Abstract
To better understand the role of β-catenin mutation in hepatocellular carcinoma (HCC), we correlated the gene mutation with hepatitis virus B (HBV) and hepatitis virus C (HCV) status and the clinicopathological features in 366 patients with resected primary unifocal HCC. β-Catenin mutations were also analyzed in 55 patients with multifocal HCC (68 tumors). Of the whole series, 57 (13.1%) of 434 tumors examined had β-catenin mutations, 34 occurred at the serine/threonine residues of the GSK-3β region of β-catenin. Outside the GSK-3β phosphorylation site, codons 32 and 34 were two mutational hot spots (17 tumors). The non-HBV-related HCC that was predominantly HCV related had a higher frequency of mutation (P < 0.00001) and more frequent mutations at codon 45 than HBV-related HCC. HBV-related HCC had a younger mean age (P < 0.00001), and higher male-to-female ratio (P < 0.003) and positive familial history of HCC (P < 0.014). Among 366 unifocal HCCs selected for clinicopathological analysis, β-catenin mutations were associated with grade I (P = 0.005) and stage I and II HCC (P < 0.0001), and a better 5-year survival rate (P = 0.00003). These findings suggest mechanisms for β-catenin mutations differ between HBV-related and non-HBV-related HCCs, and that β-catenin mutation is a favorable prognostic factor related to low stage. β-Catenin mutation was associated with nuclear expression of the protein (P < 0.00001), but we failed to detect point or large fragment deletion mutation in 39 HCCs with nuclear β-catenin expression, presumably wild-type protein. HCCs expressing mutant nuclear β-catenin had a better 5-year survival rate (P < 0.007), suggesting that mutant and wild-type nuclear β-catenin proteins are not functionally equivalent and deserve more studies for further clarification.
β-Catenin plays an important role in the cell-cell adhesion 1 and in the Wnt/wingless signaling pathway. 2-4 β-Catenin can enter the nucleus by binding the Tcf-Lef family of DNA binding proteins, and regulates transcription of target genes. 3-8 β-Catenin also forms complexes with the tumor suppressor protein APC, 9,10 leading to its own NH2-terminal phosphorylation by GSK-3β and degradation by the proteasome system. 11 The APC gene is mutated in most colorectal tumors and the decreased APC-associated degradation of β-catenin is critical to APC’s tumor suppressive effect. 5 Recently, somatic mutations of β-catenin have been demonstrated not only in malignant tumors in humans 5,8,9,12-15 and rodents, 16,17 but also in benign tumors. 18,19 But, the clinical implication of β-catenin mutations in human cancer is unclear.
Hepatocellular carcinoma (HCC) is the leading fatal cancer in Taiwan and tumor invasion is a crucial prognostic histological factor of HCC. 20 HCC is closely associated with hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, but the molecular mechanisms for the tumorigenesis of HBV- and HCV-related HCC are unclear. Although closely related to tumor invasion, mutation of tumor suppressor gene p53 is detected in only one-third of HCCs in Taiwan. 21,22 β-Catenin is frequently mutated in HCC in humans 23,24 and rodents, 23,25 but the clinical implication of β-catenin mutations in HCC needs to be clarified. In the present study, we demonstrate that β-catenin mutation is associated with low-grade, low-stage, HBV-negative HCC, and has a more favorable prognosis.
Materials and Methods
Tissue Samples
From January 1983 to December 1997, 1,033 surgically resected primary and 188 recurrent HCCs were pathologically assessed at the National Taiwan University Hospital. The tissue samples were immediately cut into small pieces, snap-frozen in liquid nitrogen, and stored in deep freezers. Of these, 421 patients who already had DNA or RNA samples taken from resected primary HCCs were analyzed for β-catenin mutations, including 366 cases of unifocal and 55 cases of multifocal HCC (68 tumors). To elucidate the clinical implication of β-catenin mutation, the 366 cases of unifocal HCC, as previously defined, 21,22,26,27 were selected for clinicopathological analysis. Multifocal HCCs were excluded from this correlation because of incomplete sampling of the tumor nodules for genetic analysis and their variation in pathological features.
Histological Study and TNM Staging
For the convenience of comparative analyses, the tumor grade was simply divided into three groups: well (grade I), moderately (grade II), and poorly differentiated HCC (grades III and IV).
We and other investigators have shown that encapsulated HCC without liver and vascular invasion has better survival than invasive HCC, regardless of tumor size. 20,28,29 Hence, resected unifocal HCC was staged according to the staging system proposed by the International Union against Cancer (UICC, 1997), but with modification according to tumor invasion and the extent of vascular spread and listed as follows: stage I: encapsulated, without evidence of liver or vascular invasion; stage II: unencapsulated or encapsulated and with liver invasion, but without vascular invasion; stage IIIA: invasion of small vessels in the tumor capsule; stage IIIB: focal invasion of portal vein branches close to the tumor; stage IV: invasion of portal veins in distal liver (1 cm away from the tumor capsule), branches of major portal vein, common bile duct, or perforation of visceral peritoneum.
Immunohistochemistry
The β-catenin was detected on formalin-fixed, paraffin-embedded sections by the labeled streptavidin-biotin method after antigen retrieval, as previously described, 30 using a monoclonal antibody against human β-catenin (Transduction Laboratories, Lexington, KY). The β-catenin immunostaining was scored according to the subcellular localization (along the membrane or in nucleus).
Mutational Analysis
DNA and RNA were extracted from fresh frozen liver tissues as previously described. 21,22 Primers for polymerase chain reaction were designed to amplify a 232-bp fragment of exon 3 of the β-catenin gene, 31 encompassing the sequence for GSK-3β phosphorylation sites that contain activating mutations. 5,6,9,13 The primers used included sense primer: 5′-AGCTGATTTGATGGAGTTGG-3′and antisense primer: 5′-ACCAGCTACTTGTTCTTGAG-3′. DNA sequencing was performed by ABI 373 Automated Sequencer, using the ABI Prism Dye Terminator Cycle Sequencing Ready Reaction Kit (Perkin Elmer, Branchburg, NJ). Each mutation was verified in both sense and antisense directions.
To detect large fragment interstitial deletions, primers flanking exons 2 to 4 of the β-catenin gene were used, as described by Koch et al. 32 The primers for DNA templates were BCAT-3, 5′-AAAATCCAGCGTGGACAATGG-3′ and BCAT-4, 5′-TGTGGCAAGTTCTGCATCATC-3′, and for cDNA were BCAT-5, 5′-GGAGGAAGGTCTGAGGAGCAG-3′ and BCAT-6, 5′-CGATGATGGGAAAGGTTATGC-3′. Polymerase chain reaction products of altered size were cloned to plasmid by the TA Cloning Kit (Invitrogen, Carlsbad, CA) and subjected to DNA sequencing.
To detect HBV DNA in tumor and nontumor liver samples negative for serum HBsAg, Southern blotting and/or polymerase chain reaction were carried out, as previously described. 26 For polymerase chain reaction, we amplified a 257-bp fragment of major S and a 433-bp fragment of C genes, respectively. The primers used were: HBV-S primer 1: 5′-ACATCAGGATTCCTAGGACCCCT-3′ and HBV-S primer 2: 5′-CATAGCAGCAGGATGAAGAGGAA-3′ (HBV DNA nucleotides 169 to 425), HBV-C primer 1: 5′-GCTTTGGGGCATGGACATTGACCC-3′, and HBV-C primer 2: TGATAAGATAGGGGCATTTGGTGG-3′ (HBV DNA nucleotides 1893 to 2325).
Follow-Up Observation
Of 366 patients who had unifocal primary HCC, 294 (80%) had been followed for more than 8 years or until death, up to 16 years. To minimize the influence of second and third recurrent primary HCC, 21,22,26 the endpoint for follow-up was set at 5 years.
Statistical Analyses
The analyses were carried out using the Statistica for Window software (Statsoft, Inc., Chicago, IL). We used two-tailed chi-square and Fisher exact tests for univariate analysis. The cumulative survival after tumor removal was calculated with log-rank test. P values <0.05 were considered statistically significant.
Results
Clinical Features of 421 Study Cases with Primary HCC
There were 339 male and 82 female patients from 10 to 88 years of age. HBsAg was detected in the sera in 280 patients (66.5%; 69.6% in the whole series of 1033 cases), and HBV genome in liver tissues in 29 HBsAg-seronegative cases. For the convenience of comparison, these 309 patients, including 44 cases also positive for anti-HCV, or co-infection, were regarded to have HBV-related HCC. In the remaining 112 non-HBV-related HCC patients, 95 (85%) were positive for anti-HCV and 17 were negative for HBV and HCV. Of these 17 cases, none was alcoholic, four had liver cirrhosis, four had chronic persistent hepatitis, and nine had normal liver histology. HBV-related HCC had younger mean age (52.7 ± 13.6 years versus 63.9 ± 9.4 years, P < 0.00001), higher male-to-female ratio (84.1% versus 70.5%, P < 0.003), more frequent serum alpha-fetoprotein (AFP) elevation above 320 ng/ml (49.5% versus 32.1%, P = 0.002), and positive familial history of HCC (14.9% versus 5.4%, P < 0.014). Liver cirrhosis was found in 40.5% and 43.8% of HBV-related and non-HBV-related HCC, respectively.
Frequency and Mutational Hotspots of β-Catenin Mutation
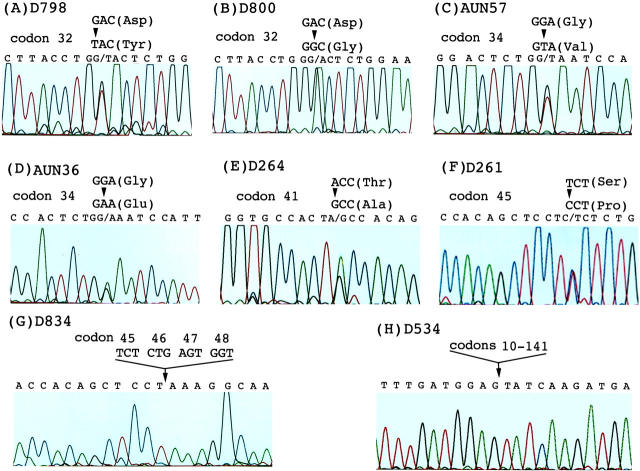
To analyze the pattern and frequency of β-catenin mutations, both unifocal and multifocal HCCs were included. β-Catenin mutations were detected in 57 (13.1%) out of 434 tumors taken from 421 HCC patients, 30 mutations in HBV-related and 27 in non-HBV-related HCC (Figures 1 and 2) ▶ ▶ . Thirty-four mutations (59.6%) were at the phosphorylation sites for the GSK-3β binding, including five at codon 33, five at codon 37, nine at codon 41, and 15 at codon 45. Nineteen mutations (33.3%) occurred outside the phosphorylation sites, including 10 at codon 32, seven at codon 34, and two novel mutations at codon 36. Deletion mutations were found in four tumors.
Figure 1.
Mutations of the β-catenin gene in hepatocellular carcinoma. Representative mutations leading to amino acid substitutions are shown. A–F: Point mutations at codons 32, 34, 41, and 45: Asp 32 → Tyr (A), Asp 32 → Gly (B), Gly 34 → Val (C), Gly 34 → Glu (D), Thr 41 → Ala (E), and Ser45 → Pro (F). A small deletion of codons 45–48 (G) and a large segment deletion of codons 10–141 (H) are indicated.
Figure 2.
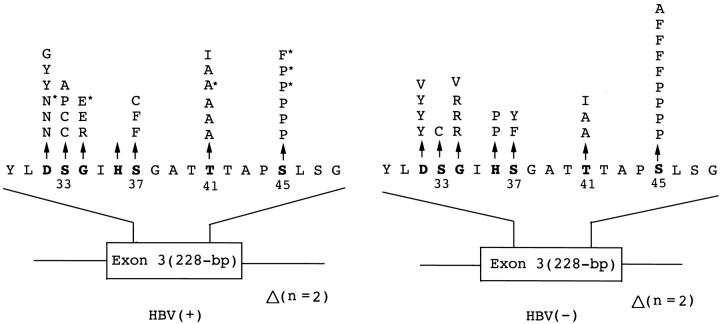
Illustration of the location of 57 mutations of the β-catenin gene in hepatitis B virus-positive HBV(+) and hepatitis B virus-negative HBV(−), primary unifocal and multifocal hepatocellular carcinoma. Of the HBV(−) HCC, 21 (87.5%) of 24 tumors were taken from patients positive for anti-HCV. The amino acid substitutions of 53 point mutations in exon 3 are indicted at the top, whereas four deletion mutations, designated as Δ, are indicated at the bottom. Bold type indicates potential phosphorylation sites on threonine and serine residues. *, designates HBV(+) patients who were also positive for anti-HCV in sera. Single-letter abbreviations for the amino acid residues are: A, Ala; C, Cys; D, Asp; E, Glu; F, Phe; G, Gly; H, His; I, Ile; K, Lys; L, Leu; M, Met; N, Asn; P, Pro; Q, Gln; R, Arg; S, Ser; T, Thr; V, Val; W, Trp; and Y, Tyr.
Correlation of β-Catenin Mutation with Hepatitis Virus Infection
β-Catenin mutations occurred more frequently in non-HBV-related HCCs in both unifocal and multifocal HCCs (P < 0.00001), regardless of presence or absence of anti-HCV (Table 1) ▶ . In addition to the difference in frequency, the pattern of β-catenin mutations also differed between HBV-related and non-HBV-related HCCs.
Table 1.
β-Catenin Mutation in Relation to Chronic Hepatitis B and C Virus Infection in 366 Patients with Unifocal and 55 Patients with Multifocal Primary Hepatocellular Carcinoma
| β-Catenin mutation in HCC (+, %) | P value | |||
|---|---|---|---|---|
| Unifocal | Multifocal | Total | ||
| HBV | ||||
| (+)* | 27/276 (9.8) | 3/47 (6.4)† | 30/323 (9.3)‡ | <0.00001 |
| (−) | 20/90 (22.2) | 7/21 (33.3)† | 27/111 (24.3)‡ | |
| Total | 47/366 (12.8) | 10/68 (14.7) | 57/434 (13.1) | |
| HBV(−) | ||||
| Anti-HCV(+) | 17/74 (23.0) | 6/18 (33.3) | 23/92 (25.0) | NS |
| Anti-HCV(−) | 3/16 (18.8) | 1/3 (33.3) | 4/19 (21.0) | |
*HBV, HCC positive for hepatitis B virus surface antigen or HBV genome in HCC and/or nontumor liver tissues. Anti-HCV, hepatitis C virus antibody in serum. The denominator designates number of tumor nodules examined.
†This includes 34 patients who were positive for serum HBsAg or had HBV genome in liver tissues, or both, and 21 patients negative for HBV.
‡Fisher exact test, two-tailed.
Non-HBV-related HCC had a higher frequency of point mutation at codon 45 (36%, or 9 out of 25 point mutations) than HBV-related HCC (21.4%, or 6 out of 28 point mutations), and three of the six HBV-related HCCs with mutations at codon 45 were also positive for HCV. In contrast, HBV-related HCCs had more frequent mutations at codons 33 and 41 (Figure 2) ▶ . Codons 32 and 34 were also frequently mutated in both groups, but the amino acid substitutions differed (Figure 2) ▶ . The nature of these base substitutions is shown in Table 2 ▶ , transitional mutations predominated in both HBV- and non-HBV-related HCCs, 79% and 64%, respectively, but the non-HBV-related HCC also had a high frequency of G:C to T:A transversion (5 tumors, or 20%).
Table 2.
Nature of Base Substitutions in the 53 Point Mutations of the β-Catenin Gene in Hepatocellular Carcinoma
| Substitutions | HBV(+) | HBV(−) |
|---|---|---|
| Transition | ||
| G:C to A:T | 10 (36) | 9 (36) |
| A:T to G:C | 12 (43) | 7 (28) |
| Transversion | ||
| G:C to T:A | 2 (7) | 5 (20) |
| G:C to C:G | 3 (11) | 1 (4) |
| A:T to C:G | 1 (3) | 2 (8) |
| A:T to T:A | 0 (0) | 1 (4) |
The numerical designates number of base substitution and the parentheses indicate percentage. HBV(+) and HBV(−) designate hepatitis virus (HBV)-related and non-HBV-related HCC, respectively.
Clinicopathological Correlation and Prognostic Significance of β-Catenin Mutations
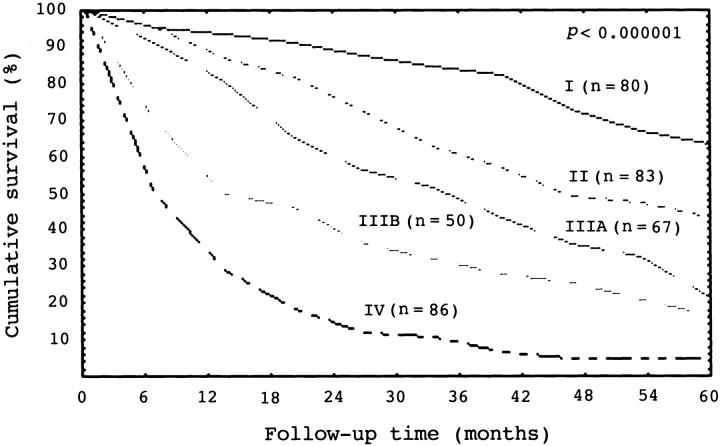
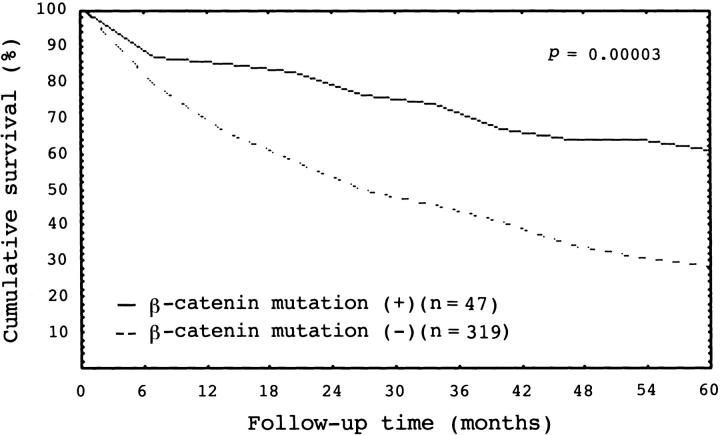
Of the 366 unifocal HCCs selected for clinicopathological analysis, the tumor was ≤2 cm in 31, 2.1 to 5 cm in 143, and larger than 5 cm in 192. Stage I, stage II, stage IIIA, stage IIIB, and stage IV HCCs had 80, 83, 67, 50, and 86 cases, respectively. There was a close correlation between tumor stage and patient’s outcome (P < 0.000001) (Figure 3) ▶ . β-Catenin mutations occurred more frequently in older patients (P = 0.0009), but inversely correlated with serum AFP elevation (P = 0.0001) (Table 3) ▶ . Histologically, β-catenin mutation was associated with grade I HCC (P = 0.005). Moreover, HCC with β-catenin mutations had a significantly higher frequency of stage I and II HCCs, but less commonly high-stage tumor with portal vein invasion (stage IIIB and IV HCCs; P < 0.0001) (Table 3) ▶ . β-Catenin mutations did not correlate with tumor size (Table 3) ▶ and liver cirrhosis (data not shown). HCC with β-catenin mutation had a significantly better 5-year survival rate than HCC without the gene mutation (P = 0.00003) (Figure 4) ▶ .
Figure 3.
Cumulative survival curves for 366 patients with unifocal hepatocellular carcinoma (HCC) according to tumor stage. The 5-year survival rates for stage I, II, IIIA, IIIB, and IV HCC are 63%, 43%, 22%, 17%, and 5%, respectively (log-rank test, P < 0.000001).
Table 3.
Correlation of β-Catenin Mutation with Clinicopathological Features in 366 Patients with Unifocal Primary Hepatocellular Carcinoma
| Feature | β-Catenin mutation | P value | |
|---|---|---|---|
| Present, n = 47 (%) | Absent, n = 319 (%) | ||
| Mean age (year) | 61.6 ± 11.0 | 54.7 ± 13.4 | 0.0009 |
| Gender: Male | 42 (89.4) | 249 (78.1) | NS |
| HBsAg in serum: (+) | 22 (46.8) | 228 (71.5) | 0.0013 |
| Serum AFP ≥ 320 ng/ml | 8 (17.0) | 155 (48.6) | <0.00001 |
| Mean tumor size (cm) | 6.31 ± 3.67 | 7.06 ± 4.49 | NS |
| Tumor grade* | |||
| I | 21 (44.7) | 69 (21.6) | 0.005† |
| II | 14 (29.8) | 131 (41.1) | |
| III | 12 (25.5) | 119 (37.3) | |
| Tumor stage* | |||
| I | 24 (51.2) | 56 (17.6) | <0.0001† |
| II | 10 (21.3) | 73 (22.9) | |
| IIIA | 7 (14.9) | 60 (18.8) | |
| IIIB | 5 (10.6) | 45 (14.1) | |
| IV | 1 (2.1) | 85 (26.6) | |
Abbreviations: HBsAg, hepatitis B surface antigen; AFP, serum α-fetoprotein level.
*See Materials and Methods for details.
†Fisher exact test, two-tailed.
Figure 4.
Cumulative survival curve for 366 patients of primary unifocal hepatocellular carcinoma (HCC). HCC with β-catenin mutations had a significantly better 5-year survival rate (P = 0.00003).
Nature and Prognostic Significance of Nuclear β-Catenin in HCC
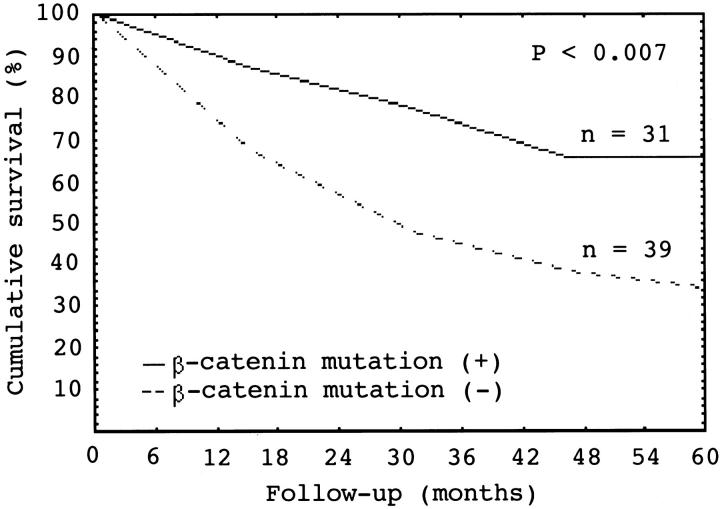
β-Catenin protein was examined by immunohistochemical stain in 282 cases with unifocal HCC. Of these, 212 expressed membranous β-catenin alone, whereas nuclear accumulation of β-catenin, ranging from diffuse to scattered cells, was detected in 70 cases (Figure 5) ▶ . β-Catenin mutation was significantly associated with nuclear expression of the protein, 83.8% (or 31 out of 37) versus 15.9% (or 39 out of 245) (P < 0.00001). We failed to detect point or large fragment deletion mutations of β-catenin gene in 39 tumors with nuclear expression of β-catenin, presumably wild-type protein. HCCs with mutant nuclear β-catenin expression had significantly higher 5-year survival rate than HCCs with wild-type nuclear β-catenin expression (66% versus 34%, P < 0.007) (Figure 6) ▶ .
Figure 5.
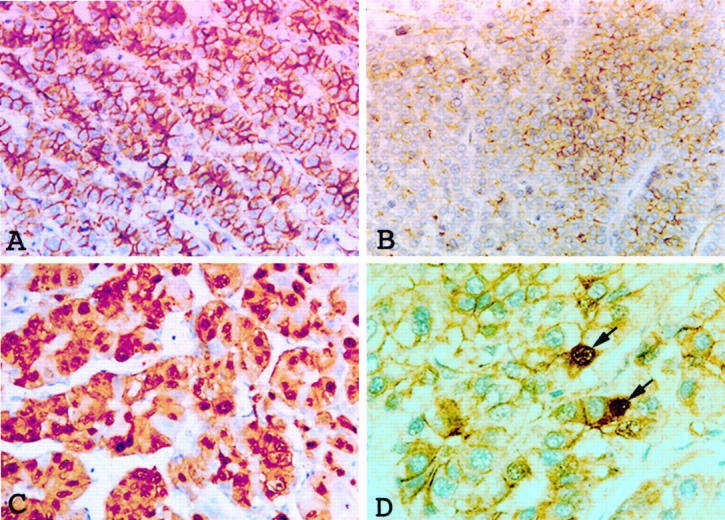
Expression of β-catenin protein. A: Diffuse staining of β-catenin along the tumor cell membranes of HCC is shown. B: Membranous staining of β-catenin of mixed positive (right) and negative areas (left) is shown. C: Abundant tumor cell nuclei are strongly positive for β-catenin. D: Scattered tumor cells are positive for nuclear β-catenin (arrows). Immunohistochemical stain for β-catenin; original magnification ×340 (A–D).
Figure 6.
Cumulative survival curve for primary unifocal hepatocellular carcinoma (HCC) that had nuclear β-catenin protein expression. HCC with nuclear β-catenin protein expression and β-catenin mutation (n = 31) had a better 5-year survival rate than HCC with nuclear β-catenin protein expression but without mutation of the gene (n = 39) (P < 0.007, log-rank test).
Discussion
In our study, β-catenin mutation was detected in 57 (13.1%) out of 434 tumors taken from 421 patients with resected primary HCC, a frequency lower than those (18.7% to 41%) reported from Japan and France, 23,24,33 where HCV is more prevalent. 34,35 HCC is known to be closely associated with HBV and HCV infection, and the two HCC populations differ in several important characteristics. 34,35 We demonstrated that HBV-related HCC had a younger mean age (P < 0.00001), higher male-to-female ratio (P < 0.003), serum AFP elevation >320 ng/ml (P = 0.002), and familial history of HCC (P < 0.014). Despite these differences, the molecular mechanisms for the HBV- and HCV-related hepatocarcinogenesis remain elusive. In contrast to the observations of Legoix et al, 36 we demonstrated that the β-catenin gene was more frequently mutated in non-HBV-related HCC, that was predominantly (84%, or 89 out of 106 cases examined) positive for anti-HCV, than in HBV-related HCC (25% versus 9.3%, P < 0.00001). Moreover, non-HBV-related HCC had predominant mutation at codon 45, particularly when HCCs with HBV and HCV co-infection were taken into consideration, but had less frequent mutations at codons 33 and 41. In contrast, HBV-related HCC had more frequent mutations at codons 33 and 41. The two groups of tumors also differed in patterns of amino acid substitution and base substitution, although not statistically significant because of the small number of cases positive for β-catenin mutation and the presence of cases with HBV and HCV co-infection. Nevertheless, our findings indicate that β-catenin mutation plays a more important role in the tumorigenesis of non-HBV-related than in HBV-related HCC and the difference in frequency and pattern of the gene mutations reflects the different etiology of the two groups of HCC. This suggestion is supported by the difference in frequency of β-catenin mutation in HCCs induced by different chemical carcinogens, ranging from 54% to 13%. 25,37 The patterns of β-catenin mutations also differed among experimental animal and human liver tumors of different etiologies. Point mutations occurred predominantly in chemically induced liver tumors, whereas deletion mutations occurred more often (58.1%) in liver tumors developing in transgenic mice harboring the c-myc oncogene 23 and in human hepatoblastoma (57.7% to 62.5%). 32,38
Of the 57 mutations, 34 (59.6%) occurred at the serine/threonine residues in the GSK-3β phosphorylation consensus motif of β-catenin, 39 four had deletion mutations, and 19 (33.3%) had mutations outside these phosphorylation residues: at codons 32 (10 tumors), 34 (seven tumors), and 36 (two tumors), flanking the serine residues at codons 33 and 37. Our findings confirm that codons 32 and 34 are mutational hot spots in both human and animal HCC. 23,24 The frequent mutations at codons 32 and 34 that are uncommon in colorectal carcinoma, 5 melanoma, 9 and endometrial carcinoma 14 provide a novel β-catenin activating mechanism in human and murine HCC. 23,24 These findings indicate that different types of β-catenin mutations reflect different etiologies of carcinogenesis in specific tissue. 24
Despite the frequent mutations of β-catenin in various types of human cancer, the clinical implication is not fully understood. To elucidate the role of β-catenin mutations, we selected the 366 cases of unifocal HCC for further statistical analysis. In contrast to the observations of Legoix et al, 36 we found that mutations of β-catenin in HCC correlated with several important clinicopathological features of HCC. In addition to having an older mean age (P = 0.0009), HCC with β-catenin mutations correlated inversely with serum AFP elevation (P < 0.00001), and positively with grade I HCC (P = 0.005). Moreover, HCC with β-catenin mutations was mostly stage I and II HCC (52.5%), whereas HCC without β-catenin mutations often had high-stage tumor with portal vein invasion (stage IIIB and IV HCC, 40.7%) (P < 0.0001). In this study, we demonstrated that HCC with β-catenin mutation had a more favorable outcome (P = 0.00003). This finding was in accord with the results that tumor stage closely correlated with prognosis (P < 0.000001). The reasons for the inverse correlation of β-catenin mutation with tumor aggression are not clear. Nevertheless, our results are in accord with the observations that mutations of β-catenin were observed in various types of benign human neoplasms, 18,19 and low-stage endometrioid ovarian carcinoma with good prognosis. 40,41 Large fragment deletion of β-catenin was detected in two out of five hepatocellular adenomas that is not a precursor of HCC (Hsu, unpublished data). N-terminally deleted β-catenin is associated with differentiated morphology and reduced invasiveness and metastasis. 42 Whether β-catenin mutations that are predominantly point mutations in HCC also possess invasion/metastasis suppressive potential in human HCC deserves more studies for clarification.
In the nucleus, β-catenin regulates transcription of target genes by binding the Tcf-Lef family of DNA binding proteins, 3-8 and enhanced wild-type β-catenin expression is critical for the tumor suppressive effect of APC. 5 We found that β-catenin mutations were strongly associated with nuclear β-catenin expression (P < 0.00001), confirming that mutations of β-catenin lead to decreased degradation and accumulation of the protein in the nucleus. 3,4,11 In addition to the close association of nuclear β-catenin expression with the gene mutation, reflecting mutant β-catenin, we also demonstrated nuclear β-catenin expression in 39 HCCs that had no point or large fragment deletion mutation of the gene, presumably reflecting wild-type β-catenin. Moreover, HCC with mutant nuclear β-catenin expression had a significantly higher 5-year survival rate (P < 0.007). These findings suggest that mutant and wild-type nuclear β-catenin proteins are not functionally equivalent. Because β-catenin mutation is associated with nuclear protein accumulation, our results also suggest that it is the qualitative change of β-catenin protein, rather than its quantity change in the nucleus, that is important for the better survival of HCC patients with the gene mutation. More studies are hence warranted to better understand the molecular mechanisms of the potential tumor invasion/metastasis suppressive effect of mutant β-catenin in HCC.
Footnotes
Address reprint requests to Dr. Hey-Chi Hsu, Department of Pathology, National Taiwan University Hospital, 7 Chung-Shan South Road, Taipei, Taiwan. E-mail: heychi@ha.mc.ntu.edu.tw.
Supported by the National Science Council of the Republic of China, Taiwan (NSC88–0419-002 to H. C. H.), and the Hauman Enterprises Co., Ltd., Taipei, Taiwan (to H. C. H.)
References
- 1.Jou TS, Stewart DB, Stappert J, Nelson WJ, Marrs JA: Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc Natl Acad Sci USA 1995, 92:5067-5071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larabell CA, Torres M, Rowning BA, Yost C, Miller JR, Wu M, Kimelman D, Moon RT: Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in β-catenin that are modulated by the Wnt signaling pathway. J Cell Biol 1997, 136:1123-1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Funayama N, Fagotto F, McCrea P, Gumbiner BM: Embryonic axis induction by the armadillo repeat domain of β-catenin: evidence for intracellular signaling. J Cell Biol 1995, 128:959-968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yost C, Torres M, Miller JR, Huang E, Kimelman D: The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev 1996, 10:1443-1454 [DOI] [PubMed] [Google Scholar]
- 5.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW: Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 1997, 275:1787-1790 [DOI] [PubMed] [Google Scholar]
- 6.Miller JR, Moon RT: Analysis of the signaling activities of localization mutants of β-catenin during axis specification in Xenopus. J Cell Biol 1997, 139:229-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W: Functional interaction of β-catenin with the transcription factor LEF-1. Nature 1996, 382:638-642 [DOI] [PubMed] [Google Scholar]
- 8.Korinek V, Barkerm N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H: Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 1997, 275:1784-1787 [DOI] [PubMed] [Google Scholar]
- 9.Rubinfeld B, Souza B, Albert I, Müller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P: Association of the APC gene product with β-catenin. Science 1993, 262:1731-1734 [DOI] [PubMed] [Google Scholar]
- 10.Su LK, Vogelstein B, Kinzler KW: Association of the APC tumor suppressor protein with catenins. Science 1993, 262:1734-1737 [DOI] [PubMed] [Google Scholar]
- 11.Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW: Serine phosphorylation-regulated ubiquitination and degradation of β-catenin. J Biol Chem 1997, 272:24735-24738 [DOI] [PubMed] [Google Scholar]
- 12.Sparks AB, Morin PJ, Vogelstein B, Kinzler KW: Mutational analysis of the APC/β-catenin/Tcf pathway in colorectal cancer. Cancer Res 1997, 58:1130-1134 [PubMed] [Google Scholar]
- 13.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P: Stabilization of β-catenin by genetic defects in melanoma cell lines. Science 1997, 275:1790-1792 [DOI] [PubMed] [Google Scholar]
- 14.Fukuchi T, Sakamoto M, Tsuda H, Maruyama K, Nozawa S, Hirohashi S: β-Catenin mutation in carcinoma of the uterine endometrium. Cancer Res 1998, 58:3526-3528 [PubMed] [Google Scholar]
- 15.Voeller HJ, Truica CI, Gelmann EP: β-Catenin mutations in human prostate cancer. Cancer Res 1998, 58:2520-2523 [PubMed] [Google Scholar]
- 16.Dashwood RH, Suzui M, Nakagama H, Sugimura T, Nagao M: High frequency of β-catenin (Ctnnb1) mutations in the colon tumors induced by two heterocyclic amines in the F344 rat. Cancer Res 1998, 58:1127-1129 [PubMed] [Google Scholar]
- 17.Takahashi M, Fukuda K, Sugimura T, Wakabayashi K: β-Catenin is frequently mutated and demonstrates altered cellular location in azoxymethane-induced at colon tumors. Cancer Res 1998, 58:42-46 [PubMed] [Google Scholar]
- 18.Chan EF, Gat U, McNiff JM, Fuchs E: A common human skin tumour is caused by activating mutations in β-catenin. Nat Genet 1999, 21:410-413 [DOI] [PubMed] [Google Scholar]
- 19.Samowitz WS, Powers MD, Spirio LN, Nollet F, van Roy F, Slattery ML: β-Catenin mutations are frequent in small colorectal adenomas than in larger adenomas and invasive carcinomas. Cancer Res 1999, 59:1442-1444 [PubMed] [Google Scholar]
- 20.Hsu HC, Wu TT, Wu MZ, Sheu JC, Lee CS, Chen DS: Tumor invasiveness and prognosis in resected hepatocellular carcinoma: clinical and pathogenetic implications. Cancer 1988, 61:2095-2099 [DOI] [PubMed] [Google Scholar]
- 21.Hsu HC, Tseng HJ, Lai PL, Lee PH, Peng SY: Expression of p53 gene in 184 unifocal hepatocellular carcinomas: association with tumor growth and invasiveness. Cancer Res 1993, 53:4691-4694 [PubMed] [Google Scholar]
- 22.Hsu HC, Peng SY, Lai PL, Chu JS, Lee PH: Mutations of p53 gene in hepatocellular carcinoma (HCC) correlate with tumor progression and patient prognosis: a study of 138 patients with unifocal HCC. Int J Oncol 1994, 4:1341-1347 [DOI] [PubMed] [Google Scholar]
- 23.de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, Perret C: Somatic mutations of the β-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA 1998, 95:8847-8851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyoshi Y, Iwao K, Nagasawa Y, Aihara T, Sasaki Y, Imaoka S, Murata M, Shimano T, Nakamura Y: Activation of the β-catenin gene in primary hepatocellular carcinomas by somatic alterations involving exon 3. Cancer Res 1998, 58:2524-2527 [PubMed] [Google Scholar]
- 25.Ogawa K, Yamada Y, Kishibe K, Ishizaki K, Tokusashi Y: β-Catenin mutations are frequent in hepatocellular carcinomas but absent in adenomas induced by diethylnitrosamine in B6C3F1 mice. Cancer Res 1999, 59:1830-1833 [PubMed] [Google Scholar]
- 26.Hsu HC, Chiou TJ, Chen ZY, Lee CS, Lee PH, Peng SY: Clonality and clonal evolution of hepatocellular carcinoma with multiple nodules. Hepatology 1991, 13:923-928 [PubMed] [Google Scholar]
- 27.Peng SY, Lai PL, Chu JS, Lee PH, Tsung PT, Chen DS, Hsu HC: Expression and hypomethylation of alpha-fetoprotein gene in unicentric and multicentric human hepatocellular carcinomas. Hepatology 1993, 17:35-41 [PubMed] [Google Scholar]
- 28.Lee CS, Sung JL, Hwang LY, Sheu JC, Chen DS, Lin TY, Beasley RP: Surgical treatment of 109 patients with symptomatic and asymptomatic hepatocellular carcinoma. Surgery 1986, 99:481-490 [PubMed] [Google Scholar]
- 29.Franco D, Capussotti L, Smadja C, Bouzari H, Meakins J, Kemeny F, Grange D, Dellepiane M: Resection of hepatocellular carcinomas: results in 72 European patients with cirrhosis. Gastroenterology 1990, 98:733-738 [PubMed] [Google Scholar]
- 30.Peng SY, Chou SP, Hsu HC: Association of downregulation of cyclin D1 and of overexpression of cyclin E with p53 mutation, high tumor grade and poor prognosis in hepatocellular carcinoma. J Hepatol 1998, 29:281-289 [DOI] [PubMed] [Google Scholar]
- 31.Nollet F, Berx G, Molemans F, van Roy F: Genomic organization of the human β-catenin gene (CTNNB1). Genomics 1996, 32:413-424 [DOI] [PubMed] [Google Scholar]
- 32.Koch A, Denkhaus D, Albrecht S, Leuschner I, von Schweinitz D, Pietsch T: Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the β-catenin gene. Cancer Res 1999, 59:269-273 [PubMed] [Google Scholar]
- 33.Huang H, Fujii H, Sankila A, Mahler-Araujo BM, Matsuda M, Cathomas G, Ohgaki H: β-Catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis C virus infection. Am J Pathol 1999, 155:1795-1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanizaki H, Ryu M, Kinoshita T, Kawano N, Konishi M, Cho A, Nakatsura T, Natsume T, Takahashi S, Sugita M, Izuishi K, Yoshino M, Furuse J, Iwasaki M, Tsubono Y: Comparison of clinical features and survival in patients with hepatitis B and C virus-related hepatocellular carcinoma. Jpn J Clin Oncol 1997, 27:67-70 [DOI] [PubMed] [Google Scholar]
- 35.Stroffolini T, Andreone P, Andriulli A, Ascione A, Craxi A, Chiaramonte M, Galante D, Manghisi OG, Mazzanti R, Medaglia C, Pilleri G, Rapaccini GL, Simonetti RG, Taliani G, Tosti ME, Villa E, Gasbarrini G: Characteristics of hepatocellular carcinoma in Italy. J Hepatol 1998, 29:944-952 [DOI] [PubMed] [Google Scholar]
- 36.Legoix P, Bluteau O, Bayer J, Perret C, Balabaud C, Belghiti J, Franco D, Thomas G, Laurent-Puig P, Zucman-Rossi J: Beta-catenin in hepatocellular carcinoma correlate with a low rate of loss of heterozygosity. Oncogene 1999, 18:4044-4046 [DOI] [PubMed] [Google Scholar]
- 37.Tsujiuchi T, Tstutumi M, Sasaki Y, Takahama M, Konishi Y: Different frequencies and patterns of β-catenin mutations in hepatocellular carcinomas induced by N-nitrosodiethylamine and a choline-deficient l-amino acid-defined diet in rats. Cancer Res 1999, 59:3904-3907 [PubMed] [Google Scholar]
- 38.Jeng YM, Wu MZ, Mao TL, Chang MH, Hsu HC: Somatic mutations of β-catenin play a crucial role in the tumorigenesis of sporadic hepatoblastoma. Cancer Lett 2000, 152:45-51 [DOI] [PubMed] [Google Scholar]
- 39.Peifer M, Pai LM, Casey M: Phosphorylation of the Drosophila adherens junction protein Armadillo: roles for wingless signal and Zeste-white 3 kinase. Dev Biol 1994, 166:543-556 [DOI] [PubMed] [Google Scholar]
- 40.Palacios J, Gamallo C: Mutations in the β-catenin (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res 1998, 58:1344-1347 [PubMed] [Google Scholar]
- 41.Gamallo C, Pacacios J, Moreno G, Calvo de Mora J, Suárez A, Armas A: β-Catenin expression pattern in stage I and II ovarian carcinomas: relationship with β-catenin mutations, clinicopathological features, and clinical outcome. Am J Pathol 1999, 155:527–536 [DOI] [PMC free article] [PubMed]
- 42.Shibata T, Ochiai A, Kanai Y, Akimoto S, Gotoh M, Yasui N, Machinami R, Hirohashi S: Dominant-negative inhibition of the association between β-catenin and c-erbB-2 by N-terminally deleted β-catenin suppresses the invasion and metastasis of cancer cells. Oncogene 1996, 13:883-889 [PubMed] [Google Scholar]