Abstract
Tangier disease (TD) and familial HDL deficiency (FHA) have recently been linked to mutations in the human ATP-binding cassette transporter 1 (hABCA1), a member of the ABC superfamily. Both diseases are characterized by the lowering or lack of high-density lipoprotein cholesterol (HDL-C) and low serum cholesterol. The murine ABCA1−/− phenotype corroborates the human TD linkage to ABCA1. Similar to TD in humans, HDL-C is virtually absent in ABCA1−/− mice accompanied by a reduction in serum cholesterol and lipid deposition in various tissues. In addition, the placenta of ABCA1−/− mice is malformed, resulting in severe embryo growth retardation, fetal loss, and neonatal death. The basis for these defects appears to be altered steroidogenesis, a direct result of the lack of HDL-C. By 6 months of age, ABCA1−/− animals develop membranoproliferative glomerulonephritis due to deposition of immunocomplexes followed by cardiomegaly with ventricular dilation and hypertrophy, ultimately succumbing to congestive heart failure. This murine model of TD will be very useful in the study of lipid metabolism, renal inflammation, and cardiovascular disease and may reveal previously unsuspected relationships between them.
The ABC superfamily comprises myriad transmembrane proteins involved in the transport of vitamins, peptides, steroid hormones, ions, sugars, and amino acids. 1 A subset of the superfamily contains four closely related proteins (ABCA1, ABCA2, ABCA3, and ABCA4). 2-4 The protein structure of this subfamily consists of two halves joined by a linker region, each with six transmembrane domains and an ATP-binding cassette site. Known genetic diseases resulting from dysfunctional ABC transporters are cystic fibrosis, Zellweger syndrome, adrenoleukodystrophy, multidrug resistance, and Stargardt macular dystrophy. 4-8 Expression of ABCA1 is inducible by cholesterol loading, and cAMP treatment and can be reduced with apolipoproteins. 9,10
The relationship between Tangier disease (TD), familial HDL deficiency (FHA), and mutation in the human ABCA1 gene has recently been established by positional cloning of hABCA1 from families of TD or FHA patients. 11-15 In each case, point mutations, deletions, or frameshifts create a nonfunctional or truncated hABCA1 protein. Individuals suffer from reduced or absent high-density lipoprotein cholesterol (HDL-C) and lower serum cholesterol; accumulate cholesteryl esters in tonsils, spleen, thymus, and liver; and may exhibit corneal clouding and peripheral neuropathy due to deposition of cholesteryl esters. 16 Fibroblasts from TD patients have a markedly reduced HDL-mediated efflux of cholesterol. 17,18 Enhanced catabolism of HDL and its precursors in vivo has also been reported in TD patients. 19 In other cholesterol metabolic diseases such as Apo A-I deficiency, lecithin-cholesterol acyltransferase deficiency, and Fish Eye disease, HDL-C is also reduced, but serum cholesterol levels remain normal. 16 Low levels of HDL-C usually indicate a high risk factor for coronary heart disease. Interestingly, the loss of HDL-C in TD patients causes only a minor predisposition to coronary heart disease. 20
Tangier disease is exceedingly rare, with only 40 reported families worldwide exhibiting the trait. As such, except for the absence of HDL-C, it is difficult to evaluate which symptoms may be directly attributed to a dysfunctional ABCA1 transporter. Our murine model of Tangier disease, which is described here, confirms the association of ABCA1 with TD and exhibits other phenotypes that have not yet been described in humans.
Materials and Methods
Disruption of the ABCA1 Gene and Generation of ABCA1-Deficient Mice
Mouse ABCA1 genomic clones were isolated from a 129/Ola mouse genomic library. The mouse ABCA1 gene contains 48 exons (GenBank X75926). 2 An 8.5-kb mouse DNA fragment containing exons 18–22 of the mouse ABCA1 gene was used to prepare the knockout construct (Figure 1A) ▶ . A cassette containing a neomycin resistance gene driven by a strong herpes simplex virus-thymidine kinase promoter and a polyadenylation signal was used to replace a 1.9-kb DNA region, which contains exons 19 and 20 and part of exon 21 of the gene. The neomycin resistance gene cassette serves as a selectable marker and truncates the transcript of the targeted gene. The deleted exons encode most of the first ATP binding cassette of the ABCA1 protein. A herpes simplex virus-thymidine kinase cassette was placed at the 3′ end of the knockout construct. The DNA construct was introduced into E14 embryonic stem cells by electroporation. Cells were cultured in the presence of 400 μg/ml G418 and 0.2 μM gancyclovir. Embryonic stem cells with the disrupted gene were detected by polymerase chain reaction (PCR) and then confirmed by Southern hybridization, using a DNA probe flanking the 3′ end of the construct. Chimeric mice were generated from embryos injected with embryonic stem cells. Germline mice were obtained from breeding of chimeric male mice with C57BL/6J females. ABCA1+/− mice were crossbred to obtain mice homozygous for the disrupted ABCA1 gene, and the lines were maintained with brother/sister crossings. Mice were maintained with Purina Formulab 5008 chow and water ad libitum.
Figure 1.
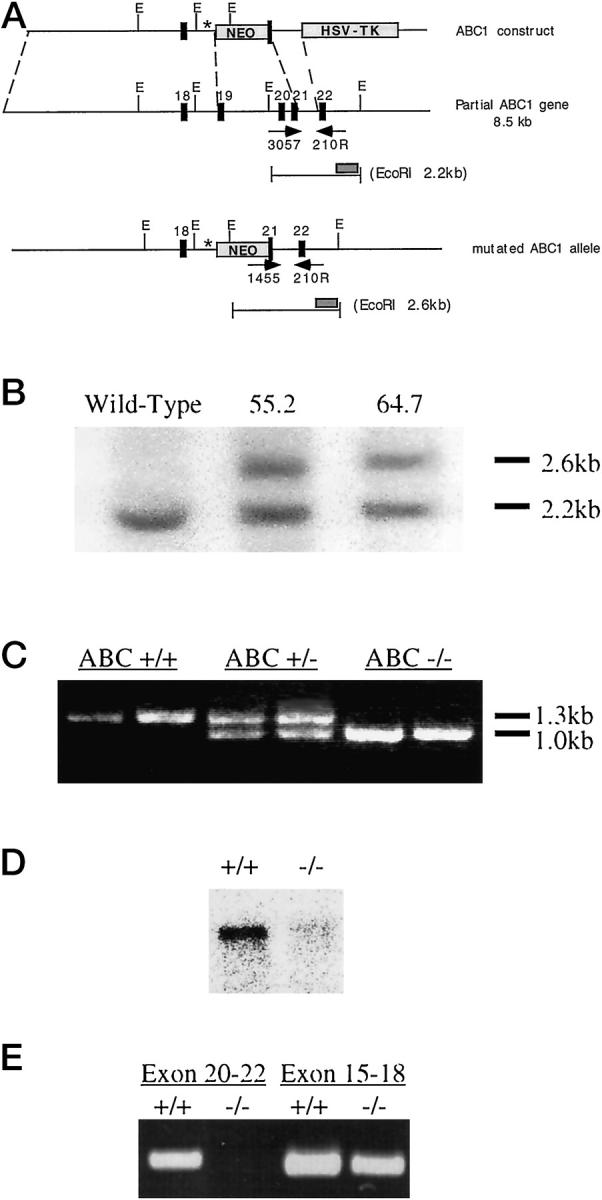
Disruption of the ABCA1 gene. A: Design of ABCA1 knockout construct. A mouse genomic DNA fragment containing exons 18–22 of the ABCA1 gene is shown. Exons 19 and 20 and part of exon 21 of the ABCA1 gene, which encode most of the first ATP binding cassette of the ABCA1 protein, was replaced with a neomycin resistance gene cassette containing stop codons (*) in all three frames at the 5′ end. Arrows denote PCR primers used to verify genotypes. The hybridization probe used in Southern blot analysis is also shown as a gray bar. E, EcoRI. B: Southern blot confirmation of the disrupted ABCA1 gene. DNA extracted from the ES cell lines 55.2 and 64.7 was digested with EcoRI and hybridized with the DNA probe shown in A. A 2.2-kb DNA band from the functional ABCA1 gene and a 2.6-kb DNA band from the disrupted ABCA1 gene hybridized to the probe. C: PCR genotyping of blood lysates from mice. The functional ABCA1 gene was amplified by oligonucleotides 3057 and 210R and resulted in a 1.3-kb PCR band. The disrupted ABCA1 gene was detected by oligonucleotides 1455 and 210R to yield a 1.0-kb PCR band. D: Northern Blot analysis of ABCA1 expression in ABCA1+/+ and ABCA1−/− mice. An ABCA1 cDNA probe (LM243) covering exons 26–31 of the ABCA1 gene, a region downstream of the disruption site, was used to detect the 7.8-kb ABCA1 mRNA in ABCA1+/+ and ABCA1−/− peritoneal macrophages. E: Detection of truncated ABCA1 mRNA in ABCA1−/− mice. ABCA1 expression was detected by reverse transcription-PCR amplification of RNA from ABCA1+/+ and ABCA1−/− placenta. A region covering exons 15–18, which is 5′ to the disrupted site, was detected as a 456-bp PCR band. A region covering exons 20–22, which is 3′ to the disrupted site, was detected by PCR as a 455-bp PCR band.
PCR and Southern and Northern Blot Analysis
Three oligonucleotides were used in PCR amplification to detect both the endogenous and altered ABCA1 genes simultaneously. The sense primers ABC3057 (5′-GAGCACATCTGGTTCTATGC-3′), located in exon 20, and Neo1455 (5′-CGCTTCCTCGTGCTTTACGGTAT-3′) are specific for the endogenous and altered ABCA1 genes, respectively. Primer ABC210R (5′-AAGACACGGTGCTGCTACTGTT-3′) lies downstream of the knockout region in exon 22. PCR amplification results in a 1.3-kb band from the wild-type gene and a 1.0-kb band from the knockout gene. PCR amplification was carried out as follows: 94°C 1 minute, 54°C 1 minute, 72°C 2 minutes for two cycles; 94°C 15 seconds, 54°C 40 seconds, 72°C 1 minute 30 seconds for 40 cycles followed by a 5-minute elongation period at 72°C. Southern blot analysis was carried out by digestion of genomic DNA with EcoRI. Digested DNA was then run on a 1% agarose gel and vacuum-blotted according to manufacturer’s instructions (BioRad, Hercules, CA). Southern blots were then hybridized to a probe that lies 3′ to the region covered by the knockout construct. The probe hybridizes to a 2.2-kb band from EcoRI-digested ABCA1 gene and to a 2.6-kb band from a knockout ABCA1 gene. Northern Blot analysis was carried out by extracting total RNA from thioglycolate-treated macrophages derived from the peritoneum of wild-type or ABCA1−/− mice. Total RNA was run on a denaturing formaldehyde gel and blotted overnight with standard methods. Northern Blots were then hybridized to a probe named LM243, which covers ABCA1 exons 26–31. Total RNA from gestation day 16 placenta derived from wild-type or ABCA1−/− mice was extracted and reverse transcribed to cDNA with standard methods. Primers ABC2370 (5′-CTGTGCGTAGCCTGGCAGGACTAT-3′) and ABC2826R (5′-CAACCTTCATGCCATCTCGGTAA-3′) or ABC3057 and ABC210R (see above) were used in PCR to amplify exons 15–18 (456 bp) and exons 20–22 (455 bp), respectively, of the ABCA1 cDNA. PCR amplification was carried out as follows: 94°C 1 minute, 57°C 2 minutes, 72°C 2 minutes for two cycles; 94°C 45 seconds, 62°C 45 seconds, 72°C 2 minutes for 40 cycles followed by a 10-minute elongation period at 72°C.
Lipid and Steroid Hormone Analysis
Cholesterol and HDL-C from six mice of each genotype were directly measured by standard enzymatic means on an Olympus AU5200 multichannel analyzer. Tissues taken for lipid analysis were frozen in OCT, cut, stained with Oil Red O, and counterstained with hematoxylin (San Diego Pathology Laboratory). Tissues taken for birefringence analysis of free cholesterol crystals were frozen in OCT, cut, and immediately viewed at 25°C with a pair of polarizing lenses purchased from Fisher (no. 12-572PA).
Tissues taken for cholesteryl ester staining by the perchloric acid-naphthoquinone (PAN) method were frozen in OCT, freshly cut, and fixed in formol-calcium (3.7% formaldehyde and 1% CaCl2) for 1 minute and allowed to air dry. Slides were treated with 1% FeCl3 for 4 hours, washed with distilled water, then painted with freshly made PAN reagent (1 mg/ml 1:2 naphthoquinone-4-sulfonic acid, 50% ethanol, 15% perchloric acid, 1% formaldehyde), using a camel hair brush. Slides were then heated on a surface at 70°C for 1–2 minutes until the color developed, with occasional replenishment of the PAN reagent. Tissues were then mounted with a drop of perchloric acid, coverslipped, and immediately photographed.
Tissues taken for free cholesterol staining using the digitonin-PAN method were cut, fixed, and dried as described above. Free cholesterol was precipitated in tissues with 0.5% digitonin in 40% ethanol for 2 hours. Cholesterol esters were then extracted by incubation with acetone for 1 hour at room temperature. Slides were then treated with FeCl3 and PAN as described above.
Progesterone was analyzed by a standard chemiluminescence assay and estrogen by a standard extraction radioimmune assay. All clinical chemistry assays were performed at Quest Diagnostics (San Diego, CA).
Tail-Flick and Hot-Plate Test
Ten ABCA1+/+ and 11 ABCA1−/− mice were tested for peripheral neuropathy by standardized methods. 21,22 The tail-flick test consisted of immersion of the tail in a 55°C water bath, and latency to a rapid tail flick was measured. Withdrawal of the tail from the water terminated the test with a maximum cut-off time of 10 seconds. The hot-plate test consisted of placing the mouse on a surface heated to 53°C. Stamping of the hind feet, washing of the front paws, or jumping from the heat source terminated the test with a maximum cut-off time of 30 seconds.
In Situ Hybridization
Placenta from gestation day 14 pregnancies was fixed in buffered formalin and paraffin embedded. In situ hybridization was performed with 35S-riboprobes on this tissue with an adapted protocol. 23 The tissue was then put on x-ray film for 6 days, after which the tissue was dipped in NBT2 nuclear emulsion (Kodak) and kept desiccated in the dark at 4°C for 22 days. Slides were developed, stained with hematoxylin and eosin (H&E), and studied under the microscope to observe the distribution of ABCA1 mRNA detected by the LM243 cRNA probe.
RNA Probes
[35S]UTP-labeled antisense and sense probes for LM243 were synthesized after linearization with EcoRI or HindIII, using T7 or T3 RNA polymerase, respectively. The labeled sense strands served as controls and did not show any specific labeling of cellular localization (data not shown). Each slide received 100 μl of hybridization mixture, containing the [35S]UTP-labeled probe (10 7 cpm/ml). All restriction enzymes and phage RNA polymerases were obtained from Boehringer Mannheim (Indianapolis, IN).
Tissue Fixation and Staining
Tissue was either fixed in buffered formalin, paraffin embedded, and stained with hematoxylin and eosin or quick frozen in OCT and fixed with acetone at −20°C. Frozen tissue sections were processed and stained using VectaStain (catalog no. PK-6100) according to manufacturer’s instructions (Vector Labs, Burlingame, CA). Rat anti-CD71 (catalog no. 015191D; Pharmingen, San Diego, CA) was used at a concentration of 5 μg/ml, sheep anti-mouse immunoglobulin (catalog no. 1092 618; Boehringer Mannheim) at a concentration of 0.5 μg/ml, and goat anti-mouse C3 (catalog no. 55444; ICN, Aurora, OH) at a dilution of 1:500. Biotinylated rabbit anti-rat IgG (catalog no. BA-4001), biotinylated rabbit anti-sheep IgG (catalog no. BA-6000), and biotinylated rabbit anti-goat IgG (catalog no. BA-5000) were used at a dilution of 1:200 (Vector Labs). Frozen sections were stained with 3-amino-9-ethylcarbazole, counterstained with hematoxylin, and mounted.
Results
Verification and Screening of ABCA1 Functional Knockout
Gene targeting technology was used to disrupt the ABCA1 gene. Most of the N-terminal ATP binding cassette of the ABCA1 gene was replaced with the neomycin resistance gene cassette (Figure 1A) ▶ . The neomycin resistance cassette harbors stop codons in all three reading frames at the 5′ end, blocking translation of the ABCA1 protein 3′ to the insertion. Two separate lines of mice capable of germline transmission were generated from two independent embryonic stem cell clones with the disrupted ABCA1 gene (55.2 and 64.7). Southern blot and PCR analysis identified the functional and disrupted ABCA1 alleles, confirming the mutation (Figure 1, B and C) ▶ . The ABCA1 mRNA 3′ to the disruption is not detectable in ABCA1−/− animals, as demonstrated in Northern blot analysis of RNA extracted from ABCA1−/− macrophages (Figure 1D) ▶ .
Using primers either upstream or downstream of the site of disruption, reverse transcription-PCR was performed to investigate the possibility of truncated ABCA1 mRNA being expressed in ABCA1−/− mice. Total RNA was extracted from placenta derived from either ABCA1−/− or wild-type animals and was reverse transcribed by standard methods. PCR primers that amplify exons 15–18, which are directly upstream of the disrupted region, clearly indicate that this portion of the ABCA1 transcript is present in both wild-type and homozygous animals. PCR primers that amplify exons 20–22, however, fail to amplify the ABCA1 transcript in homozygous animals (Figure 1E) ▶ . These results suggest that RNA transcribed from the disrupted ABCA1 gene is truncated at the site of the neomycin resistance cassette insertion.
HDL Loss and Lipid Deposition Is the Direct Result of ABCA1 Dysfunction
The primary hallmark of Tangier disease is the lack of HDL-C, lowered serum cholesterol, and deposition of cholesteryl esters in various tissues. TD is inherited in an autosomal recessive fashion, though the HDL-C and cholesterol levels found in heterozygotes are half the normal values. 11,13,14 The more common disease, FHA, is inherited as an autosomal dominant trait characterized by a low HDL-C phenotype, but without the clinical manifestation of TD. Four FHA familial cohorts have been found bearing mutations in the ABCA1 gene. 12,15 As in TD patients, HDL-C levels in both lines of ABCA1−/− mice are minimal, while in heterozygous mice HDL-C levels are roughly one-half of the levels found in normal mice (Table 1 ▶ , paired t-test, P < 0.002). Cholesterol levels are also significantly reduced in a similar manner (paired t-test, P < 0.008).
Table 1.
HDL-C and Cholesterol Levels in ABCA1−/− Mice Are Significantly Reduced
| Genotype | HDL-C (mg/dl) | Cholesterol (mg/dl) |
|---|---|---|
| ABCA1+/+ | 77.3 ± 6.1 | 100.7 ± 6.2 |
| ABCA1+/− | 41.0 ± 8.9 | 60.2 ± 13 |
| ABCA1−/− | 2.3 ± 0.5 | 22.6 ± 9.8 |
Also reminiscent of human TD, lipid deposition is prevalent in the thymus, liver, and testes of ABCA1−/− mice as determined by Oil Red O staining (Figure 2) ▶ . Lipid in the ABCA1−/− thymus appears mainly in the medulla and heavily borders the interface between the medulla and cortex (Figure 2A) ▶ . The lipid appears specific to macrophages, as determined by immunohistochemical staining with anti-F4/80 (data not shown). Faint lipid staining can be observed in some macrophages in the ABCA1+/+ thymus. Lipid staining is minimal in the ABCA1+/+ liver. In contrast, lipid is heavily deposited in large vacuoles throughout the liver of ABCA1−/− mice (Figure 2B) ▶ . Lipid deposition in both wild-type and ABCA1−/− testes is present primarily in the Leydig cells, the site of testosterone synthesis. However, in ABCA1−/− mice additional lipid deposition is seen in the Sertoli cells lining the seminiferous tubules (Figure 2C) ▶ . These cells contain Oil Red O-positive material, as well as large vacuoles, giving the seminiferous tubules a “Swiss cheese” appearance.
Figure 2.
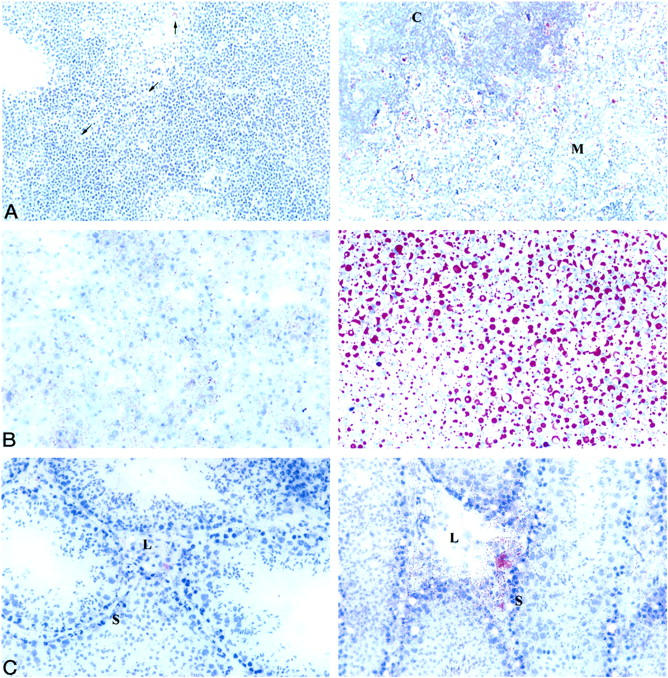
Lipid deposition in ABCA1−/− thymic, hepatic, and testicular tissues. A: Oil red O staining of lipid in thymus derived from ABCA1+/+ (left) and ABCA1−/− mice (right). Original magnification, ×250. Arrows indicate lipid-stained macrophages in ABCA1+/+ thymus. C, cortex; M, medulla; n = 6 ABCA1+/+ and 9 ABCA1−/− mice. B: Oil red O staining of lipid in liver derived from ABCA1+/+ (left) and ABCA1−/− (right) mice. Original magnification, ×400. n = 4 ABCA1+/+ and 4 ABCA1−/− mice. C: Oil red O staining of lipid in testes derived from ABCA1+/+ (left) and ABCA1−/− mice (right). Original magnification, ×400. L, Leydig cells; S, Sertoli cells; n = 4 ABCA1+/+ and 3 ABCA1−/− mice. Sections were cut from frozen, unfixed tissue.
Several other tissues were also tested for lipid deposition. With a minimum of four wild-type and ABCA1−/− animals, we found no evidence of lipid deposition in the spleen, lung, heart, or kidney of ABCA1−/− mice (data not shown). In addition, five aged ABCA1−/− mice (>6 months) were checked for the presence of lipid in the sciatic nerve and aorta. No abnormalities were found (data not shown). Taken together, the data suggest that the murine version of Tangier disease is identical to the human disease with regard to HDL-C loss, the lowering of serum cholesterol, and lipid deposition in specific tissues.
Another characteristic of Tangier disease is peripheral neuropathy. We used the tail-flick test and hot-plate test to determine whether the peripheral nervous system of ABCA1−/− mice was affected by any lipid deposition that we had failed to observe. 21,22 No significant difference was found in the withdrawal of either the tail or paws of ABCA1−/− mice from the heat source when compared to wild-type mice (data not shown). These results suggest that a peripheral sensory neuropathy is not present in our ABCA1−/− model.
Nonproductive Mating and Neonatal Death Are Prevalent in ABCA1−/− Mice
Only 8.4% of the offspring from crosses of ABCA1+/− mice are ABCA1−/− mice compared to the expected Mendelian rate of 25% (Table 2 ▶ , χ 2 test, P < 0.0005). In heterozygote/homozygote crosses, only 28% of the pups are ABCA1−/− mice, as opposed to the expected Mendelian rate of 50% (χ 2 test, P < 0.0005).
Table 2.
Mendelian Ratio of ABCA1 Litters are Biased Against ABCA1−/− Mice
| Cross Female × male | Offspring genotype, No. of mice | ||
|---|---|---|---|
| ABCA1+/+ | ABCA1+/− | ABCA1−/− | |
| ABCA1+/−× ABCA1+/− | 77 (30.8%) | 152 (60.8%) | 21 (8.4%) |
| ABCA1+/−× ABCA1−/− | — | 54 (71.0%) | 22 (28.9%) |
ABCA1−/− pups are less likely to survive to be weaned at 3 weeks of age (Table 3 ▶ , paired t-test, P < 0.0005). ABCA1−/− pups often succumbed in the first 24–48 hours after birth. Necropsy indicated the lungs were congested with blood accompanied by extensive bronchopulmonary dysplasia, consistent with severe respiratory distress (Figure 3) ▶ . In addition, eight neonates from eight different litters runted as early as 5 days after birth and died from similar causes by 2.5 weeks.
Table 3.
Litter Size Changes Significantly after Birth in ABCA1−/− Litters
| Female × male | Litter size at birth | Litter size at weaning |
|---|---|---|
| ABCA1+/+× ABCA1+/+ | 4.7 ± 1.8 | 4.6 ± 1.7 |
| ABCA1+/−× ABCA1−/− | 4.6 ± 2.5 | 4.0 ± 2.5 |
| ABCA1−/−× ABCA1−/− | 4.2 ± 1.5 | 1.9 ± 1.8 |
Figure 3.
Neonatal lungs of ABCA1−/− mice are severely congested. Lungs from 1-day-old neonates, wild type (left), and ABCA1−/− (right) mice were H&E stained. Original magnification, ×100.
ABCA1−/− females are also difficult to breed. Mating of ABCA1+/− or ABCA1−/− female mice with ABCA1−/− male mice was monitored daily for the presence of a vaginal plug to determine that mating had taken place (Table 4) ▶ . ABCA1−/− females failed 55% of the time to become pregnant after mating, as determined by visual inspection throughout the 3-week term of pregnancy (χ 2 test, P < 0.0005). No fertility problems were observed with male ABCA1−/− mice.
Table 4.
Mating of ABCA1−/− Female Mice Sporadically Results in Pregnancy
| Genotype | Vaginal plug | Plug/no pregnancy | Pregnancy |
|---|---|---|---|
| ABCA1+/− (n = 13) | 31 | 1 | 30 |
| ABCA1−/− (n = 19) | 38 | 21 | 17 |
Functional Loss of ABCA1 Results in Severe Placental Malformation and Fetal Distress
The marked loss of neonates shortly after birth and the biased Mendelian ratios led to an examination of fetal development. ABCA1 mRNA expression in murine placenta was studied using in situ hybridization. In normal mice, prominent ABCA1 mRNA expression is clearly seen in the lining of decidual maternal blood vessels and is present throughout the labyrinthine trophoblast layer (Figure 4, A and B) ▶ . In contrast, ABCA1 mRNA was observed only in the deciduae of an ABCA1−/− placenta taken from an ABCA1+/− female.
Figure 4.
ABCA1 mRNA distribution in maternal deciduae and placenta. A: In situ hybridization showed that ABCA1 mRNA was expressed in the lining of maternal decidual blood vessel derived from a 64.7 ABCA1+/− female at day 14 gestation. Original magnification, ×250. B: Autoradiograph of ABCA1+/+ (top) and ABCA1−/− (bottom) placentas harvested from the same 64.7 ABCA1+/− female, hybridized to LM243 ABCA1 cDNA probe. L, labyrinth; D, deciduae.
Severe malformations were often noted in multiple ABCA1−/− and ABCA1+/− placentas. DNA was extracted from embryos to determine genotype, and the placentas were examined by H&E staining or immunohistochemistry. In wild-type placenta, a well-formed labyrinth is composed of a lacy, open structure with a consistent symmetry as shown in Figure 5A ▶ (left). Most ABCA1−/− placentas exhibited a more cellular labyrinth, displaying distorted symmetry by day 14 of gestation (Figure 5A ▶ , right). Disrupted placental architecture with hemorrhages, cell debris, and ragged inclusions of the spongiotrophoblast are prevalent (Figure 5B ▶ , right). All ABCA1+/− placentas presented with milder structural abnormalities in the labyrinthine trophoblast (Figure 5B ▶ , middle). No abnormalities were noted in the wild-type placentas (Figure 5B ▶ , left). Intrauterine growth retardation is evident for ABCA1−/− embryos, accompanied by fetal death and resorption in utero at gestation day 14 (Figure 5C) ▶ . As pregnancy progresses, fetal ABCA1−/− distress becomes even more apparent. The uterus of ABCA1−/− females appears inflamed on gross examination, with thick green-brown mucous. Multiple resorptions, runts, dead fetuses, and necrotic-appearing placenta are also apparent (Table 5) ▶ . The amniotic sacs are stained brown, consistent with the release of meconium into the amniotic fluid (Figure 5D ▶ , right). Often half of the remaining pups are dead in utero, as measured by lack of reflexive movement. Microscopic analysis of the placenta demonstrated the same features as those described for gestation day 14 (Figure 5E) ▶ . In contrast, all wild-type fetuses at gestation day 19 appeared healthy and normal.
Figure 5.
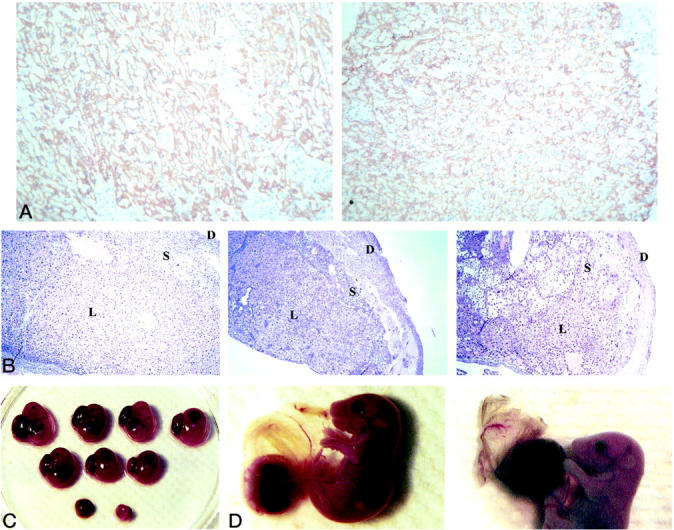

Placenta malformation and fetal distress in ABCA1−/− mice. A: Placental labyrinth from day 14 of gestation was immunostained with anti-CD71 (specific for trophoblasts). Placentas were ABCA1+/+ (left) and 64.7 ABCA1−/− (right). Original magnification, ×100. B: Placental labyrinth from day 14 of gestation was H&E stained. Placentas were ABCA1+/+ (left), ABCA1+/− (middle), and ABCA1−/− (right). Original magnification, ×63. L, labyrinth; S, spongiotrophoblast; D, deciduae; n = 20 ABCA1−/−, 13 ABCA1+/−, and 22 ABCA1+/+ placentas assayed. C: Intrauterine growth retardation of 64.7 ABCA1−/− embryos. Embryos at gestation day 14 were collected from an ABCA1−/− cross and are arranged by size. Sizes of the ABCA1−/− embryos vary from normal (top left) to resorption (bottom). D: Brown-stained amniotic sac observed in ABCA1−/− (right) but not ABCA1+/+ (left) embryos at day 19 gestation. E: Placenta labyrinth from ABCA1+/+ (left), ABCA1+/− (middle), and ABCA1−/− (right) day 19 gestation embryos was H&E stained. Original magnification, ×63. L, labyrinth; S, spongiotrophoblast; D, deciduae; n = 4 ABCA1−/−, 5 ABCA1+/−, and 7 ABCA1+/+ placentas. F: Resorbing embryos were collected from ABCA1+/+ (left) or ABCA1−/− (right) cross at day 19 of gestation. G: Resorbing placenta from ABCA1+/+ (left) or ABCA1−/− (right) embryos at day 19 of gestation were H&E stained. Original magnification, ×100.
Table 5.
Placental Malformation Results in Increased Fetal Mortality at Gestation Day 19
| Cross | Live/runt | Resorption/dead in utero |
|---|---|---|
| ABCA1−/− | 4 /2 | 15 /3 |
| ABCA1+/+ | 7 /0 | 1 /0 |
Resorbing tissues from both gestation day 14 and day 19 ABCA1−/− animals appear very different from the wild-type resorption that was found (Figure 5F) ▶ . The placental disk and fetal remnants of the wild-type absorption are easily distinguished. On microscopic examination the various cell layers characteristic of the placenta are still present, although they are clearly undergoing autolysis (Figure 5G ▶ , left). In contrast, the ABCA1−/− resorptions are much smaller brown and red balls or, if the resorption is advanced enough, tiny yellow-brown pellets. When microscopically examined, the less advanced resorptions appeared necrotic, with extensive hemorrhage, large numbers of inflammatory cells, cellular debris, and little to no evidence of residual placental tissue (Figure 5G ▶ , right). The yellow-brown pellets consisted of clot only (data not shown).
Thus loss of functional ABCA1 consequently results in a severe developmental defect of the placenta, leading to intrauterine growth retardation and neonatal death, and markedly reduces female ability to bear young.
Lipid and Steroid Hormone Levels Are Significantly Altered in ABCA1−/− Mice
The low level of HDL-C and serum cholesterol, combined with morphological defects in the placenta, led to the speculation that steroidogenesis was altered in ABCA1−/− mice. Steroidogenic tissues were examined for the presence of cholesterol, which is required for the biosynthesis of steroids. The adrenal glands and ovaries of ABCA1+/+ and ABCA1−/− females were analyzed for lipid content (including cholesteryl esters) by Oil Red O staining. Lipid is clearly seen in the adrenal cortex of ABCA1+/+ females, most particularly in the layers that synthesize glucocorticoids (Figure 6A) ▶ . In the ovaries, lipid was found in the cells surrounding the developing follicles and in the corpus luteum of ABCA1+/+ females, regions in which estrogen and progesterone, respectively, are synthesized (Figure 6B) ▶ . Lipid is distinctly absent from ABCA1−/− female adrenal glands and is vastly reduced in the ovaries, most notably in the corpus luteum. ABCA1+/+ and ABCA1−/− placentas were also examined with Oil Red O. Neither the ABCA1+/+ nor the ABCA1−/− placentas stained for the presence of lipid (data not shown).
Figure 6.
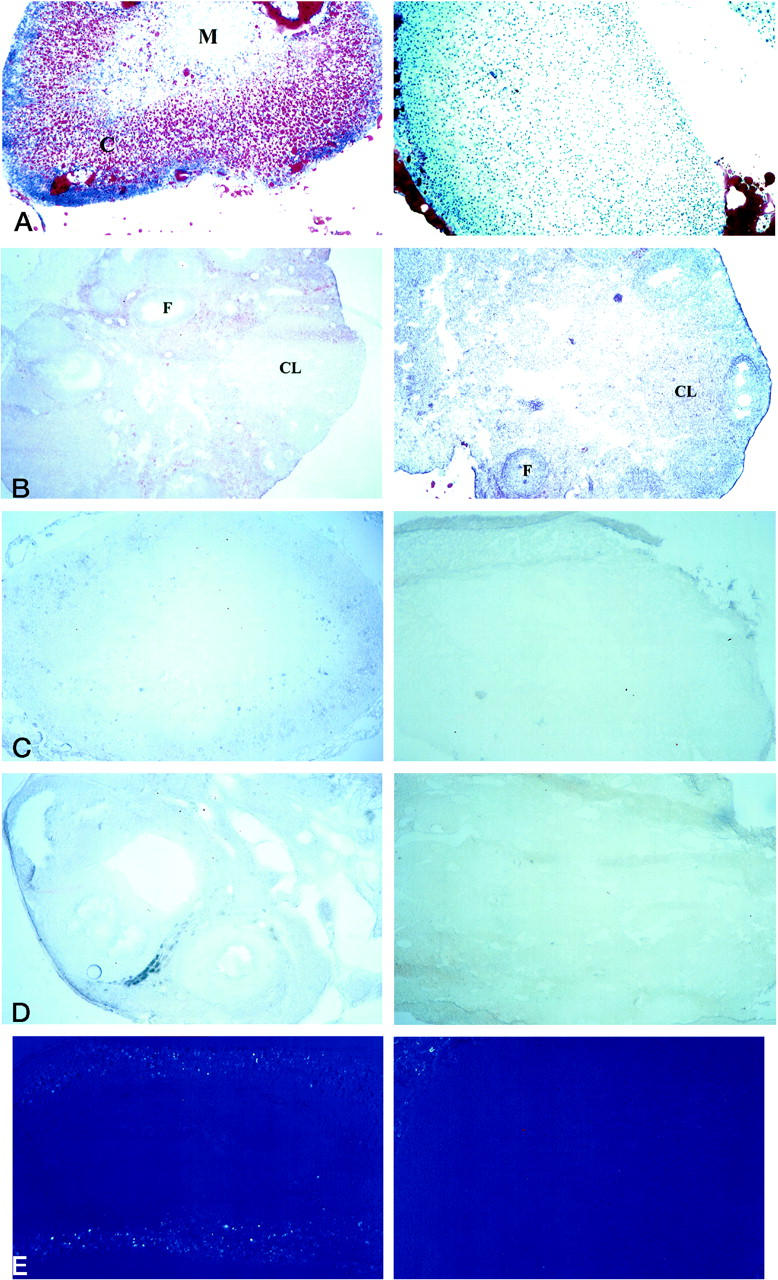
Dramatic decrease of lipid storage in ABCA1−/− adrenals and ovaries. Sections of adrenal glands and ovaries from ABCA1 +/+ (left) and ABCA1−/− (right) are shown. A: Oil red O staining of lipid in adrenal gland. Original magnification, ×100. C, cortex; M, medulla. B: Oil red O staining of lipid in ovary. Original magnification, ×63. F, follicle; CL, corpus luteum. C: PAN staining of cholesteryl esters in adrenal gland. Original magnification, ×63. D: PAN staining of cholesteryl esters in ovary. Original magnification, ×100; n = 4 ABCA1+/+ and 5 ABCA1−/− females. E: Birefringence of free cholesterol crystals in adrenal gland. Original magnification, ×100. The minor amount of birefringence that is visible at the edge of the ABCA1−/− adrenal gland is from attached fat. Sections were cut from frozen, unfixed tissue.
Oil red O stains a wide variety of lipids but is not specific for cholesteryl esters. Adrenal glands and ovaries from the same ABCA1+/+ and ABCA1−/− females described above were therefore examined using the PAN method or birefringence to specifically assay the level of cholesteryl esters and free cholesterol, respectively. Similar to the pattern seen with the Oil Red O staining described above, the PAN method creates a blue stain of cholesteryl esters, which are visible in the adrenal cortex and surround the ovary follicles of wild-type females (Figure 6, C and D) ▶ . No cholesteryl esters were visible in either the adrenal glands or ovaries of ABCA1−/− female mice. Birefringence of free cholesterol crystals was observed in the adrenal cortex of ABCA1+/+ females and was absent from ABCA1−/− females (Figure 6E) ▶ . Ovaries from both groups failed to exhibit any cholesterol birefringence (data not shown). A variation of the PAN method that utilizes digitonin to stain specifically for free cholesterol was used to confirm the birefringence data. Free cholesterol was visible in the adrenal cortex and ovary follicles of wild-type females in a similar pattern observed with Oil Red O or normal PAN staining, but not in the adrenal glands or ovaries derived from ABCA1−/− female mice (data not shown).
The distinct alterations of lipid content in both adrenal glands and ovaries in ABCA1−/− females suggest a possible change in steroidogenesis in ABCA1−/− mice. Progesterone and estrogen are of particular interest, as both are required and very tightly regulated during pregnancy. 24, 25 Serum was taken from ABCA1+/+ and ABCA1−/− females at gestation day 16 for hormonal analysis. Progesterone and estrogen levels are 53% and 62.8% lower than normal, respectively, in ABCA1−/− females compared to ABCA1+/+ females (Table 6 ▶ , paired t-test, P < 0.02 and P < 0.01, respectively).
Table 6.
Progesterone and Estrogen Levels Are Diminished in ABCA1−/− Pregnant Females
| Genotype | Progesterone (ng/ml) | Estrogen (pg/ml) |
|---|---|---|
| ABCA1+/+ (n = 8) | 86.7 ± 36.6 | 589.75 ± 147.4 |
| ABCA1−/− (n = 5) | 40.7 ± 17.7 | 219.6 ± 47.4 |
In summary, cholesterol and cholesteryl esters are lacking in the adrenal glands and ovaries of ABCA1−/− female mice as determined by Oil Red O staining, birefringence, and PAN staining. The plasma levels of the steroid hormones progesterone and estrogen in ABCA1−/− females are also reduced during late gestation pregnancy.
Loss of ABCA1 Results in Immune Complex Deposition in Kidney Glomeruli and Congestive Heart Failure
ABCA1−/− mice that survive to be weaned appear to develop normally and mature into apparently healthy adults. Between 4 and 6 months of age the ABCA1−/− mice of both lines begin to develop respiratory distress and shed granular casts into their urine. ABCA1+/− females at 1 year of age also exhibit similar distress. Necropsy examination of all distressed animals reveals lungs heavily filled with blood and cardiomegaly with dilated and hypertrophied left and right ventricles. There is occasional evidence of vasculitis around the cardiac vessels. The kidneys are pale tan in color. Microscopic examination reveals boxcar nuclei in the heart consistent with cardiac hypertrophy with frank pulmonary hemorrhages, as well as severe congestion of the lungs, liver, and spleen and scarred kidney glomeruli. The glomeruli show evidence of inflammatory infiltrates, thickened and “split” glomerular basement membranes, and proliferation of mesangial cells (Figure 7A) ▶ . Immunohistochemistry confirms the deposition of both immunoglobulin and C3 complement components in the glomeruli characteristic of membranoproliferative glomerulonephritis type I (Figure 7B) ▶ .
Figure 7.
Kidney glomerulonephritis and immunoglobulin deposition in ABCA1−/− mice. A: H&E-stained kidney sections from healthy ABCA1+/+ (left), inflammatory cell-infiltrated ABCA1−/− (middle), and diseased 55.2 ABCA1−/− (right) mice. Original magnification, ×250. Scarring of glomeruli was visible when sections were stained with trichrome (data not shown). G, glomerulus. B: Heavy deposition of immunoglobulin was found in glomeruli of 64.7 ABCA1−/− animal (right) but was not present in ABCA1+/+ glomeruli (left). Original magnification, ×100.
Conclusion
Loss of ABCA1 function in mice results in loss of HDL-C and lowered serum cholesterol. This clearly demonstrates the link between Tangier disease and FHA in humans with ABCA1 dysfunction and establishes that ABCA1 plays a role in cholesterol metabolism. The presence of heavy lipid deposition in the liver of ABCA1−/− mice as well as in tissues with a high rate of cell turnover such as the thymus and testes further confirms this relationship. We would describe the phenotype in ABCA1 mice as being autosomal dominant, because heterozygosity confers a marked reduction of HDL-C and cholesterol, malformed placenta and congestive heart failure. Furthermore, the lowered serum cholesterol distinguishes the ABCA1 mice as a model of Tangier disease, distinct from the other cholesterol metabolic diseases, and may further elucidate why the loss of HDL-C causes only a minor predisposition to coronary heart disease in TD patients.
After submission of this manuscript for publication, another murine model of Tangier disease was described by Orso et al. 26 Consistent with our model, a marked loss of HDL-C and serum cholesterol was observed. Decreased body weight and platelet aggregation, hemorrhagic diathesis, fat-soluble vitamin deficiency, and an increase in cholesterol birefringence in the adrenal glands were also noted by Orso et al. We have not observed any decrease in body weight or platelet aggregation in our TD model. We have also observed a distinct lack of cholesterol and cholesteryl esters in the adrenal glands of our TD model, in contrast to the model described by Orso et al, and verified our results by Oil Red O staining, birefringence, and the PAN method. The variation between these models may be attributable to the C57BL/6 genetic background of our model versus the DBA/1 genetic background of the model described by Orso et al. Dietary differences may also have contributed to these variations. A comparison between the Formulab 5008 diet used by our facility and the Altromin 1324 standard feed used by Orso et al reveals that the Formulab diet to be richer in fat and protein and supplemented with multiple fat-soluble vitamins, A, D, E, and K1. The richer diet fed to our murine model may have prevented some of the phenotypes noted by Orso et al. Further study directly comparing the two diets may be warranted.
Human TD has not previously been described as causing developmental problems or defective morphogenesis of the placenta. This may be due to differing physiologies because, unlike humans, mice have low LDL and high HDL and apparently have a limited ability to compensate for the loss of HDL. 27, 28 High-level expression of ABCA1 mRNA in the placenta has been noted in humans. 10 We have shown here for the first time that ABCA1 mRNA expression in placenta is specific to the labyrinthine trophoblast layer. It is not surprising, then, to discover in the murine model of TD that major structural defects exist in the placenta.
Alterations in cholesterol metabolism due to ABCA1 loss may directly affect the normal production of steroids. In the ApoAI knockout mouse, HDL-C levels are extremely low, and staining with Oil Red O demonstrated a distinct lack of lipid in the adrenal glands and ovaries, suggesting reduced cholesteryl ester storage. 27, 28 Adrenal stress response was blunted in ApoAI knockout animals as determined by cold water swimming or the administration of ACTH. The SR-BI (scavenger receptor-class BI) knockout mouse also lacks lipid in the adrenal glands and ovaries, as determined by Oil Red O stain. 29, 30 The primary pathway by which steroidogenic cells acquire cholesterol is apparently by the uptake of HDL. 31, 32 Our analysis of the adrenal glands and ovaries of ABCA1−/− mice indicates a phenotype very similar to that of the ApoAI and SR-BI knockout models and suggests that the marked reduction of pregnancies in ABCA1−/− females may be attributed to altered steroidogenesis. In addition, we have provided evidence demonstrating a distinct reduction of progesterone and estrogen levels in pregnant ABCA1−/− females, supporting the hypothesis that impaired steroidogenesis results in malformation of the placenta.
The overload of the reticuloendothelium system with cholesteryl esters is the basic cause of disease in human TD. Tissues that have a high rate of cell turnover should therefore contain lipid-overloaded macrophages because of an equally high rate of phagocytosis. Hence the phagocytotic cells of the testes and thymus are prime candidates for lipid overload. The lipid-laden Sertoli cells and thymic macrophages of ABCA1−/− mice suggest that this is true in murine TD. Previous research with murine macrophages indicates a possible dysfunction in phagocytosis associated with lack of ABCA1 function. 33 We postulate that lipid overload of ABCA1−/− macrophages may prevent normal phagocytosis of immune complexes. Alternatively, evidence exists that the major component of HDL, ApoAI, associates with clusterin, a complement lysis inhibitor. 34 The severe alteration of HDL-C in ABCA1−/− mice may in turn affect the normal formation of immune complexes and result in a similar phenotype. Indeed, several complement deficiency diseases eventually manifest with glomerulonephritis. 35 Failure to phagocytose immune complexes or to activate complement would result in a build-up of immune complexes in the blood of ABCA1−/− mice. Immune complexes are clearly present in the kidney glomeruli of ABCA1−/− mice, as are inflammatory infiltrates, classic hallmarks of glomerulonephritis. The dilated cardiomyopathy may result from a failure of the normal renal homeostatic mechanisms that control blood pressure due to the heavy scarring of the kidney glomeruli. However, the presence of vasculitis in the vessels of the heart suggests that the loss of ABCA1 function may lead to a general dysregulation of immune complex formation and/or clearance, resulting in immune complex deposition in multiple organs.
The murine model of Tangier disease demonstrates that loss of ABCA1 function may be more detrimental than previously suspected. The marked female inability to bear young, impaired steroidogenesis, and placental malformation may indicate severe developmental consequences in humans if normal ABCA1 function is affected. The renal failure due to immune complex deposition in homozygous animals may also indicate a previously unsuspected risk factor among patients with FHA or TD. Further study of the ABCA1−/− mouse model will allow better dissection of lipid metabolism and its connection with early development and the immune system.
Acknowledgments
We thank Philip Ginsburg, Irene Bressanutti, the Clinical Chemistry staff of Quest Diagnostics, Shelly Courtney, and Julie Culver for technical assistance.
Footnotes
Address reprint requests to Dr. Wai-Ping Fung-Leung, R. W. Johnson Pharmaceutical Institute, 3210 Merryfield Row, San Diego, CA 92121.
Dr. Voland’s current address: Department of Immunopathology, BD PharMingen, San Diego, CA 92121.
References
- 1.Higgins CF: ABC-transporters: from microorganisms to man. Annu Rev Cell Biol 1992, 8:67-113 [DOI] [PubMed] [Google Scholar]
- 2.Luciani MF, Denizot F, Savar S, Mattei MG, Chimini G: Cloning of two novel ABC transporters mapping on human chromosome 9. Genomics 1994, 21:150-159 [DOI] [PubMed] [Google Scholar]
- 3.Connors TD, Van Raay TJ, Petry LR, Klinger KW, Landes GM, Burn TC: The cloning of a human ABC gene (ABC3) mapping to chromosome 16p13.3. Genomics 1997, 39:231–234 [DOI] [PubMed]
- 4.Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, Rattner A, Smallwood P, Li Y, Anderson KL, Lewis RA, Nathans J, Leppert M, Dean M, Lupski JR: A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet 1997, 15:236-246 [DOI] [PubMed] [Google Scholar]
- 5.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL: Identification of the cystic fibrosis gene—cloning and characterization of complementary DNA. Science 1989, 245:1066-1072 [DOI] [PubMed] [Google Scholar]
- 6.Gartner J, Moser H, Valle D: Mutations in the 70k peroxisomal membrane-protein gene in Zellweger syndrome. Nat Genet 1992, 1:16-23 [DOI] [PubMed] [Google Scholar]
- 7.Mosser J, Douar AM, Sarde CO, Kioschis P, Feil R, Moser H, Poustka AM, Mandel JL, Aubourg P: Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 1993, 361:726-730 [DOI] [PubMed] [Google Scholar]
- 8.Gottesman MM, Patan I: Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem 1993, 62:385-428 [DOI] [PubMed] [Google Scholar]
- 9.Lawn RM, Wade DP, Garvin MR, Wang X, Schwartz K, Porter JG, Seilhamer JJ, Vaughan AM, Oram JF: The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J Clin Invest 1999, 104:R25-R31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langmann T, Klucken J, Reil M, Liebisch G, Luciani MF, Chimini G, Kaminski WE, Schmitz G: Molecular cloning of the human ATP-binding cassette transporter 1 (hABC1): evidence for sterol-dependent regulation in macrophages. Biochem Biophys Res Commun 1999, 257:29-33 [DOI] [PubMed] [Google Scholar]
- 11.Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G: The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet 1999, 22:347-351 [DOI] [PubMed] [Google Scholar]
- 12.Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J, Hayden MR: Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet 1999, 22:336-345 [DOI] [PubMed] [Google Scholar]
- 13.Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denefle P, Assmann G: Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet 1999, 22:352-355 [DOI] [PubMed] [Google Scholar]
- 14.Remaley AT, Rust S, Rosier M, Knapper C, Naudin L, Broccardo C, Peterson KM, Koch C, Arnould I, Prades C, Duverger N, Funke H, Assman G, Dinger M, Dean M, Chimini G, Santamarina-Fojo S, Fredrickson DS, Denefle P, Brewer HB, Jr: Human ATP-binding cassette transporter 1 (ABC1): genomic organization and identification of the genetic defect in the original Tangier disease kindred. Proc Natl Acad Sci USA 1999, 96:12685-12690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcil M, Brooks-Wilson A, Clee SM, Roomp K, Zhang LH, Yu L, Collins JA, van Dam M, Molhuizen HO, Loubster O, Ouellette BF, Sensen CW, Fichter K, Mott S, Denis M, Boucher B, Pimstone S, Genest J, Jr, Kastelein JJ, Hayden MR: Mutations in the ABC1 gene in familial HDL deficiency with defective cholesterol efflux. Lancet 1999, 354:1341-1346 [DOI] [PubMed] [Google Scholar]
- 16.Assmann G, von Eckardstein A, Brewer HB, Jr: Familial high density lipoprotein deficiency: Tangier disease. Scriver CR Beaudet AL Sly WS Valle D eds. The Metabolic and Molecular Basis of Inherited Disease. 1995, :pp 2053-2072 McGraw-Hill, New York [Google Scholar]
- 17.Francis GA, Knopp RH, Oram JF: Defective removal of cellular cholesterol and phospholipids by apolipoprotein A-I in Tangier disease. J Clin Invest 1995, 96:78-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogler G, Trumbach B, Klima B, Lackner KJ, Schmitz G: HDL-mediated efflux of intracellular cholesterol is impaired in fibroblasts from Tangier disease patients. Arterioscler Thromb Vasc Biol 1995, 15:683-690 [DOI] [PubMed] [Google Scholar]
- 19.Bojanovski D, Gregg RE, Zech LA, Meng MS, Bishop C, Ronan R, Brewer HB, Jr: In vivo metabolism of proapolipoprotein A-I in Tangier disease. J Clin Invest 1987, 80:1742-1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serfaty-Lacrosniere C, Civeira F, Lanzberg A, Isaia P, Berg J, Janus ED, Smith MP, Jr, Pritchard PH, Frohlich J, Lees RS: Homozygous Tangier disease and cardiovascular disease. Atherosclerosis 1994, 107:85-98 [DOI] [PubMed] [Google Scholar]
- 21.Polt R, Porreca F, Szabo LZ, Bilsky EJ, Davis P, Abbruscato TJ, Davis TP, Harvath R, Yamamura HI, Hruby VJ: Glycopeptide enkephalin analogues produce analgesia in mice: evidence for penetration of the blood-brain barrier. Proc Natl Acad Sci USA 1994, 91:7114-7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheardown MJ, Shannon HE, Swedberg MD, Suzdak PD, Bymaster FP, Olesen PH, Mitch CH, Ward JS, Sauerberg P: M1 receptor agonist activity is not a requirement for muscarinic antinociception. J Pharmacol Exp Ther 1997, 281:868-875 [PubMed] [Google Scholar]
- 23.Simmons DM, Arriza JL, Swanson LW: A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radiolabeled single-stranded RNA probes. J Histotechnol 1989, 12:169-181 [Google Scholar]
- 24.Miller BG: Effects of ovarian hormones on foetal and placental growth in the mouse. Aust J Biol Sci 1978, 31:641-648 [DOI] [PubMed] [Google Scholar]
- 25.Milligan SR, Finn CA: Minimal progesterone support required for the maintenance of pregnancy in mice. Hum Reprod 1997, 12:602-607 [DOI] [PubMed] [Google Scholar]
- 26.Orso E, Broccardo C, Kaminski WE, Bottcher A, Liebisch G, Drobnik W, Gotz A, Chambenoit O, Diederich W, Langmann T, Spruss T, Luciani MF, Rothe G, Lackner KJ, Chimini G, Schmitz G: Transport of lipids from Golgi to plasma membrane is defective in Tangier disease patients and ABC1-deficient mice. Nat Genet 2000, 24:192-196 [DOI] [PubMed] [Google Scholar]
- 27.Voyiaziakis E, Goldberg IJ, Plump AS, Rubin EM, Breslow JL, Huang LS: ApoA-I deficiency causes both hypertriglyceridemia and increased atherosclerosis in human apoB transgenic mice. J Lipid Res 1998, 39:313-321 [PubMed] [Google Scholar]
- 28.Plump AS, Erickson SK, Weng W, Partin JS, Breslow JL, Williams DL: Apolipoprotein A-I is required for cholesteryl ester accumulation in steroidogenic cells and for normal adrenal steroid production. J Clin Invest 1996, 97:2660-2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M: A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci USA 1997, 94:12610-12615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trigatti B, Rayburn H, Vinals M, Braun A, Miettinen H, Penman M, Hertz M, Schrenzel M, Amigo L, Rigotti A, Krieger M: Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci USA 1999, 96:9322-9327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gwynne JT, Maheffee D, Brewer HB, Jr, Ney RL: Adrenal cholesterol uptake from plasma lipoproteins: regulation by corticotropin. Proc Natl Acad Sci USA 1976, 73:4329-4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kovanen PT, Schneider WJ, Hillman GM, Goldstein JL, Brown MS: Separate mechanisms for the uptake of high and low density lipoproteins by mouse adrenal gland in vivo. J Biol Chem 1979, 254:5498-5505 [PubMed] [Google Scholar]
- 33.Luciani MF, Chimini G: The ATP binding cassette transporter ABC1 is required for the engulfment of corpses generated by apoptotic cell death. EMBO J 1996, 15:226-235 [PMC free article] [PubMed] [Google Scholar]
- 34.Jenne DE, Lowin B, Peitsch MC, Bottcher A, Schmitz G, Tschopp J: Clusterin (complement lysis inhibitor) forms a high density lipoprotein complex with apolipoprotein A-I in human plasma. J Biol Chem 1991, 266:11030-11036 [PubMed] [Google Scholar]
- 35.Winkelstein JA, Sullivan KE, Colten HR: Genetically determined disorders of the complement system. Scriver CR Beaudet AL Sly WS Valle D eds. The Metabolic and Molecular Basis of Inherited Disease. 1995, :pp 3911-3941 McGraw-Hill, New York [Google Scholar]





