Abstract
Alkaloids in the skin glands of poison frogs serve as a chemical defense against predation, and almost all of these alkaloids appear to be sequestered from dietary arthropods. Certain alkaloid-containing ants have been considered the primary dietary source, but dietary sources for the majority of alkaloids remain unknown. Herein we report the presence of ≈80 alkaloids from extracts of oribatid mites collected throughout Costa Rica and Panama, which represent 11 of the ≈24 structural classes of alkaloids known in poison frogs. Forty-one of these alkaloids also occur in the dendrobatid poison frog, Oophaga pumilio, which co-occurs with the collected mites. These shared alkaloids include twenty-five 5,8-disubstituted or 5,6,8-trisubstituted indolizidines; one 1,4-disubstituted quinolizidine; three pumiliotoxins; and one homopumiliotoxin. All but the last of these alkaloid classes occur widely in poison frogs. In addition, nearly 40 alkaloids of unknown structure were detected in mites; none of these alkaloids have been identified in frog extracts. Two of these alkaloids are homopumiliotoxins, five appear to be izidines, four appear to be tricyclics, and six are related in structure to poison frog alkaloids that are currently unclassified as to structure. Mites are common in the diet of O. pumilio, as well as in the diets of other poison frogs. The results of this study indicate that mites are a significant arthropod repository of a variety of alkaloids and represent a major dietary source of alkaloids in poison frogs.
Keywords: chemical defense, dendrobatid frogs, indolizidines, myrmicine ants, pumiliotoxins
Chemical defenses are widespread in nature and represent some of the most diverse and complex adaptations for avoiding predation, yet our understanding of the ecological and chemical nature of these defenses remains relatively incomplete (1, 2). Although animals generally biosynthesize chemical defenses, in some cases the defenses are acquired from external sources, which can include symbiotic relationships with other organisms or sequestration from dietary sources (2, 3). Animals that sequester chemical defenses are dependent on specific dietary sources, and this generally results in complex ecological interactions and evolutionary relationships among organisms (e.g., ref. 4). The chemical properties and biological occurrence of defensive compounds that mediate trophic interactions between organisms are fundamental to the ecological and evolutionary understanding of these systems.
The term “poison frogs” has been applied to lineages of anurans that are characterized by their ability to sequester an alkaloid-based chemical defense from dietary arthropods (5). Poison frogs include certain species from four anuran families worldwide, which include the dendrobatids from Central and South America, mantellids from Madagascar, bufonids from South America, and myobatrachids from Australia. Over the past 30 years, >800 lipophilic alkaloids from the skin of poison frogs have been characterized (6), a number that appears to directly reflect the diversity of alkaloids present in dietary arthropods.
Histrionicotoxins, pumiliotoxins, decahydroquinolines, and various izidine alkaloids make up most of the poison frog skin alkaloids and are thought to originate from ants and mites (6). Batrachotoxins occur in melyrid beetles (7), spiropyrrolizidines in siphonotid millipedes (8), and tricyclics in coccinellid beetles (9). However, only a small number of frog skin alkaloids have been associated with a specific putative dietary source. These include 26 alkaloids from ants, 5 from mites, 5 from beetles, and 6 from millipedes (refs. 8 and 10–12, and the references therein), which represent only 12 of the >20 structural classes reported from poison frogs (6).
Dietary specialization is hypothesized to play a major role in the evolution of alkaloid sequestration and aposematism in dendrobatid poison frogs (13–19). Certain species have been considered “ant–mite specialists” (13, 14), and ants are currently considered the primary dietary source for alkaloids in dendrobatids and other poison frogs. Identifying the specific dietary sources for poison frog alkaloids is necessary to fully understand the ecological and evolutionary complexity of this chemical defense system.
Alkaloids have been well studied in the dendrobatid poison frog Oophaga [formerly Dendrobates (20)] pumilio (21–23). More than 30 years of research with this species throughout its natural geographic range has resulted in the detection of >230 alkaloids from 21 different structural classes (R.A.S., M.A.D., P. Jain, H.M.G., T.F.S., and J.W.D., unpublished data); many of these alkaloids are shared with other poison frogs. The diet of O. pumilio consists mainly of ants and mites (24). O. pumilio is found throughout the Caribbean lowlands of southern Nicaragua, through Costa Rica, and into the northwestern portions of Panama (25), and alkaloid profiles differ considerably among populations throughout this range (22, 23). In an attempt to identify arthropod sources for these alkaloids, we collected arthropods throughout Costa Rica and Panama from locations where O. pumilio occurs. Our study led to the detection of a high diversity of alkaloids from a variety of oribatid mites; many of these alkaloids were also found in O. pumilio collected at the same site. The results suggest that oribatid mites are a dietary source for a wide variety of poison frog alkaloids.
Results
Alkaloids were detected only in extracts of ants, millipedes, and mites. Ants contained pyrrolidine and piperidine alkaloids, most of which were found rarely in O. pumilio. The spiropyrrolizidine alkaloid 236, which occurs in some populations of O. pumilio, was identified in samples of the siphonotid millipede Rhinotus purpureus, as reported previously (8). In contrast, mites contained a wide variety of alkaloids, many of which are found in O. pumilio.
GC–MS analyses detected 79 alkaloids, representing at least 11 structural classes, from a variety of adult oribatid mites (see Table 1 for alkaloids and Table 2 for identification of the mites). Poison frog alkaloids have been assigned individual code names that consist of bold numbers equivalent to the nominal mass and bold letters for identification of alkaloids having the same nominal mass (6). The sites of collection are shown on the map in Fig. 1. Representative poison frog alkaloids that were detected in mite extracts are shown in Fig. 2. Forty-four of these alkaloids have been detected previously in poison frogs; 35 others are alkaloids that will require further characterization. A complete listing of all alkaloids from each mite sample, in the order of GC elution, is presented in supporting information (SI) Data Set 1. In addition, mass spectral and other data on each of the uncharacterized alkaloids are presented in SI Data Set 2. Two representative GC–MS chromatograms of two different oribatid mites from one collection site are presented in SI Fig. 3. Most of the poison frog alkaloids that were identified in mites were also identified from O. pumilio collected at the same site (Table 1). Ants and mites made up the majority of arthropods identified in stomach flushings of O. pumilio. The complete results of the alkaloid and dietary analyses for individual frogs from each of these sites will be reported elsewhere.
Table 1.
Detection of poison frog alkaloids of 11 structural classes in mite extracts
| Site | Sample no. | Structural class |
Unclassifed |
||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5,8-I |
d-5,8-I |
5,6,8-I |
PTX |
hPTX |
1,4Q |
4,6-Q |
3,5-I |
Pyr |
Spiro |
Tri |
|||||||||||||||||||||||||||||||||
| 195I | 203A | 205A | 207A | 209S | 219F/L | 223D | 223V | 225D | 231C | 235B″ | 237D | 261D | 205L | 243F | 269D | 195G | 223A | 235E | 237A | 237C | 237L | 251T | 253H | 259C | 251D | 307F | 307A | 251R | 233A | 237I | 223AB | 183B | 253I | 236 | 193C | 181C | 209G | 227 | 265K | 279I | 323I | ||
| Panama | ○ | ● | ○ | ○ | ○ | ● | ○ | ○ | ○ | ○ | ○ | ||||||||||||||||||||||||||||||||
| Isla Escudo | 1 | ● | |||||||||||||||||||||||||||||||||||||||||
| 2 | ○ | ○ | |||||||||||||||||||||||||||||||||||||||||
| Cayo Agua | 1 | ● | ● | ||||||||||||||||||||||||||||||||||||||||
| 2 | ● | ● | |||||||||||||||||||||||||||||||||||||||||
| 3 | ● | ● | ○ | ● | ○ | ||||||||||||||||||||||||||||||||||||||
| Isla Popa | 1 | ||||||||||||||||||||||||||||||||||||||||||
| 2 | ● | ● | ● | ● | ● | ○ | |||||||||||||||||||||||||||||||||||||
| 3 | ● | ● | ● | ● | ● | ● | ● | ||||||||||||||||||||||||||||||||||||
| 4 | ● | ○ | ● | ||||||||||||||||||||||||||||||||||||||||
| 5 | ● | ○ | |||||||||||||||||||||||||||||||||||||||||
| 6 | ● | ● | ● | ||||||||||||||||||||||||||||||||||||||||
| Cerro Brujo | 1 | ● | ○ | ||||||||||||||||||||||||||||||||||||||||
| 2 | ● | ● | |||||||||||||||||||||||||||||||||||||||||
| 3 | ● | ||||||||||||||||||||||||||||||||||||||||||
| 4 | ● | ● | |||||||||||||||||||||||||||||||||||||||||
| 5 | ○ | ● | |||||||||||||||||||||||||||||||||||||||||
| Cayo Nancy | 1 | ● | ● | ● | ○ | ● | ● | ||||||||||||||||||||||||||||||||||||
| 2 | ● | ● | ● | ||||||||||||||||||||||||||||||||||||||||
| Isla Bastimentos | 1 | ● | ○ | ||||||||||||||||||||||||||||||||||||||||
| 2 | ● | ● | |||||||||||||||||||||||||||||||||||||||||
| 3 | ● | ||||||||||||||||||||||||||||||||||||||||||
| 4 | ● | ● | ○ | ● | ● | ||||||||||||||||||||||||||||||||||||||
| 5 | ● | ● | |||||||||||||||||||||||||||||||||||||||||
| Mainland S. of Pastores | 1 | ○ | ○ | ||||||||||||||||||||||||||||||||||||||||
| Costa Rica | ● | ● | ● | ● | |||||||||||||||||||||||||||||||||||||||
| Rio Sand Box | 1 | ● | ● | ○ | |||||||||||||||||||||||||||||||||||||||
| Roja Maca | 1 | ||||||||||||||||||||||||||||||||||||||||||
| Isais | 1 | ● | ● | ● | ● | ● | ● | ● | |||||||||||||||||||||||||||||||||||
| La Selva | 1 | ● | |||||||||||||||||||||||||||||||||||||||||
| Tortuguero | 1 | ● | ● | ● | |||||||||||||||||||||||||||||||||||||||
| 2 | ● | ||||||||||||||||||||||||||||||||||||||||||
| 3 | ○ | ○ | ○ | ○ | |||||||||||||||||||||||||||||||||||||||
| 4 | ● | ||||||||||||||||||||||||||||||||||||||||||
The filled circles indicate the presence of alkaloids in extracts of mites and O. pumilio from the same site; open circles indicate the presence of alkaloids only in mite extracts from that site. Only alkaloids previously reported from poison frogs (see ref. 6) are included. For the mite alkaloids not reported from poison frogs, see SI Data Set 2. Three isomers of 207A were detected in mites (see SI Data Set 1). The identity of the 5,8-Is 219F and 219L could not be determined from the GC–MS data. PTX, pumiliotoxin; Pyr, pyrrolidine; Spiro, spiropyrrolizidine; Tri, tricyclic.
Table 2.
Taxonomic classification of mites
| Site | Sample no. | Family | Genus/species |
|---|---|---|---|
| Panama | |||
| Isla Escudo | 1 | Scheloribatidae | Genus near Megascheloribates sp. |
| 2 | Drymobatidae | Drymobates sp. A | |
| Cayo Agua | 1 | Mochlozetidae | Dynatozetes amplus (Grandjean) |
| 2 | Unknown A | Unknown A | |
| 3 | Unknown A; Oribotritiidae | Unknown A; Oribotritia didyma (Niedbala & Schatz) | |
| Isla Popa | 1 | Scheloribatidae | Genus near Megascheloribates sp., Scheloribates sp. B |
| 2 | Galumnidae; Drymobatidae | Galumna sp. 1; Drymobates sp. B | |
| 3 | Scheloribatidae; Haplozetidae | Scheloribates sp.; Rostrozetes glaber (Beck), Rostrozetes carinatus (Beck) | |
| 4 | Scheloribatidae; Haplozetidae | Scheloribates sp. B (one was immature); Rostrozetes glaber (Beck) | |
| 5 | Unknown A; Oppiidae | Unknown A; Lanceoppia sp. sensu lato | |
| 6 | Unknown A | Unknown A | |
| Cerro Brujo | 1 | Scheloribatidae; Mochlozetidae | Genus near Megascheloribates sp.; Dynatozetes amplus (Grandjean) |
| 2 | Unknown A; Oppiidae | Unknown A; Ramusella sp. A, immature Ramusella sp. A | |
| 3 | Unknown A | Unknown A | |
| 4 | Unknown A | Unknown A | |
| 5 | Scheloribatidae | Unknown genus A | |
| Cayo Nancy | 1 | Galumnidae; Mochlozetidae | Acrogalumna sp., Galumna sp. A; Dynatozetes amplus(Grandjean); unidentified |
| 2 | Trhypochthoniidae; Tectocepheidae; Epactozetidae; Mesostigmata* | Afronothrus incisivus (Wallwork); Tegeozetes tunicatus (Berlese); Truncozetes sp. | |
| Isla Bastimentos | 1 | Trhypochthoniidae; Hypochthoniidae | Afronothrus incisivus (Wallwork); Eohypochthonius sp., cf gracilis (Jacot) |
| 2 | Unknown A; Dampfiellidae; Mochlozetidae | Unknown A; Beckiella sp., Unguizetes incertus (Balogh & Mahunka) | |
| 3 | No identification | No identification | |
| 4 | Mochlozetidae; Scheloribatidae; Austrachipteriidae | Unguizetes incertus (Balogh & Mahunka); unknown genus A, Hemileius sp.; Lamellobates sp. | |
| 5 | Galumnidae; Scheloribatidae | Galumna sp. A; genus near Megascheloribates sp. | |
| Pastores | 1 | Scheloribatidae; Haplozetidae; Galumnidae | Scheloribates sp. A; Rostrozetes glaber (Beck); Pergalumnasp. |
| Pastores Mainland | 1 | Mesostigmata: Uropodidae* | Unidentified |
| Costa Rica | |||
| Rio Sand Box | 1 | Mochlozetidae; Scheloribatidae; Galumnidae; Haplozetidae | Dynatozetes amplus (Granjean); genus near Megascheloribates sp.; Acrogalumna sp. 2, Galumna sp. 1, unknown genus |
| 2 | Drymobatidae | Drymobates sp. B | |
| Roja Maca | 1 | Scheloribatidae | Genus near Megascheloribates sp. |
| Isais | 1 | Mochlozetidae | Uracrobates (sensu lato): new species |
| La Selva | 1 | Scheloribatidae; Galumnidae | Genus near Megascheloribates sp.; Acrogalumna sp., Galumna sp. B |
| Tortuguero | 1 | Lohmanniidae; Hypochthoniidae; Oppiidae | Meristacarus sp. cf. longisetosus (Mahunka); Malacoangelia remigera (Berlese); Brachioppia sp. |
| 2 | Unknown A; Oppiidae | Unknown A; Kokoppia sp., Brachioppia sp. B | |
| 3 | Galumnidae | Galumna sp. 1 | |
| 4 | Unknown A; Oppiidae | Unknown A; Kokoppia sp., Brachioppia sp. B, Pulchroppia sp. | |
*Not an oribatid mite.
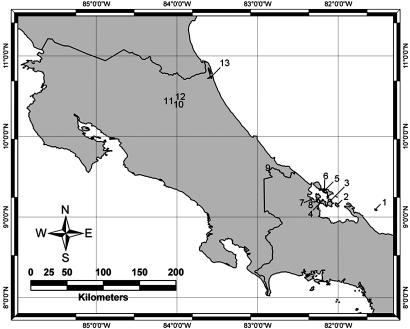
Fig. 1.
Map of sites where alkaloid-containing mites were collected. Site 1, Isla Escudo; site 2, Cayo Agua; site 3, Isla Popa; site 4, Cerro Brujo; site 5, Cayo Nancy; site 6, Isla Bastimentos; site 7, Isla Pastores; site 8, mainland south of Isla Pastores; site 9, Rio Sand Box; site 10, La Selva; site 11, Isais; site 12, Roja Maca; site 13, Tortuguero.
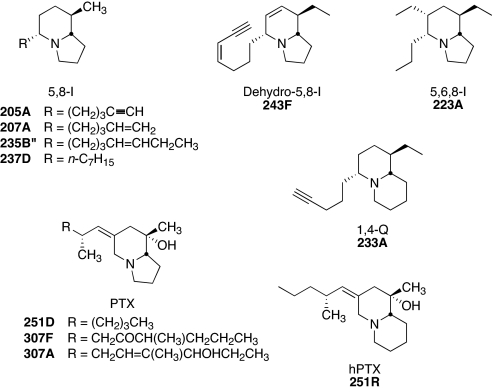
Fig. 2.
Structures of alkaloids representative of six classes of poison frog alkaloids detected in oribatid mites from Costa Rica and Panama (see Table 1). All contain branched carbon skeletons. The reported position of the ring double bond in 243F (6) is a topic requiring further investigation.
Most alkaloids extracted from oribatid mites were izidines, which contain branch points in their carbon skeletons. These izidines included thirteen 5,8-disubstituted indolizidines (5,8-Is); three dehydro-5,8-Is; nine 5,6,8-trisubstituted indolizidines (5,6,8-Is); and two 1,4-disubstituted quinolizidines (1,4-Qs) (Table 1), all of which have been detected previously in poison frogs. Five additional branched-chain izidines found in mites have not been detected in poison frogs (see SI Data Set 2). The branched-chain izidine alkaloids were identified in multiple mite families (Table 2). A 5,6,8-I and a 1,4-Q have been reported recently in extracts of a scheloribatid mite cultured in by Takada et al. in Japan (12). Branched-chain izidines are the largest group of defensive compounds found in O. pumilio; they comprise ≈30% of all alkaloids in this species, which is the same proportion as for poison frogs in general (6).
Izidines with linear carbon skeletons, lacking branch points, are well known from myrmicine ants (10). Only two branched-chain izidines have been reported from ants: a 5,6,8-I detected in a mixed sample of myrmicine ants from Panama (8) (that sample probably also contained mites) and a 5,8-I from a Madagascan myrmicine ant of the genus Tetramorium (11). Although all unbranched-chain poison frog alkaloids have been presumed to derive from myrmicine ants (10), here we report one 3,5-disubstituted indolizidine (3,5-I) and one 4,6-disubstituted quinolizidine (4,6-Q) from oribatid mites (Tables 1 and 2). Unbranched-chain izidines account for ≈7% of the alkaloids in O. pumilio and 6% of all reported poison frog alkaloids (6).
Four frog alkaloids from the pumiliotoxin family, including three pumiliotoxins and one homopumiliotoxin, were detected in oribatid mites (Tables 1 and 2). Two additional homopumiliotoxins, not yet detected in poison frogs, were present in one mite extract (see SI Data Set 1 and SI Fig. 3). Pumiliotoxins have been detected previously in two species of formicine ants (18) and in oribatid mites of the genus Scheloribates cultured in Japan (12); however, to our knowledge homopumiliotoxins have never before been detected in an arthropod. We identified pumiliotoxins from members of Scheloribatidae and an apparently undescribed family of oribatid mites and from mixed samples of mites that contained one of these two taxa (Table 2). We identified homopumiliotoxins from members of Scheloribatidae (Table 2). Collectively, the pumiliotoxins, all of which have branched carbon skeletons, are major alkaloids in many populations of O. pumilio and account for ≈16% of all alkaloids in this species. The pumiliotoxins represent the most widespread group of poison frog alkaloids and are found in all poison frog lineages worldwide.
The tricyclic alkaloid precoccinelline was detected in a mixed sample of oribatid mites containing species from Galumnidae and Mochlozeteidae (Tables 1 and 2). Precoccinelline is a well known alkaloid found in coccinellid beetles (9). Precoccinelline and another tricyclic alkaloid were recently reported from a Scheloribates species cultured in Japan (12). The most common tricyclic alkaloid in O. pumilio is 205B, which was not detected in any mite extract. Four alkaloids that appear to be tricyclics were detected in mites but not yet in frogs (see SI Data Set 1 and SI Data Set 2). Tricyclics represent ≈7% of the alkaloids in O. pumilio and 8% of those in poison frogs generally.
The spiropyrrolizidine alkaloid 236 was detected in a mixed oribatid mite sample (Tables 1 and 2) and has been identified previously in siphonotid millipedes (8, 11). Spiropyrrolizidine 236 is found as a major alkaloid in some populations of O. pumilio, and spiropyrrolizidines are known from most poison frog families but do not make up a large percentage of known alkaloids.
Several of the so-named “unclassified” poison frog alkaloids, those with as yet undefined structures, were also identified in oribatid mites (Tables 1 and 2). Interestingly, one unclassified alkaloid was also detected in a member of the mite family Uropodidae, a non-oribatid mite (Tables 1 and 2). Unclassified alkaloids represent 13% of the alkaloids in O. pumilio and >20% of the alkaloids in poison frogs.
Discussion
Our results indicate that oribatid mites represent a previously unsuspected repository of a wide variety of alkaloids and a significant dietary source for the alkaloids found in poison frogs. Alkaloids have only recently been reported in mites, namely in two different species of scheloribatid mites cultured in Japan (12). Our study adds to these findings and illustrates that oribatid mites possess a tremendous diversity of alkaloids, many of which also occur in poison frogs and some that have not previously been reported in nature. Many of the major structural classes of alkaloids found in poison frogs have now been identified in oribatid mites, suggesting that oribatid mites are a major dietary source for the alkaloids present in poison frogs.
Dietary specialization is common among organisms that sequester chemical defenses from dietary sources (e.g., ref. 26) and has been proposed as an important component in the evolution of sequestered defenses and aposematism in dendrobatid frogs (13–17, 19). Some dendrobatid frogs consume ants and/or mites in higher proportions than are locally available in the leaf-litter and have been considered “ant–mite specialists” (13, 14, 27). On the basis of previous data, ants had been assumed to be the main source of alkaloids in dendrobatids (10, 18), and myrmecophagy has been presumed to be the main focus of dietary specialization in dendrobatids (13–17, 19). Our findings suggest instead that mites exceed ants in importance as dietary sources of alkaloids in poison frogs. Certainly, mites are a major dietary component of O. pumilio (ref. 24 and this study) and other dendrobatids (27).
Oribatid mites are well known to be among the most abundant and diverse arthropods in soil and leaf-litter, in both temperate and tropical regions (28–30). Their food generally consists of decaying higher plant material and saprophytic fungi (31, 32), although stable isotope studies indicate that necrophagy or predation on small invertebrates is common, especially in tropical soils (32, 33). Oribatid mites posses paired exocrine glands (34), called opisthonotal or oil glands, that secrete a wide range of organic compounds, including monoterpenes, sesquiterpenes, aromatics, aliphatic aldehydes, a ketone, fatty acids, fatty acid esters, an alkyl formate, and hydrocarbons (12, 35–42). The functions of these compounds have been little studied but include alarm signals and chemical defenses (34, 38). In the better-studied and closely related mite group Astigmata (not occurring in our samples), compounds from homologous glands also function as aggregation signals and sex pheromones (40). Such glands are almost certainly the source of extractable alkaloids as well. In poison frogs, alkaloids are present in the skin glands as defensive compounds and in some cases [e.g., pumiliotoxins (21, 43, 44)] are highly toxic. It seems likely that alkaloids also provide defense for oribatid mites against predation, but further research is needed to confirm this.
Certain oribatid mites apparently contain many different structural classes of alkaloids. For instance, scheloribatid mites from Isla Escudo (Table 1, Isla Escudo sample 1) contain eight indolizidines, three homopumiliotoxins, a pyrrolidine, and an unclassified alkaloid. Several classes of alkaloids have also been detected from two species of Scheloribates cultured in Japan (12). One species contained a pumiliotoxin and tricyclic alkaloids, and the other species contained a different pumiliotoxin, a deoxypumiliotoxin, a 5,6,8-I, and a 1,4-Q; four other alkaloids were detected but not identified (12). In some of our cases, alkaloids are shared by different oribatid mites. For example, the 5,8-I 207A was identified from two different mite samples on Isla Escudo, each of which contained mites from a different family (Scheloribatidae and Drymobatidae). Takada et al. (12) reported that alkaloids in Scheloribates were adult-specific and not detectable in either the larval or nymphal instars, even though all shared the same diet. In the present study, alkaloids were only detected in adult mites. Thus, it seems likely that alkaloids are produced by scheloribatid mites (and possibly other oribatid mites), rather than being obtained from their diet. However, this does not preclude the possibility of a symbiotic microorganism.
It now appears that some of the alkaloid classes found in poison frogs can originate from more than one taxon of dietary arthropods. These classes include pumiliotoxins that have been identified in formicine ants and oribatid mites; certain pyrrolidines and pyrrolizidines that have been identified from myrmicine, formicine, and ponerine ants; certain indolizidines that have been identified in myrmicine ants and now in oribatid mites; tricyclics that have been identified in coccinellid beetles and oribatid mites; and spiropyrrolizidines that have been identified in siphonotid millipedes and now in oribatid mites. The presence of the same or similar alkaloids in different arthropod groups raises an interesting question: Are different arthropod groups producing identical compounds, or are certain compounds being transferred between different arthropods?
A summary of the alkaloids found in poison frogs that have now been detected in putative dietary arthropods is presented in Table 3. Eight classes of poison frog alkaloids with unbranched carbon skeletons include 148 alkaloids; of these, only 4 have now been detected in oribatid mites, whereas 23 have been reported from ants. Eleven classes of poison frog alkaloids with branched carbon skeletons include 372 alkaloids; of these, 36 have now been detected in oribatid mites, whereas only 3 have been reported from an ant. Investigation of alkaloids in ants has a long history (45–47), whereas the investigation of the presence, distribution, chemical nature, and function of mite alkaloids has just begun. It promises to be a fruitful area of research.
Table 3.
Alkaloids detected in poison frogs and in putative arthropod sources
| Structural class | No. of alkaloids |
|||||
|---|---|---|---|---|---|---|
| Poison frogs |
Arthropod sources |
|||||
| All frogs | O. pumilio | Oribatid mites | Ants | Coccinellid beetles | Siphonotid millipedes | |
| Unbranched* | ||||||
| HTX | 16 | 7 | 0 | 0† | 0 | 0 |
| DHQ | 35 | 18 | 0 | 3 | 0 | 0 |
| 3,5-P | 23 | 8 | 0 | 3 | 0 | 0 |
| 3,5-I | 20 | 5 | 1 | 8 | 0 | 0 |
| 4,6-Q | 6 | 2 | 1 | 1 | 0 | 0 |
| Pyr | 10 | 7 | 2 | 5 | 0 | 0 |
| Pip | 29 | 14 | 0 | 3 | 0 | 0 |
| Lehm | 9 | 3 | 0 | 0† | 0 | 0 |
| Branched‡ | ||||||
| PTX | 36 | 17 | 4 | 2 | 0 | 0 |
| Deoxy-PTX | 12 | 0 | 1 | 0 | 0 | 0 |
| aPTX | 22 | 13 | 0§ | 0 | 0 | 0 |
| hPTX | 18 | 2 | 1 | 0 | 0 | 0 |
| Deoxy-hPTX | 4 | 3 | 0§ | 0 | 0 | 0 |
| 5,8-I | 78 | 31 | 14 | 1 | 0 | 0 |
| Dehydro-5,8-I | 33 | 6 | 3 | 0 | 0 | 0 |
| 5,6,8-I | 74 | 26 | 9 | 0 | 0 | 0 |
| 1,4-Q | 22 | 9 | 2 | 0 | 0 | 0 |
| Spiro | 7 | 4 | 1 | 0 | 0 | 6 |
| Tri | 66 | 15 | 1 | 0 | 2 | 0 |
| Total | 520 | 190 | 40 | 26 | 2 | 6 |
Only alkaloids that have been reported previously in poison frogs (6) are included. The batrachotoxin alkaloids are not included. Six unclassified alkaloids were detected in oribatid mites but are not included in the table (see SI Data Set 1). HTX, histrionicotoxin; DHQ, decahydroquinoline; Pyr, pyrrolidine; Pip, piperidine; Lehm, lehmizidine; PTX, pumiliotoxin; Spiro, spiropyrrolizidine; Tri, tricyclic.
*Alkaloids with unbranched carbon skeletons.
†Likely of myrmicine ant origin.
‡Alkaloids with branched carbon skeletons.
§Likely of oribatid mite origin.
Materials and Methods
Arthropod and Frog Collection.
Arthropods were collected from leaf-litter at multiple sites in Costa Rica and Panama during the dry and wet seasons of 2005 and 2006 (February/March and July/August, respectively; see Fig. 1) by using Berlese funnel extractors. At each site, the extractors were run for ≈12 h, and all arthropods were allowed to fall into empty plastic bags (without solvent). Arthropods were separated by morphospecies under a dissecting microscope and placed in taxon-specific vials containing methanol. The collected arthropods included mainly mites, ants, beetles, millipedes, spiders, pseudoscorpions, opilionids, termites, springtails, and flies. At each site, 10 individuals of O. pumilio were also collected for analysis of skin alkaloids, and an additional 20 individuals were stomach-flushed to obtain dietary information. Voucher specimens are located at Florida International University.
Alkaloid Analysis.
Methanol extracts of arthropods and alkaloid fractions of O. pumilio were analyzed by GC–MS. Alkaloid fractions were prepared from methanol for individual frog skins, as described in Saporito et al. (23). GC–MS analyses were performed on a Polaris Q instrument (Thermo Electron, San Jose, CA) with a 30 m × 0.25 mm i.d. Rtx-5MS Restex fused silica column in a Focus gas chromatograph programmed to increase in temperature from 100 to 280°C at a rate of 10°C per minute. Some extracts were also analyzed by using GC–MS coupled with FTIR spectroscopy on a Hewlett–Packard (Palo Alto, CA) instrument with a 5971 series mass selective detector using a similar GC column as above. High-resolution mass data were obtained in a Micromass (Manchester, U.K.) GCT spectrometer using a similar GC column and program. Each extract was analyzed by using electron impact and chemical ionization (NH3) mass spectrometry. Previously documented alkaloids were identified based on comparison of retention times, mass spectral data, and in some cases vapor-phase FTIR spectra, with that of data for known poison frog alkaloids (see ref. 6). Some of the identifications are tentative.
Supplementary Material
Acknowledgments
We thank Autoridad Nacional del Ambiente, Republic of Panama (Permit SEX/A-97-06), Ministerio del Ambiente y Energia, and the Republic of Costa Rica (Permits 010-ACCVC-05 and 099-2006-SINAC) for permission to collect and export the samples used in this study; the Smithsonian Tropical Research Institute (STRI), the Organization for Tropical Studies (OTS), O. Arosemena, and M. Sasa for assistance in obtaining these permits; OTS La Selva Biological Station, Reserva Biologica La Tirimbina Rainforest Center, Hotel Rio Palmas, Isais Paniagua, Finca Chimuri, and Finca Calderon for permission to work and collect the samples that were used in this study; A. Madden, S. Namoff, O. Vargas, and J. Watling for assistance in the field; S. Whitfield for assistance with the map; and J. Snyder for valuable comments on this manuscript. This research was supported in part by STRI, OTS, Florida International University (FIU), the National Geographic Society, and multiple Courtesy Associate appointments by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Research at the National Institutes of Health was supported by the intramural funds of NIDDK. This is contribution 123 to the Tropical Biology Program at FIU.
Abbreviations
- 3,5-I
3,5-disubstituted indolizidine
- 5,8-I
5,8-disubstituted indolizidine
- 5,6,8-I
5,6,8-trisubstituted indolizidine
- 1,4-Q
1,4-disubstituted quinolizidine
- 4,6-Q
4,6-disubstituted quinolizidine.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702851104/DC1.
References
- 1.Berenbaum MR. Proc Natl Acad Sci USA. 1995;92:2–8. doi: 10.1073/pnas.92.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruxton GD, Sherratt TN, Speed MP. Avoiding Attack: The Evolutionary Ecology of Crypsis, Warning Signals, and Mimicry. Oxford: Oxford Univ Press; 2004. [Google Scholar]
- 3.Duffey SS. Annu Rev Entomol. 1980;25:447–477. [Google Scholar]
- 4.Berenbaum MR, Zangerl AR. Proc Natl Acad Sci USA. 1998;95:13743–13748. doi: 10.1073/pnas.95.23.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daly JW. Proc Natl Acad Sci USA. 1995;92:9–13. doi: 10.1073/pnas.92.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daly JW, Spande TF, Garraffo HM. J Nat Prod. 2005;68:1556–1575. doi: 10.1021/np0580560. [DOI] [PubMed] [Google Scholar]
- 7.Dumbacher JP, Wako A, Derrickson SR, Samuelson A, Spande TF, Daly JW. Proc Natl Acad Sci USA. 2004;101:15857–15860. doi: 10.1073/pnas.0407197101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saporito RA, Donnelly MA, Hoffman RL, Garraffo HM, Daly JW. J Chem Ecol. 2003;29:2781–2786. doi: 10.1023/b:joec.0000008065.28364.a0. [DOI] [PubMed] [Google Scholar]
- 9.Daloze D, Braekman JC, Pasteels JM. Chemoecology. 1995;5/6:173–183. [Google Scholar]
- 10.Jones TH, Gorman JST, Snelling RR, Delabie JHQ, Blum MS, Garraffo HM, Jain P, Daly JW, Spande TF. J Chem Ecol. 1999;25:1179–1193. [Google Scholar]
- 11.Clark VC, Raxworthy CJ, Rakotomalala V, Sierwald P, Fisher BL. Proc Natl Acad Sci USA. 2005;102:11617–11622. doi: 10.1073/pnas.0503502102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takada W, Sakata T, Shimano S, Enami Y, Mori N, Nishida R, Kuwahara Y. J Chem Ecol. 2005;31:2403–2415. doi: 10.1007/s10886-005-7109-9. [DOI] [PubMed] [Google Scholar]
- 13.Toft CA. Herpetologica. 1995;51:202–216. [Google Scholar]
- 14.Caldwell JP. J Zool. 1996;240:75–101. [Google Scholar]
- 15.Vences M, Glaw F, Bohme M. Zool Anz. 1998;236:217–230. [Google Scholar]
- 16.Summer K, Clough ME. Proc Natl Acad Sci USA. 2001;98:6227–6232. doi: 10.1073/pnas.101134898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos JC, Coloma LA, Cannatella DC. Proc Natl Acad Sci USA. 2003;100:12792–12797. doi: 10.1073/pnas.2133521100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saporito RA, Garraffo HM, Donnelly MA, Edwards AL, Longino JT, Daly JW. Proc Natl Acad Sci USA. 2004;101:8045–8050. doi: 10.1073/pnas.0402365101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darst CR, Menendez-Guerrero PA, Coloma LA, Cannatella DC. Am Nat. 2005;165:56–69. doi: 10.1086/426599. [DOI] [PubMed] [Google Scholar]
- 20.Grant T, Frost DR, Caldwell JP, Gagliardo R, Haddad CFB, Kok PJR, Means DB, Noonan BP, Schargel WE, Wheeler WC. Bull Am Mus Nat Hist. 2006;299:1–262. [Google Scholar]
- 21.Daly JW, Myers CW. Science. 1967;156:970–973. doi: 10.1126/science.156.3777.970. [DOI] [PubMed] [Google Scholar]
- 22.Daly JW, Myers CW, Whittaker N. Toxicon. 1987;25:1023–1095. doi: 10.1016/0041-0101(87)90265-0. [DOI] [PubMed] [Google Scholar]
- 23.Saporito RA, Donnelly MA, Garraffo HM, Spande TF, Daly JW. J Chem Ecol. 2006;32:795–814. doi: 10.1007/s10886-006-9034-y. [DOI] [PubMed] [Google Scholar]
- 24.Donnelly MA. Copeia. 1991;3:723–730. [Google Scholar]
- 25.Savage JM. The Amphibians and Reptiles of Costa Rica: A Herpetofauna Between Two Continents, Between Two Seas. Chicago: Univ Chicago Press; 2002. [Google Scholar]
- 26.Nishida R. Annu Rev Entomol. 2002;47:57–92. doi: 10.1146/annurev.ento.47.091201.145121. [DOI] [PubMed] [Google Scholar]
- 27.Simon MP, Toft CA. Oikos. 1991;61:263–278. [Google Scholar]
- 28.Peterson H, Luxton M. Oikos. 1982;39:287–388. [Google Scholar]
- 29.Maraun M, Scheu S. Ecography. 2000;23:374–383. [Google Scholar]
- 30.Franklin E, Hayek T, Fagundes EP, Silva LL. Braz J Biol. 2004;64:59–72. doi: 10.1590/s1519-69842004000100008. [DOI] [PubMed] [Google Scholar]
- 31.Schuster R. Z Morph Okol Tiere. 1956;45:1–33. [Google Scholar]
- 32.Schneider K, Renker K, Scheu S, Maraun M. Phytophaga. 2004;14:247–256. [Google Scholar]
- 33.Illig J, Langel R, Norton RA, Scheu S, Maraun M. J Trop Ecol. 2005;21:589–593. [Google Scholar]
- 34.Raspotnig G, Schuster R, Krisper G. Zoomorphology. 2003;122:105–112. [Google Scholar]
- 35.Sakata T, Tagami K, Kuwahara Y. J Acarol Soc Jpn. 1995;4:69–75. [Google Scholar]
- 36.Sakata T, Norton RA. Int J Acarol. 2001;27:281–292. [Google Scholar]
- 37.Raspotnig G, Schuster R, Krisper G, Fauler G, Leis HJ. Exp App Acarol. 2001;25:933–946. doi: 10.1023/a:1020634215709. [DOI] [PubMed] [Google Scholar]
- 38.Shimano S, Sakata T, Mizutani Y, Kuwahara Y, Aoki J. J Chem Ecol. 2002;28:1831–1837. doi: 10.1023/a:1020517319363. [DOI] [PubMed] [Google Scholar]
- 39.Sakata T, Shimano S, Kuwahara Y. Exp App Acarol. 2003;29:279–291. doi: 10.1023/a:1025882214375. [DOI] [PubMed] [Google Scholar]
- 40.Kuwahara Y. In: Advances in Chemical Ecology. Carde RT, Millar JG, editors. Cambridge, UK: Cambridge Univ Press; 2004. pp. 76–109. [Google Scholar]
- 41.Raspotnig G, Krisper G, Schuster R. Exp App Acarol. 2005;35:47–58. doi: 10.1007/s10493-004-2027-z. [DOI] [PubMed] [Google Scholar]
- 42.Raspotnig G, Krisper R, Schuster R, Fauler G, Leis HJ. J Chem Ecol. 2005;31:419–430. doi: 10.1007/s10886-005-1350-0. [DOI] [PubMed] [Google Scholar]
- 43.Daly JW, Tetsuo K, Wilham J, Garraffo HM, Spande TF, Espinosa A, Donnelly MA. Proc Natl Acad Sci USA. 2002;99:13996–14001. doi: 10.1073/pnas.222551599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weldon PJ, Kramer M, Gordon S, Spande TF, Daly JW. Proc Natl Acad Sci USA. 2006;103:17818–17821. doi: 10.1073/pnas.0608646103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blum MS. J Toxicol Toxin Rev. 1992;11:115–164. [Google Scholar]
- 46.Numata A, Ibuka T. In: The Alkaloids. Brossi A, editor. Vol 31. San Diego: Academic; 1987. pp. 193–315. [Google Scholar]
- 47.Jones TH, Blum MS. In: Alkaloids: Chemical and Biological Perspectives. Pelletier SW, editor. Vol 1. New York: Wiley; 1983. pp. 33–84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.