Abstract
Vascular endothelial cells are continuously exposed to mechanical and chemical stimuli, such as shear stress and VEGF, respectively. It is still not clear how cells perceive these stimuli and orchestrate their responses. Studying the molecular mechanism by which shear stress and VEGF regulate the signaling pathways in bovine endothelial aortic cells, we found that VEGF induced a rapid association of VEGF receptor 2 (Flk-1) with Nckβ, but shear stress did not have such an effect. SU1498 (a specific inhibitor of Flk-1) and Nckβnm (a negative mutant of Nckβ) blocked the VEGF-induced ERK and JNK activities. Only SU1498, but not Nckβnm, inhibited the shear-induced ERK activity. Furthermore, neither SU1498 nor Nckβnm had significant effects on the shear-induced JNK activity, which can be blocked by inhibitors of Src family kinase and ROCK kinase. Therefore, mechanical (shear stress) and chemical (VEGF) stimuli diverge at the receptor Flk-1 in terms of the recruitment of the adapter protein Nckβ, and they employ different components of the complex signaling network in regulating downstream molecules, e.g., ERK and JNK.
Keywords: endothelial cells, ERK, fibronectin, Flk-1, Nckβ
Vascular endothelial cells (ECs) are continuously exposed to mechanical (e.g., shear stress) and chemical (e.g., VEGF) stimuli, which are important modulators of vascular cell functions in physiological and pathophysiological conditions. Shear stress, as the tangential component of hemodynamic force, has been demonstrated to be involved in atherogenesis (1, 2), whereas VEGF is recognized as the key regulator of angiogenesis and vascular permeability (3). In vitro studies have shown that both shear stress and VEGF activate similar signaling molecules, including membrane receptors (integrins and VEGF receptor 2) and downstream molecules (ERK and JNK) (4–7). There is increasing evidence that proteins associate into a complex network and that signaling inside the cell involves convergent and divergent pathways in response to differential stimuli to result in integrated cellular functions. It still remains unclear as to the molecular mechanisms by which cells convert these mechanical or chemical stimuli into biological signaling and orchestrate these signaling pathways to elicit a fine-tuned signaling network, which ultimately leads to appropriate cellular functions.
VEGF receptor 2 (Flk-1) belongs to the receptor tyrosine kinase family and is a major receptor mediating most of the functional signaling pathways in response to VEGF (8). On its activation, Flk-1 has been reported to associate with a number of adapter proteins that contain src homology 2 (SH2) domain, including Grb2, Nck, phosphatidylinositol 3-kinase, and Shc (8, 9). Nckβ, an adapter protein consisting of three N-terminal juxtaposed SH3 domains and a C-terminal SH2 domain, is homologous to Nck (10). The SH2 domain in Nckβ is well documented to associate with tyrosine-phosphorylated sites and has been reported to bind a variety of receptor tyrosine kinases, including EGF receptor and PDGF receptor (10). The presence of three distinct SH3 domains suggests the capability of Nckβ to associate with multiple proteins containing proline-rich domain. Indeed, the p21-activated kinase (PAK) has been reported to constitutively associate with Nckβ (11). Therefore, by binding to receptor tyrosine kinases, Nckβ may serve as the docking protein bringing PAK to the cell membrane, where it can be exposed to its upstream activators, including the Rho family members Cdc42 and Rac1. The activated PAK can ultimately induce the JNK activation (12). The SH3 domains of Nckβ have also been shown to bind a guanine exchange factor Sos (11), which could further activate Ras and ERK (13).
Both shear stress and VEGF have been reported to induce the tyrosine phosphorylation of Flk-1 (14) and the activation of JNK and ERK (5, 15, 16). However, it remains unclear whether and how Flk-1 and its associated adaptor proteins, e.g., Nckβ, are involved in regulating JNK and ERK. In this study, we demonstrated that VEGF, but not shear stress, induced the association of Flk-1 and Nckβ. The inhibition of either Flk-1 or Nckβ blocked the JNK and ERK activations in response to VEGF. In the case of shear stress, however, ERK activation is mediated by only Flk-1 but not Nckβ, and JNK activation is mediated by neither. Therefore, mechanical (shear stress) and chemical (VEGF) stimuli may distinctively regulate the membrane receptor Flk-1 in its association with adapter proteins to differentially regulate downstream signaling events and cellular functions. Because ECs are exposed to both VEGF and shear stress, our results shed light on the molecular mechanism by which ECs perceive different chemical/physical cues and coordinate signaling pathways to regulate physiological processes, e.g., angiogenesis and vascular remodeling.
Results
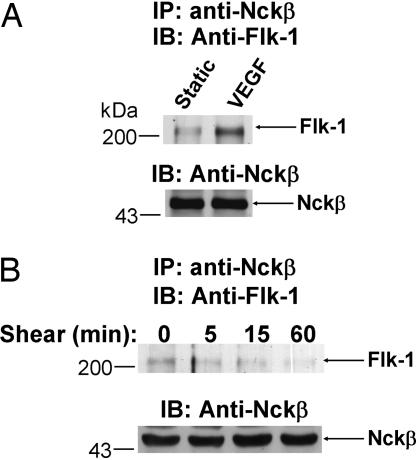
VEGF, but Not Shear Stress, Induced the Flk-1·Nckβ Association.
VEGF induced a maximal tyrosine phosphorylation of Flk-1 and its association with adapter protein Nck at 5 min (9, 14). A recently identified protein, Nckβ, has been shown to be homologous to Nck in structure and have similar functions as Nck, including the binding to EGF receptor, PDGF receptor, Grb2, and PAK (10, 11). We examined whether Flk-1 can also associate with Nckβ in response to VEGF, as in the case for Nck. Bovine aortic endothelial cells (BAECs) were challenged with VEGF or kept as static control for 5 min. Cell lysates were immunoprecipitated with an anti-Nckβ antibody, followed by immunoblotting with anti-Flk-1 antibodies. As shown in Fig. 1A, VEGF induced the Flk-1·Nckβ association. We further examined whether shear stress, as a mechanical stimulation, can also induce the Flk-1·Nckβ association. Contrary to VEGF, shear stress (up to 60 min) did not cause any detectable induction of the Flk-1·Nckβ association (Fig. 1B). These results suggest that mechanical stimulation may induce Flk-1 in a way that is mechanistically different from that of VEGF.
Fig. 1.
VEGF, but not shear stress, induced the Flk-1·Nckβ association. BAECs were subjected to (A) VEGF (10 ng/ml) for 5 min or (B) shear stress (12 dyn/cm2) for various time periods as indicated or kept as static control (time 0). The cell lysates were subjected to IP with an anti-Nckβ antibody, followed by IB with an anti-Flk-1 antibody (Upper) (arrow, Flk-1) or an anti-Nckβ antibody (Lower) (arrow, Nckβ). The results are representative of three separate experiments.
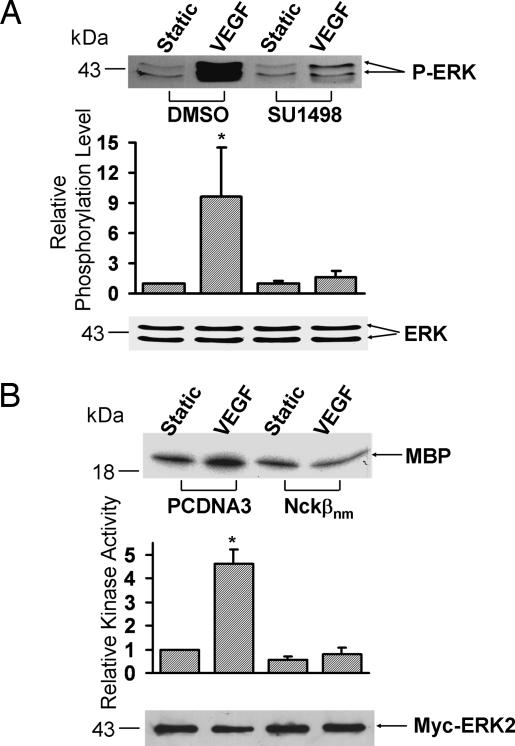
Flk-1 and Nckβ Mediate the VEGF-Induced ERK Activation.
VEGF is known to induce Flk-1 and ERK activation (8, 17). Because VEGF can induce the Flk-1·Nckβ association, we tested the possibility that Flk-1 and Nckβ may mediate the VEGF-induced ERK activation. To examine the effect of Flk-1 in the VEGF-induced ERK activation, BAECs were treated with SU1498 (a specific inhibitor of Flk-1) to inhibit the Flk-1 activation before they were challenged with VEGF. As shown in Fig. 2A, SU1498 blocked the VEGF-induced phosphorylation of ERK. To study the role of Nckβ in regulating the VEGF-activated ERK, Myc-ERK2 was cotransfected with PCDNA3 or negative mutant of Nckβ (Nckβnm) into BAECs. To assess ERK activity in the transfected cells, Myc-ERK2 was immunoprecipitated by an anti-Myc antibody and subjected to kinase assay. Kinase assay revealed that Nckβnm abolished the VEGF-induced ERK activation (Fig. 2B). These results suggest that both Flk-1 and Nckβ are involved in the regulation of the VEGF-induced ERK activation.
Fig. 2.
Flk-1 and Nckβ mediate the VEGF-induced ERK activation. (A) BAECs were treated with 0.1% DMSO (the solvent of SU1498) as control or 5 μM SU1498 for 1 h. (B) Myc-ERK2 was cotransfected with control vector PCDNA3 or Flag-Nckβnm into BAECs. These cells were then subjected to VEGF (10 ng/ml) for 15 min or kept under static incubation. (A) The upper gel band shows the phosphorylated ERK levels, and the lower gel band represents the loaded ERK proteins under different conditions as indicated. (B) The cell lysates were immunoprecipitated with anti-Myc antibodies for immunocomplex kinase assays by using myelin basic protein (MBP) as the substrate. The upper gel band is phosphorylated MBP, which indicates the level of ERK activation, and the lower gel band shows IB with an anti-Myc antibody to indicate that the levels of the expressed exogenous Myc-tagged ERK proteins were comparable among the various samples. The bar graphs are the results of densitometry analysis showing mean ± SEM from three separate experiments. The asterisks indicate significant differences (P < 0.05) between the SU1498-treated sample and DMSO control after VEGF activation in A and between cells transfected with PCDNA3 and Nckβnm after VEGF activation in B.
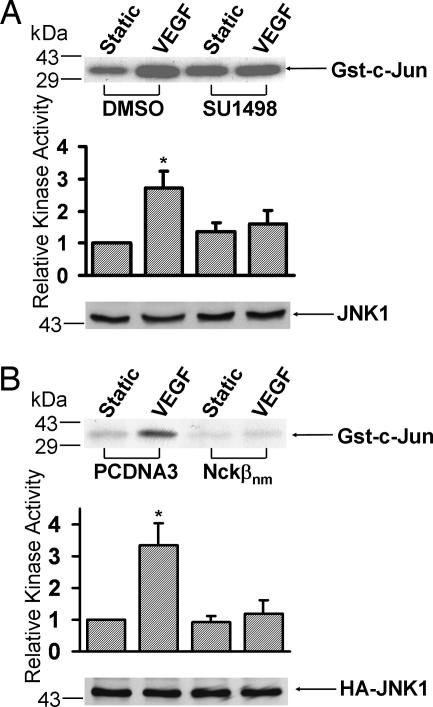
Flk-1 and Nckβ Mediate the VEGF-Activated JNK.
VEGF has been reported to induce JNK activation (6). In addition, Nckβ can bind to PAK, which has been shown to play an important role in regulating JNK activation (18). We therefore examined whether Flk-1 and Nckβ mediate the VEGF-induced JNK activation. As shown in Fig. 3A, the inhibition of Flk-1 by SU1498 blocked the VEGF-induced JNK activity. HA-JNK1 was further cotransfected with PCDNA3 or Nckβnm to assess the role of Nckβ in regulating the VEGF-activated JNK. The expression of Nckβnm blocked the VEGF-induced JNK activation (Fig. 3B), suggesting that both Flk-1 and Nckβ are essential for the VEGF-induced JNK activation.
Fig. 3.
Flk-1 and Nckβ mediate the VEGF activation of JNK. (A) BAECs were treated with 0.1% DMSO (the solvent of SU1498) as control or 5 μM SU1498 for 1 h. (B) HA-JNK1 was cotransfected with the control vector PCDNA3 or Flag-Nckβnm into BAECs. These cells were then subjected to VEGF (10 ng/ml) for 15 min or kept under static incubation. The cell lysates were immunoprecipitated with anti-JNK1 antibodies (A) or anti-HA antibodies (B) for immunocomplex kinase assays by using Gst-c-Jun as the substrate. The upper gel bands are phosphorylated Gst-c-Jun, which indicates the level of JNK activation, and the bottom gel bands show IB with an anti-JNK1 (A) or anti-HA antibody (B) to indicate that the levels of the loaded protein (A) or the expressed exogenous HA-tagged proteins (B) were comparable among the various samples. The bar graphs and asterisks are the same as in Fig. 2.
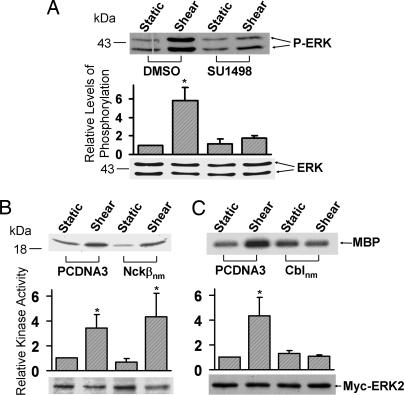
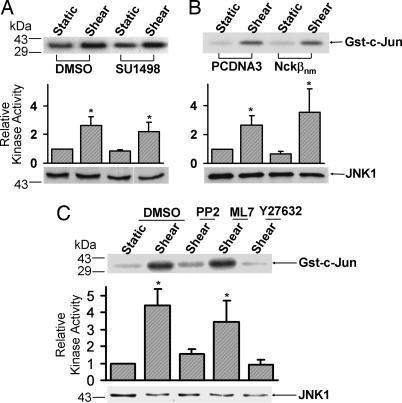
Flk-1, but Not Nckβ, Is Essential for the Shear-Activated ERK.
Shear stress has been proven to activate ERK activation in a variety of cell types (19, 20). To examine the effect of Flk-1 in the shear-induced ERK activation, BAECs were pretreated with SU1498 to inhibit Flk-1 before shear stress application. As shown in Fig. 4A, SU1498 significantly inhibited the shear-induced ERK phosphorylation. However, kinase assay revealed that Nckβnm did not block the shear-induced ERK activation (Fig. 4B). In contrast, the negative mutant of Cbl (Cblnm) blocked the shear-activated ERK (Fig. 4C), consistent with our previous finding that Cbl associates with Flk-1 on shear stress application (21). Therefore, whereas VEGF regulates ERK via Flk-1 and Nckβ, the shear-induced ERK depends on Flk-1 and Cbl, but not on Nckβ.
Fig. 4.
Flk-1, but not Nckβ, is essential for the shear-activated ERK. (A) BAECs were treated with 0.1% DMSO (the solvent of SU1498) as control or 5 μM SU1498 for 1 h. (B and C) Myc-ERK2 was cotransfected into BAECs with control vector PCDNA3 or Flag-Nckβnm (B) or HA-Cblnm (C). These cells were then subjected to shear stress (12 dyn/cm2) for 10 min or kept under static incubation. (A) The upper gel band shows the phosphorylated ERK levels, and the lower gel band represents the loaded ERK proteins under different conditions as indicated. (B and C) The cell lysates were immunoprecipitated with anti-Myc antibodies for immunocomplex kinase assays by using MBP as the substrate. The upper gel bands are phosphorylated MBP, which indicates the level of ERK activation, and the bottom gel bands show IB with an anti-Myc antibody to indicate that the levels of the expressed exogenous Myc-tagged ERK proteins were comparable among the various samples. The bar graphs are the results of densitometry analysis showing mean ± SEM from three separate experiments. The asterisks indicate significant differences (P < 0.05) between the various samples and static controls.
Neither Flk-1 nor Nckβ Is Essential for the Shear-Activated JNK.
We then examined whether Flk-1 and Nckβ are involved in the shear-induced JNK activation. As shown in Fig. 5 A and B, neither SU1498 nor Nckβnm had any significant effect on the shear-induced JNK activity. Further experiments indicate that the inhibition of Src kinase by PP2 and of Rho-associated kinase (ROCK) kinase by Y27632, but not of myosin light chain kinase (MLCK) by ML7, blocked the shear-induced JNK activation. PP2, Y27632, or ML7 did not cause a significant change of the basal JNK activity before shearing (data not shown). Therefore, whereas VEGF regulates JNK via Flk-1 and Nckβ, our results indicate that the shear-induced JNK relies on other signaling pathways independent of Flk-1, e.g., Src and ROCK kinases.
Fig. 5.
Neither Flk-1 nor Nckβ is essential for the shear activation of JNK. (A) BAECs were treated with 0.1% DMSO (the solvent of SU1498) as control or 5 μM SU1498 for 1 h. (B) HA-JNK1 was cotransfected with the control vector PCDNA3 or Flag-Nckβnm into BAECs. (C) BAECs were treated with 0.1% DMSO, 10 μM PP2, 5 μM ML7, or 10 μM Y27632 for 1 h. The treated cells were then subjected to shear stress (12 dyn/cm2) for 30 min or kept under static incubation. The cell lysates were immunoprecipitated with anti-JNK1 antibodies (A and C) or anti-HA antibodies (B) for immunocomplex kinase assays by using Gst-c-Jun as the substrate. The upper gel bands are phosphorylated Gst-c-Jun, which indicates the level of JNK activation, and the bottom gel bands show IB with anti-JNK1 (A and C) or anti-HA (B) antibodies to indicate that the levels of the loaded protein (A and C) or the expressed exogenous HA-tagged JNK proteins (B) were comparable among the various samples. The bar graphs and asterisks are the same as in Fig. 4.
Discussion
Both VEGF and shear stress induced Flk-1 phosphorylation, but only VEGF induces Flk-1·Nckβ association (Fig. 1). This distinct effect of VEGF from shear stress may be attributed to their different mechanisms in activating Flk-1. VEGF, as a growth factor, has been shown to form dimers by linking the monomers through disulfide bridges in a “head-to-tail” fashion. This spatial arrangement of VEGF dimer can bring two Flk-1 monomers together to form a homodimer of Flk-1. This ligand-stimulated receptor dimerization of Flk-1 can cause the autotyrosine phosphorylation of the intracellular kinase domain of Flk-1 (8). These tyrosine phosphorylation sites, with their appropriate context of neighboring amino acids, may turn on the catalytic activity of Flk-1, as well as creating potential binding sites for adapter proteins that contain SH2 domain, e.g., Nckβ and Shc (9). The detailed mechanism by which shear stress induces the tyrosine phosphorylation of Flk-1, however, remains unclear. It has been postulated that shear stress imposes its effect on the cytoskeletal network to regulate cellular functions (22). The resultant cytoskeletal deformation may help to put the kinases and their substrates into juxtapositions, thus altering the three-dimensional conformation of signaling molecules to activate the downstream signaling pathways. It is possible that this ligand-independent phosphorylation of Flk-1 in response to shear stress may result from the conformational change of Flk-1 as well as the binding of tyrosine kinases that can phosphorylate Flk-1, such as Src. Therefore, the differences in the activation mechanisms and resultant conformations of Flk-1 in response to mechanical (shear stress) or chemical (VEGF) stimuli may explain the fact that, whereas both types of stimuli induced the tyrosine phosphorylation of Flk-1 (9, 14), only VEGF was able to enhance the Flk-1·Nckβ association. This difference in the state of association of Flk-1 with adapter proteins in response to VEGF and shear stress may lead to the variations in signaling cascades and the ultimate cellular functions. Further studies on the phosphorylation of Flk-1 by VEGF and shear stress at subcellular levels may also help to determine whether differential phosphorylation is responsible for the recruitment of Nckβ.
VEGF is a strong activator of ERK (23). The activation of Flk-1 induced by VEGF may result in the activation of the guanine exchange factor Sos through Nckβ and other adaptor proteins (13). Sos has been known to stimulate Ras, which subsequently activates its downstream signaling molecules MEK1 and ERK (24). Hence, the recruitment of Nckβ by Flk-1 may serve as an important step in the VEGF-regulated ERK activation. VEGF is also an important growth factor that can promote EC proliferation involving JNK (25). In addition to the fact that JNK participates in cellular proliferation via the transcriptional factor AP-1 (26), recent studies indicate that JNK is indeed an important effecter of VEGF to stimulate cyclin D1 synthesis and EC proliferation (6). The detailed mechanism by which VEGF challenge leads to JNK activation is, however, not well defined. Whereas RAFTK (related adhesion focal tyrosine kinase) has been suggested to be involved in the VEGF-induced JNK activation (27, 28), the cross-talk between ERK and JNK appears to underlie the VEGF-induced signaling transduction, with ERK serving as the upstream molecule to JNK (6). In our study, both Flk-1 and Nckβ are essential for the VEGF-induced JNK (Fig. 3). Given that the SH2 domain of Nckβ may recruit PAK through its proline-rich domain and that PAK is well documented to enact JNK activation (12, 18), we hypothesize that the Flk-1·Nckβ association in response to VEGF may recruit PAK to regulate JNK activation. Therefore, VEGF may induce the Flk-1·Nckβ association and subsequently regulate ERK through Sos and JNK via PAK.
A myriad of signaling molecules have been reported to mediate the shear-induced ERK, including integrins (29), PECAM-1 (30), FAK (5), Src (31), Caveolae (32), and PKC-ε (33). Flk-1 is also essential for the shear-induced ERK activation (Fig. 4A). It is possible that Flk-1 can interact with other signaling molecules, e.g., integrins, PECAM-1, or Src, in regulating ERK activation. In fact, integrins have been shown to interact with and regulate Flk-1 (21), and PECAM-1 has been shown to form a complex with Flk-1 in regulating NF-κB and downstream inflammatory genes in response to shear stress (34). It will be interesting to examine whether Flk-1 also interacts with integrins or PECAM-1 in regulating the shear activation of ERK. In contrast to the action of VEGF, shear-induced ERK activation depends on Cbl, but not Nckβ (Fig. 4 B and C), which is consistent with the finding that Cbl, but not Nckβ, associates with Flk-1 on shear application (Fig. 1B) (21). For the shear-induced JNK activation, neither Flk-1 nor Nckβ is involved (Fig. 5 A and B). It has previously been shown that the cytoskeleton integrity and its related signaling molecules, e.g., Rho small GTPases and phosphatidylinositol 3-kinase, are essential for this shear-induced JNK (5, 35–38). Consistently, our results also indicate that Src and ROCK kinases, which are known to regulate cytoskeletal network (39), mediate the shear-induced JNK activation (Fig. 5C). It is interesting to note that the inhibition of MLCK, which is a downstream effecter of ROCK kinase and a known regulator of myosin phosphorylation and actomyosin contractility (40), did not block the shear-induced JNK activity (Fig. 5C). It is possible that an alternative signaling pathway other than MLCK mediates the action of ROCK on JNK in response to shear stress. In fact, ROCK has been shown to directly phosphorylate MLC (myosin light chain) when MLCK is inhibited (41). Therefore, the shear-induced JNK activation may be dependent on signaling molecules related to cytoskeletal functions, but not on Flk-1 or Nckβ. Src and ROCK are also involved in the VEGF-induced signaling transduction (42, 43). It will be interesting to examine in the future whether Src, ROCK, and Nckβ form a coordinated network in regulating downstream molecules in response to VEGF.
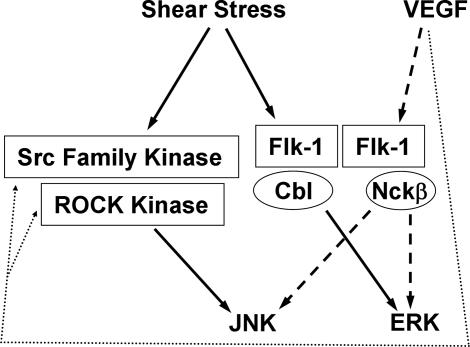
In conclusion, our results suggest that mechanical (ligand-independent: shear stress) and chemical (ligand-dependent: VEGF) stimuli, by inducing the selective associations of Flk-1 with different adapter proteins, diverge at the membrane receptor Flk-1 in regulating ERK activation. Our results also indicate that these two types of stimuli activate JNK through different molecular mechanisms (Fig. 6). These results highlight the complexity of cell signaling transduction in response to external cues and the importance of the system biology approach to investigate the molecular mechanism regulating pathophysiological processes.
Fig. 6.
A proposed model showing the selective recruitment of adaptor proteins by Flk-1 and the differential signaling pathways regulating ERK and JNK in response to VEGF and shear stress. VEGF induces the Flk-1·Nckβ association to regulate ERK and JNK activation (broken arrows), whereas shear stress regulates ERK through Flk-1 and Cbl, but activates JNK through Src and ROCK (solid arrows) independent of Flk-1. The dashed arrows represent possible actions of VEGF on Src and ROCK.
Materials and Methods
Cell Culture.
Cell culture reagents were obtained from GIBCO/BRL (Grand Island, NY). BAECs were isolated from bovine aorta with collagenase and cultured in a humidified 95% air/5% CO2 incubator at 37°C. The culture medium was DMEM supplemented with 10% FBS, 2 mM l-glutamine, 1 unit/ml penicillin, 100 μg/ml streptomycin, and 1 mM sodium pyruvate. All experiments were conducted with BAEC cultures before passage 10.
Shear Stress and VEGF Treatment.
A flow system was used to impose shear stress on the cultured ECs (44). In all experiments, BAECs were incubated for 12 h in 0.5% serum and for a subsequent 2 h in serum-free medium before they were subjected to shear stress (12 dyne/cm2) (1 dyne = 10 μN) or VEGF (10 ng/ml).
Inhibitors, DNA Plasmids, and Transient Transfection.
SU1498 (5 μM) and Y27632 (10 μM), inhibitors of Flk-1 and ROCK, respectively, were purchased from Calbiochem (La Jolla, CA). PP2 (10 μM) and ML7 (5 μM), inhibitors of Src family kinase and MLCK, respectively, were from Sigma–Aldrich (St. Louis, MO). The HA-tagged plasmid-containing JNK1 (HA-JNK1), the Myc-tagged plasmid-containing ERK2 (Myc-ERK2), and the HA-tagged Cblnm, in which the main tyrosine sites 700, 731, and 774 responsible for phosphorylation have been mutated to phenylalanine, were described previously (5, 45). The first and second N-terminal SH3 domains of Nckβ were deleted to generate the Flag-tagged Nckβnm (10). The various plasmids were transfected into BAECs at 80% confluence by using the lipofectamine method as described by the vendor (GIBCO/BRL).
Immunoprecipitation (IP) and Immunoblotting (IB).
The antibodies used for IP and IB were polyclonal anti-Nckβ (Upstate Biotechnology, Lake Placid, NY), anti-Cbl, anti-Flk-1, anti-JNK1, anti-HA, and anti-Myc antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), monoclonal anti-phospho-ERK, polyclonal anti-ERK antibodies (Cell Signaling Technology, Beverly, MA). For IP, the cells were lysed in a buffer containing 25 mM Tris·HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 5 mM NaF, 1 mM Na3VO4, 1 mM PMSF, and 10 μg/ml leupeptin. The lysate was centrifuged, and the supernatant was immunoprecipitated with appropriate antibodies and protein A-Sepharose beads (Amersham Biosciences, Piscataway, NJ) at 4°C overnight. The immunoprecipitated complexes were washed and used for either kinase activity assays or IB. For IB, proteins separated on the SDS/PAGE were transferred to a nitrocellulose membrane. The membrane was then blocked with 5% BSA, followed by incubation with the primary antibody. The bound primary antibodies were detected by using a goat anti-rabbit IgG-horseradish peroxidase conjugate (Santa Cruz Biotechnology) and the ECL detection system (Amersham Biosciences).
Kinase Activity Assay.
Endogenous JNK1, exogenous HA-JNK1 or Myc-ERK2, was immunoprecipitated from the cell lysate by using protein A-Sepharose beads and anti-JNK1, anti-HA, or anti-Myc antibodies, respectively. The immunocomplex was resuspended in the buffer containing [γ-32P]ATP and GST-c-Jun or MBP as the substrate for JNK or ERK, respectively. The kinase reaction mixture was resolved on SDS/PAGE after incubation at 30°C for 30 min, and the phosphorylated GST-c-Jun or MBP was detected by autoradiography.
Acknowledgments
We thank Dr. Shunichi Usami for valuable discussions and Suli Yuan, Phu Nguyen, and Gerard Norwich for the excellent technical assistance. This work was supported in part by National Institutes of Health Research Grants HL43026, HL64382, HL80518 (to S.C.), and GM65188 (to C.W.).
Abbreviations
- EC
endothelial cell
- Flk-1
VEGF receptor 2
- SH2
src homology 2
- PAK
p21-activated kinase
- BAEC
bovine aortic endothelial cell
- Nckβnm
negative mutant of Nckβ
- ROCK
Rho-associated kinase
- MLCK
myosin light chain kinase
- IP
immunoprecipitation
- IB
immunoblotting
- MBP
myelin basic protein.
Footnotes
The authors declare no conflict of interest.
References
- 1.Caro CG, Fitz-Gerald JM, Schroter RC. Proc R Soc Lond B Biol Sci. 1971;177:109–159. doi: 10.1098/rspb.1971.0019. [DOI] [PubMed] [Google Scholar]
- 2.Giddens DP, Zarins CK, Glagov S. J Biomech Eng. 1993;115:588–594. doi: 10.1115/1.2895545. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N. Am J Physiol. 2001;280:C1358–C1366. doi: 10.1152/ajpcell.2001.280.6.C1358. [DOI] [PubMed] [Google Scholar]
- 4.Byzova TV, Goldman CK, Pampori N, Thomas KA, Bett A, Shattil SJ, Plow EF. Mol Cell. 2000;6:851–860. [PubMed] [Google Scholar]
- 5.Li S, Kim M, Hu YL, Jalali S, Schlaepfer DD, Hunter T, Chien S, Shyy JY. J Biol Chem. 1997;272:30455–30462. doi: 10.1074/jbc.272.48.30455. [DOI] [PubMed] [Google Scholar]
- 6.Pedram A, Razandi M, Levin ER. J Biol Chem. 1998;273:26722–26728. doi: 10.1074/jbc.273.41.26722. [DOI] [PubMed] [Google Scholar]
- 7.Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. EMBO J. 2001;20:4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 9.Kroll J, Waltenberger J. J Biol Chem. 1997;272:32521–32527. doi: 10.1074/jbc.272.51.32521. [DOI] [PubMed] [Google Scholar]
- 10.Tu Y, Li F, Wu C. Mol Biol Cell. 1998;9:3367–3382. doi: 10.1091/mbc.9.12.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braverman LE, Quilliam LA. J Biol Chem. 1999;274:5542–5549. doi: 10.1074/jbc.274.9.5542. [DOI] [PubMed] [Google Scholar]
- 12.McCarty JH. BioEssays. 1998;20:913–921. doi: 10.1002/(SICI)1521-1878(199811)20:11<913::AID-BIES6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 13.Buday L, Wunderlich L, Tamas P. Cell Signal. 2002;14:723–731. doi: 10.1016/s0898-6568(02)00027-x. [DOI] [PubMed] [Google Scholar]
- 14.Chen KD, Li YS, Kim M, Li S, Yuan S, Chien S, Shyy JY. J Biol Chem. 1999;274:18393–18400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- 15.Li YS, Shyy JY, Li S, Lee J, Su B, Karin M, Chien S. Mol Cell Biol. 1996;16:5947–5954. doi: 10.1128/mcb.16.11.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedram A, Razandi M, Levin ER. J Biol Chem. 2002;277:44385–44398. doi: 10.1074/jbc.M202391200. [DOI] [PubMed] [Google Scholar]
- 17.Cho CH, Lee CS, Chang M, Jang IH, Kim SJ, Hwang I, Ryu SH, Lee CO, Koh GY. Am J Physiol. 2004;286:H1881–H1888. doi: 10.1152/ajpheart.00786.2003. [DOI] [PubMed] [Google Scholar]
- 18.Bagrodia S, Derijard B, Davis RJ, Cerione RA. J Biol Chem. 1995;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- 19.Pearce MJ, McIntyre TM, Prescott SM, Zimmerman GA, Whatley RE. Biochem Biophys Res Commun. 1996;218:500–504. doi: 10.1006/bbrc.1996.0089. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi M, Berk BC. J Clin Invest. 1996;98:2623–2631. doi: 10.1172/JCI119083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Miao H, Li S, Chen KD, Li YS, Yuan S, Shyy JY, Chien S. Am J Physiol. 2002;283:C1540–C1547. doi: 10.1152/ajpcell.00222.2002. [DOI] [PubMed] [Google Scholar]
- 22.Ingber DE. Gravit Space Biol Bull. 1997;10:49–55. [PubMed] [Google Scholar]
- 23.Zachary I. Biochem Soc Trans. 2003;31:1171–1177. doi: 10.1042/bst0311171. [DOI] [PubMed] [Google Scholar]
- 24.Birge RB, Knudsen BS, Besser D, Hanafusa H. Genes Cells. 1996;1:595–613. doi: 10.1046/j.1365-2443.1996.00258.x. [DOI] [PubMed] [Google Scholar]
- 25.Zachary I, Gliki G. Cardiovasc Res. 2001;49:568–581. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- 26.Angel P, Hattori K, Smeal T, Karin M. Cell. 1988;55:875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- 27.Liu ZY, Ganju RK, Wang JF, Ona MA, Hatch WC, Zheng T, Avraham S, Gill P, Groopman JE. J Clin Invest. 1997;99:1798–1804. doi: 10.1172/JCI119344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu ZY, Ganju RK, Wang JF, Schweitzer K, Weksler B, Avraham S, Groopman JE. Blood. 1997;90:2253–2259. [PubMed] [Google Scholar]
- 29.Shyy JY, Chien S. Circ Res. 2002;91:769–775. doi: 10.1161/01.res.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- 30.Osawa M, Masuda M, Kusano K, Fujiwara K. J Cell Biol. 2002;158:773–785. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jalali S, Li YS, Sotoudeh M, Yuan S, Li S, Chien S, Shyy JY. Arterioscler Thromb Vasc Biol. 1998;18:227–234. doi: 10.1161/01.atv.18.2.227. [DOI] [PubMed] [Google Scholar]
- 32.Boyd NL, Park H, Yi H, Boo YC, Sorescu GP, Sykes M, Jo H. Am J Physiol. 2003;285:H1113–H1122. doi: 10.1152/ajpheart.00302.2003. [DOI] [PubMed] [Google Scholar]
- 33.Traub O, Monia BP, Dean NM, Berk BC. J Biol Chem. 1997;272:31251–31257. doi: 10.1074/jbc.272.50.31251. [DOI] [PubMed] [Google Scholar]
- 34.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 35.Go YM, Boo YC, Park H, Maland MC, Patel R, Pritchard KA, Jr, Fujio Y, Walsh K, Darley-Usmar V, Jo H. J Appl Physiol. 2001;91:1574–1581. doi: 10.1152/jappl.2001.91.4.1574. [DOI] [PubMed] [Google Scholar]
- 36.Go YM, Park H, Maland MC, Darley-Usmar VM, Stoyanov B, Wetzker R, Jo H. Am J Physiol. 1998;275:H1898–H1904. doi: 10.1152/ajpheart.1998.275.5.H1898. [DOI] [PubMed] [Google Scholar]
- 37.Jo H, Sipos K, Go YM, Law R, Rong J, McDonald JM. J Biol Chem. 1997;272:1395–1401. doi: 10.1074/jbc.272.2.1395. [DOI] [PubMed] [Google Scholar]
- 38.Li S, Chen BP, Azuma N, Hu YL, Wu SZ, Sumpio BE, Shyy JY, Chien S. J Clin Invest. 1999;103:1141–1150. doi: 10.1172/JCI5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frame MC. J Cell Sci. 2004;117:989–998. doi: 10.1242/jcs.01111. [DOI] [PubMed] [Google Scholar]
- 40.Matsumura F, Totsukawa G, Yamakita Y, Yamashiro S. Cell Struct Funct. 2001;26:639–644. doi: 10.1247/csf.26.639. [DOI] [PubMed] [Google Scholar]
- 41.Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, Kaibuchi K, Ito M. J Biol Chem. 1997;272:12257–12260. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- 42.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Mol Cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 43.Sun H, Breslin JW, Zhu J, Yuan SY, Wu MH. Microcirculation. 2006;13:237–247. doi: 10.1080/10739680600556944. [DOI] [PubMed] [Google Scholar]
- 44.Frangos JA, Eskin SG, McIntire LV, Ives CL. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 45.Feshchenko EA, Langdon WY, Tsygankov AY. J Biol Chem. 1998;273:8323–8331. doi: 10.1074/jbc.273.14.8323. [DOI] [PubMed] [Google Scholar]