Abstract
Helicobacter pylori is a Gram-negative spiral bacterium that causes gastritis and peptic ulcer and has been implicated in the pathogenesis of gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma. Although Th1 immunity is involved in gastritis and the accumulation of H. pylori-specific CD4+ T cells in the H. pylori-infected gastric mucosa in human patients, how T cells are primed with H. pylori antigens is unknown because no apparent lymphoid tissues are present in the stomach. We demonstrate here that Peyer's patches (PPs) in the small intestine play critical roles in H. pylori-induced gastritis; no gastritis is induced in H. pylori-infected mice lacking PPs. We also observed that the coccoid form of H. pylori is phagocytosed by dendritic cells in PPs. We propose that H. pylori converts to the coccoid form in the anaerobic small intestine and stimulates the host immune system through PPs.
Keywords: CD4 T cells, coccoid form, dendritic cells, gastric epithelial cells, inflammation
Helicobacter pylori is a Gram-negative microaerophilic bacterium that infects human gastric epithelial cell (gEC) surfaces and the overlying gastric mucin. More than 50% of the world's population is infected by H. pylori, although most patients have no remarkable symptoms (1). However, in some of patients, H. pylori infection leads to active chronic gastritis or peptic ulcer (2). In addition, H. pylori has also been implicated in the pathogenesis of gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma (3). When H. pylori colonizes gastric mucosa, effector molecules are injected into gastric epithelial cells or the submucosal area through the type IV secretion system (1, 4). For example, the CagA effector is phosphorylated in the target cells and activates a signaling pathway to elicit growth factor-like responses. Another effector molecule, VacA, causes the massive vacuolar degradation of epithelial cells, thus disrupting the gastric epithelial barrier. VacA also interferes with the activation and proliferation of T lymphocytes within the gastric lamina propria (gLP) (5).
It was originally proposed that effector molecules, including CagA, trigger the secretion of chemokines such as IL-8 and RANTES from gECs, which attract neutrophils and mononuclear cells into the gLP (4). However, it was later shown that H. pylori did not induce gastritis in lymphopenic SCID mice, although gastritis was induced after adoptive transfer of naive CD4+ T cells (6). The importance of CD4+ T cells was underscored by the fact that H. pylori is not eliminated from gastric mucosa in MHC class II-deficient mice (7).
Gastritis is more severe in Th1-prone mice than Th2-prone mice on infection with the mouse-adapted H. pylori strain SS1 (8). Furthermore, the accumulation of H. pylori-specific CD4+ T cells in the H. pylori-infected gastric mucosa in human patients (9) suggests that CD4+ T cell-mediated Th1 immune responses play a critical role in H. pylori-induced gastritis. However, how CD4+ T cells are primed by H. pylori antigens in the stomach where no apparent lymphoid tissues are present and how the H. pylori-induced chronic inflammation is maintained by T cells is unknown.
Although H. pylori forms an actively dividing, spiral-shaped morphology in the stomach, it is able to convert to a nonculturable, but viable, coccoid form under unfavorable conditions such as an anaerobic environment, increased oxygen tension, and long-term culture (10, 11). The coccoid form is thought to be important for transmission to new hosts by an oral–oral or oral–feces route because this form is more resistant to environmental stresses. Although the coccoid form is not culturable in vitro, transcription and translation actively take place in the coccoid form (12, 13). However, it is unknown whether the coccoid form is involved in the pathogenesis of H. pylori-induced gastritis.
In this study, we demonstrate that H. pylori antigen-specific CD4+ T cells are necessary and sufficient for the induction of gastritis by H. pylori. We also demonstrate that CD4+ T cells are likely primed with H. pylori antigens captured in the small intestine, where the coccoid form of H. pylori is taken up by dendritic cells (DCs) in Peyer's patches (PPs).
Results
Adoptive Transfer of Naive CD4+T Cells Induces Gastritis in H. pylori-Infected Rag2−/− Mice.
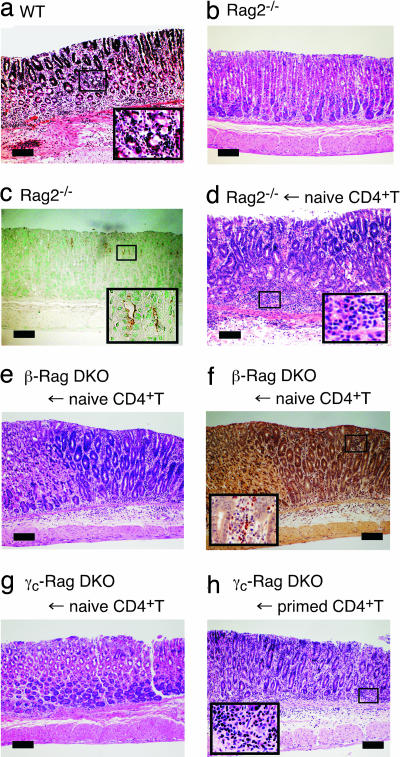
The H. pylori SS1 strain induces more severe gastritis in Th1-prone C57BL/6 than Th2-prone BALB/c mice as demonstrated by the infiltration of neutrophils and lymphocytes into the gLP and the submucosal area (Fig. 1a and data not shown). In contrast, when C57BL/6-Rag2−/− mice lacking T and B cells were infected with H. pylori, no gastritis was observed (Fig. 1b), as previously shown with SCID mice (6). The clearance of bacteria in Rag2−/− mice was impaired because >107 cfu/g tissues of H. pylori colonized the gastric mucosa (Table 1), and the colonization of H. pylori was readily detected by anti-H. pylori antibody staining (Fig. 1c). However, adoptive transfer of naive splenic CD4+ T cells into Rag2−/− mice 2 months after H. pylori infection induced severe gastritis, with massive infiltration of neutrophils and lymphocytes into the gLP and the submucosal area (Fig. 1d). This massive infiltration resulted in the exclusion of colonized H. pylori from gastric mucosa (Table 1).
Fig. 1.
Naive CD4+ T cells did not induce gastritis in H. pylori-infected γc-Rag double knockout (DKO) mice. (a–c) Wild-type (a) or Rag2−/− (b and c) mice were infected with H. pylori. Two months after the infection, gastric specimens were prepared. (d–h) Rag2−/− (d), β-Rag DKO (e and f), and γc-Rag DKO (g and h) mice were infected with H. pylori. Two months after the infection, naive (d–g) or primed (h) splenic CD4+ T cells were transferred. Two months after the cell transfer, gastric specimens were prepared. Specimens were stained with H&E (a, b, d, e, g, and h), anti-H. pylori antisera (brown) (c), or chloroacetate esterase (red) for infiltrated neutrophils and mast cells (f). (Scale bars: 200 μm.)
Table 1.
PP-dependent bacterial clearance in H. pylori infection
| Mouse* | n | Cells transferred† | Bacterial colonization,‡ cfu/g tissue × 10−6 | Neutrophils, average (range) | Active inflammation, average (range) | GAIS,§ average (range) |
|---|---|---|---|---|---|---|
| Wild type | 7 | None | 2.2 ± 1.3 | 1.6 (0–3) | 1.4 (0–3) | 13.6 (0–34) |
| Wild type | 4 | CD4+ T cell-depleted | 25 ± 7 | 0 (0) | 1.0 (0–2) | 0 (0) |
| Rag2−/− | 4 | None | 14 ± 4 | 0 (0) | 0 (0) | 0 (0) |
| Rag2−/− | 5 | Naive CD4+ T | 0.15 ± 0.17 | 2.0 (2) | 2.0 (2) | 5.0 (2–8) |
| Rag2−/− | 5 | OT-II-Rag CD4+ T | 34 ± 11 | 0 (0) | 0 (0) | 0 (0) |
| β-Rag DKO | 4 | Naive CD4+ T | 0.67 ± 0.39 | 1.5 (1–2) | 1.3 (1–2) | 9.2 (3–18) |
| γc-Rag DKO | 6 | Naive CD4+ T | 18 ± 11 | 0 (0) | 0 (0) | 0 (0) |
| γc-Rag DKO | 6 | Primed CD4+ T | 0.22 ± 0.39 | 0.66 (0–1) | 1.5 (0–2) | 1.6 (0–5) |
| γc-Rag DKO | 3 | Primed CD4+ T from PPs | <0.01 | 1.0 (1) | 2.0 (2) | 1.0 (0–2) |
| γc-Rag DKO | 3 | Primed CD4+ T from mLN | 0.88 ± 0.78 | 0.5 (0–1) | 1.5 (1–2) | 4.0 (3–5) |
| γc-Rag DKO | 3 | Primed CD4+ T by coccoid form | 1.8 ± 1.6 | 0.33 (0–1) | 0.66 (0–1) | 2.0 (0–6) |
| PP-null-wild type | 8 | None | 16 ± 9 | 0 (0) | 0.37 (0–1) | 0 (0) |
| PP-null-Rag2−/− | 3 | Naive CD4+ T | 5.3 ± 4.0 | 1.0 (0–2) | 1.0 (0–2) | 4 (0–7) |
| PP-null-Rag2−/− | 3 | Primed CD4+ T | 0.53 ± 0.28 | 2.6 (2–3) | 2.6 (2–3) | 21 (13–33) |
*All mice were on a C57BL/6 background. Although not shown, the degrees of bacterial colonization in β-Rag DKO, γc-Rag DKO, and PP-null-Rag2−/− mice without CD4+ T cell transfer were similar to those of Rag2−/− mice.
†Splenocytes were used for cell transfer unless otherwise stated. Five million cells were transferred except for the transfer of PP-derived cells, where 5 × 105 cells were used.
‡Mean ± SD.
§Gland active inflammatory score. See Materials and Methods.
H. pylori Antigen-Specific CD4+ T Cells Are Indispensable for Induction of Gastritis.
Primary gECs secrete MIP-2, a functional homolog of IL-8, on H. pylori infection in vitro, and the amount produced by Rag2−/− gECs was comparable to that produced by wild-type gECs [supporting information (SI) Fig. 5a]. CD4+ T cells isolated from the gLP of H. pylori-infected mice were able to produce larger amounts of MIP-2 than splenic CD4+ T cells from the same mice in response to H. pylori antigens (SI Fig. 5b). Moreover, the amounts of MIP-2 produced by gLP CD4+ T cells were much larger than those produced by gECs (compare SI Fig. 5 a and b). The importance of CD4+ T cells for neutrophil infiltration on H. pylori infection was further confirmed by the depletion of CD4+ T cells from wild-type mice that had already developed gastritis by H. pylori infection. After depleting CD4+ T cells by the i.v. injection of anti-CD4 mAb, the gastritis became milder (Table 1 and SI Fig. 6 a and b), and the number of bacteria in the gastric mucosa increased (Table 1). These results indicate the critical role of CD4+ T cells for both triggering and maintaining gastritis. When CD4+ T cells from OT-II transgenic mice on a Rag2−/− background (OT-II-Rag mice), specific for an ovalbumin (OVA323–339 peptide on an MHC class II molecule I-Ab), were transferred into H. pylori-infected Rag2−/− mice, no gastritis was induced (SI Fig. 6c). Similarly, when OT-II-Rag mice were infected with H. pylori, no gastritis was induced despite the presence of CD4+ T cells (Table 1 and SI Fig. 6d). Furthermore, when OVA protein or OVA323–339 peptide was administered into H. pylori-infected OT-II-Rag mice, no inflammation was observed, although CD4+ T cells were activated in these mice (Table 1, SI Fig. 6e, and data not shown). These results collectively indicate the importance of H. pylori antigen recognition by CD4+ T cells in the induction of gastritis.
CD4+ T Cells Are Not Primed with H. pylori Antigen in γc-Rag DKO Mice.
IFNγ, a key cytokine for Th1 immune responses, is important for the pathogenesis of H. pylori-induced gastritis (14). Natural killer (NK) cells and antigen-presenting cells (APCs) including DCs are able to produce IFNγ to prevent bacterial infection (15). The interaction between DCs and NK cells enhances the production of IFNγ during H. pylori infection (16, 17). To test the importance of DC–NK interaction in the H. pylori-induced inflammatory response, we transferred splenic CD4+ T cells into H. pylori-infected IL-2 receptor β chain (IL-2Rβ)−/−Rag2−/− (β-Rag DKO) mice and cytokine receptor common γ chain (γc)−/−Rag2−/− DKO (γc-Rag DKO) mice. These mice lack NK cells because of impaired IL-15 signaling, which is critical for NK cell development (18, 19). In addition, the production of IL-12 and IFNγ by APCs from these mice is impaired (20). As shown in Fig. 1 e and f, gastritis was induced in H. pylori-infected β-Rag DKO mice when naive CD4+ T cells were transferred. Clearance of bacteria was also achieved by the naive CD4+ T cell transfer (Table 1), indicating that NK cells and NK–DC interaction are dispensable for the induction of gastritis by H. pylori infection.
Surprisingly, there was no gastritis induced in γc-Rag DKO mice even after the transfer of naive CD4+ T cells (Fig. 1g), NK cells (SI Fig. 7a), or NK cells with naive CD4+T cells (SI Fig. 7b), suggesting that γc-Rag DKO mice have additional defects compared with β-Rag DKO mice. Interestingly, when splenic CD4+ T cells isolated from H. pylori-infected wild-type mice were transferred, gastritis was induced in H. pylori-infected γc-Rag DKO mice (Fig. 1h), and the clearance of bacteria was evident (Table 1). These results suggest that CD4+ T cells were not primed in γc-Rag DKO mice. In fact, splenocytes from these mice did not respond to DCs preincubated with H. pylori lysate, whereas splenocytes from wild-type mice infected with H. pylori strongly responded and produced IFNγ in response to the same DC preparation (data not shown). It should be noted that there were no apparent defects in DCs from γc-Rag DKO mice compared with those from wild-type mice with regard to their ability to induce T cell activation and present antigen as examined by the induction of CD69 expression and IFNγ production by splenic CD4+ T cells (SI Fig. 8).
PPs Are a Critical Tissue for Priming CD4+T Cells with H. pylori Antigen.
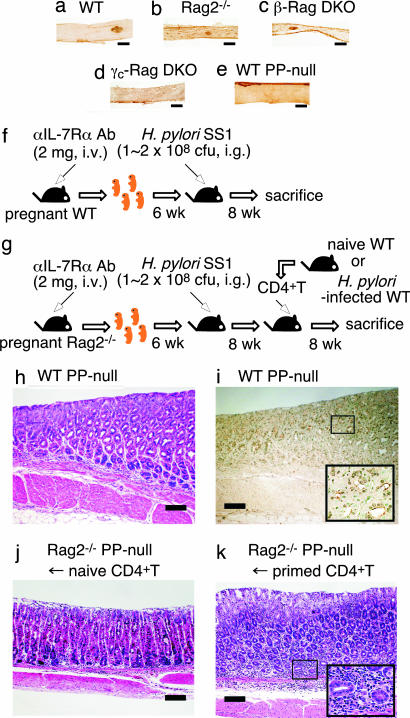
One difference between β-Rag DKO and γc-Rag DKO mice is that the latter lack gut-associated lymphoid tissues (GALT) such as PPs and isolated lymphoid follicles (ILFs) due to impaired IL-7 signaling (21) (Fig. 2 a–d). Thus, we hypothesized that CD4+ T cells are primed in GALT such as PPs or ILFs. To test this possibility, we generated PP-null mice by administration of anti-IL-7Rα mAb in utero (22) (Fig. 2 e and f). As observed in γc-Rag DKO mice, no gastritis was induced in PP-null mice 2 months after H. pylori infection, and a large number of H. pylori was detected in the gastric mucosa (Fig. 2 h and i and Table 1). We also generated PP-null mice on a Rag2−/− background (PP-null-Rag2−/− mice) (Fig. 2g). The adoptive transfer of CD4+ T cells from H. pylori-infected wild-type mice, but not naive CD4+ T cells, induced strong inflammation in PP-null-Rag2−/− mice just as in the γc-Rag DKO mice (Fig. 2 j and k and Table 1). These results strongly suggest that PPs are critical for priming CD4+ T cells in H. pylori infection, but dispensable for the effector phase.
Fig. 2.
PPs are critical for the priming of CD4+ T cells in H. pylori infection. (a–e) Small intestines from wild-type (WT) (a) and PP-null (e) mice were stained with anti-B220 mAb, and small intestines from Rag2−/− (b), β-Rag DKO (c), and γc-Rag DKO (d) mice were stained with anti-CD45 mAb. (f and g) Schemes of the generation of PP-null WT (f) or PP-null-Rag2−/− (g) mice. (h and i) PP-null WT mice were infected with H. pylori. Two months after the infection, gastric specimens were prepared. (j and k) PP-null-Rag2−/− mice were infected with H. pylori. Two months after the infection, naive (j) or primed (k) splenic CD4+ T cells were transferred. Two months after the cell transfer, gastric specimens were prepared. Specimens were stained with H&E (h, j, and k) or anti-H. pylori antisera (brown) (i). (Scale bars: 200 μm.)
The Coccoid, but Not Helical, Form of H. pylori Is Phagocytosed by DCs in PP.
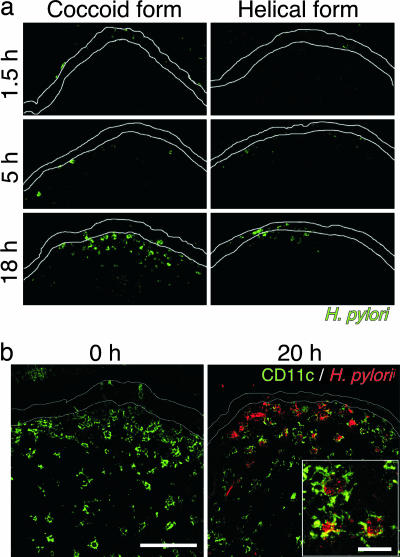
Although H. pylori is helical in the stomach, it transforms to the coccoid form under anaerobic conditions, such as in the small intestine (23). Interestingly, the coccoid form of H. pylori induced higher IL-12 production from bone marrow-derived cells (BMDCs) than the helical form (SI Fig. 9). There is a possibility that H. pylori transforms to the coccoid form in the intestine and is then captured by DCs present in PPs to induce a Th1 response. To test this possibility, the coccoid and helical forms of H. pylori were inoculated into ligated small intestinal loops. As shown in Fig. 3a, immunofluorescence staining detected H. pylori in the subepithelial dome (SED) region of PPs in a time-dependent manner, and the number of bacteria in the SED region inoculated with the coccoid form was larger than that inoculated with the helical form of H. pylori. In addition, double immunofluorescence staining with anti-H. pylori antibody and anti-CD11c mAb demonstrated that the bacteria were captured by CD11c+ DCs in the SED region (Fig. 3b). Although the helical form of H. pylori kept the rod shape 1.5 h after inoculation (SI Fig. 10 a and b), the bacteria phagocytosed by CD11c+ DCs in PPs were round (SI Fig. 10 c and d). These results suggest that the coccoid, but not the helical, form of H. pylori is captured by DCs in PPs and activates immune responses by generating H. pylori-specific pathogenic CD4+ T cells. Consistent with this observation, CD4+ T cells from the PPs as well as mesenteric lymph node (mLN) of H. pylori-infected wild-type mice were also able to eliminate the bacteria in γc-Rag DKO mice infected with H. pylori (Table 1).
Fig. 3.
The coccoid form of H. pylori is captured by DCs in PPs. The coccoid or helical form of H. pylori was inoculated into the ligated small intestines of wild-type mice. (a) After the indicated incubation times, PPs were stained with anti-H. pylori antibody. (b) Twenty hours after inoculation of the coccoid form, PPs were stained with anti-CD11c mAb (green) and anti-H. pylori antibody (red). (Scale bars: 0 h,100 μm; 20 h, 20 μm.)
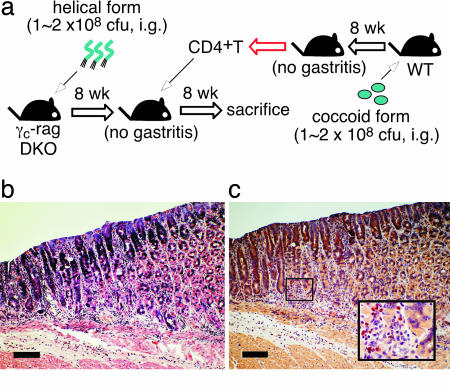
When wild-type mice were infected with the coccoid form of H. pylori, gastritis was not induced because the coccoid form of H. pylori was unable to colonize in the stomach (Fig. 4a and data not shown). However, CD4+ T cells from these mice induced gastritis in γc-Rag DKO mice infected with the helical form of H. pylori (Fig. 4 b and c and Table 1). These results indicate that CD4+ T cells primed with the coccoid form of H. pylori in the intestine are sufficient to induce inflammation in the stomach infected with the helical form of H. pylori.
Fig. 4.
Gastritis is induced by CD4+ T cells primed by the coccoid form of H. pylori. Two months after infection of γc-Rag DKO mice with the helical form of H. pylori, splenic CD4+ T cells from wild-type (WT) mice orally infected by the coccoid form of H. pylori were transferred to the infected γc-Rag DKO mice (a). Two months after the cell transfer, gastric specimens were prepared. Specimens were stained with H&E (b) or chloroacetate esterase (red) for infiltrated neutrophils and mast cells (c). (Scale bars: 200 μm.)
Discussion
We showed here that H. pylori antigen-specific CD4+ T cells are required to induce and maintain gastritis on infection with H. pylori. Because H. pylori interacts with and injects pathological molecules into gECs, it is generally thought that neutrophils infiltrating the gLP are attracted by chemokines produced by gECs. However, neutrophil infiltration was not observed in Rag2−/− mice, although gECs of Rag2−/− mice were able to secret MIP-2 on H. pylori infection. Thus, the secretion of chemokines by gECs seems insufficient for the induction of gastritis. In addition, adoptive transfer of CD4+ T cells recognizing H. pylori-independent antigens did not induce gastritis, suggesting that H. pylori-specific CD4+ T cells directly or indirectly regulate production of chemokines that attract neutrophils. In fact, a large amount of MIP-2 was produced by activated CD4+ T cells derived from the gLP of H. pylori-infected mice. In addition, infiltrated neutrophils were located around CD4+ T cells in the gLP of H. pylori-infected mice (data not shown). It is known that another keratinocyte-derived chemokine is able to recruit neutrophils. However, the amounts of keratinocyte-derived chemokine produced by both gECs and CD4 T cells were much lower than those of MIP-2 (data not shown).
Oral or intra-PP immunization with H. pylori antigens was effective in enhancing H. pylori-specific CD4+ T cell responses and reducing H. pylori colonization in the stomach (24, 25). These reports are consistent with our current observation that PPs play critical roles in priming CD4+ T cells, and H. pylori is indeed captured by DCs in PPs. H. pylori antigen-specific CD4+ T cells would be primed by DCs in PPs or mLNs where DCs migrate after capturing antigens. Interestingly, CD4+ T cells cannot be primed by DCs in the gLP or gEC, both of which are capable of expressing class II MHC and presenting antigens. The lack of antigen presentation is partly due to the fact that the helical form of H. pylori is resistant to phagocytosis in a type IV secretion system-dependent manner, although the molecular mechanisms of antiphagocytic activity remain to be determined (26). It should be noted that the transformation of the helical to the coccoid form is accompanied by changes in the composition of surface proteins and/or carbohydrates, which may make the bacteria susceptible to phagocytosis (27, 28), a subject worthy of further studies. It has been shown that mast cells are able to present H. pylori antigens to H. pylori-specific CD4+ T cells, which in turn activate mast cells to degranulate (29). When we infected W/Wv and Sl/Sld mice lacking mast cells with H. pylori, gastritis was readily induced in both strains of mice on infection (S.N., T.Y., Y.B., and S.K., unpublished data), indicating that mast cells are not essential in priming CD4+ T cells. In an in vitro experiment, BMDCs infected with the coccoid form of H. pylori produced larger amounts of IL-12 than those infected with the helical form of H. pylori, suggesting that the coccoid form of H. pylori easily induces Th1 immune responses on H. pylori infection.
Importantly, CD4+ T cells primed with the coccoid form of H. pylori were able to induce gastritis in H. pylori-infected GALT-null γc-Rag DKO mice where CD4+ T cells are not primed with H. pylori antigen at all. Thus, the following scenario emerges from our results: H. pylori transforms to the coccoid form when entering the intestinal tract and is captured by DCs in PPs. H. pylori antigens presented by DCs are recognized by CD4+ T cells in PPs or mLN, and activated T cells migrate to the gastric mucosa to induce and maintain inflammatory responses. We noted that PP-null-Rag2−/− mice exhibited modest inflammation in the stomach after naive CD4+ T cell transfer, compared with γc-Rag DKO mice, which showed no sign of inflammation. Because treatment by anti-IL-7Rα mAb in utero suppresses PP development, but leaves ILFs and recently discovered villous M cells (30) intact, ILFs and/or villous M cells may also participate in the capture of H. pylori in the intestine. Although luminal antigens can be taken up by M cells located over the follicular epithelia of ILF, the tissue is predominantly occupied by B cells (31). Furthermore, in a separate study, we have shown that no apparent organized lymphoid structure is developed under villous M cells (data not shown).
H. pylori is also implicated in the cause of other diseases such as idiopathic thrombocytopenic purpura (32) and Sjögren syndrome (33). Indeed, it has been shown that T cells migrate from the intestine to the salivary gland in Sjögren syndrome patients (34). It will be of interest to examine the functional relationship between these diseases and the coccoid form of H. pylori captured via PPs.
Materials and Methods
Mice.
All mice used in this study were on a C57BL/6 background and were maintained at Taconic (Germantown, NY) or in our animal facility under specific pathogen-free conditions. Wild-type C57BL/6 mice were purchased from Sankyo Labo Service (Shizuoka, Japan) and CLEA Japan (Tokyo, Japan). Rag2−/− mice, γc-Rag DKO mice, and OT-II-Rag mice were obtained from Taconic. IL-2Rβ−/− mice (35) were generously provided by T. W. Mak (Ontario Cancer Institute, Toronto, ON, Canada). β-Rag DKO mice were obtained by crossing IL-2Rβ−/− with Rag2−/− mice (20). All experiments were approved by the Animal Care and Use Committee of the Keio University School of Medicine and were performed in accordance with institutional guidelines.
Antibodies.
Fluorescein-conjugated antibodies for flow-cytometric analysis and biotin anti-mouse CD2c (HL3) were purchased from BD Bioscience (San Jose, NJ). Anti-H. pylori antibodies were purchased from Biomeda (Foster City, CA) or DAKO (Glostrup, Denmark).
Bacteria.
H. pylori strain SS1, a mouse-adapted human isolate, was used for all experiments. To prepare the helical form of H. pylori, SS1 was grown on 5% sheep blood agar plates for 2 days. Before inoculating into mice, bacteria were grown in Brucella broth with 5% FCS overnight at 37°C under microaerobic conditions with gentle agitation. To prepare the coccoid form, SS1 was grown on 5% sheep blood agar plates under microaerobic conditions for 3 days at 37°C and then cultured under anaerobic conditions for 7 days at 37°C.
In Vivo Infection of Mice.
Bacteria were prepared from logarithmic phase cultures. Mice were intragastrically infected with 1–2 × 108 cfu H. pylori in 0.15 ml of broth. After the indicated time period, mice were killed and the stomach was aseptically removed. The stomach was then bisected along the greater and lesser curvatures. Half of the stomach was homogenized for the determination of bacterial colonization by a plate-dilution method. The rest of the stomach was sectioned transversely into two strips for frozen and paraffin-embedded sections.
In Situ Infection of Intestinal Loop.
Wild-type mice (6-week-old females) were anesthetized by an i.m. injection of 2 mg of ketamine hydrochloride (Sankyo, Tokyo, Japan) and 0.1 mg of Xylazine per mouse. An ≈4-cm-long piece of the small intestine containing one or two PPs was ligated at both ends with surgical thread. H. pylori (1 × 109) suspended in 0.2 ml of saline was inoculated into the loop. After the indicated time periods, PPs were removed and extensively washed with PBS. After fixation in 4% paraformaldehyde in PBS, specimens were processed for histopathological examination.
Generation of PP-Null Mice.
PPs were depleted from a small intestine as previously described (22). Briefly, 14.5 days postcoitum pregnant wild-type or Rag2−/− mice were i.v. injected with 2 mg of anti-IL-7Rα mAb (A7R34; kindly provided by S.-I. Nishikawa, RIKEN CDB, Kobe, Japan). To confirm the depletion of PPs, a dissected small intestine from one of the offspring was fixed with acetone and stained with anti-B220 or anti-pan CD45 mAb (BD Biosciences).
Adoptive Transfer of CD4+ T Lymphocytes.
Naive or H. pylori antigen-primed CD4+ T cells were purified from splenocytes, mLN, and PPs by using anti-mouse CD4-microbeads and AutoMACS (Miltenyi Biotech, Sunnyvale, CA) according to the manufacturer's instruction. The purity of isolated cells was >95%. Isolated cells (5 × 106 per mouse for splenocytes and mLN, 5 × 105 per mouse for PP-derived cells) were injected i.v. into recipient mice infected with H. pylori for 8 weeks. Eight weeks after the transfer, mice were killed for the indicated analyses.
Histological Analysis.
An excised stomach was fixed in a neutral-buffered 10% formalin solution and cut into four strips. Samples were processed by standard methods, embedded in paraffin, and sectioned at 4 to 5 μm. Specimens were stained with H&E or used for cytochemical and immunohistochemical studies. The Leder method was used to assess naphtol-AS-d-chloroacetate esterase detection (36). Immunohistochemical analysis was performed with formalin-fixed and paraffin-embedded tissue sections by using heat-induced epitope retrieval and the ABC (Vectastain ABC kit; Vector Laboratories, Burlingame, CA) method. Anti-H. pylori serum from DAKO was used for H. pylori staining. In some cases, frozen sections (7 μm) were prepared, fixed with 4% paraformaldehyde in PBS, and blocked with 2% BSA-PBS, and immunofluorescence was performed using the tyramide amplication method (TSA-Plus Fluorescein System; PerkinElmer Life and Analytical Sciences, Boston, MA) and then incubated with anti-H. pylori antibody from Biomeda, followed by Cy5 (GE Healthcare Bioscience AB, Uppsala, Sweden) or TRITC-linked rabbit IgG (Sigma–Aldrich, St. Louis, MO). The specimens were mounted with Vectashield (Vector Laboratories) and examined with a confocal laser-scanning microscope LSM510 by using version 3.2 software (Carl Zeiss, Thornwood, NY). The zymogenic zone of middle corpus ≈3 mm from the FS/Z transition zone was examined in each sample.
Histological Score.
For assessment of gastric histopathology, blinded sections stained with H&E were examined by light microscopy. Neutrophil infiltration was assessed by the presence of neutrophils in the gastric mucosa. Active inflammation was assessed by the degree and area of damages of mucosal tissue and muscular layers because of infiltrations of neutrophils, lymphocytes, and/or macrophages. The scoring was graded as 0 (no), 1 (mild), 2 (moderate), or 3 (severe). The total number of glands with neutrophil infiltration in the crypt and lumen was also counted to produce a gland active inflammatory score.
Whole-Mount Immunohistochemistry.
Small intestines were removed and stained with antibodies as described previously (37). Briefly, small intestines were washed, incubated twice in HBSS containing 5 mM EDTA at 37°C for 20 min, and fixed with ice-cold formalin for 1 h. After blocking, specimens were incubated with biotin-conjugated anti-B220 mAb for wild-type mice or anti-CD45 mAb for mice on a Rag2−/− background in Solution A containing 0.6% Triton X-100 and 0.1% BSA for 1 h, incubated with ABC reagent (Vector Laboratories) at room temperature for 2 h, and reacted with diaminobenzidene.
Supplementary Material
Acknowledgments
We thank Drs. S.-I. Nishikawa and T. W. Mak for valuable materials; Dr. L. K. Clayton for valuable discussion and critical reading of the manuscript; and M. Motouchi and N. Yumoto for animal care. This work was supported by the Waksman Foundation of Japan (S.N.), the Uehara Memorial Foundation, the Tokyo Biochemical Research Foundation, Japan Society for the Promotion of Science Grant-in-Aid 18659141, Scientific Research on Priority Areas Grant-in-Aid 14021110, a National Grant-in-Aid for the Establishment of a High-Tech Research Center in a Private University, a grant for the Promotion of the Advancement of Education and Research in Graduate Schools, and a Scientific Frontier Research Grant from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Abbreviations
- DKO
double knockout
- LP
lamina propria
- gLP
gastric LP
- gEC
gastric epithelial cell
- DC
dendritic cell
- PPs
Peyer's patches
- OVA
ovalbumin
- NK
natural killer
- APC
antigen-presenting cell
- β-Rag DKO
IL-2 receptor β chain (IL-2Rβ)−/−Rag2−/− DKO
- γc-Rag DKO
cytokine receptor common γ chain (γc)−/−Rag2−/− DKO
- GALT
gut-associated lymphoid tissue
- ILF
isolated lymphoid follicle
- BMDC
bone marrow-derived cell
- SED
subepithelial dome
- mLN
mesenteric lymph node.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609014104/DC1.
References
- 1.Covacci A, Telford JL, Del Giudice G, Parsonnet J, Rappuoli R. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 2.Ernst PB, Gold BD. Annu Rev Microbiol. 2000;54:615–640. doi: 10.1146/annurev.micro.54.1.615. [DOI] [PubMed] [Google Scholar]
- 3.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 4.Baldari CT, Lanzavecchia A, Telford JL. Trends Immunol. 2005;26:199–207. doi: 10.1016/j.it.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Cover TL, Blanke SR. Nat Rev Microbiol. 2005;3:320–332. doi: 10.1038/nrmicro1095. [DOI] [PubMed] [Google Scholar]
- 6.Eaton KA, Ringler SR, Danon SJ. Infect Immun. 1999;67:4594–4602. doi: 10.1128/iai.67.9.4594-4602.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pappo J, Torrey D, Castriotta L, Savinainen A, Kabok Z, Ibraghimov A. Infect Immun. 1999;67:337–341. doi: 10.1128/iai.67.1.337-341.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panthel K, Faller G, Haas R. Infect Immun. 2003;71:794–800. doi: 10.1128/IAI.71.2.794-800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundgren A, Trollmo C, Edebo A, Svennerholm AM, Lundin BS. Infect Immun. 2005;73:5612–5619. doi: 10.1128/IAI.73.9.5612-5619.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi H, Osaki T, Takahashi M, Taguchi H, Kamiya S. FEMS Microbiol Lett. 1999;175:107–111. doi: 10.1111/j.1574-6968.1999.tb13608.x. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds DJ, Penn CW. Microbiology. 1994;140:2649–2656. doi: 10.1099/00221287-140-10-2649. [DOI] [PubMed] [Google Scholar]
- 12.Narikawa S, Kawai S, Aoshima H, Kawamata O, Kawaguchi R, Hikiji K, Kato M, Iino S, Mizushima Y. Clin Diag Lab Immunol. 1997;4:285–290. doi: 10.1128/cdli.4.3.285-290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bumann D, Habibi H, Kan B, Schmid M, Goosmann C, Brinkmann V, Meyer TF, Jungblut PR. Infect Immunol. 2004;72:6738–6742. doi: 10.1128/IAI.72.11.6738-6742.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obonyo M, Guiney DG, Harwood J, Fierer J, Cole SP. Infect Immun. 2002;70:3295–3299. doi: 10.1128/IAI.70.6.3295-3299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frucht DM, Fukao T, Bogdan C, Schindler H, O'Shea JJ, Koyasu S. Trends Immunol. 2001;22:556–560. doi: 10.1016/s1471-4906(01)02005-1. [DOI] [PubMed] [Google Scholar]
- 16.Ferlazzo G, Munz C. J Immunol. 2004;172:1333–1339. doi: 10.4049/jimmunol.172.3.1333. [DOI] [PubMed] [Google Scholar]
- 17.Hafsi N, Voland P, Schwendy S, Rad R, Reindl W, Gerhard M, Prinz C. J Immunol. 2004;173:1249–1257. doi: 10.4049/jimmunol.173.2.1249. [DOI] [PubMed] [Google Scholar]
- 18.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, et al. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohteki T, Suzue K, Maki C, Ota T, Koyasu S. Nat Immunol. 2001;2:1138–1143. doi: 10.1038/ni729. [DOI] [PubMed] [Google Scholar]
- 21.Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, Drago J, Noguchi M, Grinberg A, Bloom ET, et al. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida H, Honda K, Shinkura R, Adachi S, Nishikawa S, Maki K, Ikuta K, Nishikawa SI. Int Immunol. 1999;11:643–655. doi: 10.1093/intimm/11.5.643. [DOI] [PubMed] [Google Scholar]
- 23.Bode G, Mauch F, Malfertheiner P. Epidemiol Infect. 1993;111:483–490. doi: 10.1017/s0950268800057216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunkley ML, Harris SJ, McCoy RJ, Musicka MJ, Eyers FM, Beagley LG, Lumley PJ, Beagley KW, Clancy RL. FEMS Immunol Med Microbiol. 1999;24:221–225. doi: 10.1111/j.1574-695X.1999.tb01286.x. [DOI] [PubMed] [Google Scholar]
- 25.Ramarao N, Gray-Owen SD, Backert S, Meyer TF. Mol Microbiol. 2000;37:1389–1404. doi: 10.1046/j.1365-2958.2000.02089.x. [DOI] [PubMed] [Google Scholar]
- 26.Nystrom J, Raghavan S, Svennerholm AM. Microbes Infect. 2006;8:442–449. doi: 10.1016/j.micinf.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Bergman MP, Engering A, Smits HH, van Vliet SJ, van Bodegraven AA, Wirth HP, Kapsenberg ML, Vandenbroucke-Grauls CM, van Kooyk Y, Appelmelk BJ. J Exp Med. 2004;200:979–990. doi: 10.1084/jem.20041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emilia G, Longo G, Luppi M, Gandini G, Morselli M, Ferrara L, Amarri S, Cagossi K, Torelli G. Blood. 2001;97:812–814. doi: 10.1182/blood.v97.3.812. [DOI] [PubMed] [Google Scholar]
- 29.Velin D, Bachmann D, Bouzourene H, Michetti P. Gastroenterology. 2005;29:142–155. doi: 10.1053/j.gastro.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Jang MH, Kweon MN, Iwatani K, Yamamoto M, Terahara K, Sasakawa C, Suzuki T, Nochi T, Yokota Y, Rennert PD, et al. Proc Natl Acad Sci USA. 2004;101:6110–6115. doi: 10.1073/pnas.0400969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S, Kaminogawa S, Takahashi-Iwanaga H, Iwanaga T, Kiyono H, et al. J Immunol. 2002;168:57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- 32.Khin MM, Hua JS, Ng HC, Wadstrom T, Bow H. World J Gastroenterol. 2000;6:202–209. doi: 10.3748/wjg.v6.i2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Vita S, Ferraccioli G, Avellini C, Sorrentino D, Dolcetti R, Di Loreto C, Bartoli E, Boiocchi M, Beltrami CA. Gastroenterology. 1996;110:1969–1974. doi: 10.1053/gast.1996.v110.pm8964425. [DOI] [PubMed] [Google Scholar]
- 34.Kroneld U, Jonsson R, Carlsten H, Bremell T, Johannessen AC, Tarkowski A. Scand J Rheumatol. 1998;27:215–218. doi: 10.1080/030097498440831. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki H, Duncan GS, Takimoto H, Mak TW. J Exp Med. 1997;185:499–505. doi: 10.1084/jem.185.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leder LD. Am J Dermatopathol. 1979;1:39–42. [PubMed] [Google Scholar]
- 37.Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, Newberry RD. J Immunol. 2003;170:5475–5482. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.