Abstract
The response of tropical forests to climate change will depend on individual plant species' nutritional strategies, which have not been defined in the case of the nitrogen nutrition that is critical to sustaining plant growth and photosynthesis. We used isotope natural abundances to show that a group of tropical plant species with diverse growth strategies (trees and ferns, canopy, and subcanopy) relied on a common pool of inorganic nitrogen, rather than specializing on different nitrogen pools. Moreover, the tropical species we examined changed their dominant nitrogen source abruptly, and in unison, in response to precipitation change. This threshold response indicates a coherent strategy among species to exploit the most available form of nitrogen in soils. The apparent community-wide flexibility in nitrogen uptake suggests that diverse species within tropical forests can physiologically track changes in nitrogen cycling caused by climate change.
Keywords: global change, isotope, community ecology
Strategies of plant nitrogen (N) acquisition control many different aspects of ecological systems (1–3), with important implications for modeling and predicting ecosystem responses to climate change, rising levels of atmospheric CO2, and N pollution (4, 5). Whether a given plant species can adjust to different N sources will determine its ability to adapt to environmental change. For instance, if species specialize on a particular form of N in the soil, either nitrate, ammonium, or dissolved organic N (DON) (6–11), then any changes in the N cycle could trigger marked changes in community composition and species distributions. Alternatively, if plants are less specialized (12, 13), environmental changes to the N cycle may not result in dramatic species turnover, but instead could induce increased competition for N together with more subtle changes in plant communities.
Studies of extratropical land plant communities (6–11) and theories of plant competition have since Hutchinson's “paradox of the plankton” (14, 15) largely emphasized the first strategy, that species coexist by partitioning nutrient sources into relatively specialized “niches.” Little is known, however, about the sources of N that support plants in tropical forests, the sensitivity of N sources to climate change, and the resulting links between plant diversity and the N cycle.
Here, we use natural stable isotopes to constrain the sources of N that fuel the growth of a community of functionally diverse tropical plant species in response to differences in precipitation climates. We make use of six well characterized sites of montane tropical forest from the windward slopes of Mt. Haleakala on the island of Maui, Hawaii (16), across which mean annual precipitation (MAP) changes from 2,200 to 5,050 mm. Although this range in precipitation spans that observed for many tropical rainforests globally (17), other state factors such as mean annual temperature (16°C), geologic substrate age (≈400,000 years), and biotic composition (dominated by native species) are relatively constant across this sequence (16, 18).
At each of our sites, Schuur and Matson (16) measured the 15N/14N of foliage from four different plant species that together contribute >80% of total aboveground biomass and productivity of the forests. When combined, the species also encompass the growth strategies that characterize forest ecosystems more generally: Metrosideros polymorpha, a dominant canopy tree; Cheirodendron trigynum, a subdominant canopy tree; Cibotium glaucum, a tree fern; and Melicope clusiifolia, an understory woody plant. These data show only slight (≈1–3‰) differences in the δ15N of species' leaves within a given site (Table 1) [δ15N in units of per mil (‰) vs. air = (15N/14Nsample/15N/14Nair − 1) × 1,000], and a broad decline in plant δ15N with increasing precipitation (see Fig. 2a), similar to the pattern identified for plant communities worldwide (19, 20). Although bulk soil δ15N also decreases across the precipitation gradient, the δ15N decrease in the foliage is nearly twice as great (Table 1).
Table 1.
Nitrogen concentration and isotope data
| MAP, mm | Dry soil, μmol/g |
‰ vs. air |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NO3− | NH4+ | DON | NO3− δ15N | NH4+ δ15N | DON δ15N | SOM δ15N | Metro. δ15N | Meli. δ15N | Cheir. δ15N | Cibot. δ15N | Leaf δ15N range | |
| 2,200 | 0.95 | 0.21 | 3.44 | 3.5 | 7.3 | 6.9 | 5.79 | 1.0 | 3.21 | 1.38 | 2.4 | 2.21 |
| (0.37) | (0.05) | (0.61) | (0.42) | (1.34) | (0.19) | (0.64) | (0.18) | (0.44) | (0.25) | |||
| n = 11 | n = 11 | n = 6 | n = 11 | n = 11 | n = 6 | |||||||
| 2,450 | 0.38 | 0.22 | 1.95 | 1.6 | 8.1 | 6.6 | 6.12 | 0.72 | 2.4 | −0.28 | 0.86 | 2.68 |
| (0.08) | (0.03) | (0.41) | (0.90) | (0.77) | (0.52) | (0.23) | (0.3) | (0.11) | (0.21) | |||
| n = 11 | n = 11 | n = 6 | n = 11 | n = 11 | n = 6 | |||||||
| 2,750 | 0.29 | 0.28 | 3.32 | −0.1 | 6.4 | 5.7 | 4.87 | −1.18 | −0.52 | −1.89 | −0.04 | 1.86 |
| (0.05) | (0.05) | (0.51) | (1.30) | (0.75) | (0.30) | (0.27) | (0.63) | (0.26) | (0.3) | |||
| n = 6 | n = 11 | n = 6 | n = 6 | n = 11 | n = 6 | |||||||
| 3,350 | 0.38 | 0.15 | 2.68 | 2.5 | 5.7 | 6.8 | 7.35 | −0.1 | 1.34 | −0.28 | 0.41 | 1.62 |
| (0.03) | (0.02) | (0.20) | (0.62) | (0.65) | (0.51) | (0.34) | (0.3) | (0.21) | (0.17) | |||
| n = 11 | n = 11 | n = 6 | n = 11 | n = 11 | n = 6 | |||||||
| 4,050 | 0.03 | 0.56 | 6.33 | 56.2 | −0.7 | 5.0 | 3.2 | −3.98 | −1.13 | −4.11 | −2.78 | 2.98 |
| (0.03) | (0.05) | (0.90) | (35.52) | (0.97) | (0.72) | (0.39) | (0.55) | (0.67) | (0.35) | |||
| n = 11 | n = 11 | n = 6 | n = 4 | n = 8 | n = 6 | |||||||
| 5,050 | 0.01 | 0.75 | 8.01 | 20.0 | −2.8 | 4.9 | 2.95 | −4.45 | −4.93 | −6.89 | −4.4 | 2.49 |
| (0.001) | (0.08) | (0.45) | (N/A) | (1.04) | (0.042) | (0.67) | (0.1) | (0.61) | (0.31) | |||
| n = 9 | n = 9 | n = 9 | n = 1 | n = 9 | n = 9 | |||||||
Means (SE) are shown. Plant leaf (n = 5) and bulk soil organic matter (SOM) isotope data are from Schuur and Matson (8). SOM δ15N is the composite of four samples of the top 10 cm of soil at each site. Metro., Metrosideros polymorpha; Meli., Melicope clusiifolia; Cheir., Cheirodendron trigynum; Cibot., Cibotium glaucum.
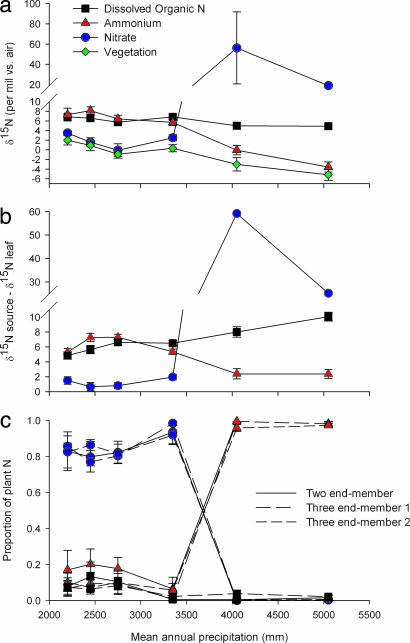
Fig. 2.
N isotope data and calculations of source attribution. (a) Site-averaged δ15N of nitrate, ammonium, and DON sources, and plant foliar N. (b) The difference between foliar δ15N and soil N sources for all possible soil N source pools. (c) Mixing analysis of the proportion of vegetation N derived from N sources. Results of the two end-member calculation (with nitrate and ammonium) and three end-member calculations (inclusion of DON) are shown. Two different scenarios were examined for DON uptake: scenario 1 is based on the measured δ15N of bulk DON; scenario 2 is based on an estimate of amino acid δ15N (see Methods). All three approaches yield the same basic pattern. Error bars are ±SE, except for the three end-member results, which are ±SD.
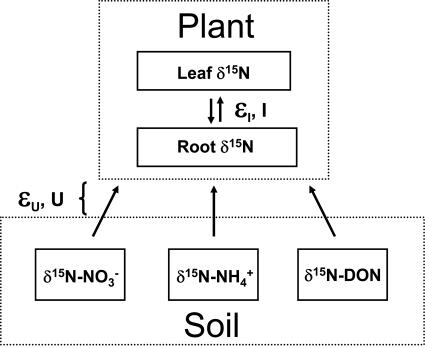
Regardless of whether individual plants are at steady state with respect to the environment, the δ15N of their leaves should be close to that of their N source(s) (Fig. 1). Plant N uptake does not express an appreciable isotope effect under natural soil conditions (21–25). Thus, this process causes a minimal isotopic difference between a plant and its N source, and it similarly does not significantly modify the isotopic ratio of the acquired N source. In addition, isotopic discrimination is minimal during the major loss processes, leaf fall and root turnover (26, 27). Internal plant fractionation has been shown to cause ≈2‰ differences between roots and shoots across a diversity of ecosystems (23, 25, 33) that include tropical rainforests (34). Finally, whereas ectomycorrhizae may influence plant δ15N relative to a soil N source (28, 29), they are virtually absent from native Hawaiian flora (30–32). The dominant type of mycorrhizae in Hawaiian soils, arbuscular mycorrhizae (AM) (30–32), may impart a slight additional fractionation of 2‰ (35) or less (28). We therefore assume a combined isotopic effect of 4‰ owing to plant N allocation and arbuscular mycorrhizae, causing leaves to be ≈2‰ lower than the preferred N source (Fig. 1 and Methods).
Fig. 1.
Conceptual model of the plant–soil N isotope system under steady-state conditions. U, plant uptake flux; I, internal plant allocation flux. εu and εi are effective isotope effects resulting from plant uptake and root-to-shoot allocation, respectively [ε (‰) = (14k/15k − 1) × 1,000]. Under natural soil conditions, εu is negligible (21–25); plant uptake does not impart a major fractionation of N isotopes, so that the 15N/14N of soil N sources is not impacted by plant uptake processes. Based on previous observations (23, 25, 33) and allowing for a potential effect from arbuscular mycorrhizae (35), we assume an εi of 4‰. Assuming that N is lost via leaf and root litter equally, one-half of the isotope effect of 4‰ is expressed on plant foliage, yielding a 2‰ difference of leaves from their soil N sources (see text and Methods for further explanation and equations). See also Fig. 2 for evidence of εi in our rainforests.
Given the relatively minor fractionation during plant uptake, the impact of plants on isotopic differences among available soil N pools is largely caused by competition between plant uptake and the more fractionating microbial transformations (e.g., nitrification and denitrification). For instance, a lower ratio of plant nitrate uptake relative to denitrification will increase the δ15N of soil nitrate owing to discrimination against the heavier isotope of N by denitrifying bacteria, all else held constant. Similarly, to the extent that plants alter the relative importance of leaching vs. gaseous N losses, these effects will feed back on the integrated δ15N of bulk soil N (18). However, these indirect roles of plant uptake in setting the δ15N of the various soil N pools do not impact the isotopic link between plants and their soil N sources, which is the focus here.
Adopting these constraints on our plant–soil systems, one possible interpretation for the close correspondence in δ15N across plant species in a given site is that all plants are supported by a common source of N. Alternatively, if the δ15N of N sources are similar to one another in these forests (e.g., roughly within 3‰ of one another), the δ15N of the vegetation foliage would not provide a significant constraint on N source attribution. With regard to the decline in plant δ15N with increasing precipitation, one plausible explanation is that the δ15N of a single dominant N source for plants decreases systematically from the driest to wettest climates. Alternatively, the proximal N source to all plants may change with increasing precipitation, such that the observed decrease in plant δ15N represents a change in the dominant N source. Finally, both dynamics (changes in the δ15N of a given source and switches in plant preference) may contribute to the δ15N changes in plants.
Results and Discussion
To distinguish among the above competing explanations for the similarity of δ15N among plant types and the change in δ15N with precipitation, we extracted pools of nitrate, ammonium, and DON from the top 15 cm of soil, within which ≈80% of plant root biomass is located (16). Nitrate and ammonium displayed unique and contrasting patterns of change in δ15N with increasing precipitation. Ammonium decreased in δ15N with increasing MAP, whereas nitrate δ15N increased dramatically with increasing MAP; the δ15N of both forms changed abruptly at ≈3,500 mm of MAP (Fig. 2a). These isotopic trends correlated inversely with the abundance of each N source: nitrate was most abundant in soils at the dry end of the sequence, whereas ammonium was most abundant in wetter soils (Table 1). We have previously shown that these trends in δ15N with rainfall are caused by differences in N isotope fractionation imparted by nitrifying and denitrifying bacteria (18, 36). Across this climate gradient, N mineralization and nitrification rates decrease monotonically (16, 37), whereas denitrification consumes virtually all nitrate produced in forests receiving >3,350 mm of MAP (18). In contrast to inorganic N isotopes, DON, the largest of the extractable N pools (Table 1), showed only slight decreases in δ15N from dry to wet climates (Fig. 2a).
A comparison of the average δ15N in plant leaves with nitrate and ammonium pools indicates a shift in the N sources for plants across the sites. At the driest sites (≤2,700 mm of MAP), leaf δ15N is within 1‰ of nitrate δ15N, although differing substantially (5–8‰) from ammonium δ15N (Fig. 2b and Table 1). At 3,350 mm of MAP, foliar δ15N is within 2‰ of nitrate, but differs from ammonium by 7‰. This pattern changes dramatically at >3,350 mm of MAP: foliar δ15N is within ≈2‰ of ammonium δ15N, but differs from nitrate by 30‰ or more (Fig. 2b).
These results indicate that the dominant source of N for vegetation growth changes sharply with precipitation, from nitrate at the drier sites to ammonium at the wetter sites. The observation that the δ15N of forest foliage is ≈1–2‰ less than the δ15N of either nitrate or ammonium across all sites is consistent with the expression of a small internal plant fractionation (discussed above) (Fig. 1). In addition, this inferred shift in proximal N source closely tracks the shift in N availability as measured by concentrations of extractable nitrate and ammonium (Table 1). In contrast, the site-to-site pattern in δ15N of extractable DON did not correspond to that of plant δ15N across the gradient (Fig. 2 a and b, and Table 1).
We placed quantitative constraints on the contribution of N sources to plant species uptake across the precipitation gradient by using isotopic mixing analysis (Figs. 2c and 3a). Based on the qualitative patterns of the N isotopes, we first assumed that plants feed on two sources, ammonium or nitrate, in our calculations. We corrected plant foliage for a 2‰ internal isotope effect as discussed above (Figs. 1 and 2b; see also Methods). Considering the four plant species together, our two end-member calculation revealed a major shift in the proportion of plant N derived from nitrate and ammonium sources as a function of precipitation climate (Fig. 2c). Plants fed almost exclusively (>80%) on nitrate in forests with ≤3,350 mm of MAP where nitrate is more abundant than ammonium. In contrast, >95% of plant growth requirements are met by ammonium in the wetter climates, where nitrate is scarce but ammonium is abundant.
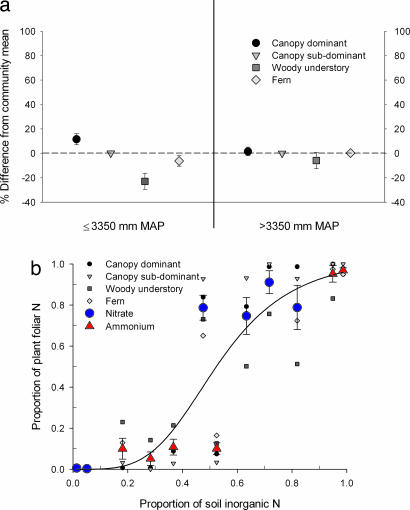
Fig. 3.
Species responses to precipitation differences and changing sources of N. (a) Species' preference for N sources compared with the dominant N source supporting the community based on the three end-member 1 scenario in Fig. 2c. The percentage difference is calculated for each species as follows: ≤3,350 = (species proportion of nitrate uptake − average proportion of nitrate uptake by all species)/average proportion of nitrate uptake by all species × 100; >3,350 = (species proportion of ammonium uptake − average proportion of ammonium uptake by all species)/average proportion of ammonium uptake by all species × 100. (b) Plant N uptake vs. N source abundance. There were no significant differences between species; a single logistic regression model is able to capture the shift in N uptake. Error bars are ±SE.
This isotopic evidence for a switch in N sources was not sensitive to our treatment of DON as a potential source of N for plants. We specifically examined two additional scenarios: that plants (i) also feed on bulk extractable DON at the N isotopic ratio observed at each site, and (ii) acquire DON that is ≈2.8‰ higher in δ15N than bulk soil organic matter (38) (see Methods). These approaches introduced a third end-member, causing our system of equations to be mathematically underdetermined. We treated the underdetermined mixtures by a standard statistical method (39) (see Methods).
Neither our isotopic measures of bulk DON (scenario 1) nor expectations for amino acid δ15N (scenario 2) imply DON as the dominant N source for any of the plants at any of the sites (Figs. 2 a and c, and 3b). Although DON is the largest pool of extractable N at each site (Table 1), the δ15N of plant leaves is never as close to the δ15N of DON as it is to either nitrate or ammonium (Fig. 2b), hence the low DON uptake proportions throughout our gradient.
Applying our isotope-based approach across sites, we also could not find major differences among plant functional types in their preference for N sources (Fig. 3a). In the driest sites, all species appear to have preferred nitrate, although apparently with lower fidelity in the case of the woody-understory species. In the wettest sites, our analysis suggests that ammonium almost single-handedly supported the growth of all plant species examined. Consequently, a simple logistic regression model relating plant N proportions to the abundance of inorganic N sources captures the observed shift in nutrient acquisition by the vegetation (Fig. 3b). This relationship is highly significant (z < 0.001) and displays no statistical evidence for species-dependent interactions (ANCOVA; in all cases, z > 0.1). The overlap at ≈50% of soil N abundance indicates that plants appear to prefer nitrate in environments where its abundance is approximately equal to that of ammonium, perhaps because nitrate bonds less strongly to the soil exchange complex (40).
Our results imply that a functionally diverse group of dominant species in Hawaiian tropical forest are inherently flexible in their capacity to grow on different N forms, consistently accessing the most abundant form of inorganic N in the soil. The observed switch in N source mirrored changes in soil N forms, which followed changes in microbial N mineralization, nitrification, and denitrification rates across the climate gradient (18, 36). However, the threshold character of the switch in all investigated species from nitrate to ammonium at >3,350 mm of MAP (Fig. 3b), together with preference for nitrate over ammonium when both N forms are equally abundant, argues against a passive physiological response of plants to these changes in N availability. Instead, these species seem to share a coherent and tightly regulated strategy for addressing changes in the abundance of N forms in their environment. The existence of such a strategy is consistent with the significant energetic costs in plant growth associated with the uptake and assimilation of N. Because our approach relies on interpretation of natural isotope abundances across intact forests, these findings are not subject to problems associated with either isotopic enrichment studies or manipulation of plant communities.
From an evolutionary perspective, our results are consistent with the idea that these tropical plant species have evolved a uniformly plastic ability to switch among different N sources. The different species and growth forms all sought to forage on the form of N that was most abundant in each local environment. This does not support the idea that natural selection has caused species to diverge into highly specialized niches for N consumption (7, 14, 15); rather, it is consistent with the notion that the species have evolved similar strategies to capitalize on the locally most abundant N form in order to most fully exploit available soil resources.
Finally, our findings raise the possibility that coexistence among functionally diverse species in tropical forest is not necessarily linked to the particular form of N available. This observation may have implications for predicting the response of individual species within diverse tropical communities to changes in climate and other environmental parameters. Moreover, the “threshold” behavior we documented, with plants switching abruptly from one N source to another (Fig. 3b), implies that even gradual changes in tropical ecosystem conditions [such as precipitation (41)] may lead to abrupt changes in forest N cycles and plant N nutrition.
Methods
Study Sites.
Located over a geographic distance of <10 km, our sites cut across a sharp rain shadow on the northern flank of Mt. Haleakala, Maui, Hawaii (16). There is no evidence that humans cleared any of the forests; all six sites are located in mature, old-growth stands. Soils are classified as Inceptisols and Andisols developed from lava ash deposits, and all sites are on relatively flat soil surfaces (relief <5%).
Sampling and Analysis.
We sampled the top 15 cm of soil of all but the wettest site of the sequence during 2003 (the wettest site being inaccessible during that time) and all sites in 2004. Soil samples were collected in plastic bags; coarse and fine roots were removed by sieving and forceps within 3–4 h of collection. We performed 2 M KCl soil extractions within 3–5 h of sampling. We used filter apparatuses made of acid-washed high-density polyethylene to expedite filtration through precombusted 1.0-μm GFB (muffled at 550°C for 2 h). In sum, we present results of 96 separate soil extractions (data in Table 1).
Chemical Analysis.
Chemical analysis included the following: nitrate (and any trace nitrite) by vanadium reduction followed by chemiluminescence detection (42); ammonium by colorimetry; total dissolved nitrogen by persulfate oxidation (43); and DON as the difference between total dissolved N and the sum of inorganic N. The 15N/14N of nitrate was analyzed by using the denitrifier method (44); 15N/14N of total dissolved nitrogen was determined by persulfate oxidation followed by the denitrifier method (43); and 15N/14N of ammonium was measured by ammonia diffusion (45), followed by persulfate oxidation and the denitrifier method.
Calculations and Statistics.
We performed three sets of isotopic mixing calculations. In all cases, we corrected foliar δ15N for isotope fractionation imparted during internal plant N allocation and/or during arbuscular mycorrhiza transport (Fig. 1). This correction was motivated by comprehensive reviews of terrestrial δ15N (23, 25, 33), and was supported by our observation of 1–2‰ elevation of leaf δ15N relative to N sources (Fig. 2 a and b). If individual plants are characterized by a steady state between plant N uptake and losses by way of below-ground decay (roots and arbuscular mycorrhiza) and leaf fall, foliar δ15N is determined by the isotopic signature of the N source to the plant and the proportion of above- vs. below-ground losses:
where εi is the effective isotope effect of the internal fractionation, defined so that a positive value indicates preferential 15N enrichment in the roots. If above- and below-ground N losses are equal and if εi is 4‰ (including the effects of arbuscular mycorrhiza), the δ15N of leaves will be 2‰ lower than N sources. Our calculations thus assume that leaves should have a δ15N that is 2‰ lower than the plant N source. These calculations are relatively insensitive to uncertainty in εi: variation of as much as 4‰ in εi yields only modest effects on source apportionment [supporting information (SI) Fig. 4].
Our two end-member calculation with nitrate and ammonium takes on the following form:
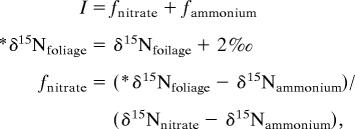 |
where the δ15N of sources and foliage are the values averaged for each site and/or species, and the *δ15Nfoliage is the measured foliage corrected for the internal isotope effect. Our second set of calculations involved the inclusion of DON:
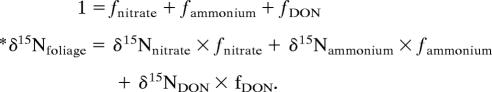 |
To resolve this mathematically underdetermined set of equations, we used the Iso-source model (39). This model iteratively generates source isotopic mixtures whose proportions (values of f) sum to 1, while comparing each calculation against a known mixture, and retaining only those mixtures that satisfy the known value (within some mass-balance tolerance) as defined by a data set of feasible solutions. Although this model can only generate feasible solutions (presented here as the average probability), it nevertheless provides a systematic way of constraining the attribution of N sources in an underdetermined system. In our case, the calculated mixtures reflected combinations of the δ15N of nitrate, ammonium, and DON, and the known was that of plant foliage; we applied a mass-balance tolerance of 0.5‰ to our calculations, which is consistent with the analytical uncertainties in the combined N isotope abundance measures. The δ15N of plant available DON was taken as either the average of bulk DON measured for each site (Table 1) or that estimated for amino acids. For the latter, we treated the δ15N of amino acids as the δ15N of soil organic matter (Table 1) measured for the top 10 cm of soil (16) plus 2.8‰. Previous work has shown that the δ15N of extractable amino acids is either equivalent to or elevated above that of bulk soil N δ15N by up to 2.8‰ across a range of soil conditions (38). Combining these approaches allowed us to conduct a sensitivity analysis of the importance of DON sources for plants.
We performed statistical tests of significance by using “R.” Logistic regression equations for each species in Fig. 3b were examined by ANCOVA; all proportion data were logit-transformed according to standard statistical procedures (46).
Supplementary Material
Acknowledgments
We thank Angie Knapp, Heraldo Farrington, Gordon Holtgrieve, Jon Benner, Alex Barron, Duncan Menge, and Jennifer Houlton for assistance in sample collection. Paul Singleton and Herald Keyser provided laboratory space in Maui; the East Maui Irrigation Company graciously provided access to the field sites; and Moritz Lehman helped devise the method for analyzing ammonium δ15N. This work was supported by a grant from the Andrew W. Mellon Foundation (to L.O.H.) and National Science Foundation Grants DEB-0083566 (to L.O.H.) and OCE-0447570 (to D.M.S.).
Abbreviations
- DON
dissolved organic nitrogen
- MAP
mean annual precipitation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609935104/DC1.
References
- 1.Parton WJ, Mosier AR, Schimel DS. Biogeochemistry. 1988;5:109–131. [Google Scholar]
- 2.Aber JD, Nadelhoffer KD, Steudler P, Melillo JM. BioScience. 1989;39:378–386. [Google Scholar]
- 3.Rastetter EB, Shaver GR. Ecology. 1992;73:1157–1174. [Google Scholar]
- 4.Reich PB, Hobbie SE, Lee T, Ellsworth DS, West JB, Tilman D, Knops JMH, Naeem S, Trost J. Nature. 2006;440:922–925. doi: 10.1038/nature04486. [DOI] [PubMed] [Google Scholar]
- 5.van Groenigen KJ, Six J, Hungate BA, de Graaff MA, van Breemen N, van Kessel C. Proc Natl Acad Sci USA. 2006;103:6571–6574. doi: 10.1073/pnas.0509038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulze ED, Chapin FS, Gebauer G. Oecologia. 1994;100:404–412. doi: 10.1007/BF00317862. [DOI] [PubMed] [Google Scholar]
- 7.McKane RB, Johnson LC, Shaver GR, Nadelhoffer KJ, Rastetter EB, Fry B, Giblin AE, Kielland K, Kwiatkowski BL, Laundre JA, et al. Nature. 2002;415:68–71. doi: 10.1038/415068a. [DOI] [PubMed] [Google Scholar]
- 8.Weigelt A, Bol R, Bardgett RD. Oecologia. 2005;142:627–635. doi: 10.1007/s00442-004-1765-2. [DOI] [PubMed] [Google Scholar]
- 9.Miller AE, Bowman WD. Plant Soil. 2003;250:283–292. [Google Scholar]
- 10.Nadelhoffer K, Shaver G, Fry B, Giblin A, Johnson L, McKane R. Oecologia. 1996;107:386–394. doi: 10.1007/BF00328456. [DOI] [PubMed] [Google Scholar]
- 11.Nordin A, Schmidt IK, Shaver GR. Ecology. 2004;85:955–962. [Google Scholar]
- 12.Warren CR. Funct Plant Biol. 2006;33:653–660. doi: 10.1071/FP06045. [DOI] [PubMed] [Google Scholar]
- 13.Kielland K, McFarland J, Olson K. Plant Soil. 2006;288:297–307. [Google Scholar]
- 14.Hutchinson GE. Am Nat. 1961;95:137–145. [Google Scholar]
- 15.Tilman D. Resource Competition and Community Structure. Princeton, NJ: Princeton Univ Press; 1982. [PubMed] [Google Scholar]
- 16.Schuur EAG, Matson PA. Oecologia. 2001;128:431–442. doi: 10.1007/s004420100671. [DOI] [PubMed] [Google Scholar]
- 17.Molles MC. Ecology: Concepts and Applications. New York: WCB/McGraw–Hill; 1999. [Google Scholar]
- 18.Houlton BZ, Sigman DM, Hedin LO. Proc Natl Acad Sci USA. 2006;103:8745–8750. doi: 10.1073/pnas.0510185103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Handley LL, Austin AT, Robinson D, Scrimgeour CM, Raven JA, Heaton THE, Schmidt S, Stewart GR. Aust J Plant Physiol. 1999;26:185–199. [Google Scholar]
- 20.Amundson R, Austin AT, Schuur EAG, Yoo K, Matzek V, Kendall C, Uebersax A, Brenner D, Baisden WT. Global Biogeochem Cycles. 2003;17:1031. [Google Scholar]
- 21.Mariotti A, Mariotti F, Champigny ML, Amarger N, Moyse A. Plant Physiol. 1982;69:880–884. doi: 10.1104/pp.69.4.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson BJ, Fry B. Annu Rev Ecol Syst. 1987;18:293–320. [Google Scholar]
- 23.Hogberg P. New Phytologist. 1997;137:179–203. doi: 10.1046/j.1469-8137.1997.00808.x. [DOI] [PubMed] [Google Scholar]
- 24.Evans RD, Bloom AJ, Sukrapanna SS, Ehleringer JR. Plant Cell Environ. 1996;19:1317–1324. [Google Scholar]
- 25.Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP. Annu Rev Ecol Syst. 2002;33:507–559. [Google Scholar]
- 26.Garten CT. Ecology. 1993;74:2098–2113. [Google Scholar]
- 27.Kolb KJ, Evans RD. New Phytologist. 2002;156:57–64. [Google Scholar]
- 28.Michelsen A, Quarmby C, Sleep D, Jonasson S. Oecologia. 1998;115:406–418. doi: 10.1007/s004420050535. [DOI] [PubMed] [Google Scholar]
- 29.Hobbie EA, Macko SA, Shugart HH. Oecologia. 1999;118:353–360. doi: 10.1007/s004420050736. [DOI] [PubMed] [Google Scholar]
- 30.Gemma JN, Koske RE, Flynn T. Am J Bot. 1992;79:843–852. [Google Scholar]
- 31.Koske RE, Gemma JN, Flynn T. Am J Bot. 1992;79:853–862. [Google Scholar]
- 32.Treseder KK, Vitousek PM. Ecology. 2001;82:946–954. [Google Scholar]
- 33.Shearer G, Kohl DH. Aust J Plant Physiol. 1986;13:699–756. [Google Scholar]
- 34.Kitayama K, Iwamoto K. Plant Soil. 2001;229:203–212. [Google Scholar]
- 35.Pate JS, Stewart GR, Unkovich M. Plant Cell Environ. 1993;16:365–373. [Google Scholar]
- 36.Houlton BZ. Princeton: Princeton Univ; 2005. PhD dissertation. [Google Scholar]
- 37.Holtgrieve GW, Jewett PK, Matson PA. Oecologia. 2006;146:584–594. doi: 10.1007/s00442-005-0222-1. [DOI] [PubMed] [Google Scholar]
- 38.Ostle NJ, Bol R, Petzke KJ, Jarvis SC. Soil Biol Biochem. 1999;31:1751–1755. [Google Scholar]
- 39.Phillips DL, Gregg JW. Oecologia. 2003;136:261–269. doi: 10.1007/s00442-003-1218-3. [DOI] [PubMed] [Google Scholar]
- 40.Gutschick VP. Am Nat. 1981;118:607–637. [Google Scholar]
- 41.Folland CK, Karl TR. Climate Change 2001: The Scientific Basis; Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge Univ Press; 2001. pp. 101–165. [Google Scholar]
- 42.Braman RS, Hendrix SA. Anal Chem. 1989;61:2715–2718. doi: 10.1021/ac00199a007. [DOI] [PubMed] [Google Scholar]
- 43.Knapp AN, Sigman DM, Lipschultz F. Global Biogeochem Cycles. 2005;19:GB4024. [Google Scholar]
- 44.Sigman DM, Casciotti KL, Andreani M, Barford C, Galanter M, Bohlke JK. Anal Chem. 2001;73:4145–4153. doi: 10.1021/ac010088e. [DOI] [PubMed] [Google Scholar]
- 45.Sigman DM, Altabet MA, Michener R, McCorkle DC, Fry B, Holmes RM. Mar Chem. 1997;57:227–242. [Google Scholar]
- 46.Chatterjee S, Hadi AS, Price B. Regression Analysis by Example. New York: Wiley; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.