Abstract
Epoxyeicosatrienoic acids (EETs), as metabolites of arachidonic acid, may function as antihypertensive and antiatherosclerotic mediators for vasculature. EETs are degraded by soluble epoxide hydrolase (sEH). Pharmacological inhibition and genetic ablation of sEH have been shown to increase the level of EETs, and treating angiotensin II (Ang II)-infused hypertension rats with sEH-selective inhibitors increased the levels of EETs, with attendant decrease in systolic blood pressure. To elucidate the mechanisms by which Ang II regulates sEH expression, we treated human umbilical vein endothelial cells (ECs) and bovine aortic ECs with Ang II and found increased sEH expression at both the mRNA and protein levels. Transient transfection assays showed that the activity of the human sEH promoter was increased in ECs in response to Ang II. Further analysis of the promoter region of the sEH gene demonstrated that treatment with Ang II, like overexpression of c-Jun/c-Fos, activates the sEH promoter through an AP-1-binding motif. The binding of c-Jun to the AP-1 site of the sEH promoter was confirmed by chromatin immunoprecipitation assays. In contrast, adenovirus overexpression of the dominant-negative mutant of c-Jun significantly attenuated the effects of Ang II on sEH induction. An elevated level of sEH was found in the aortic intima of both spontaneously hypertensive rats and Ang II-infused Wistar rats. Blocking Ang II binding to Ang II receptor 1 by losartan abolished the sEH induction. Thus, AP-1 activation is involved in the transcriptional up-regulation of sEH by Ang II in ECs, which may contribute to Ang II-induced hypertension.
Keywords: endothelial cells, arachidonic acid, AP-1, promoter, hypertension
Arachidonic acid (AA) derived from membrane phospholipids plays a key role in vascular inflammatory and/or antiinflammatory responses. AA can be converted to eicosanoids by three major enzymatic pathways, namely, cyclooxygenase, lipoxygenase, and CYP 450 epoxygenase. Exerting autocrine effects on vascular endothelial cells (ECs), four epoxyeicosatrienoic acids (EETs) regioisomers 5,6-, 8,9-, 11,12-, and 14,15-EET are the major metabolites generated by CYP 450 epoxygenase (1). EETs can be released by ECs to act as paracrine mediators on neighboring cells such as vascular smooth muscle cells (VSMCs) (2). EETs exert membrane potential-independent effects and modulate several signaling cascades that affect EC proliferation and angiogenesis. EETs also function as endothelium-derived hyperpolarizing factors (3). By increasing intracellular Ca2+ concentration, EETs activate large conductance Ca2+-activated K+ channel (BKCa) in the smooth muscle. The activation of BKCa then causes hyperpolarization of VSMCs and subsequent vasodilation, which lowers the blood pressure (4). As well, EETs inhibit cytokine-induced inflammatory responses in ECs (5, 6). Treating ECs with 11,12-EET or overexpression of CYP2J2 attenuated the TNFα-, IL-1α-, and LPS-induced expression of adhesion molecules in ECs, thus decreasing leukocyte adhesion to the vascular wall (7).
Epoxide hydrolases (EHs) convert epoxides to the corresponding diols. Under physiological conditions, EETs can be enzymatically hydrolysed to dihydroxyeicosatrienoic acids (DHETs) by EHs (1). Two major EHs in the α/β hydrolase family exist in mammalian cells: soluble EH (sEH), which primarily presents in the cytosol and peroxisomes, and microsomal EH, which binds to the intracellular membranes (8). Highly expressed in the liver, kidney, intestine, and vasculature, sEH is the main enzyme that converts 5,6-, 8,9-, 11,12-, 14,15-EET to 5,6-, 8,9-, 11,12-, 14,15-DHET, respectively. The mammalian sEH is a homodimer, and each subunit contains a C- and an N-terminal domain. The active site is located in the C-terminal domain in which the residues Asp-333, Asp-495, and His-523 form the catalytic triad (9). DHETs are much more polar than EETs and are generally considered as biologically inactive products of EETs. However, their roles are not fully understood.
Angiotensin II (Ang II), a potent vessel constrictor, elevates blood pressure in various animal models. i.p. injection of sEH-selective inhibitors to Ang II-infused hypertensive rats greatly increased the level of EETs and lowered systolic blood pressure (10). Thus, augmentation of EET levels with enhanced production by CYP450s or decreased hydrolysis by sEH seems to control blood pressure in vivo. In line with this hypothesis, recent studies demonstrated that the selective sEH inhibitor N-cyclohexyl-N-dodecyl urea reversed the hypertensive phenotype in the spontaneously hypertensive rat (SHR) (11).
We have previously shown that laminar shear stress, an atheroprotective flow, decreased the expression of sEH. Further, the increased level of EETs because of attenuated sEH increased binding to peroxisome proliferator-activated receptor γ nuclear receptor, which contributes in part to the antiinflammatory effect of laminar shear stress (12). In this study, we aimed to examine the proximal sEH promoter region for the mechanism of Ang II-increased sEH transcriptional activation to provide an explanation for the control of blood pressure by the endothelium-derived hyperpolarizing factors-sEH system.
Results
Ang II Up-Regulates sEH in ECs.
Because sEH hydrolysis of EETs is associated with Ang II-induced hypertension (10), we investigated first the effect of Ang II on sEH expression in ECs. Human umbilical vein ECs (HUVECs) and bovine aortic ECs (BAECs) were treated with Ang II, up to 100 nM, for 24 h, and cell lysates were assayed by Western blotting. Ang II increased sEH level at 1 nM, but the greatest effects were seen at 10 and 100 nM (Fig. 1 A and B). ECs treated with 100 nM Ang II for different times showed sEH level increased significantly at 12 h; the increase lasted for at least 24 h. Thus, Ang II induced sEH expression in a dose- and time-dependent manner. To explore whether the up-regulated sEH was at the level of transcription, quantitative real-time RT-PCR was performed to assess the level of sEH mRNA in HUVECs. A 2.4 ± 0.3-fold increase in sEH mRNA was found in Ang II-treated ECs as compared with untreated controls (Fig. 1C). In contrast, Ang II-treated ECs and untreated controls did not differ in level of human CYP2J2 and CYP2C9 mRNA encoding enzymes that convert AA into EETs. Thus, Ang II up-regulates sEH mRNA and protein in ECs.
Fig. 1.
Ang II induces sEH expression in ECs. (A and B) HUVECs (A) and BAECs (B) were treated with different concentrations of Ang II as indicated for 24 h or 100 nM Ang II for different periods of time. Cells were lysed, and proteins were resolved by 10% SDS/PAGE, transferred to a nitrocellulose membrane and probed with anti-sEH and anti-β-actin antibodies. Data are representative of three independent experiments. (C) HUVECs were incubated with 100 nM Ang II for 24 h. RNA was isolated and samples of total RNA analyzed by real-time RT-PCR with primers specific for human sEH, CYP2C9, or CYP2J2. β-Actin cDNA was used as an internal control. Data are means ± SD of the relative mRNA normalized to that of β-actin from three independent experiments.
Ang II Activates the sEH Promoter in ECs.
To study the regulation of sEH by Ang II at the level of transcription, we cloned a 1.1-kb genomic DNA fragment upstream of the human sEH gene from + 32 to −1091 (Fig. 2A). For better transfection efficiency, BEACs were used in the transient transfection assay with this sEH promoter construct. As shown in Fig. 2B, treatment of Ang II activated sEH promoter in BEACs. Sequence analysis revealed a number of regulatory motifs within the sEH promoter region. Of them, one NF-κB-binding site was located at −250, and three AP-1 putative binding sites were located at −156, −446, and −777, of the 1.1-kb sEH promoter (Fig. 2A). To further define the role of each of these motifs in response to Ang II, three deletion constructs at 586, 374, and 92 bp upstream of the transcription initiation site were then subcloned into pGL3-Luc. BAECs were transfected with various sEH promoter constructs and treated with Ang II for Luc induction assays. Ang II exerted a similar induction effect on sEH-1091-Luc, sEH-586-Luc, and sEH-374-Luc, which supported the transcriptional activation of sEH by Ang II (Fig. 2B). However, much higher basal and Ang II-induced luciferase activities were observed for sEH-586-Luc, which indicates that the active promoter and Ang II responsive elements are within this region. In contrast, Ang II had little effect on the transactivity of sEH-92-Luc lacking both AP-1 and NF-κB sites. Furthermore, when 60 bp of nucleotides including the AP-1 site at −446 were deleted from sEH-586, the promoter lost both basal and Ang II-induced activities (Fig. 2C).
Fig. 2.
Ang II activates the human sEH promoter in ECs. (A) Sequence of the 1.1-kb-cloned human sEH promoter and sketch of putative AP-1 and NFκB-binding sites. (B) BAECs were transfected with plasmids of sEH-Luc (−1091, −586, −374, and −92). (C) BAECs were transfected with sEH-586 or -586D. All transfected cells were then treated with 100 nM Ang II for 24 h. The β-gal plasmid was cotransfected as a transfection control. Promoter activities were measured by use of luciferase, which was normalized to β-gal. The results are expressed as the relative luciferase activities, of which activities of −1091-Luc in B and −586D-Luc in C are designed as 1. Data are mean ± SD of the relative luciferase activities from three independent experiments, each performed in triplicate (∗, P < 0.05).
Ang II Activates the sEH Promoter by an AP-1-Dependent Mechanism.
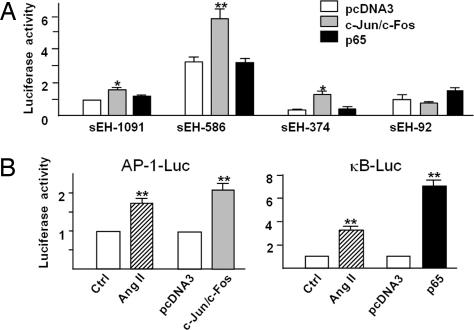
It was reported that Ang II activated AP-1 and NF-κB in cardiomyocytes and VSMCs (13, 14). We next studied the involvement of the respective AP-1 and NF-κB sites in the Ang II induction of the sEH promoter. Fig. 3A shows that cotransfection of plasmids expressing c-Jun and c-Fos induced sEH-1091-Luc, sEH-586-Luc, and sEH-374-Luc in a similar fashion as that by Ang II (Fig. 2C). Cotransfection of an expression plasmid encoding p65 NF-κB subunit had little effect on the induction of sEH deletion constructs (Fig. 2C). Furthermore, the results in Fig. 3B showed that treatment of Ang II could activate both AP-1-luc and NF-κB-luc in ECs. As a control, the expression of c-Jun/c-Fos and p65 NF-κB subunit was shown to induce AP-1-Luc and NF-κB-Luc, respectively. These data suggest that the AP-1, but not NF-κB, within the sEH promoter region is the functional element responsive for Ang II activation of sEH.
Fig. 3.
AP-1 overexpression activates the human sEH promoter in ECs. (A) Plasmids of sEH-Luc (−1091, −586, −374, and −92) were cotransfected with expression plasmids of c-Jun, c-Fos, p65, or pcDNA3 in BAECs for 48 h. (B) BAECs were transfected with plasmids of 5 × NF-κB-Luc or 7 × AP-1-Luc for 24 h and then treated with 100 nM Ang II for 24 h. In parallel experiments, cells were cotransfected with the expression plasmids of c-Jun and c-Fos, p65, or pcDNA3 for 48 h. The β-gal plasmid was cotransfected in all experiments as a transfection control. Promoter activities measurement and data analysis were the same as those in Fig. 2.
c-Jun Binds to the sEH Promoter and Induces the Gene Expression of sEH.
We performed ChIP assays to test further whether AP-1 increases its binding to the sEH promoter in response to Ang II in ECs. c-Jun was immunoprecipitated from HUVECs treated with Ang II or infected with an adenovirus overexpressing c-Jun, which was followed by PCR with a pair of oligonucleotide primers targeting the −583 to −126 region of the sEH promoter. Both Ang II treatment and c-Jun overexpression increased c-Jun binding to the sEH promoter, as compared with no treatment (Fig. 4A). To test further the hypothesis that Ang II increased AP-1 binding to the proximal promoter of the sEH gene in ECs, EMSA was performed with nuclear extracts isolated from Ang II-treated and Ad-c-Jun-infected HUVECs. Ang II treatment or c-Jun overexpression increased the binding of nuclear extracts to the consensus AP-1 binding sequence (i.e., TGACTCA) and the divergent AP-1 site at the sEH promoter (i.e., TGACACA) (data not shown).
Fig. 4.
AP-1 is involved in the Ang II-induced sEH in ECs. (A) Confluent HUVECs were infected with Ad-c-Jun for 24 h or treated with Ang II for 12 h. After cross-linking and sonication, nuclear proteins were extracted. ChIP assays involved use of anti-c-Jun antibody for IP, and normal rabbit IgG was used in control experiments. PCR involved use of sEH promoter-specific primers to detect binding of c-Jun to the sEH promoter. (B) HUVECs were infected with Ad-c-Jun at different multiplicity of infection for 48 h. (C) HUVECs were infected with Ad-TAM67 or Ad-TTA control virus for 24 h. Then, the cells were treated with 100 nM Ang II for another 24 h. Cell lysates were analyzed by Western blotting with use of anti-sEH, anti-c-Jun, or anti-β-actin antibodies. Results are representative of three independent experiments.
Because Ang II activated the transcriptional activation of sEH by specific binding of c-Jun to the AP-1 sites of the sEH promoter, as shown in Figs. 2 and 3, we further examined the effect of AP-1 on the transcriptional induction of sEH. Coinfection of HUVECs with a recombinant adenovirus overexpressing c-Jun (i.e., Ad-c-Jun) induced sEH protein expression in a dose-dependent manner (Fig. 4B), with a peak level at 20–100 multiplicity of infection. As an important subunit of AP-1, c-Jun forms heterodimers or homodimers to regulate the expression of its target genes. To test whether c-Jun is necessary for the Ang II-induced sEH, we used an adenovirus expressing TAM67, a truncated form of c-Jun lacking the transactivation domain (15), to block the effect of Ang II in ECs. In contrast to the wild-type c-Jun, TAM67 largely abolished the Ang II-induced sEH increase (Fig. 4C).
Ang II Up-Regulates sEH Expression in Aortic Intima in Vivo.
We used rat models to examine the pathophysiological relevance among Ang II, sEH expression, and hypertension. First, a high plasma level of Ang II was induced in SHRs by feeding them a saline containing 2% NaCl for 14 days. Systolic blood pressure in SHR rats was much higher than in Wistar rats (190 ± 10 mmHg vs. 110 ± 8 mmHg), and blood pressure in the high saline-fed SHRs was gradually increased to 240 ± 6 mmHg (Fig. 5A). The plasma Ang II levels were 50.4 ± 10.9 pg/ml and 46 ± 6 pg/ml in control SHR and Wistar rats, respectively (Fig. 5B). The levels were decreased to 8 ± 1.2 pg/ml in saline-treated Wistars, whereas that in saline-treated SHRs increased to 270 ± 10 pg/ml (Fig. 5B). Western blotting revealed a 2.4 ± 0.62 -fold increase in level of sEH in aortic intima of saline-treated SHRs as compared with that in control rats and 80% decrease in saline-treated Wistars (Fig. 5C). Consistent with results from the Western blot, the immunohistochemistry study in Fig. 5D showed that the expression of sEH in the aortic endothelium of saline-treated SHR was stronger than that in control rats.
Fig. 5.
Level of sEH protein is elevated in the aortic intima of SHR rats. Wistar and SHR rats (180–280 g, male, n = 6) had free access to tap water supplemented with or without 2% NaCl for 14 days. (A) Systolic blood pressure was measured every 2 days until rats were killed. (B) Plasma Ang II levels were measured by use of an RIA kit. (C) Protein extracts of aorta intima from each rat were analyzed by Western blotting with anti-sEH and anti-β-actin antibodies in three individual animals from two separate sets of experiments. (D) The cross sections of the abdominal aorta from different treated rats were subjected to immunohistochemical staining with anti-sEH antibody. The results shown are representative of the rats from two separate sets of experiments. The sections were counterstained with hematoxylin.
To further decipher the link between Ang II and sEH expression in the aortic intima, we infused Wistar rats with 450 ng/kg per min Ang II through an implanted minipump. Mean systolic blood pressure before infusion was 95 ± 3 mmHg, and it increased to 131 ± 3 and 155 ± 5 mmHg on days 2 and 3 postinfusion (Fig. 6A). As an additional control, treatment of Wistar rats with losartan, an Ang II receptor 1 (AT1) blocker, for 9 days (6 days before and 3 days during Ang II infusion), produced systolic blood pressure comparable with that of nontreated rats or those receiving losartan alone. Western blotting revealed levels of sEH in the aortic intima to be significantly higher in Ang II-infused rats than in other three groups: vehicle infused, losartan alone, and losartan plus Ang II (Fig. 6B). In contrast, the level of sEH in aortic media was not significantly different among the three control groups (Fig. 6C).
Fig. 6.
Ang II infusion increases sEH protein in the aortic intima of Wistar rats. Wistar rats (180–280 g, male) received losartan (Los, 25 mg/kg per day) or PBS (Ctrl) by oral gavage for 6 days. A minipump was then implanted in the dorsal region to deliver either Ang II at 450 ng/kg per minute (Ang II) or PBS for 3 days. (A) Systolic blood pressure was measured daily after implantation. (B and C) At day 3, rats were killed, and protein extracts from intima (B) or media (C) of aortas were analyzed by Western blotting with anti-sEH and anti-β-actin antibodies. The density of each band was quantified with a densitometer, and the quantified data are mean ± SD of the relative mRNA normalized to that of β-actin in three individual animals from two separate sets of experiments.
Discussion
An optimal level of EETs exerts several effects of cardiovascular benefit, including hyperpolarizing VSMC, dilating coronary arteries, and suppressing adhesion molecules. An imbalance in the metabolism of EETs may lead to an impaired vascular protection. Ang II causes systemic hypertension by acting on arterial VSMCs and renal microvasculature. Related to CYPs-EETs-sEH, Ang II has been reported to up-regulate sEH protein in the kidney (10). Ang II-infused hypertensive rats receiving sEH-specific inhibitors indeed showed increased level of EETs with attendant decrease in systolic blood pressure (10). As well, TNF-α-induced VCAM-1 expression in the murine carotid artery was suppressed by intraarterial infusion of 11,12-EET or 14,15-EET (7). Herein, we investigated the regulation of sEH by Ang II in ECs and the underlying mechanism. Ang II up-regulated sEH in cultured ECs at both protein and mRNA levels and increased sEH promoter activity by the AP-1 pathway, an elevated level of sEH was found in the aortic intima of both saline-treated SHR rats and Ang II-infused rats, and blockade of Ang II with the AT1 inhibitor losartan abolished the induction of sEH.
The cellular level of EETs is largely determined by their generation from AA catalyzed by CYP and their hydrolysis to DHETs by sEH. Human ECs express two CYP family members, namely, CYP2C and CYP2J (3, 7). Although Ang II increased the sEH expression in ECs, the level of mRNA encoding CYP2C9 or CYP2J2 was not affected (Fig. 1C), which resulted in a net decrease in EET level in ECs. Consequently, the paracrine effect of EETs on hyperpolarizing the neighboring VSMCs was attenuated, leading to a hypertensive state of the vessel. This hypothesized mechanism agrees with previous reports demonstrating the level of renal cortical sEH protein significantly higher in Ang II-induced hypertensive rats as compared with normotensive animals (16). Likewise, the level of urinary 14,15-DHET in proximal tubule cells was significantly increased in hypertensive versus normotensive animals (10). Interestingly, we reported that laminar shear stress, an atheroprotective mechanical stimulation, decreased the level of sEH in ECs (12). The increase in EET level results in increased binding to proliferator-activated receptor γ nuclear receptor, which contributes to the antiinflammatory effect of laminar shear stress (12). Ang II and shear stress, two physiological stimuli, likely regulate endothelial sEH to maintain vascular homeostasis in terms of hypertensive vs. antihypertensive and inflammatory vs. antiinflammatory responses. Paradoxically, both Ang II and laminar shear stress seem to activate AP-1 in ECs (ref. 17 and Fig. 2C), Ang II increasing but laminar shear stress reducing the level of sEH. One explanation is that Ang II causes greater sustained AP-1 activation than laminar shear stress (18, 19). The temporal discrepancy of AP-1 activation thus contributes to the Ang II-up-regulated sEH.
As a potent mitogen, Ang II binds to its receptor AT1 to activate several signaling pathways, with ensuing up-regulation of the corresponding downstream transcriptional factors, including AP-1, NF-κB, and Stat (13, 14, 18). Although three divergent AP-1 and one NF-κB-binding sites are located in the 1.1-kb sEH promoter, our data from promoter deletion construct transfection and ChIP demonstrate that the transcription factor and the cognate cis-binding element mediating sEH induction by Ang II is c-Jun/c-Fos binding to the AP-1 site at −446. Further analysis involving overexpressing c-Jun and its dominant-negative mutant confirmed that c-Jun is necessary and sufficient for the sEH induction by Ang II. Our data showed that sEH-586-Luc had higher basal activity than that of sEH-1091-Luc. sEH-374-Luc exhibited lowest basal activity. Possibilities include the presence of a cosuppressor binding site between −1091 and −586 and/or a coactivator-binding site existing between −586 to −374. The deletion of the −446 AP-1 site and the adjacent CA-rich region abolished not only the Ang II-induced but also the basal promoter activities. Further site-directed mutations of the CA-rich region and each of the three AP-1 sites would provide insightful information regarding the regulation of sEH transcription.
The sEH protein level was increased in aortic specimens collected from saline-fed SHRs (Fig. 5) or Ang II-infused Wistar rats (Fig. 6). Noticeably, the sEH level was increased only in the aortic intima, which contains endothelium, but not media containing mainly VSMCs. Thus, the Ang II-induced sEH may show an endothelium-selective effect. Given that VSMC is a major cell type responding to Ang II, other Ang II-elicited signaling and/or transcription seem likely to override AP-1 activation. Because Ang II infusion causes c-Jun and c-Fos activation in the rat aorta (20), AP-1 activation resulting from c-Jun/c-Fos heterodimer formation could be responsible for the Ang II induction of the sEH gene in endothelium in vivo. Ang II also up-regulates p65 NFκB through ribosomal S6 kinase and/or IκB kinase in several cell types (21, 22). Apparently, Ang II induced NF-κB in ECs in our experimental system, which was evidenced by the activation of NF-κB-Luc reporter in BAECs by Ang II treatment (Fig. 3). However, the induction of sEH does not seem to involve NF-κB, because the sEH promoter was not activated by p65 overexpression (Fig. 3). The lack of significant induction of sEH by NF-κB in our study may be due, in part, to the reduced function of the divergent NF-κB-binding site at the promoter or the poor binding capacity of p65 NF-κB to that site.
In summary, our findings provide evidence for the first time that Ang II, by AP-1, transcriptionally up-regulates sEH in the endothelium in vitro and in vivo. Inhibition of such an up-regulation by the AT-1 blocker losartan can abrogate the Ang II-induced sEH. Thus, we proposed that the binding of Ang II to AT-1 activates AP-1, which, in turn, up-regulates sEH by activating the cognate cis-element at the promoter. The increased sEH enhances the hydrolysis of EETs to become DHETs. With decreased EETs released from ECs, the paracrine effect of EETs on VSMC hyperpolarization is attenuated, which increases blood pressure. Thus, pharmacological inhibition of EET metabolizing enzyme (i.e., sEH) may provide a novel treatment for hypertension.
Materials and Methods
Cell Cultures.
HUVECs were isolated and maintained as described (23). BAECs isolated from bovine aorta were cultured in DMEM supplemented with 10% FBS and antibiotics. All experiments were performed with ECs up to passage three and cultured to confluence before treatment.
Cloning of Human sEH Promoter, Plasmid Construction, and Transient Transfection.
The human sEH promoter region was located according to the human genomic sequence of NM_001979 (Homo sapiens chr8 27402578–27404778) (24), with transcription start site (7404578) designated as 0. The promoter region (−1091 to +32) of the sEH gene was amplified by PCR from the human genomic DNA with the primer set 5′-AGACGAGCTCAAACCCACGGCTCTGGTCAATCCTG-3′; and 5′-CTCGAGATCTCAGCTAACCTGGGAGATGCGCGAAG-3′. The amplified PCR products were subcloned into the SacI and BglII sites of the pGL-3 basic vector (Invitrogen, Carlsbad, CA). The generated plasmid with the sEH promoter linked to luciferase (Luc) reporter was designated as sEH-1091. For a series of sEH promoter deletion constructs (i.e., sEH-586, sEH-374, and sEH-92), the corresponding fragments were amplified by PCR with sEH-1091 used as the template and the following primer sets: sEH-586, 5′-CTTGGAGCTCAAGAGCGTGCCTAGAGGAGTGGTCAGG-3′; sEH-374, 5′-GATCGAGCTCTTCCCAGGCATTCCAAGTC-3′; and sEH-92, 5′-GATCGAGCTCAGAGGGCGGAGTCCCGTTAA-3′. For a deletion construct of the sEH promoter, sEH-586D, the divergent AP-1 site and CA-rich sequence, 5′-TGACACACACACACACACACACACACACACACACACACACACACACACACACACACAGAG -3′ was deleted from sEH-586. The plasmid 5×NF-κB-Luc and 7×AP-1-Luc and expression vectors of c-Jun, c-Fos, and p65 were used as reported (15). For transient transfection, plasmid DNA was transfected into BAECs by use of the jetPEI method (Polyplus, San Marcos, CA). CMV-β-gal was cotransfected as a transfection control. After various treatments, ECs were lysed and the cell lysates collected for luciferase activity assays.
Western Blot Analysis.
Cultured ECs and isolated rat aortic intima were lysed, and protein concentrations were measured by use of the BCA protein assay kit. Cell lysates were resolved by 10% SDS/PAGE and transferred to a nitrocellulose membrane. sEH and β-actin proteins were detected by use of a polyclonal anti-sEH (Santa Cruz Biotechnology, Santa Cruz, CA) and an anti-β-actin followed by a HRP-conjugated secondary antibody. The protein bands were visualized by the ECL detection system (Amersham, Arlington Heights, IL), and the densities of the bands were quantified by use of Scion Image software (Scion, Frederick, MD).
Quantitative Real-Time RT-PCR.
Total RNA was isolated from cells with TRIzol reagent (Invitrogen). The isolated RNA was converted into cDNA. Quantitative RT-PCR with the Brilliant SYBR green QPCR system was performed by using β-actin as internal control (Stratagene, La Jolla, CA). The nucleotide sequences of the primers were as follows: sEH, 5′-TGCCATCCTCACCAACAC-3′ and 5′-ACGGACCCTGGGCTTTAC-3′; CYP2J2, 5′-AAGGCCAAGTGGAATGTGAC-3′ and 5′-TGACCGAAATTGCTACCACC-3′; CYP2C9, 5′-CCCTGACTTCTGTGCTAC -3′ and 5′-TCAAGGTTCTTTGGGTC-3′; β-actin, 5′-TGACCGGGTCACCCACACTGTGCCCATCTA-3′ and 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3′.
Adenoviruses and EC Infection.
The recombinant adenoviruses expressing a dominant-negative mutant of c-Jun (i.e., TAM67) and c-Jun, henceforth referred to as Ad-TAM67 and Ad-c-Jun, have been described (15). Ad-GFP was used as an infection control. Confluent ECs were infected with recombinant adenoviruses at the indicated multiplicity of infection and incubated for 24 h before experiments.
ChIP.
ChIP assays were performed as described (25). In brief, HUVECs were cross-linked and then sonicated, followed by immunoprecipitation (IP) with polyclonal anti-c-Jun (Santa Cruz Biotechnology). Normal IgG was used as an IP control, and the supernatant was an input control. After digestion with proteinase K, the resting DNA was extracted, and the sEH promoter containing the AP-1 consensus element was amplified by PCR with the primers 5′-AGCGTGCCTAGAGGAGTG-3′ and 5′- GGAATGCCTGGGAAAGAG-3′. The resulting DNA was resolved on 1% agarose gel and stained with ethidium bromide.
Animal Experiments.
All animal experimental protocols were approved by Peking University Institutional Animal Care and Use Committee. SHRs and Wistar rats [Vital River (Beijing, China) male, 180–280 g] were kept in 12-h-light—12-h-dark cycles at a controlled room temperature with free access to standard chow and tap water. To induce hypertension, four groups of rats (n = 6 in each group), SHRs, or Wistar rats drank water with or without 2% NaCl for 14 days. Systolic blood pressures were measured in conscious rats every 2 days by tail-cuff plethysmography. The plasma levels of Ang II after death were measured by use of an RIA kit (Furui, Beijing, China). For the experiment of Ang II infusion, an osmotic minipump [Alzet (Cupertino, CA) model 1003D] was implanted in the dorsal region of Wistar rats to deliver either Ang II at 450 ng/kg per min or PBS for 3 days. Four groups of rats (n = 6) were evaluated: vehicle-infused control, those fed losartan by oral gavage at 25 mg/kg per day for 9 days, Ang II-infused rats, and Ang II rats fed losartan for 9 days, with a minipump implanted at day 6 and Ang II infusion afterward for 3 days. The rats were killed, and abdominal aortic intima containing endothelium and media of VSMC layer were isolated separately and stored at −80°C until further use.
Immunohistochemistry.
The arterial tree was perfused by the left ventricle with PBS at a pressure of 100 mmHg and fixed with 4% paraformaldehyde. Cross sections 7 μm in thickness were prepared from OCT-embedded rat aortas. After endogenous peroxidase was quenched, and nonspecific reaction was blocked, the sections were immunostained with a rabbit–anti-sEH antibody and HRP-conjugated secondary antibody. Diaminobenzidine tetrahydrochloride was used for color development. The resulting images were acquired by using a digital camera. Negative controls were performed with the use of species-matched IgG.
Statistical Analysis.
Results are expressed as mean ± SD from at least three independent experiments. The significance of variability was determined by unpaired two-tailed Student's t test or ANOVA. Each experiment included triplicate measurements for each condition tested, unless indicated otherwise. P < 0.05 was considered to be statistically significant.
Acknowledgments
This study was supported in part by National Natural Science Foundation of China Grants 30470631, 30570713, and 30630032 (to Y.Z.), the Major National Basic Research Grant of China Grants 2006CB503802 (to Y.Z.) and 2006CB503906 (to N.W.), National Institutes of Health Grant HL77448 (to J.Y.S.), and National Institute of Environmental Health Sciences Grants ES02710, ES04699, and ES05707 (to B.H.).
Abbreviations
- EET
epoxyeicosatrienoic acid
- EH
epoxide hydrolase
- sEH
soluble EH
- Ang II
angiotensin II
- EC
endothelial cell
- HUVEC
human umbilical vein EC
- BAEC
bovine aortic EC
- SHR
spontaneously hypertensive rat
- AA
arachidonic acid
- VSMC
vascular smooth muscle cell
- DHET
dihydroxyeicosatrienoic acid.
Footnotes
Conflict of interest statement: B.D.H. founded Arête Therapeutics to develop sEH inhibitors.
References
- 1.Spector AA, Fang X, Snyder GD, Weintraub NL. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 2.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 3.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 4.Hu S, Kim HS. Eur J Pharmacol. 1993;230:215–221. doi: 10.1016/0014-2999(93)90805-r. [DOI] [PubMed] [Google Scholar]
- 5.Pokreisz P, Fleming I, Kiss L, Barbosa-Sicard E, Fisslthaler B, Falck JR, Hammock BD, Kim IH, Szelid Z, et al. Hypertension. 2006;47:762–770. doi: 10.1161/01.HYP.0000208299.62535.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiecker M, Liao JK. Arch Biochem Biophys. 2005;433:413–420. doi: 10.1016/j.abb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arand M, Cronin A, Oesch F, Mowbray SL, Jones TA. Drug Metab Rev. 2003;35:365–383. doi: 10.1081/dmr-120026498. [DOI] [PubMed] [Google Scholar]
- 9.Arand M, Wagner H, Oesch F. J Biol Chem. 1996;271:4223–4229. doi: 10.1074/jbc.271.8.4223. [DOI] [PubMed] [Google Scholar]
- 10.Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Hypertension. 2002;39:690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- 11.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, et al. Circ Res. 2000;87:992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Zhang Y, Schmelzer K, Lee TS, Fang X, Zhu Y, Spector AA, Gill S, Morisseau C, et al. Proc Natl Acad Sci USA. 2005;102:16747–16752. doi: 10.1073/pnas.0508081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Browatzki M, Larsen D, Pfeiffer CA, Gehrke SG, Schmidt J, Kranzhofer A, Katus HA, Kranzhofer R. J Vasc Res. 2005;42:415–423. doi: 10.1159/000087451. [DOI] [PubMed] [Google Scholar]
- 14.Wu S, Gao J, Ohlemeyer C, Roos D, Niessen H, Kottgen E, Gessner R. Free Radic Biol Med. 2005;39:1601–1610. doi: 10.1016/j.freeradbiomed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Wang N, Verna L, Hardy S, Zhu Y, Ma KS, Birrer MJ, Stemerman MB. Circ Res. 1999;85:387–393. doi: 10.1161/01.res.85.5.387. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X, Yamamoto T, Newman JW, Kim IH, Watanabe T, Hammock BD, Stewart J, Pollock JS, Pollock DM, Imig JD. J Am Soc Nephrol. 2004;15:1244–1253. [PubMed] [Google Scholar]
- 17.Shyy JY, Lin MC, Han J, Lu Y, Petrime M, Chien S. Proc Natl Acad Sci USA. 1995;92:8069–8073. doi: 10.1073/pnas.92.17.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jalali S, Li YS, Sotoudeh M, Yuan S, Li S, Chien S, Shyy JY. Arterioscler Thromb Vasc Biol. 1998;18:227–234. doi: 10.1161/01.atv.18.2.227. [DOI] [PubMed] [Google Scholar]
- 19.Sahar S, Dwarakanath RS, Reddy MA, Lanting L, Todorov I, Natarajan R. Circ Res. 2005;96:1064–1071. doi: 10.1161/01.RES.0000168210.10358.f4. [DOI] [PubMed] [Google Scholar]
- 20.Xu Q, Liu Y, Gorospe M, Udelsman R, Holbrook NJ. J Clin Invest. 1996;97:508–514. doi: 10.1172/JCI118442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Cheng J, Ma Y, Thomas W, Zhang J, Du J. Circ Res. 2005;97:975–982. doi: 10.1161/01.RES.0000190589.52286.41. [DOI] [PubMed] [Google Scholar]
- 22.Douillette A, Bibeau-Poirier A, Gravel SP, Clement JF, Chenard V, Moreau P, Servant MJ. J Biol Chem. 2006;281:13275–13284. doi: 10.1074/jbc.M512815200. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y, Lin JH, Liao HL, Friedli OJ, Verna L, Marten NW, Straus DS, Stemerman MB. Arterioscler Thromb Vasc Biol. 1998;18:473–480. doi: 10.1161/01.atv.18.3.473. [DOI] [PubMed] [Google Scholar]
- 24.McGee J, Fitzpatrick F. J Biol Chem. 1985;260:12832–12837. [PubMed] [Google Scholar]
- 25.Boyd KE, Farnham PJ. Mol Cell Biol. 1997;17:2529–2537. doi: 10.1128/mcb.17.5.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]