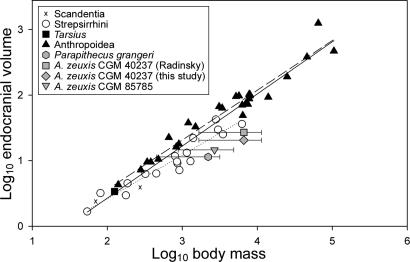
The endocranial volume for CGM 85785 falls well below previous estimates for A. zeuxis (Fig. 4). Radinsky (18) initially estimated a cranial capacity of between 30 and 34 cm3 on the basis of a partially reconstructed endocast of CGM 40237, and this was later reduced to 27 cm3 (20). Simons (19) similarly found a cranial capacity of 27 cm3 from the partial female skull DPC 5401, but this estimate was based on a partial reconstruction of the ventral part of the brain that was clearly too large. The actual dimensions of the brain case of DPC 5401 are almost the same as in CGM 85785, and therefore volume in both specimens should be nearly the same. Our reanalysis of the CGM 40237 endocranial volume by using high-resolution CT scans yielded a volume of between 20.5 and 21.8 cm3, suggesting that previous analyses overestimated the cranial capacity of this larger male individual. The ratio of male-to-female endocranial volume in A. zeuxis was thus ≈1.4–1.5, which can be easily accommodated within similarly dimorphic catarrhines, such as Gorilla (21), and a number of cercopithecid genera. The ratio of olfactory bulb volume to endocranial volume in CGM 85785 falls close to that of Parapithecus (22) and is on the lower end of the range of living strepsirrhines; however, the olfactory bulbs are not particularly small for an anthropoid of its body size.
Fig. 4.
Endocranial volume versus estimated body mass in Fayum anthropoids compared with data from extant primates and tree shrews (40). Ranges for estimated body mass of P. grangeri and male and female A. zeuxis are derived from equations for prediction of body mass from various skeletal and dental measurements [e.g., orbital area (41), bizygomatic width (28), skull length, m1 area (42), and postcranial measurements (43)]; individual plots are the average of these mean estimates.
Radinsky (18) and Simons (19) identified (on the CGM 40237 and DPC 5401 endocasts, respectively) a rostrally located central sulcus and intraparietal sulci with caudal termini located near the mediolaterally oriented lunate sulci. On the CGM 85785 endocast there is no clear evidence for a central sulcus, and the shallow intraparietal sulci extend rostrally toward the frontal lobes. Although the boundary between the primary motor and somatosensory cortices is unclear, it is obvious from CGM 85785 and other available endocasts that the frontal lobes were relatively unexpanded when compared with extant anthropoids (see also ref. 23). There is a well defined, and relatively rostrally placed, lunate sulcus delimiting the occipital lobe, confirming that Aegyptopithecus had an expanded primary visual cortex. The paths of the sylvian and superior temporal sulci identified by Radinsky (18) and Simons (19) are difficult to follow in the digitally reconstructed endocast, perhaps because of endocranial distortion.
Basicranium.
The pterygoid wings of CGM 85785 are much better preserved than those of CGM 40237. Unlike Parapithecus, the lateral wing does not contact the auditory bulla. There is some distortion to the basicranium, but the petrosals and ectotympanics are better preserved than in CGM 40237. The internal carotid foramina are located anterior to a line drawn across the anterior margin of the foramen magnum, and an enlarged promontorial branch of the internal carotid artery takes a perbullar path through the auditory bulla. As in other crown anthropoids, the anterior accessory cavity (AAC) is extensively trabeculated; in Parapithecus, there is very little evidence for AAC trabeculation. Other aspects of the petrosal anatomy of CGM 85785 are discussed by R. F. Kay, E.L.S., and J. L. Ross (unpublished manuscript).
On the left side of CGM 85785, the ectotympanic resembles that of CGM 40237 in having a ragged lateral margin, especially on the dorsal side where it is protected from breakage. The right side is more obscured and broken. The presence of an annular ectotympanic in Catopithecus (15), Parapithecus (13), Proteopithecus (24), and platyrrhines has convincingly established that this is the primitive condition within Anthropoidea and that the tubular condition in crown catarrhines is derived. In three Aegyptopithecus specimens (CGM 85785, CGM 40237, and DPC 5401) the ventral aspect of the ectotympanic has a beaded or lumpy margin, but dorsally, just behind the postglenoid process and the postglenoid foramen, it extends out laterally into a tympanic process, suggesting incipient development of the tubular auditory meatus. Zapfe (25) reported that the development of the external auditory meatus of Epipliopithecus is similarly incomplete ventrally. In Proconsul, the ectotympanic is short and has an uneven or ragged lateral margin, consisting of flange-like projections extending laterally that are longer on the dorsal side (26).
Mandibular form and lower dentition.
Several mandibles from Quarries I and M are attributable to A. zeuxis and a considerable number of them include the lower dentition from p3 through m3. Mandibular corpora of probable female A. zeuxis are distinctly smaller and the masseteric muscle insertions are less strongly developed than those of probable A. zeuxis males. In presumed males, the mandibular depth under the lower canine is typically greater, as is usually seen in anthropoids with sexually dimorphic canines (27). None of the female mandibles preserve the incisor sockets, but judging from the upper incisor breadth in CGM 85785, females probably shared the mesiodistally foreshortened incisor condition seen in male A. zeuxis. This proportion contrasts with the relatively enlarged incisors of P. chirobates, which can be determined very clearly from the enlarged incisor sockets of the type specimen of the latter species (CGM 26923) as well as in DPC 1069, in which a ratio of incisor (i1 + i2) area to m1 area is 0.73; in A. zeuxis (DPC 1112), this ratio is 0.64.
Discussion and Conclusions
The available collection of Aegyptopithecus cranial material leaves it clear that the relative brain sizes of early crown anthropoids were smaller than those of their crown platyrrhine and crown catarrhine descendants; thus, the degree of encephalization that has long been seen as a defining feature of Anthropoidea (28) evolved independently within that clade (22). A wide range of body mass estimates for male and female Aegyptopithecus unfortunately does not allow for precise estimates of relative brain size, but it appears likely that Aegyptopithecus was, like Parapithecus, at best strepsirrhine-like, and perhaps even non-primate-like, in its brain-to-body mass relationship (Fig. 4). Comparison of skull length to endocranial volume (see SI Fig. 6) similarly places Aegyptopithecus within or below the strepsirrhine range, although CGM 40237's position below strepsirrhines may be due in part to that specimen's relatively elongate rostrum. The fossil evidence reported here provides yet another striking example of detailed morphological convergence between catarrhines and platyrrhines and serves as a reminder that morphotypes derived from the anatomy of extant taxa alone may be unreliable guides for our understanding of ancient events such as the origin of crown Anthropoidea (29).
With regard to encephalization, it is now evident that hypotheses linking anthropoid neocortical expansion to diurnality, frugivory, and/or large social groups make little sense when these small-brained early anthropoids are taken into account (30). It has recently been argued that expansion of the primary visual cortex may have occurred in tandem with the evolution of high visual acuity in anthropoids and that later enlargement of the neocortex might have been driven by selection for increased visual processing associated with socioecological tasks (31). The first part of this hypothesis gains support from Aegyptopithecus endocasts, which confirm that this anthropoid had a large primary visual cortex. However one of the most striking differences between Fayum anthropoids and their extant relatives is the relatively expanded frontal lobes of the latter, which are primarily responsible for a variety of different functions related to general intelligence, such as memory, problem-solving, and forethought (32, 33). Although all of these functions are presumably more likely to be selected for in lineages with a preexisting capacity for increased visual processing, explanations for later encephalization in platyrrhines and catarrhines should probably continue to take into account selection for higher intelligence, ideally within the context of the groups' very different evolutionary histories in South America and Afro-Arabia, respectively. For instance, neontologists who are unfamiliar with the mammalian fossil record would not take into account the fact that crown catarrhines likely faced new demands on general intelligence after the influx of relatively large-brained Laurasian competitors and predators into Afro-Arabia during the early Miocene, whereas platyrrhines likely experienced similarly strong selection pressures after their arrival on a landmass with new competitors, predators, and phenological patterns.
Another surprising conclusion that can be drawn from available Aegyptopithecus material is that A. zeuxis was highly sexually dimorphic in postcanine tooth size, craniofacial morphology, brain size, and body mass. This is the earliest known example of a peculiar pattern otherwise known only from the fossil record of Miocene catarrhine evolution (9, 34), and further substantiates the already well established phylogenetic link between Aegyptopithecus and crown catarrhines. By analogy with other extant anthropoids, it is likely that A. zeuxis had a polygynous social structure and intense male–male competition for access to females (35), but such an extreme difference in male and female body mass is difficult to explain. It has been suggested that extreme sexual dimorphism could be due to selection for reduced female body mass, relaxation of constraint on male body size, or perhaps an ecological difference between males and females (35); unfortunately all such hypotheses are difficult, if not impossible, to test.
Regardless, extreme differences in the size of male and female Aegyptopithecus clearly led to striking differences in craniofacial morphology. Of particular note is the fact that female crania lack a number of facial features, such as a well developed rostrum, elongate sagittal crest, frontal trigon, and steep frontal, that have been accorded special phylogenetic significance in catarrhine systematics (16, 36). This is interesting, because of the few catarrhine faces known from the early and middle Miocene, those of males [Afropithecus turkanensis (37) and Victoriapithecus macinessi (16)] preserve the aforementioned features, whereas those of female specimens [Proconsul nyanzae or heseloni (38) and Turkanapithecus kalakolensis (39)] do not. Given that all of these taxa were sexually dimorphic (although not necessarily extremely dimorphic), more material will be required to determine whether these species conform to the pattern of craniofacial sexual dimorphism observable in A. zeuxis. In the absence of such information, these potentially size-related features should be treated with caution in phylogenetic analysis.
Materials and Methods
Aegyptopithecus crania were scanned on the Omni-X HD600 high-resolution x-ray CT system (Bio-Imaging Research, Lincolnshire, IL) at the Center for Quantitative Imaging at Pennsylvania State University. The X-TEK x-ray subsystem was used with energy settings of 160 kV and 0.150 mA and a source object distance of 183.3 mm. Serial cross-sectional slices were collected in the coronal plane for the entire cranium with a slice thickness of 0.06379 mm. Images were collected with 2,400 views, two samples per view, and 41 slices per rotation. After data collection, the image data were reconstructed as 16-bit 1,024 × 1,024 TIFF images with a field of view equal to 57.344 mm, resulting in x,y pixel sizes of 0.056 mm. A total of 1,107 slices were collected for the entire cranium.
The sandstone matrix infilling the cranial vault was sufficiently different from the fossil in density and material composition, so bone and matrix could be easily differentiated. The image data were input into the software package Amira 3.1.1 (Mercury Computer Systems, San Diego, CA) running on a Linux workstation with 8 GB RAM. To facilitate manipulation of the large data set, the entire data set was converted from 16 bit to 8 bit (reduction to 256 grayscale values) and down-sampled to a matrix size of 512 × 512. As a result of this step, the pixel size was reduced to 0.112 mm, but the slice thickness remained the same. The endocranial cavity was segmented using a combination of automatic and manual segmentation tools in Amira. The olfactory bulbs were also reconstructed on the basis of the olfactory fossa.
Supplementary Material
Acknowledgments
We thank H. Hamouda, K. Soliman, Z. el-Alfy, and the staff of the Egyptian Mineral Resources Authority and the Egyptian Geological Museum, Cairo, for supporting our work in Egypt. Field operations were managed by P. Chatrath. P. Gingerich (University of Michigan, Ann Arbor, MI) provided photographs for Fig. 1, E. C. Kirk (University of Texas, Austin, TX) provided the data for SI Fig. 6, A. Walker assisted with microCT scanning, and J. Fleagle and J. Rossie provided useful comments on the manuscript. This research was funded by National Science Foundation Grant BCS-0416164 and the Leakey Foundation. This is Duke Lemur Center publication no. 1002.
Abbreviations
- CGM
Egyptian Geological Museum, Cairo
- DPC
Duke Paleontological Collections, Duke Lemur Center
- microCT
high-resolution computed tomography
- Pn
upper premolar
- pn
lower premolar
- Mn
upper molar
- mn
lower molar.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703129104/DC1.
References
- 1.Simons EL, Rasmussen DT. In: The Geology of Egypt. Said R, editor. Rotterdam: A. A. Balkema; 1990. pp. 627–638. [Google Scholar]
- 2.Rasmussen DT. In: The Primate Fossil Record. Hartwig WC, editor. Cambridge, UK: Cambridge Univ Press; 2002. pp. 203–220. [Google Scholar]
- 3.Seiffert ER. Proc Natl Acad Sci USA. 2006;103:5000–5005. doi: 10.1073/pnas.0600689103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bown TM, Kraus MJ. US Geol Surv Prof Paper. 1988;1452:1–64. [Google Scholar]
- 5.Simons EL. J Hum Evol. 1987;16:273–289. [Google Scholar]
- 6.Fleagle JG, Kay RF, Simons EL. Nature. 1980;287:328–330. doi: 10.1038/287328a0. [DOI] [PubMed] [Google Scholar]
- 7.Kay RF, Fleagle JG, Simons EL. Am J Phys Anthropol. 1981;55:293–322. [Google Scholar]
- 8.Schrein C. J Hum Evol. 2006;50:460–468. doi: 10.1016/j.jhevol.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Kelley J, Plavcan JM. J Hum Evol. 1998;35:577–596. doi: 10.1006/jhev.1998.0253. [DOI] [PubMed] [Google Scholar]
- 10.Kelley J. J Hum Evol. 1986;15:461–495. [Google Scholar]
- 11.Kay RF. Proc Natl Acad Sci USA. 1982;79:209–212. doi: 10.1073/pnas.79.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker A, Teaford MF, Martin L, Andrews P. J Hum Evol. 1993;25:43–56. [Google Scholar]
- 13.Simons EL. Proc Natl Acad Sci USA. 2001;98:7892–7897. doi: 10.1073/pnas.051003398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleagle JG, Kay RF. J Hum Evol. 1987;16:483–532. [Google Scholar]
- 15.Simons EL, Rasmussen DT. Am J Phys Anthropol. 1996;100:261–292. doi: 10.1002/(SICI)1096-8644(199606)100:2<261::AID-AJPA7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Benefit BR, McCrossin ML. Nature. 1997;388:368–371. doi: 10.1038/41078. [DOI] [PubMed] [Google Scholar]
- 17.Horovitz I, MacPhee RDE. J Hum Evol. 1999;36:33–68. doi: 10.1006/jhev.1998.0259. [DOI] [PubMed] [Google Scholar]
- 18.Radinsky L. Am J Phys Anthropol. 1973;39:239–248. doi: 10.1002/ajpa.1330390214. [DOI] [PubMed] [Google Scholar]
- 19.Simons EL. Am J Sci. 1993;293A:383–390. [Google Scholar]
- 20.Radinsky L. J Hum Evol. 1977;6:79–86. [Google Scholar]
- 21.Lee S-H. Am J Phys Anthropol. 2005;127:263–266. doi: 10.1002/ajpa.20105. [DOI] [PubMed] [Google Scholar]
- 22.Bush EC, Simons EL, Allman JM. The Anatomical Record, Part A. 2004;281:1083–1087. doi: 10.1002/ar.a.20113. [DOI] [PubMed] [Google Scholar]
- 23.Bush EC, Allman JM. Proc Natl Acad Sci USA. 2004;101:3962–3966. doi: 10.1073/pnas.0305760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simons EL. Proc Natl Acad Sci USA. 1997;94:14970–14975. doi: 10.1073/pnas.94.26.14970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zapfe H. Schweiz Pal Abh. 1960;78:4–293. [Google Scholar]
- 26.Napier JR, Davis PR. Fossil Mammals Africa. 1959;16:1–69. [Google Scholar]
- 27.Plavcan JM, Daegling DJ. J Hum Evol. 2006;51:171–184. doi: 10.1016/j.jhevol.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Martin RD. Primate Origins and Evolution: A Phylogenetic Reconstruction. Princeton, NJ: Princeton Univ Press; 1990. [Google Scholar]
- 29.Seiffert ER, Simons EL, Simons CVM. In: Anthropoid Origins: New Visions. Ross CF, Kay RF, editors. Kluwer: New York; 2004. pp. 157–181. [Google Scholar]
- 30.Ross CF. Ann Rev Anthropol. 2000;29:147–194. [Google Scholar]
- 31.Kirk EC. J Hum Evol. 2006;51:76–90. doi: 10.1016/j.jhevol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen V-P, Huttunen M, Lönnqvist J, Standertskjöld-Nordenstam C-G, Kaprio J, Khaledy M, et al. Nat Neurosci. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- 33.Duncan J, Seitz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, Newell FN, Emslie H. Science. 2000;289:457–460. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- 34.Kelley J, Xu Q. Nature. 1991;352:151–153. doi: 10.1038/352151a0. [DOI] [PubMed] [Google Scholar]
- 35.Plavcan JM. Yrbk Phys Anthropol. 2001;44:25–53. doi: 10.1002/ajpa.10011.abs. [DOI] [PubMed] [Google Scholar]
- 36.Benefit BR, McCrossin ML. Am J Phys Anthropol. 1993;92:329–370. doi: 10.1002/ajpa.1330920307. [DOI] [PubMed] [Google Scholar]
- 37.Leakey REF, Leakey MG, Walker AC. Am J Phys Anthropol. 1988;76:289–307. doi: 10.1002/ajpa.1330760303. [DOI] [PubMed] [Google Scholar]
- 38.Le Gros Clark W, Leakey LSB. Fossil Mammals Africa. 1951;1:1–117. [Google Scholar]
- 39.Leakey REF, Leakey MG, Walker AC. Am J Phys Anthropol. 1988;76:277–288. doi: 10.1002/ajpa.1330760302. [DOI] [PubMed] [Google Scholar]
- 40.Stephan H, Frahm HD, Baron G. Folia Primatol. 1981;35:1–29. doi: 10.1159/000155963. [DOI] [PubMed] [Google Scholar]
- 41.Kappelman J. J Hum Evol. 1996;30:243–276. [Google Scholar]
- 42.Conroy GC. Int J Primatol. 1987;8:115–137. [Google Scholar]
- 43.Payseur BA, Covert HA, Vinyard CJ, Dagosto M. Am J Phys Anthropol. 1999;109:41–52. doi: 10.1002/(SICI)1096-8644(199905)109:1<41::AID-AJPA5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.