Abstract
Saccadic eye movements cause sudden and global shifts in the retinal image. Rather than causing confusion, however, eye movements expand our sense of space and detail. In macaques, a stable representation of space is embodied by neural populations in intraparietal cortex that redistribute activity with each saccade to compensate for eye displacement, but little is known about equivalent updating mechanisms in humans. We combined noninvasive cortical stimulation with a double-step saccade task to examine the contribution of two human intraparietal areas to transsaccadic spatial updating. Right hemisphere stimulation over the posterior termination of the intraparietal sulcus (IPSp) broadened and shifted the distribution of second-saccade endpoints, but only when the first-saccade was directed into the contralateral hemifield. By interleaving trials with and without cortical stimulation, we show that the shift in endpoints was caused by an enduring effect of stimulation on neural functioning (e.g., modulation of neuronal gain). By varying the onset time of stimulation, we show that the representation of space in IPSp is updated immediately after the first-saccade. In contrast, stimulation of an adjacent IPS site had no such effects on second-saccades. These experiments suggest that stimulation of IPSp distorts an eye position or displacement signal that updates the representation of space at the completion of a saccade. Such sensory-motor integration in IPSp is crucial for the ongoing control of action, and may contribute to visual stability across saccades.
Keywords: coordinate transformations, parietal cortex, spatial representation, transcranial magnetic stimulation, vision
Spatially directed behaviors, such as saccadic eye movements and reaching, require that the brain extracts the positions of objects from the available sensory information while taking into account the current or future positions of relevant body parts. At the same time, sensory inputs caused by self-movement must be distinguished from those arising from changes in the environment. A mechanism common to both of these abilities is the integration of sensory signals with internal copies of motor commands known as corollary discharge (1–11). In macaque posterior parietal cortex (PPC), for example, corollary discharge about impending saccades drives a coordinate transformation in which the internal representation of space is updated to compensate for eye displacement (7, 8). This “spatial updating” mechanism ensures that spatial codes for perception and action are not compromised by eye movements. Spatial updating occurs in the lateral intraparietal area (LIP) as well as several other cortical and subcortical regions of the monkey brain, including the frontal eye fields (FEF) (12), the parietal reach region (8, 13), extrastriate cortex (14), and the superior colliculus (15).
Functional MRI studies of the human intraparietal sulcus (IPS) have revealed changes in hemispheric activation that are consistent with the spatial updating mechanism identified in monkey PPC (16, 17). Because the blood oxygenation level-dependent signal is a correlational measure, however, it is not possible for these studies to distinguish activation that directly contributes to spatial updating from that which merely reflects its outcome, such as activation subserving subsequent directed attention or planned action (18–21). In contrast, spatial updating mechanisms may be isolated by stimulating neural populations along the IPS with transcranial magnetic stimulation (TMS) and measuring the consequent disturbances in spatial representation across saccades. In addition to permitting causal inferences, TMS can be applied at specific times relative to a saccade to probe the neural time course of the underlying coordinate transformation (22).
In the current study, we stimulated the human IPS of the right hemisphere with TMS and measured effects on spatial updating using a variant of the “double-step saccade” task (Fig. 1a). This behavioral paradigm (23–25) has been used extensively to study spatial updating in monkeys and humans and requires subjects to perform a sequence of two saccades to sequentially flashed targets (23–25). Because the second target (T2) is extinguished before the saccade to the first target (T1), the memory trace of T2 must be updated after the first-saccade to compensate for the change in eye position. The endpoint of the second-saccade thus provides a behavioral probe into the transsaccadic updating mechanism.
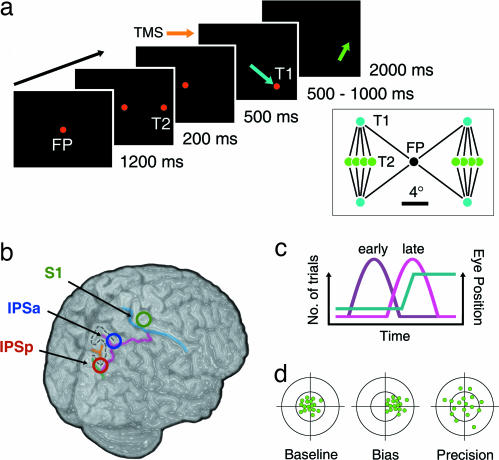
Fig. 1.
Schematic of the double-step saccade paradigm, sites of cortical stimulation, timing of stimulation, and possible effects of stimulation on second-saccade error. (a) Sequence of displays from a typical trial of the double-step saccade task used to measure spatial updating in Experiment 1. At the onset of T1, observers performed a 10° saccade from the central fixation point to T1 (blue arrow). At the offset of T1, a second-saccade (≈6°) was made to the remembered location of T2 (green arrow). The second-saccade was made in complete darkness (i.e., without feedback). (Inset) All 16 unique double-step saccade sequences (FP, fixation point; T1, first-saccade target; T2, second-saccade target). (b) TMS was applied to one of three cortical sites in the right hemisphere in each session, IPSp (red annulus), IPSa (blue annulus), and S1 (green annulus). The highlighted sulci and stippled “S” illustrate the major neuroanatomical landmarks used to identify stimulation sites in each participant's MR brain scan. Mean MNI coordinates (x, y, z ±1 SD) for IPSp, IPSa, and S1 were [27 ± 4, −84 ± 7, 48 ± 8], [30 ± 6, −67 ± 8, 62 ± 5], and [28 ± 5, −31 ± 5, 75 ± 5], respectively. (c) Timing of cortical stimulation relative to the first saccade (cyan line). A train of three TMS pulses (100 ms) was targeted to either the final 100 ms before the onset of the first saccade (“early,” purple line) or the first 100 ms after the offset of the first saccade (“late,” pink line). TMS was timed according to the predicted rather than the actual onset or offset of the first saccade. Variability in saccadic latency generated a bimodal distribution of TMS onset times. One-third of the trials did not involve cortical stimulation (“nonstimulation”). All double-step sequences and TMS timing conditions were interleaved within a session. (d) Predicted effects of TMS on second-saccades for a brain region involved in spatial updating. The distribution of second-saccade endpoints is used as a proxy for the coordinate transformation. The left schematic shows a theoretical distribution of second-saccade endpoints during the baseline condition in which TMS was applied to S1. Disrupting the spatial updating mechanism with TMS of the IPS could bias the coordinate transformation (central schematic) and/or alter its precision (right schematic).
We targeted two regions along the IPS that, like macaque area LIP, are involved in attention and eye movement control (19, 21, 26, 27): the dorsomedial bank of the IPS/transverse occipital sulcus junction (posterior IPS; IPSp), an anatomical region in the vicinity of visual areas V3a and V7 (26, 28), and a site located further along the sulcus in the rostral direction, where the IPS branches medially (anterior IPS; IPSa) (Fig. 1b). Cortical stimulation was applied either before or after the first-saccade (Fig. 1c). If a population of cortical neurons is necessary for updating the memory trace of T2 in the double-step saccade task, then stimulation of that brain site should influence performance of second-saccades (Fig. 1d). Crucially, and in contrast to a conceptually related study (29), we compared the behavioral effects of IPS stimulation with a control site in which stimulation was applied over medial primary somatosensory cortex (S1). We show that stimulation of IPSp introduces both bias and random noise into the coordinate transformation that underlies spatial updating across saccades. Furthermore, we show that spatial updating in IPSp occurs immediately after completion of the saccade. Additional control experiments confirmed that the behavioral effects of IPSp stimulation are specific to oculomotor tasks that require spatial updating.
Results
The traditional version of the double-step saccade task has some key disadvantages. First, T2 is presented for a very short duration just before onset of the first-saccade, thus reducing the quality of its encoded position. Second, because the targets are delivered close in time, the brain has available an explicit retinal signal of the vector from T1 to T2. Spatial updating mechanisms would be less important if this vector were used to direct the second-saccade. To circumvent these limitations, we used a variant of the traditional task in which T2 was presented prior to T1 (Fig. 1a). This manipulation was first used in monkeys (30) and has the advantage of allowing T1 and T2 to be separated in time. Despite this manipulation, neither the first- nor the second-saccade could be preplanned because the location of T1 could not be predicted.
A short train of TMS pulses (100 ms, three pulses) was applied around the time of the first-saccade to disrupt putative neural mechanisms that update the remembered location of T2. The first TMS pulse was delivered either 100 ms before saccade onset (“early” trials), or at saccade offset (“late” trials; Fig. 1c). Trials in which no cortical stimulation was applied were also interleaved. These “nonstimulation” trials were necessary because repetitive TMS frequently causes changes in cortical functioning that outlast the period of direct stimulation (31–34). Because nonstimulation trials are free from transient disruptive effects, they provide an uncontaminated measure of the aftereffects of stimulation on cortical representation and behavior.
For the main analysis, the data were organized according to the site of stimulation (IPSp, IPSa, or S1), the direction of the first-saccade (contralateral or ipsilateral to the stimulated hemisphere), and the timing of cortical stimulation (early, late, or nonstimulation). Trials in which the first-saccade was directed above and below the horizontal meridian were combined. Furthermore, data from each of the four possible T2 locations in each hemispace were separately pooled and treated as a single sequence.
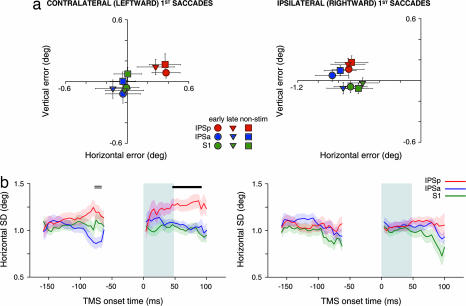
To assess whether cortical stimulation biased the coordinate transformation, the mean error of second-saccade endpoints was examined for each brain site (Fig. 2a). Stimulation of IPSp during contralateral (leftward) first-saccades caused a clear, predominantly rightward, shift in the endpoints of the second-saccades compared with stimulation of the other sites (both P < 0.01; Fig. 2a Left). This effect did not occur for sequences involving an ipsilateral (rightward) first-saccade (both P > 0.05 for Fig. 2a Right; F (2, 30) = 4.53, P < 0.05 for the cross-panel interaction between stimulation site and direction). Interestingly, the magnitude of the rightward shift for IPSp compared with IPSa and S1 was comparable for early, late, and nonstimulation trials. The rightward shift therefore reflects an enduring effect of TMS on the activity of IPSp neurons. Indeed, long-lasting effects of TMS on cortical neurons are well documented and thought to reflect molecular and cellular changes akin to long-term potentiation (LTP) and long-term depression (LTD) (22, 31–34). Furthermore, the rightward shift was similar irrespective of whether the second-saccade was directed contraversively or ipsiversively with respect to the location of T1 [see supporting information (SI) Fig. 4]. The specificity of the rightward shift for IPSp was highly consistent across the group: the mean horizontal endpoint position during IPSp stimulation was rightward compared with IPSa, and with S1, in 12 of 16 and 13 of 16 participants respectively.
Fig. 2.
Second-saccade performance in the double-step saccade task of Experiment 1. Data are plotted separately for trials in which the first-saccade was directed leftward (i.e., contralateral to the side of stimulation; Left) and rightward (ipsilateral to the side of stimulation; Right). (a) Mean second-saccade endpoint error for early, late, and nonstimulation conditions (circles, triangles, and squares, respectively) and each stimulation site (colors). Error bars represent ±1 SEM. For the purposes of pooling and plotting the data, all sequences involving an upward first-saccade were reflected along the horizontal meridian. Stimulation of IPSp caused a rightward bias in second-saccade endpoints but only on trials in which the first-saccade was directed into the contralateral (left) visual field. The shift was independent of the timing or presence of TMS on any given trial, reflecting a lasting effect of TMS on the spatial updating mechanism. (b) Horizontal dispersion (SD) of second-saccade endpoints as a function of TMS onset time for each site of stimulation. Each site is represented by a unique color (see legend) with shading to indicate ±1 SEM. The gray shaded region indicates the time of the first-saccade. Stimulation of IPSp at or shortly after the offset of contralateral first-saccades significantly increased the horizontal dispersion of the second-saccade distribution compared with both IPSa and S1 stimulation (solid black line). Although IPSp and IPSa differed significantly just before saccade onset (double line), neither site differed significantly from S1. Sections of the time course in which there were insufficient data to provide reliable estimates of dispersion are omitted.
A rightward shift in second-saccade endpoints is equivalent to an overcompensation for the change in horizontal eye position associated with the first-saccade. Overcompensation would be expected if the brain used an exaggerated representation of eye position or displacement to update the memory trace of T2. To quantify this effect further, the magnitude of the horizontal shift with respect to S1 (0.35°, averaged over early, late, and nonstimulation conditions) can be expressed as a percentage of the actual horizontal displacement of the eye during the first-saccade (M = 7.65°, SEM = 0.08°). This analysis indicates that second-saccades during IPSp stimulation overcompensated for the contralateral first-saccade by ≈5% of the actual eye displacement.
There was also a small upward shift in second-saccade endpoints during IPSp stimulation compared with IPSa and S1 [F (2, 30) = 5.21, P < 0.05, both comparisons P < 0.05], but, unlike the horizontal component, this effect did not depend on the direction of the first-saccade. Finally, the endpoint error of second-saccades during ipsilateral double-step sequences was more leftward than that observed during contralateral sequences for all stimulation sites, including the S1 baseline. This site-independent bias in performance most likely reflects an influence of the experimental configuration, in which the stimulating coil was always located over right scalp locations; its existence highlights the importance of including a control stimulation site over the same hemisphere, as we did in our study.
Next we examined whether stimulation affected the precision of the coordinate transformation. Precision in this context refers to how consistently the updating mechanism operates from trial to trial. Therefore, each participant's horizontal and vertical SD of the endpoint distributions were used as dispersion measures for the second-saccades. Unlike the bias effect reported above, a preliminary inspection of the dispersion data suggested a clear dependence on the timing of cortical stimulation. Fig. 2b shows the horizontal dispersion of second-saccade endpoints as a function of the time between the onset of TMS and the first-saccade. Horizontal and vertical endpoint SDs were calculated for all of the trials in which TMS onset fell within a moving window of 60 ms. The window started well before the onset of the first-saccade and was moved until well after saccade offset. The mean SD was calculated at each time point to provide a group dispersion time course. This analysis was undertaken separately for each participant, stimulation site, and direction of first-saccade.
Stimulation of IPSp significantly increased the horizontal dispersion of second-saccade endpoints compared with the other sites. This effect was observed exclusively on trials in which the first-saccade was directed into contralateral hemispace. Strikingly, this effect on precision occurred only when TMS was applied within ≈40 ms of first-saccade offset (Fig. 2b; t tests: P < 0.05). The precision deficit caused by IPSp stimulation was also highly consistent across participants: the mean SD for IPSp during this period was larger than for S1 and IPSa in 12 of 16 participants.
The precision effect was quantified further by expressing the mean increase in dispersion compared with S1 (0.27°) as a percentage of the mean S1 dispersion during this time window (1.02°). This measure indicated that stimulating IPSp increased the horizontal dispersion of second-saccade endpoints by 27%. Note that the site and directional specificity of this effect is consistent with the bias effect of IPSp stimulation reported in the previous section. The additional temporal specificity of this precision effect is important because it indicates the time at which the coordinate transformation is implemented. The same analyses revealed no significant effects of cortical stimulation on the vertical dispersion of second-saccade endpoints (all P > 0.05; data not shown).
In sum, we have shown that stimulation of right IPSp causes a rightward shift in the endpoints of second-saccades that is independent of the time of stimulation as well as an increase in dispersion that is time-locked to the offset of the first-saccade. Both of these effects occur only when the first-saccade is directed into the contralateral (left) hemispace. These findings suggest that IPSp stimulation affects spatial updating by disturbing the integration of corollary information about the first-saccade. Corollary discharge, however, is just one component of the coordinate transformation. Moreover, performing the second-saccade requires more than just spatial updating mechanisms.
In the following sections, we rule out several alternative explanations for our findings. First, we show that IPSp stimulation does not have a differential effect on the temporal or spatial characteristics of the first-saccade. Second, we present data from a control experiment demonstrating that IPSp stimulation does not disrupt the retinotopic memory trace of T2 (Experiment 2). Finally, we use data from a further control experiment to rule out the possibility that IPSp stimulation causes a spatial or oculomotor bias in saccade planning or execution from contralateral eye positions (Experiment 3).
First-Saccades Are Not Affected by IPSp Stimulation.
The mean and dispersion of first-saccade endpoints were examined to rule out the possibility that the effects of IPSp stimulation on second-saccades were merely sequelae of effects on contralateral first-saccades (SI Fig. 5). The mean and dispersion of contralateral first-saccade endpoints for IPSp, IPSa, and S1 did not differ for any of the stimulation timing conditions (all P > 0.05). We also examined the amplitude, peak velocity, latency, and duration of contralateral first-saccades as a function of the time between TMS onset and first-saccade onset. First-saccades during IPSp stimulation did not differ from the other stimulation sites for any of these variables at any time point (all P > 0.05; SI Fig. 6). The differences in second-saccade endpoints cannot, therefore, be explained by a differential effect of IPSp stimulation on first-saccades compared with the other sites.
The Memory Trace of T2 Is Not Affected by IPSp Stimulation.
The transformation that updates the location of T2 in eye-centered coordinates can be conceptualized as the vector combination of two quantities: the memory trace of the position of T2 in retinal coordinates, as seen during initial fixation, and a corollary discharge that represents the eye displacement caused by the first-saccade (1–6). We have attributed the effects of IPSp stimulation on second-saccade endpoints to a change in the internal representation of eye displacement, but, in principle, a change in either quantity could affect the postsaccadic retinal coordinates of T2. We therefore conducted a control experiment to measure the effect of IPSp stimulation on the stored retinal coordinates of T2.
In this task, participants ignored the onset of T1 and performed a single saccade to the remembered location of T2. The design for Experiment 2 was otherwise comparable with Experiment 1, including the time between presentation of T2 and the saccade toward its remembered location, the timing and intensity of TMS, and the temporal flow of display events. The requirement to remember the location of T2 was therefore identical to Experiment 1, but, because there was no eye displacement caused by a saccade to T1, spatial updating was not required. If the effects on second-saccades in Experiment 1 occurred because IPSp stimulation affected the memory trace of contralateral targets in retinal coordinates, changes in the saccade endpoints would also be expected in this task. Alternatively, if the effects on second-saccades instead reflected a distorted internal representation of the first-saccade, as we have suggested, there should be no effect of IPSp stimulation on performance of single saccades to T2.
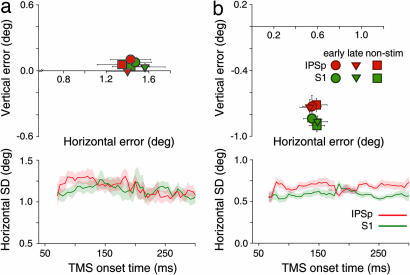
The mean and dispersion of the saccade endpoint distributions were used to measure the quality of the T2 memory trace. Fig. 3a shows that the mean and horizontal dispersion of the endpoint distributions for contralateral saccades during IPSp and S1 stimulation were equivalent in this task (all P > 0.05). There were also no significant effects of cortical stimulation on the vertical dispersion of second-saccade endpoints (all P > 0.05; data not shown). Experiment 2 thus shows that the rightward shift and increase in dispersion for contralateral saccade sequences in Experiment 1 cannot be attributed to stimulation effects on the retinotopic memory trace of T2.
Fig. 3.
Performance for single-step saccades in the control experiments. (a) Results of Experiment 2, which measured performance during stimulation of IPSp for single saccades directed contralaterally from the central fixation point to T2. (Upper) Mean saccade endpoints for IPSp and S1, plotted in the same format as in Fig. 2a. (Lower) horizontal dispersion of saccade endpoints for IPSp and S1, plotted in the same format as in Fig. 2b, except that TMS onset time is plotted relative to the onset of T1. (Ipsilateral saccade data not shown.) Performance during IPSp stimulation did not differ significantly from performance during stimulation of S1. Error bars and shading represent ±1 SEM. (b) Results of Experiment 3, which measured performance during stimulation of IPSp for single saccades directed from T1 to T2 within the contralateral hemispace. Data are presented in the same format as in a. Saccades during stimulation of IPSp and S1 were not significantly different.
For both sites, the horizontal error in this single-step task was greater than that observed in the double-step task of Experiment 1 (compare Figs. 2a and 3a). Superficially, this result seems counterintuitive but is readily explained by the fact that the required leftward saccade in Experiment 2 was large (≈6–9°) and exclusively horizontal. By contrast, the required saccade to T2 in Experiment 1 was approximately vertical and was equally often directed leftward and rightward with respect to T1.
Saccades from Contralateral Eye Positions Are Not Affected by IPSp Stimulation.
A saccade to T1 in the double-step task introduces the need to update the memory trace of T2, but it also requires the second-saccade to originate from a contralateral eye position. Eye-in-orbit position modulates the activity of neurons in area LIP and many other attentional and oculomotor areas of the monkey (35–39). If IPSp stimulation disturbed neurons with similar properties, this could have effects on saccade planning or memory that depend on eye position and might thus explain the effects on second-saccades observed in the double-step saccade task. This possibility was tested in a further control experiment (Experiment 3).
The task required a single memory-guided saccade to T2, but, rather than fixating centrally at the start of a trial, as in the previous two experiments, T1 was used as the initial fixation point. The saccade required in this task was therefore identical to the second-saccade in Experiment 1, but because no saccade intervened between the appearance of T2 and the saccade toward it, there was no need to update the memory trace of T2. If the bias and dispersion effects observed in the double-step task were caused by effects of IPS stimulation on planning saccades from contralateral eye positions, then such effects should also be evident in this control task. Fig. 3b shows the mean error (Upper) and horizontal dispersion (Lower) of the endpoint distribution for contralateral saccades for IPSp and S1. Single saccades originating from T1 during IPSp stimulation were not significantly different from those during S1 stimulation, even though the saccade required in this task was identical to the second-saccade in the double-step experiment (P > 0.05). Thus, the bias and precision effects observed in Experiment 1 cannot be explained by effects of IPSp stimulation on executing memory-guided saccades from a contralateral eye position.
Discussion
This study investigated the neural bases of spatial updating across saccades by using noninvasive cortical stimulation (TMS) and a double-step saccade task. Stimulation over the posterior termination of the right IPS (IPSp) during contralateral (leftward) saccades impaired spatial updating of a remembered target location. Specifically, participants overcompensated for the leftward horizontal displacement of the eye caused by the first-saccade, as shown by a rightward shift in the endpoints of the second-saccades. Stimulation of IPSp also increased the dispersion of second-saccade endpoints, but only when TMS was applied immediately after the offset of the first-saccade. Crucially, neither of these effects was observed when TMS was applied to an alternative IPS site located just 22 mm further along the sulcus in the rostral direction (based on the difference in mean MNI coordinates for IPSp and IPSa). Moreover, we showed that the effects on second-saccades were not merely a consequence of changes in the execution of first-saccades; nor were they attributable to a disruption of the memory trace of T2 in retinal coordinates; nor were saccades from T1 to the remembered location of T2 affected when there was no preceding saccade. Taken together, these experiments indicate that stimulation of IPSp introduces bias and random noise into the coordinate transformation that underlies spatial updating across saccades.
Only one previous study has combined TMS and a double-step saccade task to examine spatial updating in human PPC (29). The data from this former study were interpreted as evidence for a time-locked involvement of the PPC in spatial updating; however, this conclusion is subject to two important criticisms. First, the authors applied TMS to a single parietal site only with a relatively nonfocal circular coil; hence, it cannot be determined whether the findings arose directly from PPC stimulation or were merely a nonspecific consequence of right hemisphere disruption. More critically, however, this former study did not assess the influence of TMS on second-saccades in the absence of any requirement for spatial updating. It is therefore unclear whether stimulation of the PPC influenced spatial updating, or simply affected the planning or execution of second-saccades in the double-step task. Indeed, consistent with this latter conclusion is the authors' observation that the largest saccadic deficit occurred when TMS was applied just before the onset of ipsilateral second-saccades (<100 ms). Our observed effects of IPS stimulation on the dispersion of the second-saccade endpoints suggest instead that spatial updating occurs immediately after the offset of the first-saccade, well before the onset of the second-saccade (>500 ms).
In contrast to the transient effects of IPSp stimulation on saccadic dispersion, the rightward bias in second-saccade endpoints occurred independently of the timing and presence of stimulation on any given trial. This observation implies a modulation of neural processing that outlasts the direct period of cortical stimulation and endures sufficiently to propagate across trials (i.e., >5 s). Such aftereffects are common in repetitive TMS protocols and are attributed to changes in the excitability of neurons in the stimulated region and/or connected structures (31–34). Animal studies suggest that such changes in excitability may be traced to long lasting modulatory effects of TMS on synaptic transmission, gene expression, and neurotransmitter functioning (33, 34).
The finding that second-saccades overcompensated for leftward first-saccades suggests that an exaggerated representation of contralateral eye position or displacement is used in the transsaccadic coordinate transformation. In humans and monkeys, it remains controversial as to which of these potential sources of extraretinal information is used to update the internal representation of space. Indeed, the primary characteristic that divides current models of spatial updating is whether the transformation is considered to be driven by an eye-in-orbit position signal or a position-free eye displacement signal (7, 11, 12, 38, 40–42). Our results cannot distinguish between these two possibilities because the direction of eye displacement and the final eye-in-orbit position covaried in the saccade sequences used here. Future studies could identify the crucial extraretinal signal by exploring which of these variables determines the behavioral effects of TMS as reported here. This could be achieved by adding a manipulation of eye position to the double-step task. A similar approach has been used to study spatial updating in monkeys during inactivation of the PPC (30) and in patients with damage to the parietal cortex (43).
More broadly, our findings have implications for understanding disorders of spatial representation associated with parietal damage, such as unilateral neglect (44) and optic ataxia (45). Several investigators have suggested that deficient transsaccadic spatial updating mechanisms contribute to the symptoms observed in these patients (43, 46, 47). Because unilateral neglect is particularly common after damage to the parietal lobe of the right hemisphere (48), our findings provide a plausible neuroanatomical substrate for these deficits. Whether similar hemispheric asymmetries exist for the stimulation effects reported here remains to be determined.
In sum, we have demonstrated a crucial role for the posterior intraparietal area in updating representations of space across saccades, a characteristic that, in our view, asserts this cortical area as the most probable of several recently identified potential homologs of monkey LIP (19–21, 26, 27).
Experimental Procedures
Participants.
Sixteen right-handed volunteers participated in each of the three experiments. Thirteen of the 16 participants from Experiment 1 also completed both control experiments. The mean age was 25.4 years for Experiment 1 (11 males) and 26.6 years for the control experiments (12 males). All participants had normal or corrected-to-normal vision and gave informed written consent before participation. All aspects of this research were approved by the Human Research Ethics Committee at the University of Melbourne.
Stimuli and Procedure.
Stimuli were presented on a γ-corrected 19-in CRT monitor (frame rate, 120 Hz) and viewed from a distance of 53 cm. All fixation and target stimuli were red spots (diameter, 0.3°) presented against a uniform black background. A red filter (71° × 71°) placed over the screen ensured that the faint luminance of the background and edges of the CRT image could not be seen. All saccade tasks were therefore performed without any visual references other than fixation and target stimuli.
The double-step task used in Experiment 1 is shown in Fig. 1a. The same sequence of stimuli was used for Experiment 2 except that the central fixation was extinguished at the same time as T1, which cued the participants to execute a saccade to the remembered location of T2. In Experiment 3, each trial began with a fixation stimulus located in one of the four positions used for T1 in Experiment 1. The central stimulus used as the initial fixation point in the first two experiments remained in Experiment 3, as shown in Fig. 1a, but it was irrelevant to the participant's task.
Eye position was sampled at 240 Hz by using an ASL 504 remote infrared eye tracker (ASL, Bedford, MA) and custom software. The eye tracker was calibrated before each block of 24 trials if necessary. A chin rest was used to prevent head movement.
TMS and MRI Parameters.
Stimulation sites were identified on an individual basis before participation in the behavioral sessions by using T1-weighted MR brain scans. Sites of stimulation were defined by their position with respect to sulcal landmarks (Fig. 1b). Standardized coordinates were obtained for each stimulation site by spatially normalizing each participant's anatomical image to the Montreal Neurological Institute template by using SPM2 software (Wellcome Department of Imaging Neuroscience, London, U.K.; www.fil.ion.ucl.ac.uk). Note that normalization was performed only after completion of the experiment for the purpose of relating the stimulated sites to other TMS and fMRI studies; it was not used to identify cortical loci or position the TMS coil.
TMS was delivered by using a Magstim Rapid system (2.2 T; Magstim, Whitland, U.K.) and a 70-mm figure-of-eight induction coil held in position on the scalp surface by a clamp and tripod. The intensity of stimulation was set to the maximum comfortable level, expressed as a percentage of motor threshold, and adjusted for each site to control for differences in the distance between the scalp and cortex (49). This protocol yielded a mean TMS intensity that was 104% of the distance-adjusted motor threshold (62% of maximum stimulator output, 1.36 T). Consecutive testing sessions were separated by at least 24 h.
For the double-step saccade task in Experiment 1, TMS was timed according to the predicted onset or offset of the first-saccade, rather than at fixed times relative to a display event. Predictions for leftward and rightward first-saccades were generated independently by using an exponentially weighted average of previous saccade latencies. This procedure ensured that the distribution of TMS onset times would be comparable within and between different testing sessions. For the control tasks in Experiments 2 and 3, in which there was a single saccade to T2 only, TMS was timed on each trial according to a randomly selected sample from the participant's first-saccade latency distribution from Experiment 1. This “virtual saccade” ensured that the timing of stimulation in the three experiments was comparable. Data from a representative participant were used for the three participants that did not participate in Experiment 1.
Data Analysis.
Eye position data were filtered offline by using a nonlinear exponential smoothing algorithm (50). Saccades were automatically detected by using a combination of velocity (>40°/s) and acceleration (a period of positive and negative acceleration >1,000°/s2) criteria. The output of the algorithm for each trial was inspected visually for accuracy. Trials that contained blinks, incorrect behavioral responses (e.g., breaking fixation, anticipatory saccades) or other artefacts were rejected (SI Table 1). Early and late TMS conditions were obtained by parsing the bimodal distribution of TMS onset times into two separate clusters. The tails of each cluster were truncated to remove sections of the time courses where parameter estimates were unreliable. Only the central 100-ms period of each cluster was analyzed.
Mean endpoint error of saccades was analyzed for each experiment by using repeated measures ANOVAs (α = 0.05) with factors of stimulation site (IPSp, IPSa, S1), direction/hemispace of (first) saccade (leftward, rightward), and TMS condition (early, late, nonstimulation; n = 16). One participant was removed from the analyses for Experiment 2 because his mean endpoint error was almost 3 SD beyond the group mean. Simple main effects were examined by using pairwise comparisons with Bonferroni correction where appropriate. The dispersion of saccade endpoints was analyzed as a function of TMS onset time and stimulation site. Time points where the stimulated sites differed significantly were identified by using ensembles of t tests with the α-level adjusted to ensure that the average false-discovery rate in the early and late periods was not >0.05 (51).
Additional details of methodology and data analysis are provided in SI Text.
Acknowledgments
We thank Marc Sommer, Yves Rossetti, and Marc Kamke for comments on the manuscript and Jacqueline Snow, Charles Liu, Ian Gould, and Geoff Stuart for helpful discussions. This research was supported by the National Health and Medical Research Council (J.B.M. and C.D.C.).
Abbreviations
- IPS
intraparietal sulcus
- IPSa
anterior intraparietal sulcus
- IPSp
posterior intraparietal sulcus
- PPC
posterior parietal cortex
- S1
primary somatosensory cortex
- TMS
transcranial magnetic stimulation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610508104/DC1.
References
- 1.von Helmholtz H. Helmholtz's Treatise on Physiological Optics. Bristol, UK: Thoemmes; 2000. [Google Scholar]
- 2.von Holst E. B J Anim Behav. 1954;2:89–94. [Google Scholar]
- 3.Sperry RW. J Comp Physiol Psychol. 1950;43:482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- 4.Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. J Neurophysiol. 1985;54:714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- 5.Gnadt JW, Andersen RA. Exp Brain Res. 1988;70:216–220. doi: 10.1007/BF00271862. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg ME, Bruce CJ. J Neurophysiol. 1990;64:489–508. doi: 10.1152/jn.1990.64.2.489. [DOI] [PubMed] [Google Scholar]
- 7.Duhamel JR, Colby CL, Goldberg ME. Science. 1992;255:90–92. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- 8.Batista AP, Buneo CA, Snyder LH, Andersen RA. Science. 1999;285:257–260. doi: 10.1126/science.285.5425.257. [DOI] [PubMed] [Google Scholar]
- 9.Sommer MA, Wurtz RH. J Neurophysiol. 2004;91:1381–1402. doi: 10.1152/jn.00738.2003. [DOI] [PubMed] [Google Scholar]
- 10.Sommer MA, Wurtz RH. J Neurophysiol. 2004;91:1403–1423. doi: 10.1152/jn.00740.2003. [DOI] [PubMed] [Google Scholar]
- 11.Sommer MA, Wurtz RH. Nature. 2006;444:374–377. doi: 10.1038/nature05279. [DOI] [PubMed] [Google Scholar]
- 12.Umeno MM, Goldberg ME. J Neurophysiol. 2001;86:2344–2352. doi: 10.1152/jn.2001.86.5.2344. [DOI] [PubMed] [Google Scholar]
- 13.Batista AP, Andersen RA. J Neurophysiol. 2001;85:539–544. doi: 10.1152/jn.2001.85.2.539. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura K, Colby CL. Proc Natl Acad Sci USA. 2002;99:4026–4031. doi: 10.1073/pnas.052379899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker MF, Fitzgibbon EJ, Goldberg ME. J Neurophysiol. 1995;73:1988–2003. doi: 10.1152/jn.1995.73.5.1988. [DOI] [PubMed] [Google Scholar]
- 16.Merriam EP, Colby CL, Thulborn KR, Luna B, Olson CR, Sweeney JA. NeuroImage. 2001;13:794–800. doi: 10.1006/nimg.2000.0742. [DOI] [PubMed] [Google Scholar]
- 17.Medendorp WP, Goltz HC, Vilis T, Crawford JD. J Neurosci. 2003;23:6209–6214. doi: 10.1523/JNEUROSCI.23-15-06209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kastner S, Ungerleider LG. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- 19.Schluppeck D, Glimcher P, Heeger DJ. J Neurophysiol. 2005;94:1372–1384. doi: 10.1152/jn.01290.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silver MA, Ress D, Heeger DJ. J Neurophysiol. 2005;94:1358–1371. doi: 10.1152/jn.01316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sereno MI, Pitzalis S, Martinez A. Science. 2001;294:1350–1354. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- 22.Walsh V, Cowey A. Nat Rev Neurosci. 2000;1:73–79. doi: 10.1038/35036239. [DOI] [PubMed] [Google Scholar]
- 23.Hallett PE, Lightstone AD. Vision Res. 1976;16:107–114. doi: 10.1016/0042-6989(76)90084-5. [DOI] [PubMed] [Google Scholar]
- 24.Mays LE, Sparks DL. J Neurophysiol. 1980;43:207–232. doi: 10.1152/jn.1980.43.1.207. [DOI] [PubMed] [Google Scholar]
- 25.Becker W, Jurgens R. Vision Res. 1979;19:967–983. doi: 10.1016/0042-6989(79)90222-0. [DOI] [PubMed] [Google Scholar]
- 26.Tootell RB, Hadjikhani N, Hall EK, Marrett S, Vanduffel W, Vaughan JT, Dale AM. Neuron. 1998;21:1409–1422. doi: 10.1016/s0896-6273(00)80659-5. [DOI] [PubMed] [Google Scholar]
- 27.Koyama M, Hasegawa I, Osada T, Adachi Y, Nakahara K, Miyashita Y. Neuron. 2004;41:795–807. doi: 10.1016/s0896-6273(04)00047-9. [DOI] [PubMed] [Google Scholar]
- 28.Tootell RB, Mendola JD, Hadjikhani NK, Ledden PJ, Liu AK, Reppas JB, Sereno MI, Dale AM. J Neurosci. 1997;17:7060–7078. doi: 10.1523/JNEUROSCI.17-18-07060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Donkelaar P, Muri R. Proc R Soc London Ser B. 2002;269:735–739. doi: 10.1098/rspb.2001.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li CS, Andersen RA. Exp Brain Res. 2001;137:45–57. doi: 10.1007/s002210000546. [DOI] [PubMed] [Google Scholar]
- 31.Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Brain. 1994;117(Pt 4):847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- 32.Siebner HR, Rothwell J. Exp Brain Res. 2003;148:1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- 33.Levkovitz Y, Marx J, Grisaru N, Segal M. J Neurosci. 1999;19:3198–3203. doi: 10.1523/JNEUROSCI.19-08-03198.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Wang X, Scheich H. NeuroReport. 1996;7:521–525. doi: 10.1097/00001756-199601310-00035. [DOI] [PubMed] [Google Scholar]
- 35.Andersen RA, Bracewell RM, Barash S, Gnadt JW, Fogassi L. J Neurosci. 1990;10:1176–1196. doi: 10.1523/JNEUROSCI.10-04-01176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersen RA, Mountcastle VB. J Neurosci. 1983;3:532–548. doi: 10.1523/JNEUROSCI.03-03-00532.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snyder LH. Curr Opin Neurobiol. 2000;10:747–754. doi: 10.1016/s0959-4388(00)00152-5. [DOI] [PubMed] [Google Scholar]
- 38.Andersen RA, Buneo CA. Annu Rev Neurosci. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- 39.Andersen RA, Snyder LH, Li CS, Stricanne B. Curr Opin Neurobiol. 1993;3 doi: 10.1016/0959-4388(93)90206-e. [DOI] [PubMed] [Google Scholar]
- 40.Bruce CJ, Goldberg ME. J Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- 41.Zipser D, Andersen RA. Nature. 1988;331:679–684. doi: 10.1038/331679a0. [DOI] [PubMed] [Google Scholar]
- 42.Xing J, Andersen RA. J Neurophysiol. 2000;84:651–665. doi: 10.1152/jn.2000.84.2.651. [DOI] [PubMed] [Google Scholar]
- 43.Heide W, Sprenger A, Sackerer B, Rottach K, Gaebel C, Kompf D. Ann NY Acad Sci. 2003;1004:465–468. [Google Scholar]
- 44.Driver J, Mattingley JB. Nat Neurosci. 1998;1:17–22. doi: 10.1038/217. [DOI] [PubMed] [Google Scholar]
- 45.Rossetti Y, Pisella L, Vighetto A. Exp Brain Res. 2003;153:171–179. doi: 10.1007/s00221-003-1590-6. [DOI] [PubMed] [Google Scholar]
- 46.Pisella L, Mattingley JB. Neurosci Biobehav Rev. 2004;28:181–200. doi: 10.1016/j.neubiorev.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Khan AZ, Pisella L, Vighetto A, Cotton F, Luaute J, Boisson D, Salemme R, Crawford JD, Rossetti Y. Nat Neurosci. 2005;8:418–420. doi: 10.1038/nn1425. [DOI] [PubMed] [Google Scholar]
- 48.Vallar G, Perani D. Neuropsychologia. 1986;24:609–622. doi: 10.1016/0028-3932(86)90001-1. [DOI] [PubMed] [Google Scholar]
- 49.Stokes MG, Chambers CD, Gould IC, Henderson TR, Janko NE, Allen NB, Mattingley JB. J Neurophysiol. 2005;94:4520–4527. doi: 10.1152/jn.00067.2005. [DOI] [PubMed] [Google Scholar]
- 50.Goot RE, Mahlab U, Cohen R. HAIT J Sci Eng. 2007 in press. [Google Scholar]
- 51.Benjamini Y, Hochberg Y. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]