Abstract
Entrainment of the circadian pacemaker to the light:dark cycle is necessary for rhythmic physiological functions to be appropriately timed over the 24-h day. Nonentrainment results in sleep, endocrine, and neurobehavioral impairments. Exposures to intermittent bright light pulses have been reported to phase shift the circadian pacemaker with great efficacy. Therefore, we tested the hypothesis that a modulated light exposure (MLE) with bright light pulses in the evening would entrain subjects to a light:dark cycle 1 h longer than their own circadian period (τ). Twelve subjects underwent a 65-day inpatient study. Individual subject's circadian period was determined in a forced desynchrony protocol. Subsequently, subjects were released into 30 longer-than-24-h days (daylength of τ + 1 h) in one of three light:dark conditions: (i) ≈25 lux; (ii) ≈100 lux; and (iii) MLE: ≈25 lux followed by ≈100 lux, plus two 45-min bright light pulses of ≈9,500 lux near the end of scheduled wakefulness. We found that lighting levels of ≈25 lux were insufficient to entrain all subjects tested. Exposure to ≈100 lux was sufficient to entrain subjects, although at a significantly wider phase angle compared with baseline. Exposure to MLE was able to entrain the subjects to the imposed sleep–wake cycles but at a phase angle comparable to baseline. These results suggest that MLE can be used to entrain the circadian pacemaker to non-24-h days. The implications of these findings are important because they could be used to treat circadian misalignment associated with space flight and circadian rhythm sleep disorders such as shift-work disorder.
Keywords: light, melatonin, phase angle of entrainment, phase response curve, sleep
Entrainment of a circadian rhythm by a zeitgeber fulfills biological purpose in providing a very distinct phase relationship between the periodicity of the organism and that of the environment.
J. Aschoff (1)
To be of functional significance for the organism, circadian rhythms must be entrained to the 24-h day. For nearly all species studied, the light:dark cycle is the most powerful circadian synchronizer. The resetting capacity of light depends on its intensity, timing, duration, temporal pattern, and spectral composition (2–7). In totally blind people, the circadian timekeeping system often loses synchrony with the earth's 24-h light:dark cycle (8). Well described in animals (9–11) and only recently confirmed in humans (12), entrainment of the circadian system depends on (i) its intrinsic period (τ), (ii) the light:dark cycle to which it is exposed (T; T-cycle), and (iii) the strength of the entraining stimulus (zeitgeber, from German for “time giver”). The generally accepted nonparametric model of circadian entrainment predicts immediate phase shifting in response to light, according to a phase response curve (PRC) (3, 9, 13). In humans, for whom the intrinsic period is on average ≈24.2 h (14), entrainment to the solar day of earth (T = 24 h) requires that the biological clock be “reset” by on average of ≈0.2 h per day in the advance direction. At the individual level, persons with τ < 24 h require a daily phase delay (−Δφ), whereas individuals with τ > 24 h require a daily advance (+Δφ) to synchronize to T = 24 h. Entrainment is achieved when T = τ − Δφ with a stable phase relationship (or phase angle, ψ) between a phase marker for the synchronizing cycle (e.g., light offset) and a phase marker of the driven rhythm [e.g., melatonin onset (MELon)]. In animals, there is a well known quantitative relationship between ψ and T, such that ψ generally widens as T differs from τ, and ψ narrows when the strength of the zeitgeber increases (13). Additionally, the response to a resetting stimulus is correlated with τ, such that animals with short τ (fast pacemakers) tend to be more phase delayed and less phase advanced by light than animals with longer τ (slow pacemakers) (9).
In the present study, we evaluated entrainment of the human circadian system to longer-than-24-h entraining T-cycles in response to three zeitgebers of different strengths: dim light of 25 lux, room light of 100 lux, and a modulated light exposure (MLE) protocol consisting of dim light of 25 lux for the first 10 h of the waking day, room light of 100 lux for the remainder of the waking day, and exposure to two bright light pulses near the end of the waking day (Fig. 1). Based on the mathematical model of the effect of light on the human circadian pacemaker developed by Kronauer and colleagues (15–17), we hypothesized that the resetting effect of the MLE would entrain the biological clock of subjects to a τ + 1 h T-cycle, whereas the resetting effect of 25 and 100 lux would be insufficient to entrain the biological clock to a τ + 1 h T-cycle.
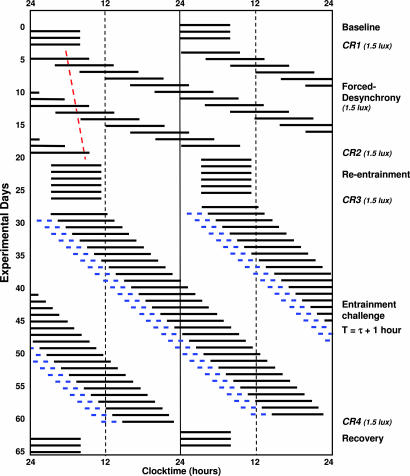
Fig. 1.
Double raster plot of experimental protocol. After three baseline days, during which time subjects continued to sleep (black bars) and wake at their habitual times, subjects were scheduled to a CR (CR1). The FD procedure was used to estimate the intrinsic circadian period (τ). The red dashed line illustrates the drift in phase corresponding to a 24.2-h period. A second CR (CR2) was used to reassess circadian phase, and subjects were then scheduled to sleep at their habitual phase angle of entrainment (assessed from CR1) for five 24-h days. Reentrainment was verified by measurement of phase during CR3. Participants were then scheduled to a “30-day” entrainment segment [assigned to one of three light conditions described in supporting information (SI) Fig. 6], a subject-dependent T-cycle of τ + 1 h, calculated by adding 1 h to each subject's τ. The two blue bars displayed at the end of the waking day show the relative timing of bright light pulses received by subjects assigned to the MLE condition. CR4 was used to assess the circadian phase after the entrainment segment, and participants were discharged after three 24-h recovery days.
Results and Discussion
Range of Circadian Periods Observed.
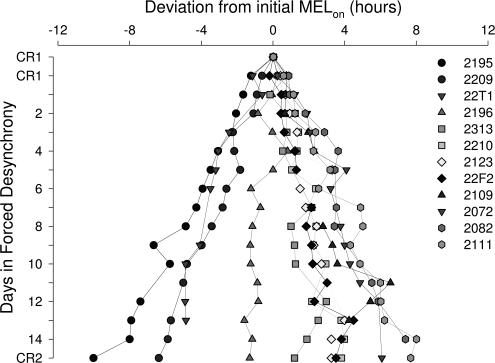
A 1-h range of intrinsic circadian periods was observed in our subjects (from 23.47 h to 24.48 h) (Fig. 2 and Table 1) consistent with that of prior forced desynchrony (FD) studies (14, 18). This confirms the importance of having customized the circadian entrainment challenge to which each subject was exposed (τ + 1 h) particularly because there was a difference (P < 0.05) in circadian periods between groups to which subjects were assigned (Table 1). The average composite period was 24.07 ± 0.33 h, and 4 of 12 subjects had periods <24 h (Table 1). Although there was a small statistical difference between the average estimated temperature period (τt) and melatonin period (τm) (24.04 ± 0.32 h vs. 24.10 ± 0.34 h, respectively; Student t test = −3.5; P < 0.005), the two estimates were highly correlated (rpearson = 0.99; P < 0.0001) in this cohort, and no difference on average was found in other cohorts (19). The scalloping of the MELon observed across the FD protocol (Fig. 2) is consistent with relative coordination (20, 21).
Fig. 2.
Daily melatonin phase estimates (MELon) throughout the FD protocol.
Table 1.
Circadian period estimates
| Subject | Experimental conditions | FD |
Entrainment trial |
Entrainment status | ||||
|---|---|---|---|---|---|---|---|---|
| τt ± SD | τm ± SD | τ | T-cycle | τobs | τobs (CI 95%) | |||
| 2195 | 25 lux | 23.47 ± 0.03 | 23.47 ± 0.01 | 23.47 | 24.47 | 24.46 | 24.43–24.50 | + |
| 2209 | 25 lux | 23.57 ± 0.04 | 23.59 ± 0.01 | 23.58 | 24.58 | 24.31 | 24.25–24.36 | − |
| 22T1 | 25 lux | 23.73 ± 0.07 | 23.76 ± 0.04 | 23.75 | 24.75 | 23.75 | 23.70–23.79 | − |
| 2313 | 25 lux | 24.02 ± 0.04 | 24.09 ± 0.02 | 24.05 | 25.05 | 24.74 | 24.71–24.77 | − |
| 2123 | 100 lux | 24.24 ± 0.04 | 24.24 ± 0.01 | 24.24 | 25.24 | 25.25 | 25.23–25.27 | + |
| 22F2 | 100 lux | 24.24 ± 0.07 | 24.24 ± 0.01 | 24.24 | 25.24 | 25.26 | 25.22–25.30 | + |
| 2109 | 100 lux | 24.24 ± 0.05 | 24.36 ± 0.02 | 24.30 | 25.30 | 25.27 | 25.24–25.31 | + |
| 2072 | 100 lux | 24.23 ± 0.06 | 24.42 ± 0.05 | 24.33 | 25.33 | 25.33 | 25.28–25.39 | + |
| 2196 | MLE | 23.82 ± 0.07 | 23.91 ± 0.01 | 23.87 | 24.87 | 24.88 | 24.85–24.91 | + |
| 2210 | MLE | 24.22 ± 0.04 | 24.28 ± 0.02 | 24.25 | 25.25 | 25.25 | 25.20–25.30 | + |
| 2082 | MLE | 24.33 ± 0.03 | 24.37 ± 0.03 | 24.35 | 25.49 | 25.49 | 25.47–25.51 | + |
| 2111 | MLE | 24.44 ± 0.03 | 24.52 ± 0.01 | 24.48 | 25.48 | 25.48 | 25.46–25.51 | + |
Circadian periods measured during FD on core body temperature rhythms (τt) and plasma melatonin rhythms (τm). Subjects were considered as entrained (+) when the 95% confidence interval (CI) of their observed period (τobs) measured during the entrainment trial included the period of the imposed T-cycle. They were considered nonentrained (−) otherwise.
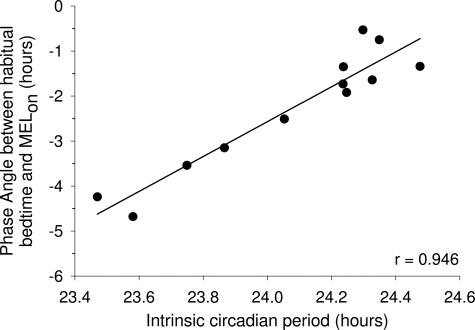
Fig. 3 reveals that the phase angle of entrainment, as determined by the relationship between MELon and habitual bedtime (ψMELon), is strongly correlated with τ such that participants with shorter circadian periods have an earlier ψMELon than those with longer periods. This is consistent with correlations from temperature data collected immediately after entrainment to a 24-h day (22) and even after entrainment to a variety of daylength and lighting conditions (23). The very high correlation demonstrated in Fig. 3 indicates that the phase estimates collected on a single day may provide a tool for estimating an intrinsic circadian period. Further research will be needed to define the specific conditions that are required to maintain the high correlation between circadian phase observed and the measure of circadian period. It should be noted that, in the present cohort, this high correlation was observed in subjects who maintained a wake:sleep schedule with a light:dark ratio of 2:1 for at least 3 weeks before entry in the study. In addition, once they entered the laboratory, stringent control of lighting conditions were maintained for 3 consecutive days, and the transition from ordinary room light (≈90 lux) to dim light (≈1.5 lux) was made on day 3 (D3), 10 h after habitual waketime, at a phase that is on average of minimal sensitivity for photic resetting.
Fig. 3.
Relationship between intrinsic circadian period and phase angle of entrainment measured on CR1 as the difference in time between habitual bedtime (lights off) and MELon. Subjects with a shorter circadian period showed a larger phase angle. The high correlation coefficient (r = 0.946 and P < 0.001) indicates that a single phase estimate may provide a tool for estimating an intrinsic circadian period.
Reestablishment of a Normal ψ Before the Entrainment Trial to T = τ + 1 h.
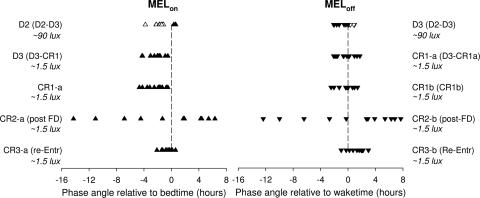
Fig. 4 shows the timing between lights off and MELon (ψMELon) and between lights on and melatonin offset (ψMELoff) at different times during the protocol. From D2 to constant routine (CR) 1, subjects showed a consistent ψMELoff [no significant change in ψ MELoff from D3 to the second day of the first CR (CR1b); ANOVA, P > 0.05]. MELon occurred later on D2, when subjects were exposed to ≈90 lux in the angle of gaze, than on D3 and CR1, when light levels were ≈1.5 lux (P < 0.0001 for both days). This is consistent with the masking effect of room light on melatonin (MELon). As illustrated in Fig. 4, reestablishment of ψ after FD within a normal range was largely successful in all of our subjects. That is, the wide distribution of ψ is compressed into a normal range of ψ on CR3, after five 24.0-h days scheduled at a normal phase angle under 450-lux background light. Note that both MELon and MELoff phases were slightly delayed on CR3 compared with D2, D3, and CR1 (ANOVA, Student–Newman–Keuls, P < 0.0001). This change, which likely occurred in response to the phase-delaying effect of 5 days in 450 lux, is consistent with previous finding in animals (9, 13) and humans (23), showing that the phase angle narrows with increased light intensities.
Fig. 4.
Timing between lights off and MELon and between lights on and MELoff at different times during the protocol. Open symbols are used on D2 and D3 for masked MELon and MELoff (occurring under ≈90 lux of light). The successful reentrainment after FD is clear (as measured by a phase angle in CR3 comparable to that in D2, D3, and CR1) despite the wide range of phase angles achieved immediately at the end of FD (CR2).
Entrainment to T = τ + 1 h with Zeitgebers of Different Strengths.
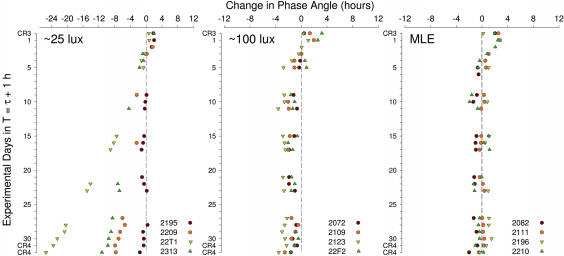
Fig. 5 illustrates the change in phase angle of entrainment between MELoff and the T-cycle (waketime) for subjects in the three zeitgeber light conditions. Because of the shorter phase angles that occurred after reentrainment under a strong zeitgeber (≈450 lux), changes in phase angle throughout the T-cycle were expressed relative to the phase angle on D3. As shown in Table 1, three of four subjects in the 25-lux condition were not entrained to the τ + 1 h T-cycle. In the three nonentrained subjects, MELoff gradually drifted to an earlier time relative to the light:dark cycle (Fig. 5 Left). These subjects were classified as not entrained because the 95% confidence interval of their observed period (τobs) did not include the period of the T-cycle (Table 1). Strikingly, one subject (2195) remained entrained to the τ + 1 h T-cycle under the same low light levels of 25 lux. This subject had the shortest τ of all subjects (23.47 h). Subjects with short τ require a daily phase delay to entrain to the 24-h light:dark cycle of Earth. By contrast, subjects with a longer-than-24-h τ require a daily advance shift. One might expect that an individual with such a short τ might require enhanced delay sensitivity. This notion would be consistent with one aspect of the Pittendrigh and Daan entrainment model (24), which, to explain stable phase angle of entrainment despite photoperiodic changes across the year, requires that night active species with a short period have an asymmetric PRC with higher sensitivity in the delay region (larger range and higher amplitude), whereas day-active animals with a period >24 h have higher sensitivity in the advance region (24). Therefore, one might hypothesize that individuals with short circadian periods could present a PRC asymmetry with a very sensitive delay region. However, the two other subjects with a short τ were misaligned (subject 2209, τ = 23.58 h, average phase delay during the T-cycle = 0.73 h per cycle; subject 22T1, τ = 23.75 h, average phase delay during the T-cycle = 0 h). This does not necessarily contradict the hypothesis of PRC asymmetry. Indeed, the amplitude and shape of the PRC vary between species and individuals, as does the range of entrainment (9). In two of the three subjects who did not entrain to T = τ + 1 h, the imposed T-cycle still exerted an effect on the circadian clock (τobs), but the synchronizing stimulus was of insufficient strength to entrain it (Table 1).
Fig. 5.
Change in phase angle of entrainment between MELoff and the T-cycle in the three light conditions. Phase angles are plotted relative to the phase angle measured on D3. Three of the four subjects in the 25-lux condition did not maintain entrainment, as MELoff gradually drifted to an earlier time. After a transitory drift, subjects in the 100-lux and the MLE conditions entrained to the τ + 1 h T-cycle (Table 1), although at a different phase angle.
All four subjects exposed to 16 h of 100 lux showed a transitory drift in MELoff to an earlier time. After the first week in the T-cycle, the timing of MELoff was stable for all subjects (Fig. 5). These subjects were classified as entrained to the T-cycle (Table 1). On average, by the end of the T-cycle, ψ had widened by −1.26 ± 0.36 h in DL100 condition compared with baseline (paired t test, P = 0.038; Wilcoxon matched-pairs test, P = 0.0678, marginal effect). All four subjects showed a widened ψ compared with baseline (binomial test, P < 0.0001).
Subjects exposed to MLE also showed a transitory drift in MELoff to an earlier time (Fig. 5). However, this segment was shorter than in the two other groups because MELoff appeared to reach a stable ψ after only ≈5 days in the T-cycle. All four MLE subjects were classified as entrained to the T-cycle (Table 1). On average for this group of subjects, ψ was not significantly different at the end of the T-cycle from that measured at the beginning of the study (mean change, −0.19 ± 0.41 h; paired t test, P = 0.67; Wilcoxon matched-pairs test, P = 0.47). Interestingly, even subject 2082, who was mistakenly scheduled to τ + 1.14 h, successfully entrained (τobs = T) at a normal ψ despite the additional phase delay challenge of 0.14 h (8 min) per day.
SI Fig. 7 illustrates the dynamics of the melatonin rhythm in three individuals exposed to 25 lux, 100 lux, or MLE.
Entrainment or Masking?
The last CR of our protocol (CR4) was carried out to distinguish entrainment of the circadian pacemaker from masking of the circadian phase marker by light. Once released into CR4, the phase of the circadian system corresponded to that expected from the previous cycle (Fig. 5) and did not show an abrupt change in phase (“jump”) that is characteristic of masking. In addition, our choice of using MELoff as an appropriate unmasked phase marker of the circadian system is supported by the result that the change in phase (MELoff) from the last day in the T-cycle to CR4 was not significantly different among the three groups (−1.08 ± 0.40 in 25 lux, −1.04 ± 0.87 in 100 lux, and −0.45 ± 1.17 in MLE; Kruskall–Wallis, P = 0.69) despite different lighting conditions during daytime under the entraining T-cycle (25 or 100 lux).
Phase Angle and Strength of the Zeitgeber.
As shown both in nonhuman species (13, 25) and humans (22, 23), the phase angle of entrainment between the imposed T-cycle and the phase marker of an entrained rhythm is a function of the difference in their period [ψ = f(T − τ)] and of the strength of the zeitgeber. Phase angle widens as T differs from τ, and ψ narrows as zeitgeber strength increases (13). In our study, the difference in the period of T and τ was equal for each subject (1 h by protocol design). Therefore, the difference in phase angle of entrainment between conditions is likely due to a difference in the respective zeitgeber strength, i.e., the light intensity. As predicted by entrainment theory, a larger phase angle was observed with a decrease in zeitgeber strength in our human subjects, as observed in other mammals (26) (see also our expanded discussion in SI Text).
High Sensitivity to Moderate Light Intensities of 25 and 100 lux.
The mathematical model developed by Kronauer and colleagues (15–17) was used as a guide to the design of these experiments. The present results imply a higher sensitivity to 100 lux than could have been inferred from previous data. The model has been modified to incorporate these and other recent findings on nonphotic stimuli (27). It can be considered as a “continuous” model, designed to accommodate even brief (few minutes) stimuli.
Physiologically, the higher-than-expected sensitivity to 25- and 100-lux light could be related to the recently described effects of prior light history, which revealed that the response to light may be enhanced after background light exposure of low intensity (28, 29). The mechanisms explaining the effects of prior light history are unknown, but they could involve the recently described modulation of intrinsically photosensitive retinal ganglion cells (ipRGC) sensitivity by background light levels (30). When exposed to a constant bright background, the background evoked response of ipRGC decay, and their responses to superimposed flashes suggest light adaptation of those photosensitive cells. Additionally, after extinction of a light-adapting background, sensitivity recovered progressively, indicating dark adaptation. On the other hand, it has been recently shown that the ipRGCs adapt their expression of the photopigment melanopsin to environmental light and darkness in such a way that prolonged exposure to darkness increases melanopsin mRNA levels, whereas exposure to constant light decreases melanopsin mRNA levels (31). Therefore, the increase in ipRGC sensitivity during the course of CR3 and the T-cycle in 25- and 100-lux light conditions could explain, at least in part, why the response drive is greater in relatively low light levels than expected.
Conclusions and Perspectives
Our findings demonstrate that the human circadian system shares the basic properties of entrainment biology that have been described in other species: the phase angle of entrainment (i) widens as the period of the imposed T-cycle deviates further from τ (11) and (ii) narrows as the strength of the zeitgeber increases (13).
Nonphotic stimuli have been reported to exert a small but significant synchronizing effect on the human circadian system in both sighted (32, 33) and blind (34, 35) subjects. Therefore, in our protocol, we cannot exclude that nonphotic cues, such as showers (pulses of temperature), meals, and sleep–wake schedule, exerted a weak synchronizing effect on the clock.
Only outputs of the central circadian clock (core body temperature and melatonin) were evaluated to assess entrainment in our study. The concept of multiple oscillators normally synchronized with each other but that can become desynchronized under “free-running” conditions was developed 40 years ago (36). It has been shown that the central circadian pacemaker acts as a master clock, synchronizing a multitude of peripheral clocks (37). Therefore, we may have classified as entrained subjects whose peripheral clocks were misaligned with each other and/or with the central clock. Such a misalignment between peripheral and central clock has been shown to occur in aged rodents (38). The occurrence of partial entrainment and its impact in humans remain to be investigated.
Failure to entrain to the required sleep–wakefulness schedule occurs in numerous situations in real-life conditions. Jet-lag, shift work, and other circadian rhythm sleep disorders such as advanced and delayed sleep phase types and free-running type are all associated, to different extents, with a condition in which the circadian system is out of synchrony with the light:dark/rest–activity cycle. The generally associated symptoms range from cognitive and psychomotor impairment to sleep disruptions (18, 39–42) and endocrine disturbances (43). The resulting inappropriate functioning, consistent with the hypothesized circadian regulation of brain metabolism (44), may be an important factor contributing to the increased risk of accident associated with circadian misalignment (45). Evidence of significant sleep loss and disruption of circadian rhythms in astronauts (42) and associated performance decrements are also reported during space missions. In these situations, sleep and circadian disruptions could have serious consequences on the effectiveness, health, and safety of astronaut crews (46, 47). Moreover, long-duration exploration class space missions may require astronauts to be scheduled to non-24-h light:dark periods for extended durations of time in conditions in which gravity could additionally impact circadian physiology (48). The above issues highlight the importance of developing effective countermeasures to maintain circadian entrainment. Our findings suggest that appropriately timed light exposure can be used as an effective means to maintain the circadian clock in synchrony with a rest–activity cycle different from 24 h or under insufficient light conditions. A lighting protocol such as the one tested in the current study would enable astronauts to entrain to the 24.65-h Martian day while caring for crops in a brightly lit greenhouse module (ref. 49 and NASA Mars Greenhouse, www.science.ksc.nasa.gov/biomed/marsdome/index.html) provided that those duties were performed at the appropriate circadian phase.
Methods
Subjects.
Twelve healthy young subjects participated in the study [22–33 years old, average 28.8 ± 4.1 (SD) years old, nine males and three females] after a rigorous screening procedure (see SI Text). Subjects were required to maintain a regular 8:16 h sleep:wakefulness schedule at home for at least 3 weeks before laboratory admission, verified by wrist activity and light exposure recordings.
Protocol.
Subjects were maintained in individual rooms free from external time cues during the entire 65-day study (Fig. 1). Timing of light exposure, sleep opportunities, meals, and showers was scheduled. Subjects were maintained on a 24.0-h schedule for 3 days followed by a 40-h CR protocol that was used to assess circadian phase (50). Subsequently, participants were scheduled to a FD protocol using a 28-h dim light:dark cycle. This FD protocol was used to estimate their intrinsic circadian period (τ) (14). Then, a second CR was used to reassess circadian phase. Participants were then scheduled for five 24.0-h days with sleep scheduled at the same phase of the endogenous circadian temperature cycle as that observed on the first CR. Successful reestablishment of phase was verified by a third CR. Participants were then scheduled for 30 days to a T-cycle of τ + 1 h in one of three assigned light conditions (below and SI Fig. 6). For example, a subject with a τ of 24.24 h as determined during FD would be scheduled to a 25.24-h day. A fourth CR was used to assess circadian phase after the τ + 1 h schedule and followed by three 24.0-h days.
The rationale for scheduling participants to a T-cycle based on their own intrinsic circadian period (τ + 1 h) rather than to a fixed T-cycle was to evaluate the effectiveness of three light:dark cycles of different zeitgeber strength on the circadian system challenged to a similar T-cycle relative to their circadian period. Fig. 2 and Table 1 show the range of circadian periods of the 12 subjects studied. A fixed T-cycle of, for example, 24.5 h would have required a phase delay shift of ≈1 h per day for the subject with the shortest period (2195, τ = 23.47 h), whereas subject 2110 would virtually not necessitate any phase adjustment to remain entrained to the T-cycle given its τ of 24.48 h. Therefore, in the case of a fixed T-cycle, it would be difficult, if not impossible, to assess the relative efficacy of different zeitgeber strength.
All experimental procedures were carried out in accordance with the principles of the Declaration of Helsinki (revised in 2000), and the protocol was approved by the Human Research Committee at the Brigham and Women's Hospital. Subjects provided written informed consent.
Lighting Conditions.
Experimental suites were equipped with ceiling-mounted cool-white fluorescent lamps (see SI Text and SI Fig. 8). A light:dark ratio of 2:1 was maintained for all daylengths. Light intensities and irradiances were measured at a height of ≈1.37 m to approximate the intensity received in the average angle of gaze during wakefulness (see SI Text). Light intensities were as follows: ≈90 lux during the first 2.5 baseline days (D1–3); ≈1.5 lux during the last 6 h of D3, CR1–4, and the FD segment; ≈450 lux during the reentrainment segment between CR2 and CR3; and, depending on the lighting condition, subjects were exposed to ≈25 lux, ≈100 lux, or a MLE protocol consisting of dim light of ≈25 lux for the first 10 h of the waking day and room light of ≈100 lux for the remainder of the waking day plus exposure to two bright light pulses of ≈9,500 lux near the end of the waking day (Fig. 1 and SI Fig. 6) during the entrainment segment of the protocol. Scheduled sleep occurred in total darkness. The timing, duration, intensity, and pattern of light exposure stimuli were based on the dynamic resetting model developed by Kronauer and colleagues (15–17). The model predicted a successful circadian entrainment to T = τ + 1 h for the MLE condition and failure to entrain to T = τ + 1 h for the 25- and 100-lux light conditions.
Data Collection and Analyses.
Temperature was recorded every minute by using a rectal thermistor (Yellow Springs Instrument, Yellow Spring, OH). Blood samples were collected every 10–60 min during baseline days, CR, and FD segments and every 60 min during five time windows of the entrainment segment. Plasma melatonin concentrations were assayed by using RIA techniques (ALPCO Diagnostics, Windham, NH). The assay sensitivity was ≈0.7 pg/ml. Average intra- and interassay coefficients of variation were <8% and 12%, respectively.
A dual-harmonic regression model (51) was used to assess the phase of the temperature minimum (CBTmin) during CR1 and CR2. The phase angle between CBTmin and habitual bedtime measured on CR1 (ψCBTminCR1) was used as an estimate of the baseline phase angle of entrainment. After CR2, subjects were scheduled to a sleep–wake cycle at the same ψ between CBTmin and habitual bedtime as that measured in CR1, such that ψCBTminCR1 = ψCBTminCR2.
MELon and MELoff were calculated by using a least-square regression analysis and a threshold of 25% of the peak-to-trough amplitude (4). Phase angle of entrainment was calculated as the difference in time between lights off (bedtime) and MELon (ψMELon) and between lights on (waketime) and MELoff (ψMELoff). Based on prior findings that melatonin levels are acutely suppressed by light (52), even at relatively low light levels (2), ψMELoff was chosen to assess entrainment of the circadian system during the entrainment segment of our study because we exposed subjects to bright light in the evening hours in the MLE condition.
Intrinsic circadian period was estimated on temperature (τt) and melatonin (τm) data collected during FD by using a nonorthogonal spectral analysis procedure (14). A composite estimate of the intrinsic circadian period for each subject (τ) was computed by averaging τt and τm. Subjects were classified as entrained to the T-cycle when the 95% confidence interval of their observed period (τobs) included the period of the T-cycle (12).
Unless otherwise indicated, results are reported as means ± SEM. Statistical significance is ascribed for P < 0.05. We also report as marginal effects with a P value between 0.05 and 0.10 (53).
Supplementary Material
Acknowledgments
We thank subject volunteers, subject recruiters, and research staff; Dr. Megan E. Jewett and Craig May for helpful discussions on mathematical simulations; Dr. Vincent Ricchiuti and the Core Laboratory for performance of the melatonin assays; and Joseph M. Ronda for technical support. This work was supported by National Aeronautics and Space Administration Cooperative Agreement NCC 9-58 with the National Space Biomedical Research Institute, National Aeronautics and Space Administration Grant NAG 5-3952, National Heart, Lung, and Blood Institute Grant R01 HL52992, National Center for Research Resources–National Institutes of Health Grant M01 RR02635 to the Brigham and Women's Hospital General Clinical Research Center, and National Institutes of Health Grant R01 HL081761 (to K.P.W.). C.G. is supported by FP6-EUCLOCK.
Abbreviations
- AG
angle of gaze
- MLE
modulated light exposure
- ψ
phase angle
- τ
circadian period
- MELon
melatonin onset
- MELoff
melatonin offset
- PRC
phase response curve
- FD
forced desynchrony
- Dn
day n
- CR
constant routine.
Footnotes
Conflict of interest statement: C.A.C. has received consulting fees from or served as a paid member of scientific advisory boards for Actelion, Inc.; Aventis; Avera Pharmaceuticals, Inc.; Cephalon, Inc.; Coca-Cola Co.; Fedex Kinko's; Hypnion, Inc.; Morgan Stanley; Pfizer, Inc.; Respironics; Sleep Research Society; Takeda Pharmaceuticals, Inc.; Vanda Pharmaceuticals, Inc.; and Warburg-Pincus. He owns an equity interest in Hypnion, Inc., Lifetrac; and Vanda Pharmaceuticals, Inc. He has received lecture fees from Alfresa; Cephalon, Inc., and Takeda, Inc.; Tanabe Seiyaku Co., Ltd.; Tokyo Electric Power Company; clinical trial research contracts from Cephalon, Inc., Merck, Sanofi-Aventis; and Pfizer, Inc.; an investigator-initiated research grant from Cephalon, Inc.; and unrestricted research and education funds from Cephalon, Inc., Philips Lighting; Sanofi Aventis; Sepracor; Takeda; and Resmed. He is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. Since 1985, Dr. Czeisler has served as an expert witness on various legal cases related to sleep and/or circadian rhythms. K.P.W. has received consulting fees, speaker fees, and clinical research grants from Cephalon, Inc.; received consulting fees, an investigator-initiated research grant, and educational grants from Takeda, Inc.; received consulting fees from Novartis, Inc.; and served as an equity paid member of a scientific advisory board for Axon Sleep Research Laboratories, Inc.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702835104/DC1.
References
- 1.Aschoff J. In: Circadian Clocks. Aschoff J, editor. Amsterdam: North–Holland; 1965. pp. 262–276. [Google Scholar]
- 2.Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. J Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gronfier C, Wright KP, Jr, Kronauer RE, Jewett ME, Czeisler CA. Am J Physiol. 2004;287:E174–E181. doi: 10.1152/ajpendo.00385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thapan K, Arendt J, Skene DJ. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lockley SW, Brainard GC, Czeisler CA. J Clin Endocrinol Metab. 2003;88:4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 8.Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, Klein T, Rizzo JF., III New Engl J Med. 1995;332:6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- 9.Daan S, Pittendrigh CS. J Comp Physiol A. 1976;106:253–266. [Google Scholar]
- 10.Hoffmann K, Aschoff J. In: Circadian Clocks. Aschoff J, editor. Amsterdam: North–Holland; 1965. pp. 87–94. [Google Scholar]
- 11.Enright JT, Aschoff J. In: Circadian Clocks. Aschoff J, editor. Amsterdam: North–Holland; 1965. pp. 112–124. [Google Scholar]
- 12.Wright KP, Jr, Hughes RJ, Kronauer RE, Dijk DJ, Czeisler CA. Proc Natl Acad Sci USA. 2001;98:14027–14032. doi: 10.1073/pnas.201530198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pittendrigh CS, Daan S. J Comp Physiol A. 1976;106:291–331. [Google Scholar]
- 14.Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, et al. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 15.Jewett ME, Forger DB, III, Kronauer RE. J Biol Rhythms. 1999;14:493–499. doi: 10.1177/074873049901400608. [DOI] [PubMed] [Google Scholar]
- 16.Kronauer RE, Forger D, Jewett ME. J Biol Rhythms. 1999;14:500–515. doi: 10.1177/074873099129001073. [DOI] [PubMed] [Google Scholar]
- 17.Kronauer RE, Forger DB, Jewett ME. J Biol Rhythms. 2000;15:184–186. [Google Scholar]
- 18.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Am J Physiol. 1999;277:R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 19.Duffy JF, Wright KP., Jr J Biol Rhythms. 2005;20:326–338. doi: 10.1177/0748730405277983. [DOI] [PubMed] [Google Scholar]
- 20.Wever RA. J Biol Rhythms. 1989;4:161–185. [PubMed] [Google Scholar]
- 21.Ritz-De Cecco A, Jewett ME, Wyatt JK, Kronauer RE, Czeisler CA, Dijk DJ. Sleep Res Online. 1999;2:620. [Google Scholar]
- 22.Duffy JF, Rimmer DW, Czeisler CA. Behav Neurosci. 2001;115:895–899. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- 23.Wright KP, Jr, Gronfier C, Duffy JF, Czeisler CA. J Biol Rhythms. 2005;20:168–177. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pittendrigh CS, Daan S. J Comp Physiol A. 1976;106:333–355. [Google Scholar]
- 25.Hoffmann K. Z Naturforsch C. 1963;18:154–157. [Google Scholar]
- 26.Aschoff J, Klotter K, Weaver R. In: Circadian Clocks. Aschoff J, editor. Amsterdam: North–Holland; 1965. p. 1. [Google Scholar]
- 27.St Hilaire M, Klerman EB, Czeisler CA, Kronauer RE. J Theor Biol. 2007 doi: 10.1016/j.jtbi.2007.04.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hebert M, Martin SK, Lee C, Eastman CI. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith KA, Schoen MW, Czeisler CA. J Clin Endocrinol Metab. 2004;89:3610–3614. doi: 10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- 30.Wong KY, Dunn FA, Berson DM. Neuron. 2005;48:1001–1010. doi: 10.1016/j.neuron.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Hannibal J, Georg B, Hindersson P, Fahrenkrug J. J Mol Neurosci. 2005;27:147–155. doi: 10.1385/JMN:27:2:147. [DOI] [PubMed] [Google Scholar]
- 32.Aschoff J, Fatransk M, Giedke H, Doerr P, Stamm D, Wisser H. Science. 1971;171:213–215. doi: 10.1126/science.171.3967.213. [DOI] [PubMed] [Google Scholar]
- 33.Barger LK, Wright KP, Jr, Hughes RJ, Czeisler CA. Am J Physiol. 2004;286:R1077–R1084. doi: 10.1152/ajpregu.00397.2003. [DOI] [PubMed] [Google Scholar]
- 34.Klerman EB, Rimmer DW, Dijk DJ, Kronauer RE, Rizzo JF, III, Czeisler CA. Am J Physiol. 1998;274:R991–R996. doi: 10.1152/ajpregu.1998.274.4.r991. [DOI] [PubMed] [Google Scholar]
- 35.Arendt J, Aldhous M, Wright J. Lancet. 1988;1:772–773. doi: 10.1016/s0140-6736(88)91586-3. [DOI] [PubMed] [Google Scholar]
- 36.Wever R. Int J Chronobiol. 1975;3:19–55. [PubMed] [Google Scholar]
- 37.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong H-K, Oh WJ, Yoo OJ, et al. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamazaki K, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Proc Natl Acad Sci USA. 2002;99:10801–10806. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright KP, Jr, Hull JT, Hughes RJ, Ronda JM, Czeisler CA. J Cognit Neurosci. 2006;18:508–521. doi: 10.1162/jocn.2006.18.4.508. [DOI] [PubMed] [Google Scholar]
- 40.Folkard S, Wever RA, Wildgruber CM. Nature. 1983;305:223–226. doi: 10.1038/305223a0. [DOI] [PubMed] [Google Scholar]
- 41.Monk TH, Folkard S, Hockey GRJ. Stress and Fatigue in Human Performance. New York: Wiley; 1983. p. 97. [Google Scholar]
- 42.Dijk DJ, Neri DF, Wyatt JK, Ronda JM, Riel E, Ritz-De Cecco A, Hughes RJ, Elliott AR, Prisk GK, West JB, Czeisler CA. Am J Physiol. 2001;281:R1647–R1664. doi: 10.1152/ajpregu.2001.281.5.R1647. [DOI] [PubMed] [Google Scholar]
- 43.Weibel L, Brandenberger G. J Biol Rhythms. 1998;13:202–208. doi: 10.1177/074873098129000048. [DOI] [PubMed] [Google Scholar]
- 44.Buysse DJ, Nofzinger EA, Germain A, Meltzer CC, Wood A, Ombao H, Kupfer DJ, Moore RY. Sleep. 2004;27:1245–1254. doi: 10.1093/sleep/27.7.1245. [DOI] [PubMed] [Google Scholar]
- 45.Dinges DF. J Sleep Res. 1995;4:4–14. doi: 10.1111/j.1365-2869.1995.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 46.White RJ, Averner M. Nature. 2001;409:1115–1118. doi: 10.1038/35059243. [DOI] [PubMed] [Google Scholar]
- 47.Mallis MM, DeRoshia CW. Aviat Space Environ Med. 2005;76:B94–B107. [PubMed] [Google Scholar]
- 48.Fuller PM, Jones TA, Jones SM, Fuller CA. Proc Natl Acad Sci USA. 2002;99:15723–15728. doi: 10.1073/pnas.242251499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campbell PD, Moore N. [Accessed December 6, 2006];Integration of Plant Growth into a Mars Habitat. 1992 Available at http://ares.jsc.nasa.gov/HumanExplore/Exploration/EXLibrary/DOCS/EIC016.HTML.
- 50.Duffy JF, Dijk DJ. J Biol Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- 51.Brown EN, Czeisler CA. J Biol Rhythms. 1992;7:177–202. doi: 10.1177/074873049200700301. [DOI] [PubMed] [Google Scholar]
- 52.Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Science. 1980;210:1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 53.Keppel G. Design and Analysis: A Researcher's Handbook. Englewood Cliffs, NJ: Prentice–Hall; 1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.