Abstract
Several therapeutic self-proteins elicit immune responses when administered to patients. Such adverse immune responses reduce drug efficacy. To induce an immune response, a protein must interact with different immune cells, including antigen-presenting cells, T cells, and B cells. Each cell type recognizes distinct immunogenic patterns on antigens. Mannose-terminating glycans have been identified as pathogen-associated molecular patterns that are essential for internalization of microbes by antigen-presenting cells, leading to presentation. Here, we have investigated the importance of exposed mannosylation on an immunogenic therapeutic self-protein, procoagulant human factor VIII (FVIII). Administration of therapeutic FVIII to hemophilia A patients induces inhibitory anti-FVIII antibodies in up to 30% of the cases. We demonstrate that entry of FVIII into human dendritic cells (DC) leading to T cell activation, is mediated by mannose-terminating glycans on FVIII. Further, we identified macrophage mannose receptor (CD206) as a candidate endocytic receptor for FVIII on DC. Saturation of mannose receptors on DC with mannan, and enzymatic removal of mannosylated glycans from FVIII lead to reduced T cell activation. The interaction between FVIII and CD206 was blocked by VWF, suggesting that, under physiological conditions, the intrinsic mannose-dependent immunogenicity of FVIII is quenched by endogenous immunochaperones. These data provide a link between the mannosylation of therapeutic self-proteins and their iatrogenic immunogenicity. Such a link would be of special relevance in the context of replacement therapy where mechanisms of central and peripheral tolerance have not been established during ontogeny because of the absence of the antigen.
Keywords: dendritic cells, factor VIII, hemophilia, mannose receptor, mannosylated glycans
Several therapeutic self-proteins, including products isolated from human blood, recombinant human cytokines, and recombinant growth factors, elicit immune responses when administered to patients. Such adverse immune responses to therapeutic self molecules reduce drug efficacy and potency (1). In particular, treatment of bleeding episodes in hemophilia A (HA) patients, by administration of exogenous factor VIII (FVIII) to restore normal hemostasis, results in the emergence of inhibitory anti-FVIII alloantibodies in up to 30% of the cases (2). The occurrence of FVIII inhibitors complicates the day-to-day management of the patients, hampers the clinical outcome, and represents a heavy socioeconomical burden (3).
FVIII is a heterodimeric glycoprotein composed of a heavy chain with the A1-a1-A2-a2-B structure and a light chain with the a3-A3-C1-C2 structure (4). Glycans on FVIII terminate with either galactose, fucose, or mannose residues (5, 6). Most of the glycoforms ending with galactose residues are sialated, which protects FVIII from binding to the endocytic asialoglycoprotein receptor (ASGPR) (7). Mannose-ending glycosylations are, however, not capped by sialylation. They are found on Asn2118 and on Asn239 (5, 8) and form the second most prevalent type of glycans on FVIII (6).
The nature of the FVIII-specific B and T lymphocytes involved in anti-FVIII immune responses has been elucidated (9–11). Little is known, however, on FVIII entry in professional antigen-presenting cells (APCs), an event upstream from activation of immune effectors and mandatory to the elicitation of the primary immune response to FVIII. The presence of mannose-ending glycans on FVIII is interesting in this respect. Mannosylation on non-self-antigens has been shown to enhance their endocytosis by dendritic cells (DC) and subsequent presentation to antigen-specific T lymphocytes (12–14). Indeed, DC express several endocytic C-type lectin receptors (CLRs), including macrophage mannose receptor (CD206), dendritic cell-specific ICAM3 grabbing nonintegrin (DC-SIGN) and dectin 2, which bind exposed mannose residues on glycoproteins through their carbohydrate recognition domains (CRDs) (15–17).
Here, we investigated the relevance of exposed mannosylation on FVIII as a ligand for FVIII entry into DC, the initial requirement for the induction of a specific immune response. DC were used as APCs because they are suggested to be the most critical professional APCs for induction of primary immune responses (18) and are an established model to understand the immunogenicity of mannosylated ligands (12, 14). To validate that FVIII endocytosis by DC leads to presentation to CD4+ T lymphocytes, we resorted to the activation of a FVIII-specific CD4+ T cell clone (10). Our results demonstrate that exposed mannose residues on FVIII play a significant role in its uptake by human monocyte-derived DC and that removal of exposed mannose residues leads to a significantly reduced presentation to T lymphocytes.
Results
Mannose-Sensitive Receptors Mediate FVIII Endocytosis by DC.
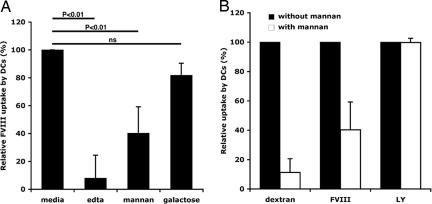
We first studied FVIII endocytosis by immature human monocyte-derived DC. Incubation of DC with FITC-conjugated FVIII (FVIII-FITC) resulted in a time- (data not shown) and dose-dependent [see supporting information (SI) Fig. 5A] labeling of the cells with FVIII. We confirmed that FVIII was internalized by using confocal microscopy (SI Fig. 5B). The high percentage of cells labeled with FVIII-FITC after 2 h of incubation and the fact that 10-fold molar excess of unconjugated FVIII inhibited the internalization of FVIII-FITC by up to 60% (data not shown) is suggestive of a receptor-mediated endocytosis of FVIII. To investigate the nature of the receptor(s) involved in FVIII endocytosis, we incubated the cells for 30 min at 37°C with either 5 mM EDTA, mannan (1 mg/ml) or galactose (1 mg/ml) before addition of FVIII-FITC. Endocytosis at 4°C was used as control.
Endocytosis of FVIII-FITC was inhibited up to 92 ± 16.5% (P < 0.01, Fig. 1A) in the case of EDTA. These data implicate a role for bivalent ion-dependent receptors in FVIII endocytosis by DC. EDTA is also known to mediate the dissociation of FVIII subunits (19). The inhibitory effect of EDTA was, however, not due to EDTA-induced FVIII dissociation, because dissociation of FVIII subunits requires higher EDTA concentrations and longer incubation periods than that used in our assays (19).
Fig. 1.
Mannose-sensitive entry of FVIII into DC. (A) DC were preincubated with 5 mM EDTA, 1 mg/ml mannan or 1 mg/ml d-galactose for 30 min at 37°C, before the addition of FVIII (40 μg/ml) for 2 h. Reported values depict relative antigen uptake defined as [(37°CMFIinh − 4°CMFImedium)/(37°CMFImedium − 4°CMFImedium)] × 100, where “MFIinh” stands for MFI detected in the presence of the inhibitor. Results are from 12 donors. Statistical significance was calculated on raw data by using unpaired Student's t test. (B) Inhibition of endocytosis in DC by mannan. Preincubation of DC with mannan (1 mg/ml) was followed by addition of dextran-FITC (50 μg/ml), FVIII-FITC (40 μg/ml) or lucifer yellow (LY, 200 μg/ml) for 2 h.
The polycarbohydrate mannan, a model competitive ligand for mannose-sensitive uptake (14, 20, 21), reduced the uptake of FVIII-FITC by 60 ± 19% (P < 0.01), whereas galactose, a competitive ligand for galactose-sensitive uptake (22), had no significant effect (Fig. 1A). The specificity of mannan for mannose-sensitive CLRs was confirmed in our experimental setup using FITC-labeled dextran, a typical ligand for mannose-sensitive CLRs, especially CD206, and lucifer yellow (LY), the internalization of which proceeds exclusively by receptor-independent macropinocytosis (20, 23). Internalization of dextran was blocked by 89 ± 9.3% in the presence of mannan, whereas that of LY was not affected (Fig. 1B). The results indicate that mannose-sensitive receptors mediate a significant part of the endocytosis of FVIII by DC.
CD206 Is a Candidate Mannose Receptor for FVIII.
We performed lectin ELISAs with Fc constructs of candidate mannose receptors expressed by DC, CD206, and DC-SIGN. FVIII bound in a dose-dependent and mannose-sensitive manner to soluble CD206, but not to DC-SIGN (data not shown). CD206 comprises several extracellular domains: a cystein-rich (CR) domain, a fibronectin type-II (FNII) domain, and 8 C-type lectin-like carbohydrate recognition domains (CTLD) (16). Direct binding assays were performed by using the mannose-binding CTLD4–7-Fc construct and the non-mannose-binding CR-FNII-CTLD1–3-Fc construct (24) (SI Fig. 6 A and B). FVIII interacted specifically with the CTLD4–7-Fc domains and not with the rest of the molecule (Fig. 2A). Mannan inhibited in a dose-dependent manner the interaction of FVIII with CTLD4–7-Fc (Fig. 2B).
Fig. 2.
Mannose-sensitive binding of FVIII to CD206. (A) Binding of FVIII to CD206 constructs CTLD4–7-Fc and CR-FNII-CTLD1–3-Fc. ELISA plates were coated with FVIII. CTLD 4–7-Fc and CR-FNII-CTLD1–3-Fc were added to the wells at 10 μg/ml. Fc portions were detected with a mouse anti-human Ig Fc-specific antibody coupled to HRP. (B) Inhibition of the binding of FVIII to CTLD4–7-Fc by mannan. FVIII (5.56 μg/ml) was coated on ELISA plates. Mannan was preincubated with CTLD4–7-Fc (10 μg/ml) for 30 min and loaded on the coated wells.
Mannose-Sensitive Endocytosis of FVIII by DC Leads to Presentation of FVIII-Derived Peptides to CD4+ T Lymphocytes.
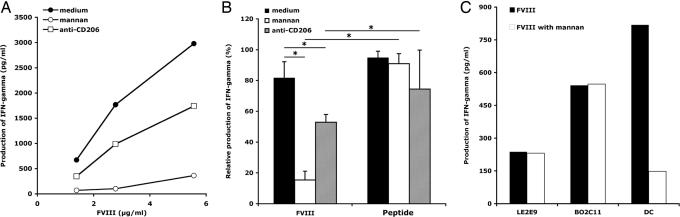
We then explored whether the endocytosis of FVIII by DC through the mannose-sensitive route results in processing of FVIII and presentation of FVIII-derived peptides to T cells. Incubation of FVIII with the FVIII-specific human CD4+ T cell clone D9E9 (10) and with DC from HLA-matched donors, resulted in a dose-dependent activation of D9E9, as measured by the release of IFN-γ in the culture supernatant (Fig. 3A). Factor IX, used as a negative control antigen, failed to activate D9E9, indicating that DC activate D9E9 in an antigen-specific manner (SI Fig. 7A). Blocking mannose-sensitive receptors by preincubation of DC with mannan before the addition of FVIII, resulted in a dramatic dose-dependant reduction (up to 80%) of the activation of D9E9 (SI Fig. 7 A and B). Blocking mannose receptors with a saturating dose of anti-CD206 IgG (10 μg/ml) (23) resulted in a significant inhibition of the IFN-γ production (Fig. 3 A and B), confirming that CD206 participates in the endocytosis of FVIII. Inhibition of FVIII endocytosis was lesser in the case of anti-CD206 IgG than in the case of mannan. The latter difference might be explained by the multivalency of mannan, which may give it the ability to mask several CRDs on CD206 as opposed to the limited spatial hindrance provided by monoclonal antibodies. Alternatively, other mannose-sensitive receptors may also be involved and synergize with CD206 in FVIII uptake.
Fig. 3.
Mannose-sensitive uptake of FVIII by DC results in the presentation FVIII-derived peptides to FVIII-specific CD4+ T cells. (A) DC from DRB1*1501/DRB5*01 healthy donors were incubated (10,000 cells per well) in medium alone or in presence of mannan (1 mg/ml) or anti-CD206 IgG (10 μg/ml), followed by incubation with the FVIII-specific T cell clone D9E9 (5,000 cells per well) in the presence of FVIII (5.56, 2.78 or 1.39 μg/ml) and 20 units/ml rhIL-2 for 20 h at 37°C. Activation of T cells was assessed by the release of IFN-γ in the culture supernatant. Results are from three to eight independent experiments. IFN-γ yield varied with different batches of D9E9 and different sources of donor DC used in separate experiments. (B) DC generated from MHC II-matched donors were preincubated with mannan (1 mg/ml) or anti-CD206 IgG (10 μg/ml), followed by the addition of FVIII (5.56 μg/ml) or peptide I2144-T2161 (2 μg/ml) and D9E9. For each treatment, the IFN-γ production was depicted relative to the maximum value obtained in each individual experiment (∗, P < 0.0001, Mann–Whitney test). Results are from three independent experiments. (C) The human FVIII-specific HLA-matched B cell lines LE2E9 and BO2C11 or DC were incubated in the presence of FVIII (10 μg/ml) and D9E9.
To rule out a direct inhibitory effect of mannan or anti-CD206 IgG on the capacity of DC to activate T cells, we used as an antigen, the FVIII-derived nonglycosylated 18-mer peptide I2144-T2161, known to be the target epitope for D9E9 (10). There is no evidence that peptide I2144-T2161 needs to be internalized by DC to activate D9E9. As depicted in Fig. 3B, D9E9 was activated by DC incubated with peptide I2144-T2161 irrespective of the presence or absence of mannan or anti-CD206 IgG. We then investigated whether mannan has a direct inhibitory effect on the capacity of T cells to be activated. To this end, we resorted to one nonspecific and two FVIII-specific human HLA-matched B cell lines, LE56, LE2E9, and BO2C11. LE2E9 and BO2C11 internalize FVIII through their B cell receptors (BCR). The three cell lines have been used as APCs for the activation of D9E9 (10). Mannan did not prevent the activation of D9E9 by any of the B cell lines incubated in the presence of FVIII (Fig. 3C and SI Fig. 8). Together, these data validate that the inhibitory effects of mannan and of anti-CD206 IgG on D9E9 activation result from the blocking of mannose-sensitive endocytosis of FVIII by DC.
Mannose-Terminating Glycans Located Outside the B Domain Mediate FVIII Endocytosis by DC and Presentation to T Cells.
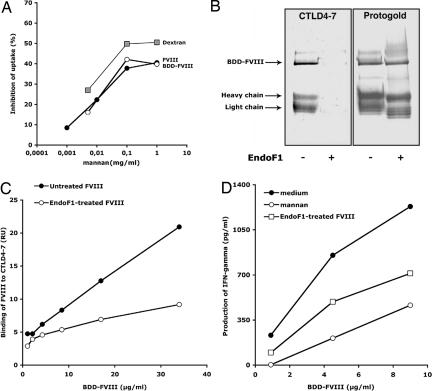
Of the 23 glycans of FVIII, 19 are located on the B domain of the molecule, 2 on the light chain, and 2 on the heavy chain (25). We first confirmed by mass-spectral analysis the presence of mannose-terminating glycans on the Asn239 and Asn2118 sites of B domain-deleted FVIII (BDD-FVIII) and their absence on the B domain of full-length FVIII (data not shown) (8). We then compared the endocytosis of full-length FVIII and BDD-FVIII by DC. Inhibition of the uptake of both forms of FVIII was saturated at the same concentration of mannan (100 μg/ml, Fig. 4A), confirming the involvement of mannose-terminating glycans located outside the B domain in the uptake of FVIII. Inhibition of the uptake of dextran, a model ligand for CD206, was saturated at the same concentration of mannan, further emphasizing the role for CD206 in mannose-sensitive endocytosis of FVIII (Fig. 4A).
Fig. 4.
Exposed mannose residues located outside the B domain play a significant role in FVIII endocytosis by DC leading to T cell activation. (A) DC were preincubated with mannan before the addition of FVIII (40 μg/ml,143 nM, filled circles) and BDD-FVIII (24.31 μg/ml,143 nM, open circles), or dextran-FITC (50 μg/ml). Antigen uptake was analyzed by flow cytometry. Percentage inhibition was calculated for each condition with respect to the condition without mannan. Representative of two individual experiments. (B) Western blot analysis of native or EndoF1-treated BDD-FVIII (3.7 μg) revealed by using Protogold or CTLD4–7-Fc using an alkaline phosphatase-conjugated anti-human IgG. The light chain (LC) and heavy chain (HC) were identified upon blotting with LC- and HC-specific monoclonal anti-FVIII IgGs (data not shown). (C) Binding of native or EndoF1-treated BDD-FVIII to CTLD4–7-Fc was assessed by surface plasmon resonance. Depicted values represent the resonance units (RU) measured 250 s after the end of the injection on a CTLD4–7-Fc-coated channel, from which the background binding to an uncoated channel has been subtracted. (D) Reduced activation of T cells upon EndoF1 treatment of BDD-FVIII. Results depict one representative of three independent experiments. Yields of IFN-γ varied with different batches of D9E9 and with the different sources of human DC used in the different experiments. To statistically compare the three sets of experiments, the production of IFN-γ was normalized with respect to the maximum value obtained in each individual experiment. Differences in normalized levels of T cell activation were statistically significant between “medium” and “EndoF1-treated” (P < 0.0001, ANOVA and Fisher's protected least-significant difference, data not shown).
We then subjected BDD-FVIII to mild enzymatic deglycosylation with EndoF1. EndoF1 cleaves oligomannose structures and hybrid oligosaccharides, but not complex oligosaccharides. The removal of oligomannose structures upon treatment with EndoF1 was indicated by a shift in the migration profile of BDD-FVIII (Fig. 4B Right). Demannosylation of BDD-FVIII was associated with a lesser binding of FVIII to the mannose-sensitive CTLD4-7 domains of CD206, as evidenced by Western blot (Fig. 4B Left) and by using the surface plasmon resonance (SPR) approach (Fig. 4C). Accordingly, DC incubated with EndoF1-treated BDD-FVIII activated D9E9 to a lesser extent than those incubated with native BDD-FVIII (Fig. 4D, P < 0.0001 in the case of DC from three different donors). Of note, demannosylation of BDD-FVIII was not as efficient in reducing T cell activation as saturation of the mannose receptors on DC using mannan (Fig. 4). The residual binding of EndoF1-treated BDD-FVIII to CTLD4–7 observed by using SPR and the residual ability of EndoF1-treated BDD-FVIII to activate D9E9 may be attributed to the presence of remaining N-acetyl-glucosamines on FVIII, which present with moderate affinity for CD206 (24).
Binding of FVIII to Mannose-Sensitive Receptors Is Inhibited by VWF.
VWF is a chaperone protein for FVIII. We have recently demonstrated that VWF prevents FVIII from being endocytosed by DC (26). VWF inhibited in a dose-dependent manner and up to 80% the binding of FVIII to CTLD4–7-Fc (SI Fig. 9). Control experiments validated (i) the absence of direct binding of VWF to CTLD4–7-Fc and (ii) the absence of inhibition of the binding of FVIII to CTLD4–7-Fc by albumin, used as an irrelevant protein at concentrations equimolar to that of VWF (data not shown).
Discussion
Entry of an antigen into APCs, and particularly into DC, may lead either to tolerance toward the antigen or, on the contrary, to an active antigen-specific immune response. The fate of the response, either tolerogenic or immunogenic, is governed by several factors: nature of the signals provided by the antigen to DC, amount of antigen internalized by DC and presented to T cells, maturation level of DC, strength and type of the signals at the DC-T cell synapse, and cytokine microenvironment. Indeed, in vivo, FVIII endocytosis is followed either by initiation of the anti-FVIII immune response, as seen in 30% of HA patients, or by an induction of active self-tolerance, as recently described in healthy individuals (27). Among the above mentioned factors, entry of the antigen into DC, although not sufficient, is mandatory for the antigen-specific priming of immune effectors. Here, we demonstrate that exposed mannose residues on FVIII, used as a model therapeutic self-protein that is immmunogenic, contribute to its entry into DC. We further demonstrate that the mannose-sensitive uptake of FVIII leads to processing and presentation of FVIII-derived peptides to CD4+ T lymphocytes. Our observations document a potential role for mannose-sensitive receptors on professional APCs and for mannose moieties on human therapeutic self-proteins, such as FVIII, in their immunogenicity.
We demonstrate the relevance of exposed mannosylation on FVIII for its interaction with APCs and its presentation to immune effectors using two complementary approaches: saturation of mannose receptors on DC using mannan and mild deglycosylation of FVIII. Glycosylation on an antigen might influence either the entry of the antigen into DC, or its intracellular processing, the docking of antigen-derived peptides in the groove of MHC II molecules, and/or the recognition of the MHC II/peptide complexes by the TCR (28). Using the endocytosis of FVIII as a read-out, we clearly show a quantitative reduction of the entry of FVIII in DC when the interaction between FVIII and mannose-sensitive receptors is perturbed. Mannosylation thus accounts for ≥60% of the uptake of FVIII. The finding of a 40% residual FVIII uptake despite the disruption of the mannose-sensitive pathway indicates the involvement of alternative endocytic processes for FVIII that remain to be identified. Our unpublished data suggest that members of the LDL receptor family and the endocytic mannose-sensitive receptor DC-SIGN on monocyte-derived DC are not implicated.
The concentration of FVIII used in the endocytosis assay was much higher than that found in patients with HA upon administration of therapeutic FVIII. We therefore resorted to a T cell activation assay, wherein DC are incubated with FVIII concentrations close to that reached in patients. The D9E9 T cell clone thus produced 26 ± 8 and 711 ± 63 pg/ml IFN-γ (mean ± SD) at 1 and 7 nM FVIII, respectively. These data confirm that, at values close to replacement therapy levels, mannose-sensitive endocytosis of FVIII by DC leads to presentation of FVIII to and activation of CD4+ T lymphocytes.
The use of the D9E9 activation assay does not allow us to decipher a direct role for mannosylated glycans in the processing, docking, or recognition of FVIII peptides. However, because D9E9 is specific for a nonglycosylated epitope of FVIII located between amino acids I2144 and T2161, these data indicate that mannosylation of the whole FVIII molecule governs the presentation of nonglycosylated epitopes. The three major clusters of B cell epitopes for FVIII inhibitors are located on the A2, A3, and C2 domains of FVIII. Interestingly, none of these domains is mannosylated. It is tempting to speculate that altering the mannosylation pattern of FVIII may reduce the presentation of peptide epitopes derived from the antigenic regions of FVIII by DC to CD4+ T lymphocytes. Indeed, removal of the mannosylated sugar at Asn-2118 on the light chain of FVIII by site-directed mutagenesis completely abrogated the activation of D9E9 upon incubation of the mutated Asn2118Ala light chain with DC as APCs (S. Dasgupta, O.C., S.V.K., and S.L.-D., preliminary unpublished data). In contrast, D9E9 was activated upon presentation of the Asn2118Ala light chain by B02C11. The use of mutated demannosylated FVIII products may reveal a previously uncharacterized strategy to reduce the risk of inhibitors in HA patients.
In this study, we have identified CD206 as candidate mannose-sensitive endocytic receptor for FVIII on DC. CD206 has been implicated in the homeostasis of several proinflammatory mannosylated serum glycoproteins (29). Conversely, CD206 has been shown to mediate the endocytosis of a number of microbial antigens by virtue of their exposed mannosylation, thus enhancing antigen-specific T cell responses (12, 30). Interestingly, the interaction of the thyroid antigens with CD206 on APCs located in the gland or draining lymph nodes has been implicated in pathological autoimmune responses to thyroid antigens (31, 32). The exacerbated expression of CD206 has been associated with several pathological situations. Thus, DC from patients with house dust mite allergy express more CD206 than that of healthy donors, and internalize Derp 1, a major mite allergen, more efficiently (23). Similarly, whereas Langerhans cells from normal human skin do not express CD206, inflammatory epidermal DC from patients with atopic dermatitis and psoriasis vulgaris, express CD206 in situ and use it for receptor-mediated endocytosis (33). Presumably, the spleen is the major immunocompetent organ where the immune response to FVIII takes place. Our preliminary data demonstrate that CD206 can be expressed by human splenic myeloid DC and suggest the functionality of the CD206-dependent endocytic pathway (M. Nascimbeni, A. Hosmalin, L. Garderet, and B. Fabiani, unpublished observations).
In addition to DC, B cells can act as efficient APCs and internalize the antigen for which they are specific through their surface B cell receptor (18). Thus, in HA patients who have already been treated with FVIII, FVIII-specific B cells may be activated and participate in the endocytosis of therapeutic FVIII and presentation to T cells (10). Unlike DC however, B cells lack other endocytic receptors such as mannose-sensitive receptors (18). Accordingly, mannan did not block the activation of T cells when FVIII-specific B cells (BO2C11 and LE2E9) and FVIII nonspecific B cells (LE56) were used as APCs. Together, these data suggest that, in a situation where FVIII-specific B cells have not been triggered (i.e., in patients that have not received exogenous FVIII as yet), mannose receptors on DC are the preferential portal of entry for FVIII into APCs.
In physiology, FVIII circulates complexed to VWF and is protected from degradation by circulating enzymes and abzymes (34, 35). VWF has been proposed to reduce the immunogenicity of FVIII in patients with HA (36, 37). Interestingly, we recently demonstrated that VWF reduces the uptake of FVIII by DC and the activation of FVIII-specific T cells (26). We report here that VWF blocks the binding of FVIII to CD206 in a dose-dependent manner. Whether VWF may reduce FVIII immunogenicity by sterically hindering the interaction of FVIII with mannose-sensitive receptors and thus preventing its uptake by DC remains to be formally demonstrated.
Here, we propose a link between the mannosylation of a therapeutic self-protein and its iatrogenic immunogenicity. The association of mannosylated proteins with endogenous chaperone molecules, such as VWF, regulates their interaction with endocytic receptors, thereby curbing the activation of immune effectors. In some situations however, the ligand/receptor equilibrium is perturbed, possibly leading to the triggering of unwanted deleterious immune reactions. Indeed, expression of mannose-sensitive receptors on DC may be up-regulated as a response to inflammatory signals (23, 33). Alternatively, the formation of complexes between mannosylated proteins and endogenous chaperones may be impaired, as seen in the case of therapeutic recombinant FVIII that incompletely associates with VWF (38). Such a perturbed equilibrium represents an aggravating factor in the context of replacement therapy where the self-antigen is absent and where mechanisms of central and peripheral tolerance have not been established during ontogeny.
Methods
FITC Conjugation of FVIII and BDD-FVIII.
Recombinant human FVIII (Kogenate SF; Bayer, Puteaux, France) or BDD-FVIII (Refacto; Wyeth, Berkshire, U.K.) was dialyzed against bicarbonate buffer (pH 9.2) containing 5 mM CaCl2 at 4°C for 2 h, followed by coupling with FITC (Sigma, Lyon, France) for 6 h at 4°C. Unconjugated FITC was removed by dialysis. FVIII-FITC was quantified by Bradford assay. The specific activity of FVIII-FITC was >1,000 IU/mg as assessed by using a chromogenic assay (Dade–Behring, Marburg, Germany), confirming functional integrity of FVIII after FITC conjugation. F/P molar ratio of FVIII-FITC ranged between 15:1 and 25:1.
Generation of DC.
Monocytes from peripheral blood mononuclear cells of healthy donors were isolated by adherence in RPMI medium 1640 supplemented with 10% human AB serum. Adherent monocytes were cultured in X-VIVO supplemented with 1% human AB serum, in presence of 500 units/106 cells human recombinant IL-4 (R & D Systems, Lille, France) and human recombinant 1,000 units/106 cells GM-CSF (ImmunoTools, Friesoythe, Germany). After 5 days, the immature status of nonadherent DC was confirmed by flow cytometry.
FVIII Uptake by DC.
DC (4 × 105 cells per well) were incubated in 100 μl of X-VIVO15 with fluorescent conjugated ligands (FVIII-FITC, BDD-FVIII-FITC) at 4°C or 37°C. Cells were then washed with ice-cold PBS and analyzed by flow cytometry. To study receptor involvement in uptake, cells were preincubated for 30 min at 37°C with mannan (Sigma–Aldrich, Lyon, France), EDTA, or d-galactose (Sigma–Aldrich) before the addition of fluorescein-conjugated ligands. Uptake was quantified as the difference in the geometric mean fluorescence intensities at 37°C and 4°C. As control ligands for mannose receptor mediated endocytosis and macropinocytosis, dextran-FITC (40,000 MW, Molecular Probes, Leiden, The Netherlands) and Lucifer Yellow (LY-CH, Sigma–Aldrich), respectively, were used.
Activation of a FVIII-Specific T Cell Clone.
Immature DC from healthy donors (MHC II haplotype: DRB1*1501/DRB5*01) were prepared in RPMI medium 1640/10% FCS. DC were resuspended in DMEM/F12 (1:1) containing 10% FCS and distributed (10,000 cells per well) in 96-well culture plates. DC were cultured with 5,000 T cells [a human FVIII-specific CD4+ T cell clone, D9E9 (10)] in DMEM/F12 (1:1) containing 10% FCS and 20 units/ml recombinant human IL-2 (Sigma), with either FVIII or BDD-FVIII (native or deglycosylated) for 20 h at 37°C. Importantly, the lowest FVIII concentrations used in our assays (i.e., 1 or 5 nM) are compatible with FVIII concentrations reached in patients' blood after replacement therapy (i.e., 1–2 nM). IFN-γ was measured in supernatants by using the human IFN-γ Duo Set (R & D Systems). When indicated, DC were preincubated with mannan or anti-mannose receptor (CD206) monoclonal antibody (PAM-1, IgG1 isotype) for 30 min at 37°C. Controls included: T cells incubated alone, or T cells incubated with DC alone or in the presence of human recombinant factor IX (Benefix; Baxter) or a synthetic FVIII peptide I2144-T2161 (NeoMPS, Strasbourg, France), reported to activate D9E9.
Deglycosylation of BDD-FVIII.
BDD-FVIII was deglycosylated by using endoglycosidases from N-DEGLY kit (Sigma-Aldrich) following the manufacturer's protocol. As revealed by the glycoprotein detection kit (Sigma-Aldrich), EndoF1 was the only endoglycosidase able to effectively cleave sugar residues on BDD-FVIII.
Lectin ELISA.
ELISA plates were coated with sugars (mannan or SO4–3-β-d-GalNAc-PAA; Lectinity Holding, Moscow, Russia), FVIII or BDD-FVIII in 154 mM NaCl. Wells were blocked with TTBS [10 mM Tris·HCl (pH 7.5)/10 mM CaCl2/154 mM NaCl/0.05% Tween 20) and 3% BSA. CD206 constructs [CTLD4–7-Fc and CR-FNII-CTLD1–3-Fc (24)] were then incubated at 10 μg/ml, followed by incubation with a mouse anti-human Ig Fc-specific antibody coupled to HRP (Southern Biotech, Birmingham, AL). Binding was measured at 492 nm after revealing with OPD.
In the case of inhibition studies using VWF, CTLD4–7-Fc (5 μg/ml) was coated, and plates were blocked with TTBS/3% BSA. FVIII (36 nM) was preincubated with VWF (1.5–357 nM, Wilfactin; LFB) in TTBS/3% BSA for 1 h at 37°C, and then transferred onto the coated plates for 1 h at 37°C. Bound FVIII was revealed after sequential incubation with a monoclonal anti-FVIII IgG, a goat anti-mouse IgG Fc fragments coupled to peroxidase and OPD.
Western Blot.
BDD-FVIII (3.7 μg) (native or EndoF1-treated) were separated on a 7.5% SDS-polyacrylamide gel. Proteins were transferred to a nitrocellulose membrane and incubated with CTLD4–7-Fc (10 μg/ml). Alkaline phosphatase-conjugated anti-human IgG was used to reveal the Fc portions of CTLD4–7-Fc. In parallel, transferred proteins were revealed by using Protogold.
Biosensor Measurement of FVIII Binding to CD206.
Real-time analysis of complex formation between CTLD4–7-Fc and FVIII was performed by using a BIAcore 2000 (Biacore, Uppsala, Sweden). CTLD4–7-Fc was dialyzed against 10 mM Hepes, 150 mM NaCl, 3.4 mM EDTA, 0.005% Tween 20, pH 7.4 (Hepes-EDTA) for 3 h at 25°C. CTLD4–7-Fc was immobilized on a CM5 sensor chip in Hepes-EDTA buffer according to the manufacturer's protocol. BDD-FVIII and deglycosylated BDD-FVIII were dialyzed against 100 mM borate, 150 mM NaCl, 5 mM CaCl2, 0.005% Tween 20, pH 7.4 (Borate–CaCl2) for 2 h. All procedures were performed at 25°C in Borate–CaCl2 buffer. BDD-FVIII (125 μl in Borate–CaCl2 buffer) was injected at a continuous flow rate of 25 μl/min, allowing a contact time with CTLD4–7-Fc of 10 min. Dissociation was monitored over a period of 5 min after the injection of Borate–CaCl2 alone. Regeneration of the surface was achieved by using 10 μl of 50 mM EDTA, 5 μl of 50 mM NaOH, and 500 mM NaCl. Background association and dissociation curves were scored under similar experimental conditions by injecting BDD-FVIII onto an uncoated channel. Background curves were subtracted from test curves. Mannan bound to immobilized CTLD4–7-Fc in a dose-dependent manner (data not shown).
Supplementary Material
Acknowledgments
We thank P. Allavena (Laboratory of Cellular Immunology, Instituto Mario Negri, Milan, Italy) and A. Hosmalin (Institut Cochin) for providing PAM-1 and for sharing unpublished data on splenocytes, respectively, Theano Irinopoulou (U706 Institut National de la Santé et de la Recherche Medicalé) and Emmanuel Nony (LFB, Les Ulis, France), for performing confocal microscopy and mass-spectral analysis of FVIII. FVIII (Kogenate SF) and FIX (Benefix) were kind gifts from BayerPharma (Puteaux, France) and Baxter (Maurepas, France), respectively. This work was supported by Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, Université Pierre et Marie Curie-Paris 6, Indo-French Center for the Promotion of Advanced Research, and Agence Nationale de la Recherche (ANR-05-MRAR-030). S. Dasgupta, J.B. and B.W. are the recipients of fellowships from Fondation de la Recherche Médicale, from Les Entreprises du Médicament (Paris) and from LFB (Les Ulis, France). T.B.H.G. was supported by Dutch Scientific Research Program Grant NWO 917-46-367.
Abbreviations
- FVIII
factor VIII
- HA
hemophilia A
- BDD-FVIII
B domain-deleted FVIII
- DC
dendritic cells
- VWF
von Willebrand factor
- CLR
C-type lectin receptor
- CTLD
C-type lectin-like domain
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702120104/DC1.
References
- 1.Chirino AJ, Ary ML, Marshall SA. Drug Discov Today. 2004;9:82–90. doi: 10.1016/S1359-6446(03)02953-2. [DOI] [PubMed] [Google Scholar]
- 2.Ehrenforth S, Kreuz W, Scharrer I, Linde R, Funk M, Güngör T, Krackhardt B, Kornhuber B. Lancet. 1992;339:594–598. doi: 10.1016/0140-6736(92)90874-3. [DOI] [PubMed] [Google Scholar]
- 3.Gringeri A, Mantovani LG, Scalone L, Mannucci PM. Blood. 2003;102:2358–2363. doi: 10.1182/blood-2003-03-0941. [DOI] [PubMed] [Google Scholar]
- 4.Fay PJ. Int J Hematol. 2006;83:103–108. doi: 10.1532/IJH97.05113. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman RJ, Wasley LC, Dorner AJ. J Biol Chem. 1988;263:6352–6362. [PubMed] [Google Scholar]
- 6.Hironaka T, Furukawa K, Esmon PC, Fournel MA, Sawada S, Kato M, Minaga T, Kobata A. J Biol Chem. 1992;267:8012–8020. [PubMed] [Google Scholar]
- 7.Bovenschen N, Rijken DC, Havekes LM, Vlijmen BJ, Mertens K. J Thromb Haemost. 2005;3:1257–1265. doi: 10.1111/j.1538-7836.2005.01389.x. [DOI] [PubMed] [Google Scholar]
- 8.Medzihradszky KF, Besman MJ, Burlingame AL. Anal Chem. 1997;69:3986–3994. doi: 10.1021/ac970372z. [DOI] [PubMed] [Google Scholar]
- 9.Reding MT, Lei S, Lei H, Green D, Gill J, Conti-Fine BM. Thromb Haemost. 2002;88:568–575. [PubMed] [Google Scholar]
- 10.Jacquemin M, Vantomme V, Buhot C, Lavend'homme R, Burny W, Demotte N, Chaux P, Peerlinck K, Vermylen J, Maillere B, et al. Blood. 2003;101:1351–1358. doi: 10.1182/blood-2002-05-1369. [DOI] [PubMed] [Google Scholar]
- 11.Misra N, Bayry J, Pashov A, Kaveri SV, D'Oiron R, Stieltjes N, Roussel-Robert V, Kazatchkine MD, Boyer O, Lacroix-Desmazes S. Thromb Haemost. 2003;90:813–822. doi: 10.1160/TH03-05-0300. [DOI] [PubMed] [Google Scholar]
- 12.Engering AJ, Cella M, Fluitsma D, Brockhaus M, Hoefsmit EC, Lanzavecchia A, Pieters J. Eur J Immunol. 1997;27:2417–2425. doi: 10.1002/eji.1830270941. [DOI] [PubMed] [Google Scholar]
- 13.Agnes MC, Tan A, Jordens R, Geluk A, Roep BO, Ottenhoff T, Drijfhout JW, Koning F. Int Immunol. 1998;10:1299–1304. doi: 10.1093/intimm/10.9.1299. [DOI] [PubMed] [Google Scholar]
- 14.Lam JS, Mansour MK, Specht CA, Levitz SM. J Immunol. 2005;175:7496–7503. doi: 10.4049/jimmunol.175.11.7496. [DOI] [PubMed] [Google Scholar]
- 15.Geijtenbeek TB, van Vliet SJ, Engering A, t Hart BA, van Kooyk Y. Annu Rev Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- 16.Taylor PR, Gordon S, Martinez-Pomares L. Trends Immunol. 2005;26:104–110. doi: 10.1016/j.it.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 17.McGreal EP, Rosas M, Brown GD, Zamze S, Wong SY, Gordon S, Martinez-Pomares L, Taylor PR. Glycobiology. 2006;16:422–430. doi: 10.1093/glycob/cwj077. [DOI] [PubMed] [Google Scholar]
- 18.Trombetta ES, Mellman I. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 19.Sudhakar K, Fay PJ. Biochemistry. 1998;37:6874–6882. doi: 10.1021/bi980084c. [DOI] [PubMed] [Google Scholar]
- 20.Sallusto F, Cella M, Danieli C, Lanzavecchia A. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 22.Kato M, Neil TK, Fearnley DB, McLellan AD, Vuckovic S, Hart DN. Int Immunol. 2000;12:1511–1519. doi: 10.1093/intimm/12.11.1511. [DOI] [PubMed] [Google Scholar]
- 23.Deslee G, Charbonnier AS, Hammad H, Angyalosi G, Tillie-Leblond I, Mantovani A, Tonnel AB, Pestel J. J Allergy Clin Immunol. 2002;110:763–770. doi: 10.1067/mai.2002.129121. [DOI] [PubMed] [Google Scholar]
- 24.Zamze S, Martinez-Pomares L, Jones H, Taylor PR, Stillion RJ, Gordon S, Wong SY. J Biol Chem. 2002;277:41613–41623. doi: 10.1074/jbc.M207057200. [DOI] [PubMed] [Google Scholar]
- 25.Lenting PJ, van Mourik JA, Mertens K. Blood. 1998;92:3983–3996. [PubMed] [Google Scholar]
- 26.Dasgupta S, Repesse Y, Bayry J, Navarrete AM, Wootla B, Delignat S, Irinopoulou T, Kamate C, Saint-Remy JM, Jacquemin M, et al. Blood. 2007;109:610–612. doi: 10.1182/blood-2006-05-022756. [DOI] [PubMed] [Google Scholar]
- 27.Kamate C, Lenting PJ, van den Berg HM, Mutis T. J Thromb Haemost. 2006 doi: 10.1111/j.1538-7836.2007.02336.x. [DOI] [PubMed] [Google Scholar]
- 28.Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Science. 2001;291:2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 29.Lee SJ, Evers S, Roeder D, Parlow AF, Risteli J, Risteli L, Lee YC, Feizi T, Langen H, Nussenzweig MC. Science. 2002;295:1898–1901. doi: 10.1126/science.1069540. [DOI] [PubMed] [Google Scholar]
- 30.Tan MC, Mommaas AM, Drijfhout JW, Jordens R, Onderwater JJ, Verwoerd D, Mulder AA, van der Heiden AN, Scheidegger D, Oomen LC, et al. Eur J Immunol. 1997;27:2426–2435. doi: 10.1002/eji.1830270942. [DOI] [PubMed] [Google Scholar]
- 31.Chazenbalk GD, Pichurin PN, Guo J, Rapoport B, McLachlan SM. Clin Exp Immunol. 2005;139:216–224. doi: 10.1111/j.1365-2249.2004.02689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linehan SA, Martinez-Pomares L, da Silva RP, Gordon S. Eur J Immunol. 2001;31:1857–1866. doi: 10.1002/1521-4141(200106)31:6<1857::aid-immu1857>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 33.Wollenberg A, Mommaas M, Oppel T, Schottdorf EM, Gunther S, Moderer M. J Invest Dermatol. 2002;118:327–334. doi: 10.1046/j.0022-202x.2001.01665.x. [DOI] [PubMed] [Google Scholar]
- 34.Nogami K, Shima M, Nishiya K, Hosokawa K, Saenko EL, Sakurai Y, Shibata M, Suzuki H, Tanaka I, Yoshioka A. Blood. 2002;99:3993–3998. doi: 10.1182/blood.v99.11.3993. [DOI] [PubMed] [Google Scholar]
- 35.Lacroix-Desmazes S, Moreau A, Sooryanarayana, Bonnemain C, Stieltjes N, Pashov A, Sultan Y, Hoebeke J, Kazatchkine MD, Kaveri SV. Nat Med. 1999;5:1044–1047. doi: 10.1038/12483. [DOI] [PubMed] [Google Scholar]
- 36.Goudemand J, Rothschild C, Demiguel V, Vinciguerrat C, Lambert T, Chambost H, Borel-Derlon A, Claeyssens S, Laurian Y, Calvez T. Blood. 2006;107:46–51. doi: 10.1182/blood-2005-04-1371. [DOI] [PubMed] [Google Scholar]
- 37.Behrmann M, Pasi J, Saint-Remy JM, Kotitschke R, Kloft M. Thromb Haemost. 2002;88:221–229. [PubMed] [Google Scholar]
- 38.Lin Y, Yang X, Chevrier MC, Craven S, Barrowcliffe TW, Lemieux R, Ofosu FA. Haemophilia. 2004;10:459–469. doi: 10.1111/j.1365-2516.2004.00957.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.