Abstract
UVB radiation-induced signaling in mammalian cells involves two major pathways: one that is initiated through the generation of DNA photoproducts in the nucleus and a second one that occurs independently of DNA damage and is characterized by cell surface receptor activation. The chromophore for the latter one has been unknown. Here, we report that the UVB response involves tryptophan as a chromophore. We show that through the intracellular generation of photoproducts, such as the arylhydrocarbon receptor (AhR) ligand 6-formylindolo[3,2-b]carbazole, signaling events are initiated, which are transferred to the nucleus and the cell membrane via activation of the cytoplasmatic AhR. Specifically, AhR activation by UVB leads to (i) transcriptional induction of cytochrome P450 1A1 and (ii) EGF receptor internalization with activation of the EGF receptor downstream target ERK1/2 and subsequent induction of cyclooxygenase-2. The role of the AhR in the UVB stress response was confirmed in vivo by studies employing AhR KO mice.
Keywords: EGF receptor; 6-formylindolo[3,2-b]carbazole; src; UVB; cyclooxygenase-2
Exposure of mammalian cells to UVB radiation (290–320 nm) results in a signaling response called the UV response (1–3). This response was shown to involve two major pathways. One that is initiated in the nucleus where UVB is absorbed by DNA and the subsequent formation of DNA photoproducts such as cyclobutane pyrimidine dimers are thought to represent the initiating signaling step (4–8). Cell enucleation experiments, however, have clearly demonstrated that a second part of the UV stress response occurs independently of nuclear DNA damage and is characterized by cell surface receptor clustering and subsequent activation of members of the MAPK (1). Activation of MAPK is relevant for UVB-induced skin inflammation and photocarcinogenesis (9–13). In particular, UVB-induced MAPK activation leads to increased expression of cyclooxygenase-2 (COX-2), the key enzyme in conversion of arachidonic acid to prostaglandins (14, 15) and COX-2 inhibition reduces UVB-induced skin tumor formation (16). The nature of the chromophore responsible for these nonnuclear UVB-induced signaling events has so far been enigmatic.
The arylhydrocarbon receptor (AhR) was discovered as a cytosolic, ligand-dependent receptor that mediates toxicity of polycyclic aromatic hydrocarbons (PAH) [e.g., benzo(a)pyrene] and halogenated PAH [e.g., tetrachlorodibenzo(p)dioxin (TCDD)] (17, 18). Numerous studies have provided conclusive evidence that all known toxic responses to TCDD are conveyed by the AhR (19, 20). Upon ligand binding, the AhR sheds its chaperones Hsp90 and associated proteins such as c-src (pp60src) (21), translocates into the nucleus where it dimerizes with its partner ARNT and activates genes including the xenobiotic metabolizing enzyme cytochrome P450 (CYP) 1A1. Dissociation of c-src from the ligand-activated receptor induces c-src translocation from the cytosol to the cell membrane, where it is thought to activate the receptor for EGF (EGFR) (22, 23). Activation of the AhR in the cytoplasm thus leads to signaling in two directions: toward the nucleus and toward the cell membrane.
We have hypothesized that AhR signaling is part of the UVB response, because not only known AhR ligands, but also exposure of rat and human skin to UVB induces AhR-dependent CYP1A activity and CYP1A1/1B1 mRNA and protein expression, respectively (24, 25). Also, transcriptional expression of CYP1A1 can be induced by UVB radiation in human cells in vitro (26). We have speculated that the AhR may transfer the UVB signal required for CYP1A1 expression from the cytoplasm to the nucleus and, e.g., via translocation of src kinase, to the cell membrane. Accordingly, src kinase activation is a prerequisite for important parts of the UV stress response, in particular the induction of c-jun (27) and the phosphorylation of extracellular signal-regulated kinase (ERK1/2) (28).
In this study, we demonstrated the intracellular formation of the AhR ligand 6-formylindolo[3,2-b]carbazole (FICZ) from the chromophore tryptophan and provide the evidence that (i) UVB irradiation translocates the AhR into the nucleus and induces CYP1A1 gene expression, (ii) the UVB-activated AhR additionally transfers the UVB signal to the cell membrane where it initiates EGFR internalization and EGFR dependent ERK1/2 phosphorylation, and (iii) this signaling pathway is of in vivo relevance because AhR KO mice show a compromised UVB responsiveness. Thus, AhR signaling is an integral part of the UVB stress response.
Results
UVB Irradiation of HaCaT Cells Causes Translocation of the AhR into the Nucleus and Induces Transcription of the AhR-Dependent Gene CYP1A1.
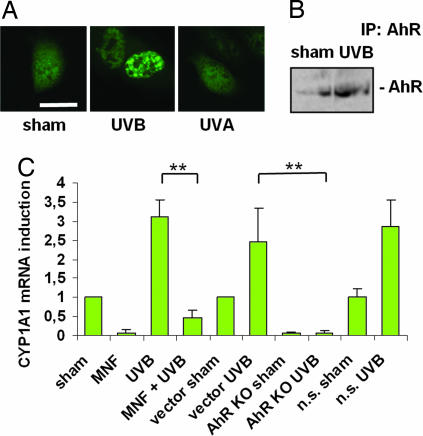
In many different cell types, AhR ligands such as TCDD instigate the translocation of the AhR from the cytoplasm into the nucleus. To test our hypothesis that also UVB irradiation, similar to known AhR ligands, translocates the AhR from the cytoplasm into the nuclear compartment, cells from the immortalized keratinocyte cell line HaCaT were transfected with a GFP-coupled AhR. Exposure of HaCaT cells to 10 mJ/cm2 UVB led to nuclear accumulation of the AhR-GFP fusion protein. In contrast to UVB, UVA radiation (30 J/cm2) did not cause AhR translocation, indicating wavelength dependency (Fig. 1A). Similarly, immunoprecipitation and Western blot analyses of nuclear proteins of UVB-irradiated HaCaT cells showed abundant amounts of native AhR protein, whereas less AhR protein was found in the nuclear compartment of sham-irradiated controls (Fig. 1B). UVB-induced AhR translocation was associated with transcriptional activation of CYP1A1 (Fig. 1C). To assess whether UVB-induced CYP1A1 expression was AhR-dependent, we next treated HaCaT cells for 1 h before irradiation with the competitive AhR inhibitor 3′methoxy-4′nitroflavone (MNF, 10 μM), that specifically targets the AhR ligand-binding site (29). MNF pretreatment caused an inhibition of UVB-induced CYP1A1 mRNA expression (Fig. 1C). To corroborate this finding, we generated AhR knockdown HaCaT (AhR KO) cells [see supporting information (SI) Fig. 7]. AhR knockdown abolished the capacity of these cells to increase CYP1A1 mRNA expression upon UVB exposure, whereas the vector and control nonsilencing (n.s.) cells remained unaffected (Fig. 1C). These data indicate that UVB radiation-induced translocation of the AhR from the cytoplasm into the nucleus leads to increased expression of an AhR-dependent gene in a ligand-dependent manner.
Fig. 1.
UVB irradiation causes translocation of the AhR into the nucleus and induces transcription of the AhR-dependent gene CYP1A1. (A) HaCaT cells were transiently transfected with a GFP-coupled AhR and irradiated with 10 mJ/cm2 UVB or 30 J/cm2 UVA. Although UVB irradiation translocated the AhR into the nucleus, UVA had no effect on AhR compartmental distribution. (Scale bar, 20 μm.) (B) AhR immunoprecipitation and Western blotting of HaCaT nuclear extracts that were UVB (10 mJ/cm2) or sham irradiated show an accumulation of AhR protein after irradiation. (C) Real-time RT-PCR analyses reveal an inhibition of UVB-induced CYP1A1 mRNA induction after treatment with the competitive AhR-inhibitor 3′methoxy-4′nitroflavone (MNF) or in AhR knockdown HaCaTs (AhR KO), whereas vector or AhR nonsilencing (n.s.) transduced cells were not impaired in CYP1A1 response. ∗∗, P < 0.01.
UVB-Induced EGFR Internalization and Downstream Signaling Is Controlled by AhR Activation in HaCaT Cells.
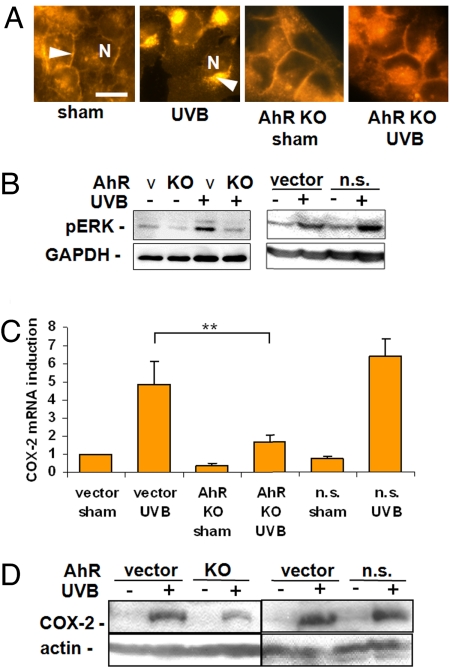
UVB radiation initiates internalization of cell surface receptors including the EGFR (2). Therefore, we next investigated the relevance of UVB-induced AhR activation for EGFR internalization by immunocytochemistry in UVB-irradiated HaCaT cells. Irradiation of AhR KO cells prevented UVB radiation-induced EGFR internalization (Fig. 2A). In addition, phosphorylation of EGFR-dependent MAPK ERK1/2 (30, 31) was antagonized by AhR knockdown (Fig. 2B), leading to inhibition of UVB-induced COX-2 mRNA and protein expression (14) (Fig. 2 C and D). The same results were obtained when the AhR was inhibited with MNF (SI Fig. 8 A–D). These results indicate that activation of AhR signaling triggers UVB-induced EGFR activation and subsequent downstream signal transduction events.
Fig. 2.
AhR controls epidermal growth factor receptor (EGFR) internalization and downstream signaling after UVB irradiation. (A) UVB irradiation (10 mJ/cm2) led to EGFR internalization with a disappearance from the cell membranes (arrow in sham control) and paranuclear accumulation (arrow in UVB irradiation) after 30 min. AhR knockdown (KO) prevented EGFR internalization (arrow indicates EGFR at the cell membrane; N indicates nuclei). (Scale bar, 20 μm.) (B) Western blot analyses of the EGFR downstream target ERK1/2 revealed UVB-induced ERK1/2 phosphorylation that is partially AhR-dependent because it is antagonized by AhR knockdown. Cells transduced with nonsilencing AhR shRNA (AhR n.s.) showed no effect on ERK1/2 phosphorylation compared with the vector controls. (C) Real-time RT-PCR demonstrated an inhibition of the UVB-induced COX-2 mRNA induction in AhR KO HaCaT cells compared with the vector or AhR n.s. transduced HaCaTs. (D) COX-2 Western blot shows a reduction of COX-2 protein induction after UVB irradiation in AhR KO cells compared with the vector and n.s. controls. ∗∗, P < 0.01.
The exact mechanism by which the AhR transfers the signal to the EGFR is not known. We observed that inhibition of src kinase by PP2 (10 μM) is associated with inhibition of UVB-induced EGFR internalization, ERK1/2 phosphorylation (28) and COX-2 mRNA and protein induction (see SI Fig. 9 A–D), suggesting that c-src acts as the mediator between AhR and EGFR (21–23).
The Role of Tryptophan in AhR-Activation by UVB.
It has been shown that tryptophan is a chromophore for UVB and that, under ex vitro conditions, UVB irradiation of tryptophan leads to the formation of FICZ, which is a high-affinity AhR ligand (32, 33). To determine the functional relevance of tryptophan photoproduct formation for UVB radiation-induced AhR-dependent signaling, we studied UVB-induced AhR-dependent responses in HaCaT cells that were incubated in tryptophan-free medium for 4 h before irradiation. Tryptophan starvation reduced the intracellular free tryptophan level from 0.3 μM/liter to undetectable levels (see SI Fig. 10). These tryptophan-deficient cells were compromised in their capacity to elicit a UVB response, as is shown for UVB-induced CYP1A1 (Fig. 3A) and COX-2 (see SI Fig. 11) mRNA expression as well as EGFR internalization (Fig. 3B). This failure to mount a UVB response could be overcome if tryptophan (1 mM) was added back to the culture medium of tryptophan-starved cells 1 h before irradiation (Fig. 4 A and B). GC–MS analyses revealed that this reintroduction of tryptophan led to an approximately 5-fold increase (1.6 μM) in free intracellular tryptophan concentration compared with the cells grown in normal medium (see SI Fig. 10). These data corroborate our previous notion that UVB radiation-induced CYP1A1 induction is tryptophan-dependent (26).
Fig. 3.
Tryptophan (Trp) is the chromophore for UVB and the precursor for the photoproduct formylindolo(3,2-b)carbazole (FICZ) that activates the AhR. Trp starvation (4 h) in trp-free medium abolished CYP1A1 mRNA inducibility (A) and EGFR internalization (B) after UVB irradiation (10 mJ/cm2) in HaCaT cells that were reconstituted after introduction of 1 mM Trp 1 h before irradiation. (Scale bar, 20 μm.) ∗∗, P < 0.01.
Fig. 4.
UVB irradiation leads to an intracellular formation of FICZ. Identification of the Trp-photoproduct FICZ by HPLC-MS: Trp-starved cells were incubated with 1 mM [13C1115N2]Trp (labeled with ∗) 1 h before irradiation with 60 mJ/cm2 UVB. Control cells were sham irradiated. After 10 min, cells were harvested, and cell extracts were prepared for HPLC-MS-MS analyses. The mass analysis revealed the occurrence of [13C15N]FICZ (arrow) with the expected mass of 305.3.
Identification of the Endogenous Formation of the Tryptophan Photoproduct FICZ.
The intracellular formation of tryptophan photoproducts like FICZ may be one prerequisite for endogenous AhR activation and initiation of the AhR-dependent UVB response as described above. So far, the generation of FICZ, the photoproduct with the highest AhR affinity, has been shown only ex vitro. To assess whether UVB causes the formation of FICZ in vivo, HaCaT cells were tryptophan-starved for 6 h and subsequently incubated with [13C1115N2]tryptophan 1 h before UVB irradiation. After 10 min, cells were harvested after removal of extracellular tryptophan by thorough washing, and UVB-induced formation of FICZ was assessed in cell extracts by HPLC-MS-MS analyses. This method has a detection limit of ≈15 pM. To assure that UVB-induced FICZ formation was above this detection limit, we had to choose the experimental conditions described above, i.e., Trp-preloading and a high UVB dose, although they are nonphysiologic. As shown in Fig. 4, UVB radiation led to the generation of ≈80 pM 13C15N-labeled FICZ at the expected mass of 305.3, which corresponds to a conversion rate of 0.0001%. Thus, UVB irradiation causes the formation of the tryptophan derivative FICZ in vivo in human cells.
Cellular Signaling Induced by the Tryptophan Photoproduct FICZ in HaCaT Cells Is AhR- and c-src-Dependent.
Previous work showed that FICZ induces Cyp1a1 in mouse hepatoma cells (34). Because AhR activation is cell type-specific (35), we next asked whether this CYP induction also occurs in HaCaT cells and whether there is AhR-dependency. Therefore, we treated AhR KO cells with FICZ (100 nM) and measured CYP1A1 mRNA induction by real-time RT-PCR. In contrast to vector and n.s. transduced cells, CYP1A1 is not inducible by FICZ in AhR-deficient cells indicating that FICZ induces CYP in HaCaT cells in an AhR-dependent manner (see SI Fig. 12A).
Next, we assessed whether FICZ is also involved in membrane-dependent signal transduction. Immunocytochemical analyses in AhR KO HaCaT cells revealed that EGFR internalization after treatment with FICZ for 30 min is AhR dependent (Fig. 5A). Interestingly, this FICZ-induced EGFR translocation cannot only be inhibited by AhR KO, but also by pretreatment of the cells with PP2 (10 μM) for one hour indicating that the effect is also c-src-dependent (see SI Fig. 12B). These findings were confirmed by analyses of the EGFR downstream target COX-2: mRNA analyses of FICZ treated AhR-proficient HaCaT cells disclosed that FICZ induces COX-2 in a dose-dependent manner (see SI Fig. 12C). Experiments in AhR KO cells confirmed an AhR dependence of COX-2 induction by FICZ on mRNA and protein levels (Fig. 5 B and C) as well as a c-src dependence as shown by pretreatment of the cells with PP2 (10 μM) (see SI Fig. 12D). Moreover, exposure of HaCaT cells for 1.5 h to FICZ in the lower picomolar range caused significant CYP1A1 and COX-2 mRNA induction (see SI Fig. 12 E and F). Longer incubation periods (4 h) did not cause gene induction at these low concentrations, confirming observations that FICZ is rapidly metabolized (34). These data suggest that FICZ may be one of the photoproducts responsible for the AhR-dependent cellular signaling responses toward the nucleus and toward the cell membrane. Furthermore, the signaling effects of FICZ mimic the cellular responses observed after UVB irradiation strongly implying that the AhR serves as a cytoplasmatic target which transfers the UVB signal, e.g., FICZ as one of the photoproducts generated from cytoplasmatic free tryptophan, from the cytoplasm to the nucleus and also to the cell membrane.
Fig. 5.
FICZ causes AhR-dependent signaling. AhR-proficient and -deficient HaCaT cells were treated with FICZ (100 nM) for the indicated times. (A) Immunocytochemical analyses demonstrated an AhR-dependent EGFR internalization 30 min after FICZ treatment. (B and C) Real-time RT-PCR and Western blot analyses showed AhR dependent COX-2 mRNA at 4 h (B) and COX-2 protein expression at 6 h (C) after exposure to FICZ. ∗∗, P < 0.01.
In Vivo Relevance of UVB-Induced AhR Activation.
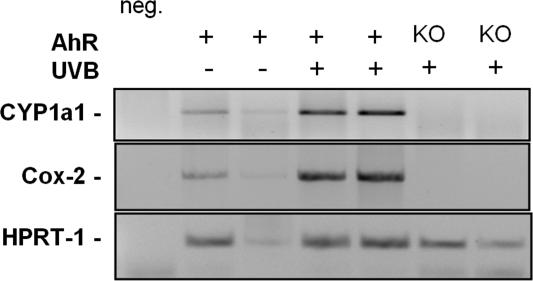
To assess the in vivo relevance of the AhR signaling pathway in the UVB response we have conducted comparative studies employing C57BL/6 mice and AhR KO mice. Two mice of each genotype were irradiated with 600 J/cm2 UVB. Twelve hours after irradiation, the skin was excised, the RNA prepared and RT-PCRs for Cyp1a1 and Cox-2 gene products performed. These end points were chosen because changes in gene expression of CYP1A1 and COX-2 can serve as suitable endpoints to reflect AhR-mediated nuclear and membranous signaling induced by UVB irradiation, respectively (Figs. 1 and 2). As is shown in Fig. 6, UVB irradiation induces Cyp1a1 and Cox-2 mRNAs in wild-type but not in AhR KO mice. Thus, AhR signaling appears to be involved in the UVB stress response in vivo as well.
Fig. 6.
AhR KO mice show a decreased UVB responsiveness. Dorsal skin of wild-type and AhR KO C57BL/6 mice was shaved 24 h before exposure. Mice were irradiated dorsally with a single exposure of UVB (20 min 40 sec; 600 J/cm2). Twelve hours after irradiation, mice were euthanized, and skin samples were excised. RT-PCR analyses indicate an AhR dependence of Cyp1a1 and Cox-2 mRNA induction in UVB-irradiated mouse skin.
Discussion
Although the consequences of the cellular stress response after UV irradiation are well described (1–3, 36, 37), the chromophore for UV that initiates cell surface receptor and subsequent MAPK activation has so far been inscrutable (38). It has previously been proposed that parts of the EGFR activation are mediated by UVB-induced reactive oxygen species, which directly inhibit protein tyrosine phosphatase activities (39). In this study, we demonstrated for the first time the intracellular generation of the endogenous AhR ligand FICZ from the chromophore tryptophan by UVB irradiation and provide evidence that FICZ may be one of the photoproducts initiating signaling events, which are transferred to the nucleus and the cell membrane via activation of the cytoplasmatic AhR. In vivo studies in AhR KO mice indicate that these in vitro findings are physiologically relevant.
The observation that UV radiation has the ability to induce Cyp1A1 enzyme activity in the skin was first made by Mukthar et al. (24) in rats. Subsequent in vitro studies in mouse liver cells demonstrated that the UVB-induced increase in Cyp1a1 was enhanced by additional offer of tryptophan before irradiation and absent in AhR deficient mouse liver cells (26). AhR signaling is highly cell and tissue specific (35, 40, 41). In the present study we confirm and extend the previous studies by showing that (i) UVB irradiation of HaCaT keratinocytes induces CYP1A1 mRNA expression, (ii) this response can be inhibited through tryptophan depletion of cells, or (iii) by directly interfering with AhR signaling, i.e., AhR knockdown or the addition of a competitive AhR antagonist. Taken together these studies clearly show that the AhR is critically involved in UVB-induced CYP induction.
Rosette and Karin (2) proposed the model of growth factor receptor activation as the initiating step of the nonnuclear part of the UV stress response. Our observations extend this model by demonstrating that part of the UVB-triggered growth factor activation is initiated in the cytoplasm of mammalian cells through activation of the AhR (Fig. 2). Growth factor activation through the “classical” AhR ligand TCDD was observed earlier in keratinocytes (42), cervical cells from macaques (43) and in rat liver epithelial cells (22). In the two latter studies, the tyrosine kinase c-src was found to be responsible for the observed growth factor receptor activation after ligand binding to the AhR complex. These findings support the observation from Enan and Matsumura (21) who identified c-src as an integral component of the cytosolic AhR complex which transduces the signal of TCDD through the protein phosphorylation pathway. EGFR activation causes the formation of prostaglandins via an induction of COX-2 protein (14, 44). We have shown earlier that COX-2 induction after TCDD exposure in vivo is AhR-dependent and that this induction is independent of specific AhR-binding elements (xenobiotic response elements, XREs) in the COX-2 promoter. Experiments with c-src KO animals, however, showed that elevation of COX-2 mRNA-levels after AhR activation is c-src dependent (45). In this respect it is of importance that also the mammalian UV response is triggered by src kinase activation (27). Our data now demonstrate that UVB irradiation, like TCDD, triggers AhR activation and thus corroborate the previous findings made after TCDD exposure. We also show that the UVB-induced EGFR activation and downstream signaling are AhR-dependent and requires src kinase. However, the precise mechanisms by which the AhR leads to EGFR activation and the integration of c-src in this signaling pathway remain to be elucidated.
Prompted by the observations that UVB irradiation induces CYP not only in the skin, but also in extracutaneous organs in vivo (24, 25, 46, 47), the intracellular generation of CYP-inducing photoproducts was proposed. Indeed, tryptophan products which were generated by irradiating an aqueous solution of tryptophan with a UVB source were found to be strong AhR ligands (32). Further characterization of these photoproducts revealed FICZ as one of the products with a very high AhR binding affinity (Kd = 7 × 10−11 M) (33) that induces CYP1A1 when administered to cells in vitro (48). So far, the formation of FICZ was shown only ex vitro in cell-free solutions, and it was not known whether FICZ can be generated in living cells and possibly trigger UVB-induced signaling. This study demonstrates the formation of FICZ in UVB-irradiated HaCaT cells (Fig. 4) and provides evidence that this may be one of the photoproducts involved in endogenous AhR activation (Fig. 5 and SI Fig. 12) (26). Accordingly, we studied signal transduction in HaCaT cells after (i) depletion of tryptophan, i.e., the FICZ precursor, before UVB irradiation and (ii) after treatment with FICZ itself. Both strategies yielded results which strongly imply that FICZ may be one of the photoproducts which can initiate UVB-induced signaling events (Fig. 3 and SI Fig. 11).
Conclusion
UVB radiation is well known to be responsible for solar radiation-induced skin damage, most importantly skin cancer and premature skin aging (photoaging) (49). Further studies are therefore needed to define the actual contribution of UVB radiation-induced AhR activation to these detrimental effects. The UVB doses used in the present study are ≈1/3 of the dose that is required to induce a visible erythema in a fair skinned individual (one minimal erythema dose) and thus of physiological relevance. In addition, in the present study we have used HaCaT cells, i.e., a spontaneously immortalized keratinocyte cell line, which has been widely used as a model for human epidermal keratinocytes, because HaCaT cells have maintained their capacity to differentiate and to form a regularly stratified epidermis (50). Also, studies employing AhR KO mice indicate a role for the AhR in UVB-induced signaling. It is thus conceivable to assume that exposure of human skin to solar radiation may have mechanistic consequences similar to those described here.
Methods
Reagents.
The AhR antagonist 3′methoxy-4′nitroflavone was kindly provided by G. Vielhaber (Symrise, Holzminden, Germany). FICZ was synthesized by J. Bergman and coworkers (Department of Biosciences and Nutrition, NOVUM, Karolinska Institutet). EGFR was activated by the addition of EGF (BioSource, Camarillo, CA) to the medium. All additional chemicals used (unless otherwise noted) were purchased from Sigma–Aldrich (Munich, Germany) and were of the highest purity available.
Cell Culture, UVB Irradiation, and Treatment with FICZ.
The immortalized keratinocyte cell line HaCaT (a kind gift of P. Boukamp, Heidelberg, Germany) was cultured in DMEM (PAA, Pasching, Austria) with 10% FCS (PAA). Cell cycle synchronization was achieved by serum starvation for 24 h. This was applied to all experiments investigating EGFR signaling. Cells were exposed to UVB through PBS. For UVB irradiation, a TL20W/12RS lamp, four tubes in parallel connection (Philips, Eindhoven, The Netherlands) was used, which emits most of its energy in the UVB range (290–320 nm) with an emission peak at 310 nm. Sham-irradiated cells were subjected to the identical procedure without being UVB-exposed. For inhibition of the AhR, cells were treated for 1 h with 10 μM MNF before irradiation. For inhibition of src kinases, cells were treated for 1 h with 10 μM PP2 (Calbiochem, Darmstadt, Germany) before irradiation. FICZ treatment was carried out for indicated times and concentrations. Controls for MNF, PP2 or FICZ were subjected to respective DMSO concentrations. Cells were starved for tryptophan (Trp) by culturing them for 4 or 6 h in Trp-free medium (special design of PAA). For some conditions, 1 mM Trp was reintroduced 1 h before UVB irradiation.
Generation of AhR KO HaCaT Cells.
A detailed description of the generation of AhR KO HaCaT cells is given in SI Methods.
Generation of pEGFP-AhR.
A detailed description of the generation of pEGFP-AhR is given in SI Methods.
Transfection of HaCaT Cells with pEGFP-AhR.
A detailed description of the transfection of HaCaT Cells with pEGFP-AhR is given in SI Methods.
RNA Preparation, cDNA Synthesis, and Real-Time RT-PCR.
A detailed description of RNA preparation, cDNA synthesis, and real-time RT-PCR is given in SI Methods.
Preparation of Nuclear Extracts and Immunopreciptation.
A detailed description of the preparation of nuclear extracts and immunopreciptation is given in SI Methods.
Western Blot Analyses.
Cells were lysed in Ripa buffer [PBS containing 1% Nonidet P-40, 0.1% SDS, 50 mM Na3VO4, and 0.2% Protease Inhibitor Mixture Set III from Calbiochem (Darmstadt, Germany)] on ice. The protein samples (cell lysates or immunoprecipitations) were subjected to SDS/10% PAGE and blotted onto nitrocellulose membranes. The blots were blocked with 5% skim milk in TBS-Tween 20 0.05% (TBS-T) at 4°C for 1 h and rinsed with TBS-T. They were incubated overnight with antibodies against AhR 1:1,000 (Affinity BioReagents, Golden, CO), phosphospecific Anti-ERK1&2 [pTpY185/187] 1:1,000 (BioSource), anti-GAPDH ab8245 1:10,000 (Abcam, Cambridge, U.K.) or COX-2-specific polyclonal antibody PG27 1:1,000 (Oxford Biomedical Research, Oxford, MI) in 5% skim milk in TBS-T at 4°C, followed by washing with TBS-T. The blots were subsequently incubated for 1 h with a 1:5,000 dilution of horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibody (Amersham Pharmacia Biotech, Buckinghamshire, U.K.) in 1% skim milk in TBS-T at room temperature, followed by washes with TBS-T. After a chemiluminescent reaction using the enhanced chemiluminescence detection system (Amersham Pharmacia Biotech), bands were visualized with the Fluor-S Multiimager (Bio-Rad, Munich, Germany).
Immunocytochemistry.
Cells were grown on chamber slides. After cell cycle synchronization by serum deprivation for 24 h, they were exposed to 10 mJ/cm2 UVB. 10, 30, 60, or 120 min after irradiation, cells were fixed for 10 min in 4% paraformaldehyde. Slides were incubated with a polyclonal anti-EGFR antibody (Upstate Biotechnology, Lake Placid, NY) for 1 h at 37°C in PBS-T (PBS containing 0,3% Triton X-100), followed by a 30-min incubation at 37°C with the rhodamine red-coupled secondary antibody. Fluorescent staining was visualized under a fluorescent microscope (Olympus, Hamburg, Germany), and photographs were taken with a ColorViewXS digital camera (Olympus).
Determination of Intracellular Tryptophan Concentration.
A detailed description of the determination of intracellular tryptophan concentration is given in SI Methods.
Identification of the AhR Ligand FICZ in Vivo.
For the detection of FICZ, HaCaT cells were tryptophan-starved for 6 h. Subsequently, 1 mM [13C1115N2]tryptophan was offered to the cells for 1 h. Cells were washed twice thoroughly with PBS to remove all extracellular tryptophan, irradiated with 60 mJ/cm2 UVB, incubated at 37°C for 10 min, and harvested in ice-cold PBS on ice. After centrifugation, pelleted cells were stored at −80°C.
The cell pellet was extracted by water followed by acetonitril. The extracts were transferred onto an RP-18-phase SPE (250 ml/5 ml), and FICZ was eluted with acetonitril. The extract was evaporated in a gentle stream of nitrogen and finally reconstituted with 150 μl of acetonitril/water (1/9). After filtration with a 0.45-μm filter, the final solution was used for analysis of FICZ by HPLC tandem mass spectrometry. Twenty-five microliters of solid-phase extract were applied to a RP amide C16 column (15 cm × 2.1 mm, 5 μm) and eluted with a water/acetonitril gradient (solvent A: water/1% formic acid; solvent B: acetonitril/1% formic acid; gradient: 10% A to 90% B within 10 min, hold 90% B for 10 min). The flow rate was 0.15 ml/min, and oven temperature was 20°C. FICZ was detected by using a micromass tandem mass spectrometer (Quattro II) with electrospray positive mode, 3.0 kV capillary voltage, 55 V cone voltage, 120°C source temperature, and 280°C desolvation temperature. The collision energy for m/z 1 ≥ m/z2 (285.2 ≥ 255.2) was 50 eV and 65 eV for 285.2 ≥ 128.4 transitions. The parameters for [C13N15]FICZ were 55 eV for 306.2 ≥ 276.5 transition and 65 eV for 306.2 ≥ 138.2 transition.
Animals.
C57BL/6 mice were obtained from Janvier (Le Genest-St-Isle, France) and housed under standard conditions. AhR KO (C57BL/6-AhrtmIBra) animals generated by Christopher Bradfield [Schmidt et al. (20)] were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred in our animal house. Dorsal skin of wild-type and AhR KO C57BL/6 mice were shaved 24 h before exposure. Mice were irradiated dorsally with a single exposure of UVB (20 min 40 sec; 600 J/cm2). Twelve hours after irradiation, mice were euthanized and skin samples excised. The animal experiments were performed according to the national animal care guidelines.
RNA Preparation and Expression of Cyp1a1 and Cox-2 mRNA in Mouse Skin.
A detailed description of RNA preparation and expression of Cyp1a1 and Cox-2 mRNA in mouse skin is given in SI Methods.
Supplementary Material
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft Grants SFB503 A11 and SFB 575.
Abbreviations
- AhR
arylhydrocarbon receptor
- COX-2
cyclooxygenase-2
- CYP
cytochrome P450
- EGFR
EGF receptor
- FICZ
6-formylindolo[3,2-b]carbazole
- TCDD
tetrachlorodibenzo(p)dioxin.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701764104/DC1.
References
- 1.Devary Y, Rosette C, DiDonato JA, Karin M. Science. 1993;261:1442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- 2.Rosette C, Karin M. Science. 1996;274:1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- 3.Herrlich P, Ponta H, Rahmsdorf HJ. Rev Physiol Biochem Pharmacol. 1992;119:187–223. doi: 10.1007/3540551921_7. [DOI] [PubMed] [Google Scholar]
- 4.Bender K, Blattner C, Knebel A, Iordanov M, Herrlich P, Rahmsdorf HJ. J Photochem Photobiol B. 1997;37:1–17. doi: 10.1016/s1011-1344(96)07459-3. [DOI] [PubMed] [Google Scholar]
- 5.Kulms D, Poppelmann B, Yarosh D, Luger TA, Krutmann J, Schwarz T. Proc Natl Acad Sci USA. 1999;96:7974–7979. doi: 10.1073/pnas.96.14.7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tyrrell RM. BioEssays. 1996;18:139–148. doi: 10.1002/bies.950180210. [DOI] [PubMed] [Google Scholar]
- 7.Tyrrell RM. EXS. 1996;77:255–271. [PubMed] [Google Scholar]
- 8.Stege H, Roza L, Vink AA, Grewe M, Ruzicka T, Grether-Beck S, Krutmann J. Proc Natl Acad Sci USA. 2000;97:1790–1795. doi: 10.1073/pnas.030528897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Abaseri TB, Fuhrman J, Trempus C, Shendrik I, Tennant RW, Hansen LA. Cancer Res. 2005;65:3958–3965. doi: 10.1158/0008-5472.CAN-04-2204. [DOI] [PubMed] [Google Scholar]
- 10.Bourcier C, Jacquel A, Hess J, Peyrottes I, Angel P, Hofman P, Auberger P, Pouyssegur J, Pages G. Cancer Res. 2006;66:2700–2707. doi: 10.1158/0008-5472.CAN-05-3129. [DOI] [PubMed] [Google Scholar]
- 11.Bode AM, Dong Z. Sci STKE. 2003;2003:RE2. doi: 10.1126/stke.2003.167.re2. [DOI] [PubMed] [Google Scholar]
- 12.Katsanakis KD, Owen C, Zoumpourlis V. Anticancer Res. 2002;22:755–759. [PubMed] [Google Scholar]
- 13.Katsanakis KD, Gorgoulis V, Papavassiliou AG, Zoumpourlis VK. Mol Med. 2002;8:624–637. [PMC free article] [PubMed] [Google Scholar]
- 14.Buckman SY, Gresham A, Hale P, Hruza G, Anast J, Masferrer J, Pentland AP. Carcinogenesis. 1998;19:723–729. doi: 10.1093/carcin/19.5.723. [DOI] [PubMed] [Google Scholar]
- 15.Ashida M, Bito T, Budiyanto A, Ichihashi M, Ueda M. Exp Dermatol. 2003;12:445–452. doi: 10.1034/j.1600-0625.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 16.Pentland AP, Schoggins JW, Scott GA, Khan KN, Han R. Carcinogenesis. 1999;20:1939–1944. doi: 10.1093/carcin/20.10.1939. [DOI] [PubMed] [Google Scholar]
- 17.Kahl GF, Friederici DE, Bigelow SW, Okey AB, Nebert DW. Dev Pharmacol Ther. 1980;1:137–162. [PubMed] [Google Scholar]
- 18.Knutson JC, Poland A. Cell. 1980;22:27–36. doi: 10.1016/0092-8674(80)90151-8. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Proc Natl Acad Sci USA. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enan E, Matsumura F. Biochem Pharmacol. 1996;52:1599–1612. doi: 10.1016/s0006-2952(96)00566-7. [DOI] [PubMed] [Google Scholar]
- 22.Kohle C, Gschaidmeier H, Lauth D, Topell S, Zitzer H, Bock KW. Arch Toxicol. 1999;73:152–158. doi: 10.1007/s002040050600. [DOI] [PubMed] [Google Scholar]
- 23.Park S, Matsumura F. Toxicology. 2006;217:139–146. doi: 10.1016/j.tox.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Mukhtar H, DelTito BJ, Jr, Matgouranis PM, Das M, Asokan P, Bickers DR. J Invest Dermatol. 1986;87:348–353. doi: 10.1111/1523-1747.ep12524446. [DOI] [PubMed] [Google Scholar]
- 25.Katiyar SK, Matsui MS, Mukhtar H. J Invest Dermatol. 2000;114:328–333. doi: 10.1046/j.1523-1747.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- 26.Wei YD, Rannug U, Rannug A. Chem Biol Interact. 1999;118:127–140. doi: 10.1016/s0009-2797(98)00118-5. [DOI] [PubMed] [Google Scholar]
- 27.Devary Y, Gottlieb RA, Smeal T, Karin M. Cell. 1992;71:1081–1091. doi: 10.1016/s0092-8674(05)80058-3. [DOI] [PubMed] [Google Scholar]
- 28.Kitagawa D, Tanemura S, Ohata S, Shimizu N, Seo J, Nishitai G, Watanabe T, Nakagawa K, Kishimoto H, Wada T, et al. J Biol Chem. 2002;277:366–371. doi: 10.1074/jbc.M107110200. [DOI] [PubMed] [Google Scholar]
- 29.Henry EC, Kende AS, Rucci G, Totleben MJ, Willey JJ, Dertinger SD, Pollenz RS, Jones JP, Gasiewicz TA. Mol Pharmacol. 1999;55:716–725. [PubMed] [Google Scholar]
- 30.Karin M, Hunter T. Curr Biol. 1995;5:747–757. doi: 10.1016/s0960-9822(95)00151-5. [DOI] [PubMed] [Google Scholar]
- 31.Fisher GJ, Talwar HS, Lin J, Lin P, McPhillips F, Wang Z, Li X, Wan Y, Kang S, Voorhees JJ. J Clin Invest. 1998;101:1432–1440. doi: 10.1172/JCI2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rannug A, Rannug U, Rosenkranz HS, Winqvist L, Westerholm R, Agurell E, Grafstrom AK. J Biol Chem. 1987;262:15422–15427. [PubMed] [Google Scholar]
- 33.Rannug U, Rannug A, Sjoberg U, Li H, Westerholm R, Bergman J. Chem Biol. 1995;2:841–845. doi: 10.1016/1074-5521(95)90090-x. [DOI] [PubMed] [Google Scholar]
- 34.Wei YD, Bergander L, Rannug U, Rannug A. Arch Biochem Biophys. 2000;383:99–107. doi: 10.1006/abbi.2000.2037. [DOI] [PubMed] [Google Scholar]
- 35.Zhang S, Qin C, Safe SH. Environ Health Perspect. 2003;111:1877–1882. doi: 10.1289/ehp.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karin M. Ann NY Acad Sci. 1998;851:139–146. doi: 10.1111/j.1749-6632.1998.tb08987.x. [DOI] [PubMed] [Google Scholar]
- 37.Krutmann J. Prog Biophys Mol Biol. 2006;92:105–107. doi: 10.1016/j.pbiomolbio.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Xu Y, Voorhees JJ, Fisher GJ. Am J Pathol. 2006;169:823–830. doi: 10.2353/ajpath.2006.050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gross S, Knebel A, Tenev T, Neininger A, Gaestel M, Herrlich P, Bohmer FD. J Biol Chem. 1999;274:26378–26386. doi: 10.1074/jbc.274.37.26378. [DOI] [PubMed] [Google Scholar]
- 40.Bernshausen T, Jux B, Esser C, Abel J, Fritsche E. Arch Toxicol. 2005;80:206–211. doi: 10.1007/s00204-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 41.Wolff S, Harper PA, Wong JM, Mostert V, Wang Y, Abel J. Mol Pharmacol. 2001;59:716–724. doi: 10.1124/mol.59.4.716. [DOI] [PubMed] [Google Scholar]
- 42.Choi EJ, Toscano DG, Ryan JA, Riedel N, Toscano WA., Jr J Biol Chem. 1991;266:9591–9597. [PubMed] [Google Scholar]
- 43.Enan E, El Sabeawy F, Scott M, Overstreet J, Lasley B. Toxicol Appl Pharmacol. 1998;151:283–293. doi: 10.1006/taap.1998.8470. [DOI] [PubMed] [Google Scholar]
- 44.Miller CC, Hale P, Pentland AP. J Biol Chem. 1994;269:3529–3533. [PubMed] [Google Scholar]
- 45.Vogel C, Boerboom AM, Baechle C, El Bahay C, Kahl R, Degen GH, Abel J. Carcinogenesis. 2000;21:2267–2274. doi: 10.1093/carcin/21.12.2267. [DOI] [PubMed] [Google Scholar]
- 46.Goerz G, Merk H, Bolsen K, Tsambaos D, Berger H. Experientia. 1983;39:385–386. doi: 10.1007/BF01963137. [DOI] [PubMed] [Google Scholar]
- 47.Goerz G, Barnstorf W, Winnekendonk G, Bolsen K, Fritsch C, Kalka K, Tsambaos D. Arch Dermatol Res. 1996;289:46–51. doi: 10.1007/s004030050151. [DOI] [PubMed] [Google Scholar]
- 48.Wei YD, Helleberg H, Rannug U, Rannug A. Chem Biol Interact. 1998;110:39–55. doi: 10.1016/s0009-2797(97)00111-7. [DOI] [PubMed] [Google Scholar]
- 49.Gilchrest BA, Krutmann J. Skin Aging. NY: Springer; 2006. [Google Scholar]
- 50.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.