Abstract
Expression of platelet-derived growth factor (PDGF)-A and PDGF-B is increased in patients with proliferative retinopathies in which traction retinal detachments occur. Previous studies have demonstrated that increased expression of PDGF-A in the retina of transgenic mice results in retinal gliosis due to proliferation of astrocytes with different retinal phenotypes based on the time of onset and location of the PDGF-A production. In this study, we investigated the effects of PDGF-B in the retina using gain-of-function transgenic mice that express PDGF-B in photoreceptors. These mice show proliferation of astrocytes, pericytes, and, to a lesser extent, endothelial cells, resulting in ectopic cells on the surface and extending into the retina. The sheets of cells exert traction on the retina resulting in traction retinal detachments similar to those seen in humans with proliferative retinopathies. These studies suggest that PDGF-B has more dramatic effects in the retina than PDGF-A, because it acts on additional cell types, in particular on pericytes, which have a highly developed contractile apparatus. These studies in the retina suggest a means that could be used in other tissues throughout the body to achieve graded PDGF effects. They also provide a new model of traction retinal detachment that can be used to investigate new treatments for patients with proliferative retinopathies.
Platelet-derived growth factor (PDGF) was isolated based on its ability to stimulate the proliferation of mesenchymal cells, and it is responsible for a substantial amount of the mitogenic activity in serum. 1 It is released from aggregated platelets at wound sites and acts as a chemoattractant and mitogen for many cell types that participate in wound repair, including glial cells, smooth muscle cells, pericytes, and fibroblasts. 2 Exogenous PDGF promotes increased wound strength, and neutralizing anti-PDGF antibodies impair wound healing. 3 Thus, PDGF plays an important role in wound repair throughout the body.
In the eye, there is low level constitutive expression of PDGFs in perivascular cells, ganglion cells, and in the retinal pigmented epithelium (RPE). 4,5 It appears to play a role in recruitment of pericytes and astrocytes into the retina, and may support the survival of these cells in adult retinas. 6,7 After retinal detachment there is marked up-regulation of PDGF expression in the RPE. 5 The retina can be reattached by surgery, but in roughly 10% of cases, scar tissue made up predominantly of RPE and glial cells forms in the vitreous and on the surfaces of the retina and results in traction that redetaches the retina. 8 There is prominent expression of PDGFs in the cells that participate in the scarring. This disease process is called proliferative vitreoretinopathy, the most common cause of failure of retinal reattachment surgery, and PDGFs have been implicated in its pathogenesis. 9,10 But PDGF also contributes to regulation of development 11-14 and expression of PDGF in neurons suggests possible neurotrophic and/or gliotrophic effects. 4,15
PDGF is a dimer made up of the products of the PDGF-A and PDGF-B genes, resulting in three isoforms, PDGF-AA, -AB, and -BB. Similarly, two gene products result in three types of dimeric receptors, PDGF receptor (PDGFR)-αα, -αβ, and -ββ. 16 PDGFR-α binds both PDGF-A and -B, while PDGFR-β binds only PDGF-B. Therefore, PDGF-A is more selective than PDGF-B and, theoretically, it might affect fewer cell types in a given tissue than PDGF-B depending on the expression of α and β receptors on various cell types.
At least two cell types appear to have very selective expression of PDGFR isoforms. Glial precursor O2-A progenitor cells from the optic nerve express PDGFR-α, but not PDGFR-β, 17 while pericytes express PDGFR-β, but not PDGFR-α. 6 Also, it has been suggested, based on in vitro data, that endothelial cells participating in angiogenesis may express PDGFR-β. 18 Because each of these cell types is located in the retina, the retina provides a good model system to explore similarities and differences in the activities of PDGF isoforms.
Recently, we coupled the cDNA of human PDGF-A to the bovine rhodopsin promoter and generated transgenic mice (rho/PDGF mice) with photoreceptor-specific expression of PDGF-A. 19 These mice developed a fairly subtle phenotype due to glial infiltration of the inner retina. The ectopic glial cells conferred a striking resistance to oxygen-induced retinal vascular nonperfusion and prevented the development of neovascularization. In this study, we used gain-of-function transgenic mice that express PDGF-B in photoreceptors to compare the effects of PDGF-B in the retina to those of PDGF-A and to determine whether these mice would exhibit a phenotype like that seen in patients with proliferative retinopathies.
Materials and Methods
Generation of Transgenic Mice
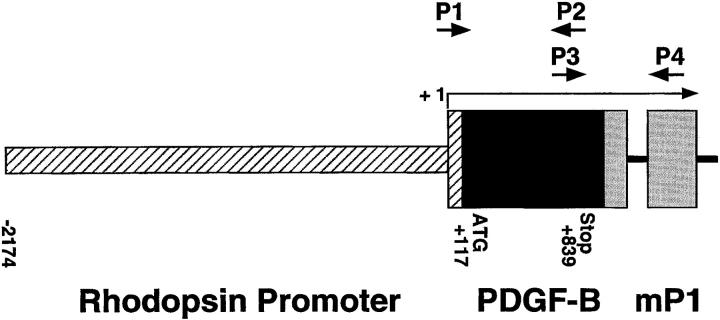
A 907-bp cDNA fragment of human PDGF-B, including 117 bp of 3′ untranslated sequence, the entire coding region, and 68 bp of untranslated 5′ sequence, was cloned into a plasmid containing the 2.2 kb HindIII/NaeI fragment from the bovine rhodopsin promoter. 20 The plasmid also contained an intron and a polyA addition site derived from the mouse protamine gene and a eukaryotic consensus ribosomal binding site. After transformation, a clone with correct orientation was selected. DNA was double CsCl purified and cut with EcoRI to provide a 3579-bp fusion gene (Figure 1) ▶ . The fusion gene was purified and transgenic mice were generated using established techniques as previously described. 20 The fusion gene was injected into the pronuclei of B6AF1 (female) × C57BL/6J (male). All offspring were backcrossed with C57BL/6J mice.
Figure 1.
Schematic map of the rhodopsin/PDGFB fusion gene. The oligonucleotide primers used to screen genomic DNA for presence of the transgene are represented by P1 and P2. The primers used for RT-PCR are represented by P3 and P4. The transcription start site is marked +1.
Mice were screened for the presence of the transgene by either Southern blot analysis or polymerase chain reaction (PCR) of tail DNA. 20 Tail pieces were digested overnight at 55°C in 50 mmol/L Tris (pH 7.5), 100 mmol/L EDTA, 400 mmol/L NaCl, and 0.5% sodium dodecyl sulfate containing 0.6 μg/μl proteinase K. PCR was done at an annealing temperature of 58°C, with primers that amplify 589 bp of transgene-specific sequence, P1 (5′-GTCCAGCCGGAGCCCCGTG-3′) and P2 (5′-CCGCACAATCTCGATCTTTCTCACC-3′) (Figure 1) ▶ .
Retinal Reverse Transcriptase PCR
At appropriate time points, mice were sacrificed, eyes were removed, and retinas were dissected. Retinal RNA was isolated using the guanidine isothiocyanate method as described by Chomczynski and Sacchi. 21 Reverse transcription was carried out with ∼0.5 μg of total RNA, reverse transcriptase (SuperScript II, Life Technologies, Gaithersburg, MD), and 5.0 μM oligo d(T) primer. Aliquots of the cDNAs were used for PCR amplification with primers for the hPDGFB/mP1 fusion gene that amplify across an intron-exon border, P3 (5′-ATAGACCGCACCAACGCCAACTTC-3′) and P4 (5′-TGTGGCGAGATGCTCTTGAAGTCTGGTA-3′) (Figure 1) ▶ . The expected PCR products for the hPDGFB/mP1 fusion gene fragment from genomic DNA and mRNA are 787 bp and 693 bp, respectively. Titrations were performed to ensure that PCR reactions were carried out in the linear range of amplification. Mouse S16 ribosomal protein primers (5′-CACTGCAAACGGGGAAATGG-3′ and 5′-TGAGATGGACTGTCGGATGG-3′) were used to provide an internal control for the amount of template in the PCR reactions.
Northern Blot Analysis
RNA blot hybridization analysis was done as previously described 5 using 10 μg of total retinal RNA. The 842 bp BamHI fragment of hPDGFB or the 598-bp BamHI fragment of hVEGF were labeled with 32P by hexanucleotide random priming and used as cDNA probes. Hybridization temperature was 65°C and the membrane was washed twice for 60 minutes at room temperature in 2× SSC, 0.1% SDS, followed by a 15 minute wash at 58°C in 1× SSC, 0.1% SDS, and a final 15-minute wash at 65°C in 0.5× SSC, 0.1% SDS.
Immunohistochemistry for PDGF-B
Transgene-positive and littermate control mice were sacrificed at various time points, and their eyes were removed and frozen in optimal cutting temperature medium (OCT, Miles Diagnostics, Elkhart, IN). Ten-micron sections were cut and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS). The sections were washed and immunohistochemical staining was performed as previously described 5 with a 1:50 dilution of a monoclonal antibody that recognizes PDGF-B. 22 Specificity of staining was assessed by substitution of nonimmune serum for primary antibody and by preabsorption of primary antibody with antigenic peptide as previously described. 23
Evaluation of the Retinal Phenotype of Transgenic Mice
At various time points, mice were sacrificed and eyes were snap-frozen in OCT or fixed in 10% buffered formalin and embedded in paraffin. Frozen or paraffin sections were stained with hematoxylin and eosin (H&E), histochemically stained with biotinylated Griffonia simplicifolia isolectin-B4 (GSA; Vector Laboratories, Burlingame, CA), or immunohistochemically stained with a 1:1000 dilution of a rabbit polyclonal antibody to glial fibrillary acidic protein (GFAP; Dako, Santa Barbara, CA), a 1:50 dilution of rabbit polyclonal antibodies to α-smooth muscle actin (Biogenix, San Ramon, CA), a 1:40 dilution of rabbit polyclonal antibody to vascular endothelial growth factor (VEGF; Santa Cruz Biotechnology, Santa Cruz, CA), or a 1:200 dilution of a rabbit polyclonal antibody directed against platelet-endothelial cell adhesion molecule (PECAM; Santa Cruz Biotechnology). PDGF-B was visualized with HistoMark Red (Gaithersburg, MD) according to the manufacturer’s instructions and other antigens were visualized with diaminobenzidine (Research Genetics, Huntsville, AL).
Staining with biotinylated GSA (Vector Laboratories), which selectively binds to vascular cells, was done as previously described. 24 Slides were incubated in methanol/H2O2 for 30 minutes at 4°C, washed with 0.05 mol/L Tris-buffered saline (TBS), pH 7.4, and incubated for 30 minutes in 10% normal porcine serum. Slides were rinsed with 0.05 Mol/L TBS and incubated 1 hour at 37°C with biotinylated lectin. After being rinsed with 0.05 Mol/L TBS, slides were incubated with avidin coupled to peroxidase (Vector Laboratories) for 45 minutes at room temperature. After being washed for 10 minutes with 0.05 mol/L Tris buffer, pH 7.6, slides were incubated with diaminobenzidine (Research Genetics) to give a brown reaction product, and mounted with Cytoseal (Stephens Scientific, Riverdale, NJ).
Results
Generation of Rho/PDGFB Transgenic Mice
Six independent lines that incorporated the rhodopsin promoter/PDGFB fusion gene (Figure 1) ▶ were obtained (designated rho/PDGFB1–6). The founders were backcrossed with C57BL/6J mice to establish transgenic lines. Mice that were heterozygous at the transgene locus were used in all analyses.
Expression of PDGF-B mRNA in the Retinas of Transgenic Mice
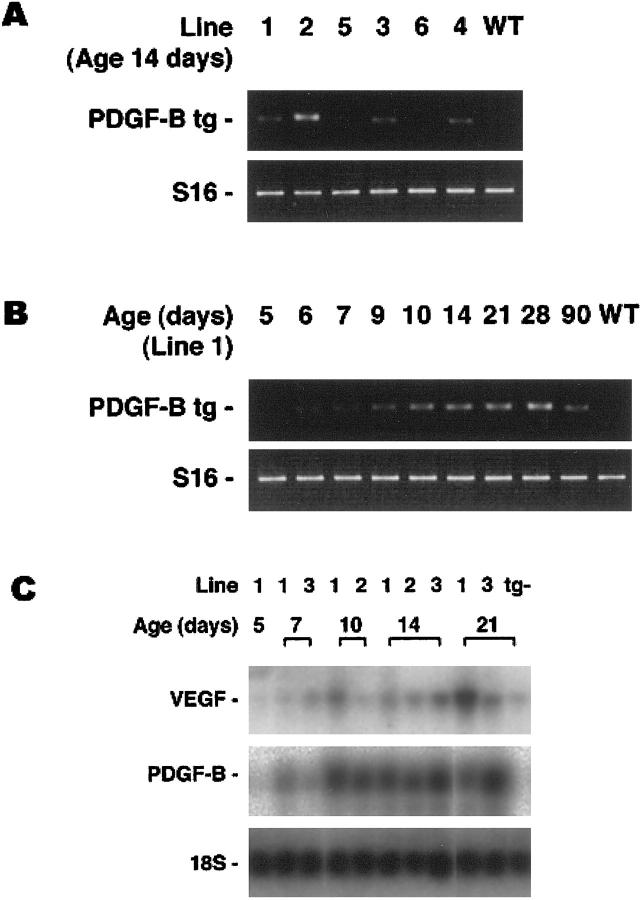
At postnatal day 14 (P14), RT-PCR, using total retinal RNA as template and primers specific for transgene mRNA, showed good expression in lines rho/PDGFB1–4, barely detectable expression in line 5, and no detectable expression in line 6 (Figure 2A) ▶ . A time course in mice from line 1 showed transgene mRNA was first detectable at P5 or P6, increased to a steady state level by about P10, and was maintained through at least 3 months after birth, the longest time point examined (Figure 2B) ▶ . Northern analysis also suggested that steady state levels of mRNA were reached by day 10, and there appeared to be similar levels of expression in lines 1, 2, and 3 (Figure 2C) ▶ .
Figure 2.
Assessment of transgene mRNA level in retinal RNA by RT-PCR and Northern blot analysis. A: Total RNA was extracted from the retinas of a transgene-negative (WT) and transgene-positive mice from 6 different lines (rho/PDGFB1–6) and RT-PCR was done for PDGFB transgene mRNA and S16 ribosomal protein mRNA. The size of the band amplified with transgene primers was compatible with the 693 bp predicted for amplification from mRNA. Lines 1–4 showed easily detectable expression, line 5 showed barely detectable expression, which reproduces poorly in this figure, and line 6 shows no detectable expression. B: RT-PCR was done for PDGFB transgene mRNA and S16 ribosomal protein mRNA on retinal RNA from different aged transgene-positive or -negative (WT) mice from line 1. Transgene mRNA is barely detectable at P5 (reproduces poorly in this figure) and increases to reach steady-state level between P10 and P14. C: Northern blot analysis shows PDGFB transgene mRNA detectable on P7, and there are similar levels on P10 and P14 in lines 1–3. There is also an increase in VEGF mRNA and at P21 transgene-positive mice show greater levels than transgene-negative mice (tg−).
Localization of PDGF-B in the Retinas of rho/PDGFB Transgenics
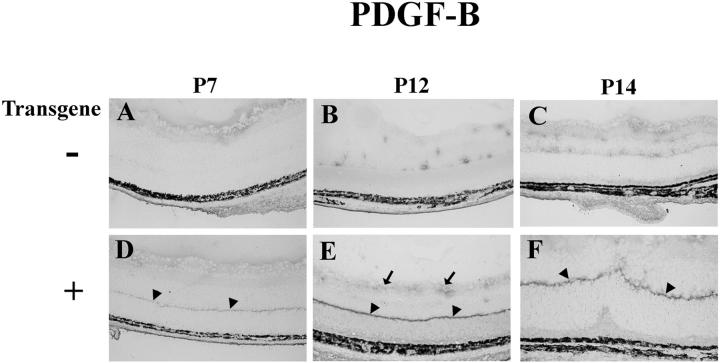
Immunohistochemistry with an antibody that specifically recognizes PDGF-B showed reaction product in photoreceptors, with an intense band of staining in the region of photoreceptor terminals (Figure 3) ▶ . Similar localization was seen for PDGF-A in several lines of rho/PDGFA mice, 19 suggesting the possibility of polarized secretion. Although rho/PDGFB mice show the greatest staining for PDGF-B in photoreceptors, there is also increased staining for PDGF-B in the inner retina compared to wild-type mice, suggesting that PDGF-BB diffuses through the retina and is accessible to cells in the inner retina.
Figure 3.
Immunohistochemical staining for PDGF-B in the retinas of transgene-negative (−) or -positive (+) rho/PDGFB1 mice. Retinal frozen sections were immunohistochemically stained for PDGF-B as described in Materials and Methods. All mice show some diffuse staining on the surface of the retina at P7. At P12 and P14 control mice show focal staining around blood vessels. Transgene-positive mice show a discrete band of staining at the inner border of the outer nuclear layer (arrowheads), the region of photoreceptor terminals. The band is faint at P7 and more intense at P12 and P14. There is also more staining at the surface of the retina in transgenics at P12.
Adult rho/PDGFB Mice Develop Traction Retinal Detachments
For each of the 6 transgenic lines, histopathological evaluation was done between 3 and 9 months of age on 8 to 10 mice selected from 3 generations. All mice from lines 1–4 showed traction detachments of the retinas in both eyes, whereas mice from lines 5 and 6 had normal appearing retinas with no detachments, presumably due to the low expression levels of transgene-derived PDGF-B in the latter two lines. In many instances, the retina became adherent to the back of the lens, forming a retrolental mass. There was striking degeneration of the detached retinas and prominent epiretinal and subretinal cellular membranes (Figure 4) ▶ . The traction detachments in rho/PDGFB mice are similar to the traction retinal detachments seen in humans with proliferative vitreoretinopathy, in which there are tractional membranes made up primarily of retinal pigmented epithelial (RPE) cells and retinal glial cells, or to the traction retinal detachments seen in retinopathy of prematurity, in which there is a prominent vasoproliferative component in addition to proliferation of RPE and glia. To explore why overexpression of PDGF-B in the retina leads to traction retinal detachment, a detailed investigation of the cellular involvement and sequence of events was performed primarily in lines 1 and 2.
Figure 4.
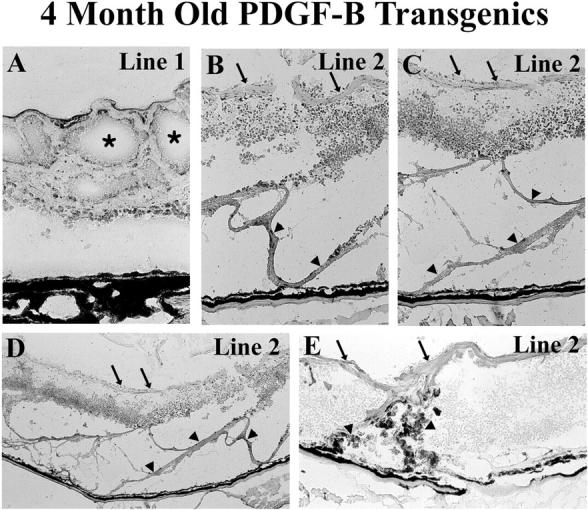
Traction retinal detachments in adult rho/PDGFB mice. Transgene-positive mice were sacrificed at 4 months of age and retinal frozen sections were immunohistochemically stained for glial fibrillary acidic protein and counterstained with hematoxylin. A: The retina of a mouse from line 1 is chronically detached and disorganized with extensive cystic change (asterisks). B-D: The retina from a line 2 mouse is atrophic from a total detachment that is chronic. There are extensive epiretinal (arrows) and subretinal (arrowheads) cellular membranes made up predominantly of glial cells. Low power view (D) shows a panoramic view that illustrates the broad extent of the sheets of ectopic cells exerting traction on the surface (arrows) and beneath (arrowheads) the retina. Higher power views (B and C) show that the disorganized retina is surrounded by complex sheets of glial cells. E: Retinal pigmented epithelial cells (darkly pigmented cells; arrowheads) are migrating through the shallowly detached retina of another line 2 mouse to participate with the sheets of glial cells on the surface (arrows) of the retina to exert traction.
Onset of Expression of PDGF-B Results in a Rapid Increase in Astrocytes in the Retina
Astrocytes are small glial cells that in adult mouse retinas are normally found in the nerve fiber layer and adjacent to retinal blood vessels. Muller cells are large glial cells extending from the inner to the outer surface of the retina. Developing astrocytes are known to have PDGFR-αs and are very responsive to PDGFs for at least 2 weeks after birth. 25 They migrate from the optic nerve into the posterior portion of the inner retina and then spread anteriorly until they populate the entire nerve fiber layer. They associate with blood vessels in the nerve fiber layer and are visualized by immunohistochemical staining for glial fibrillary acidic protein (GFAP). At P6, which is soon after transgene expression begins, rho/PDGFB1 or -2 and wild-type mice showed identical patterns of sparse GFAP-positive astrocytes at the inner surface of the retina (Figure 5 ▶ , P6+ and P6−), but by P7, GFAP staining along the inner surface of the retina was increased in transgenics compared to wild-type mice. The difference was more pronounced at P8 when there was clearly a multilayered collection of astrocytes in transgenics. At P9 and P10, in addition to continued sparse staining at the retinal surface, wild-type mice showed focal areas of GFAP staining in the inner nuclear layer, presumably due to migration of astrocytes into the retina along with penetrating retinal vessels. In contrast, rho/PDGFB1 and -2 mice showed a thick carpet of astrocytes on the surface of the retina and cords of invading cells extending into the inner nuclear layer that were quite prominent by P10. At P12 and beyond, there was often evidence of retinal folding with focal areas of retinal detachment that progressed to total retinal detachment at later time points. Perturbations of the retina, including detachment, often result in increased expression of GFAP in Muller cells; this is illustrated by the radial streaks of staining seen at P14 and beyond in transgenics (Figure 5 ▶ ; P14, P21, and P28). The carpet of astrocytes along the retinal surface became more compact and less prominent at later time points.
Figure 5.
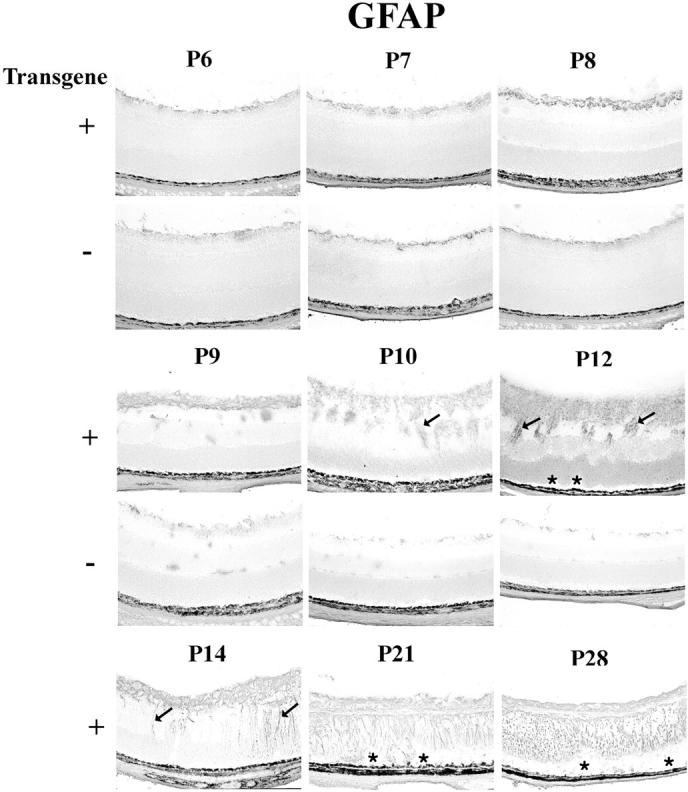
Immunohistochemical staining for glial fibrillary acidic protein (GFAP) in transgene-positive (+) and -negative (−) rho/PDGFB1 mice. There is no difference in GFAP staining at P6, but at P7 and P8 there are more stained cells at the surface of the retina in transgene-positive compared to transgene-negative mice. At P9 there is a multilayered sheet of glial cells on the surface of the retina that continues to increase in thickness at P10 and P12, when there are also cords of cells invading the retina. Folding of the outer retina, resulting in small retinal detachments (asterisks) are seen at P12, indicating that the ectopic cells are exerting traction. Muller cell processes (arrows) become more conspicuously stained in transgene-positive mice at P14. At P21 and P28, the retinas are shallowly detached (asterisks) and Muller cells spanning the retinas as well as astrocytes near the surface are expressing GFAP.
Increased Pericytes and Vascular Endothelial Cells in the Retinas of rho/PDGFB Mice
Pericytes have PDGFR-βs, 4,6 and PDGF-BB stimulates their proliferation. 26 Also, there is some suggestion that endothelial cells may express PDGFR-βs under certain conditions. 18 Therefore, we sought to determine whether pericytes and endothelial cells participate in the proliferative response in rho/PDGFB mice. GSA lectin selectively binds vascular cells and has been suggested to be an endothelial cell-specific marker, 27,28 but we have recently demonstrated that it binds to both endothelial cells and pericytes. 29
There was identical staining for GSA on the surface of the retina in wild-type and transgenic mice at P6, which is around the time of onset of PDGF-B expression in transgenics (Figure 6) ▶ . This represents the developing retinal vessels on the surface of the retina. By P7 there was more GSA staining along the inner surface of the retina in transgenics compared to wild-type mice, and the difference was more pronounced at P8 when there was clearly a multilayered collection of vascular cells in transgenics. At P9, wild-type mice show penetrating retinal vessels that form the intermediate and deep capillary beds that assume the appearance seen in normal adults between P10 and P12. In transgenics, the formation of the deep capillary beds was impaired. On P9, there were many more vascular cells on the surface of the retina in transgenic compared to wild-type mice, but fewer vascular cells had migrated into the retina. At P10, there were thick, finger-like projections extending into the inner retina from a multilayered carpet of cells on the retinal surface. The invading cords of vascular cells did not appear to be organized into vessels. At P12, much of the inner retina was infiltrated and replaced by GSA-positive cells. At P14 and later time points, there appeared to be some involution and contraction of the vascular cells, resulting in focal areas of retinal detachment that progressed and resulted in total retinal detachment.
Figure 6.
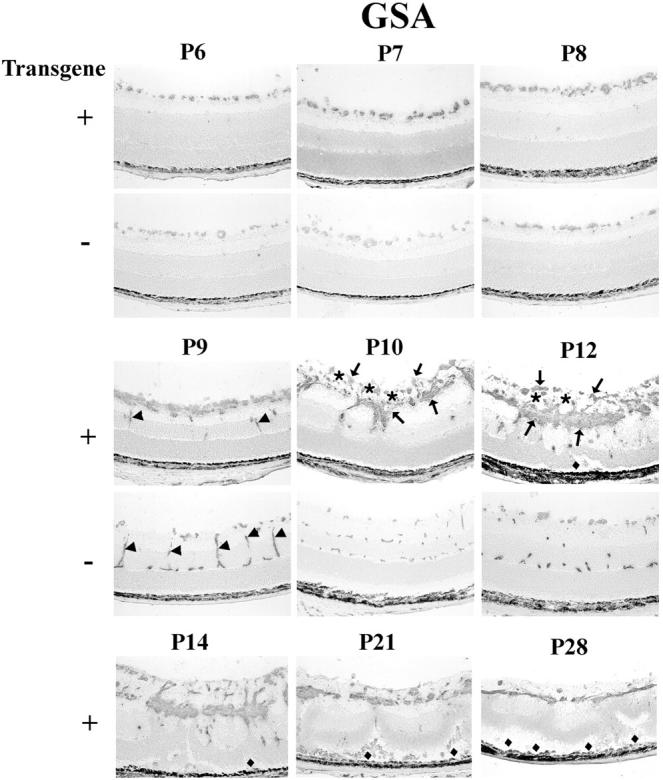
Histochemical staining for G. simplicifolia lectin (GSA) in transgene-positive (+) and -negative (−) rho/PDGFB1 mice. There is no definite difference in GSA-staining at P6 and P7, but by P8, transgene-positive mice have more stained cells on the surface of the retina. At P9, transgene-positive mice show a multilayered sheet of GSA-positive cells on the surface of the retina. They also show a few positive cells in the inner nuclear layer that could represent penetrating vessels (arrows), but they are much less extensive than the penetrating vessels (arrows) forming the deep capillary beds in transgene-negative mice. At P10 and P12, the superficial, intermediate, and deep capillary beds are seen in transgene-negative mice, but not in transgene-positive mice, which lack identifiable vessels, and instead show a disorganized mass of GSA-positive (arrows) and -negative cells (presumably astrocytes; asterisks) on the surface of the retina and cords of cells invading the retina. Focal areas of traction detachment (diamonds) are seen at P12 and P14, and more extensive detachments are seen at P21 and P28 in transgene-positive mice.
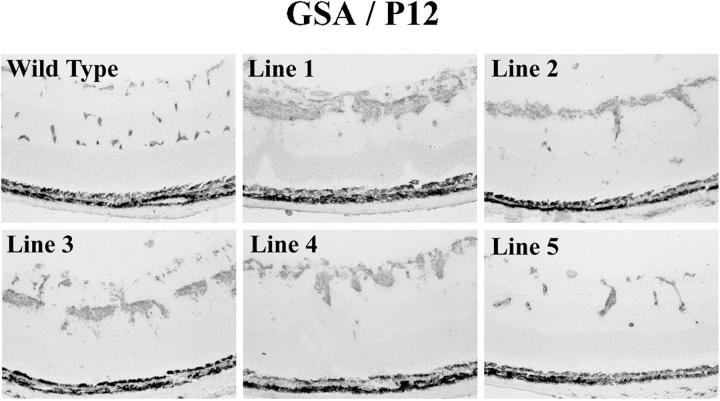
Staining for GSA was compared in retinas of P12 mice from each of the rho/PDGFB lines, except line 6, which was lost because mice from this line reproduced poorly. Mice from lines 1–4 showed similar abnormalities in each (Figure 7) ▶ . Mice from line 5, which had barely detectable transgene expression, showed the mildest phenotype with some development of the deep capillary bed and minimal increase in GSA-positive cells in the inner retina. A deficiency of normally perfused retinal vessels was confirmed in whole mounts of fluorescein-labeled dextran-perfused retinas from line 1 (Figure 8) ▶ . The capillary network was much less dense in transgenics compared to wild type mice, and there were several moderate-sized areas of nonperfusion (Figure 8B ▶ , asterisks). As expected from the disorganized appearance of the vascular cell proliferation seen in retinal sections, whole mounts of perfused retinas failed to show definite neovascularization, but did show occasional vascular malformations (Figure 8B ▶ , arrows).
Figure 7.
Histochemical staining for G. simplicifolia lectin (GSA) in transgene-positive (+) rho/PDGFB mice from lines 1–5. Transgene-positive mice from lines 1–5 and a transgene-negative mouse from line 1 (wild-type) were sacrificed at P12 and retinal frozen sections were stained with GSA. The phenotype is very similar in mice from lines 1–4, which all show proliferation of vascular cells on or near the surface of the retina and little or no development of the deep retinal capillary beds. Mice from line 5, which showed barely detectable transgene expression, showed the mildest abnormalities, with some evidence of vascular cell proliferation and some development of the deep capillary beds.
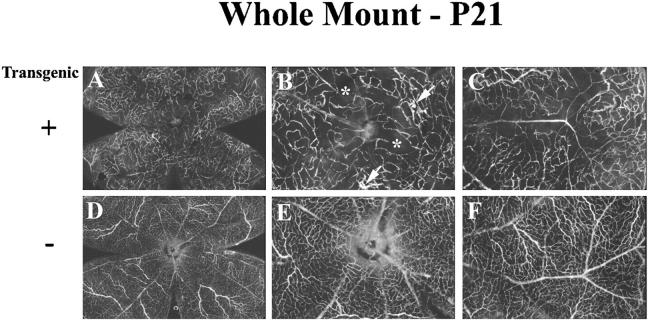
Figure 8.
Retinal whole mounts of fluorescein dextran-perfused transgene-positive (+) and -negative (−) rho/PDGFB1 mice show that transgene-positive mice have decreased retinal capillary density, vascular malformations, and no neovascularization. Mice were perfused with fluorescein-labeled dextran at P21 and retinal whole mounts were examined by fluorescence microscopy. Transgene-positive mice showed an irregular capillary bed that was less dense than that in transgene-negative mice, and showed moderate sized areas of nonperfused retina (asterisks). No neovascularization was identified, but there were vascular malformations (arrows). B and C are higher power views of the same retina shown in A, and E and F are higher power views of the same retina shown in D.
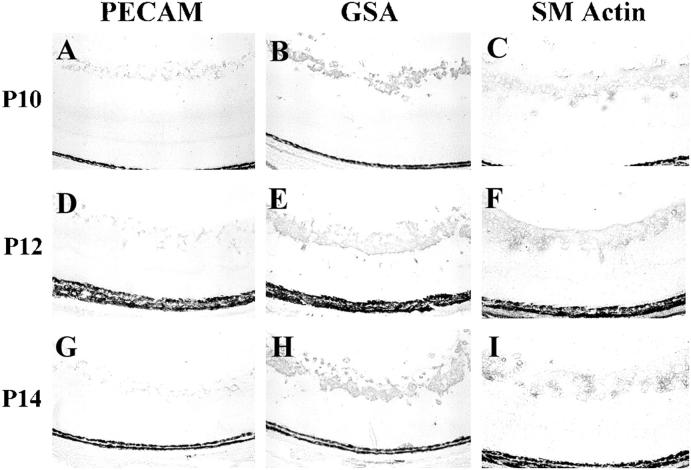
Anti-PECAM binds endothelial cells and not pericytes, 30,31 and anti-smooth muscle actin binds pericytes and not endothelial cells. 32 Immunohistochemical staining with these two antibodies was compared with GSA staining in rho/PDGFB transgenics at P10, P12, and P14 (Figure 9) ▶ . Staining for PECAM showed thin tube-like structures within the thick, PECAM-negative cell mass on the surface of the retina (Figure 9, A, D, and G) ▶ , whereas retinas stained for smooth muscle actin looked very much like GSA-stained retinas, showing large groups of cells that were not organized into recognizable structures. Collectively the immunohistochemical data suggest that the proliferating ectopic cells in the retinas of rho/PDGFB mice consist of large accumulations of pericytes and astrocytes surrounding small tube-like structures made up of endothelial cells.
Figure 9.
The increase in vascular cells in rho/PDGFB mice is due to a large increase in pericytes and a much smaller increase in vascular endothelial cells. Rho/PDGFB mice were sacrificed at P10, P12, or P14. Serial frozen sections were stained for platelet-endothelial cell adhesion molecule (PECAM), which selectively stains endothelial cells, G. simplicifolia lectin (GSA), which stains endothelial cells and pericytes, or smooth muscle actin (SM actin), which selectively stains pericytes. At each time point, SM actin staining corresponds closely to GSA staining, with PECAM staining being much less. This suggests that most of the proliferated vascular cells are pericytes.
Rho/PDGFB Mice Show Increased Expression of VEGF in the Retina
The prominent participation of vascular cells in the proliferative response of rho/PDGFB mice caused us to compare levels of VEGF mRNA in transgenic and wild-type mice. VEGF was increased in the retinas of transgenics compared to wild-type mice at P21 (Figure 2C) ▶ . This is likely to be due at least in part to poor development of the intermediate and deep capillary beds resulting in areas of nonperfused retina (Figure 8) ▶ . Based on the results of GSA-staining, it is likely that the onset of retinal ischemia occurred around P10 (Figure 6H) ▶ . Ectopic astrocytes and pericytes may have also contributed to increased VEGF mRNA in transgenics, since both of these cell types have been demonstrated to produce VEGF.
Discussion
In this study, we have demonstrated that increased expression of PDGF-B in photoreceptors of transgenic mice results in proliferation of several cell types in the retina, including astrocytes, pericytes, and endothelial cells. The cells initially proliferate on the surface of the retina, and this is associated with impaired development of the deep retinal capillary beds. Unlike the situation in wild-type mice, in which penetrating vessels extend into the retina, transgenic mice show a paucity of vessels; instead, cords of cells migrate into the inner nuclear layer. The cords exert traction on the retina, resulting in outer retinal folds and focal areas of detachment that enlarge, leading to total retinal detachment. This dramatic phenotype is strikingly different from the subtle phenotype exhibited in transgenic mice with increased expression of PDGF-A in photoreceptors. 19 The latter mice show nearly normal appearing retinas and are less prone to oxygen-induced retinal endothelial cell death than wild-type mice, and as a result do not develop oxygen-induced ischemic retinopathy.
It is intriguing that two members of the PDGF family with substantial homology have such markedly different effects in the retina. The basis for the difference appears to be the broader stimulatory effects of PDGF-B compared to PDGF-A. Rho/PDGFB mice show increased numbers of astrocytes, pericytes, and endothelial cells, whereas rho/PDGFA mice show only increased astrocytes. Other models, including mice with oxygen-induced ischemic retinopathy 33 and rho/VEGF transgenic mice, 34 show increased numbers of endothelial cells and pericytes in the retina, and they also have different phenotypes from that of rho/PDGFB mice. In the former, the endothelial cells and pericytes are increased in normal proportions and they are organized into blood vessels, whereas in rho/PDGFB mice, there are many more pericytes than endothelial cells, and they form disorganized sheets of cells. One possible interpretation is that in rho/VEGF mice and mice with oxygen-induced ischemic retinopathy, the primary stimulus, VEGF, causes migration and proliferation of endothelial cells, which in turn recruit pericytes, probably by production of PDGF-B. In rho/PDGFB mice, the primary stimulus, PDGF-B, causes excessive proliferation of pericytes and astrocytes, which in turn recruit endothelial cells, probably by the production of VEGF. The relative difference in amount of PDGF versus VEGF and/or the reverse order of their production cause major phenotypic differences resulting in organization of proliferating endothelial cells and pericytes into blood vessels in rho/VEGF mice and mice with oxygen-induced ischemic retinopathy, whereas in rho/PDGFB mice, the proliferating cells form sheets and cords with limited organizational structure. It is tempting to speculate that in rho/PDGFB mice, the massive proliferation of pericytes, which are cells with a highly developed contractile apparatus, and their failure to organize into vessels, which may serve to harness their contractile function, results in contractile sheets of cells that foreshorten the retina, initially causing folds in the outer retina and ultimately progressing to total retinal detachment.
Cultured vascular endothelial cells express PDGFR-βs during tube formation, 18 but in situ hybridization demonstrates PDGFR-β mRNA in murine brain pericytes, but not endothelial cells, including those in new vessel sprouts. 6 Pericytes originate from PDGFR-β-positive progenitors in arterial walls and migrate along capillary endothelial sprouts that express PDGF-B. PDGF-B-deficient mice lack pericytes, indicating that pericytes require PDGF-B for their development, 6 which is consistent with our observation that increased production of PDGF-B in the retina results in excessive production of pericytes.
Other in vitro 26,35 and in vivo 7 studies also suggest that endothelial cell-derived PDGF-B constitutes an important paracrine signal that directs pericyte recruitment along retinal microvessels. Ectopic exogenous PDGF-B, 7 just like ectopic endogenous PDGF-B (this study), disrupts retinal vascular development, indicating that the temporal and spatial patterning of the paracrine signal is critical.
In addition to providing data that support a critical role for PDGF-B as an important regulator of pericyte migration and proliferation in the retina, rho/PDGFB mice provide a model of spontaneous traction retinal detachment. Traction retinal detachment is a major cause of visual loss associated with both neovascular and non-neovascular proliferative retinopathies, and expression of PDGFs is increased in each of these disease processes. 5,9 Examples of the former are ischemic retinopathies, such as proliferative diabetic retinopathy and retinopathy of prematurity, and an example of the latter is proliferative vitreoretinopathy, the most common cause of failure of retinal reattachment surgery. 8 Oxygen-induced ischemic retinopathy in mice or rats mimics characteristics of the early stages of human ischemic retinopathies, such as retinal neovascularization, but unlike human diseases, there is no progression to traction retinal detachment. Therefore, that model cannot be used to study the pathogenesis and treatment of traction retinal detachment, which is the blinding component of human ischemic retinopathies.
Injection of heterologous or autologous cells into the vitreous cavity of rabbits results in traction retinal detachment. 36 This model and models that involve wounding the retina have been used extensively to investigate the pathogenesis and treatment of traction retinal detachments. 37-42 But they involve introduction of exogenous cells and/or trauma, which are potentially important differences that raise concerns regarding the relevance of these models to spontaneous traction retinal detachments that occur in human proliferative retinopathies, such as diabetic retinopathy and proliferative vitreoretinopathy. 10 The increase in expression of PDGF-B and the spontaneous occurrence of traction retinal detachment followed by the proliferation of retinal pigmented epithelial cells and retinal glia on and beneath the retina in rho/PDGFB mice are important features of traction retinal detachments in humans with these retinopathies. Therefore, rho/PDGFB mice provide a valuable new tool for the study of traction retinal detachment. Although both PDGF-A and PDGF-B are increased in proliferative retinopathies, comparison of rho/PDGF-A and rho/PDGF-B mice suggest that PDGF-B is likely to be more important in generation of the detachments. Future studies will explore in detail the cellular and molecular mechanisms responsible for the detachments and will investigate ways to prevent them. Such studies could help to provide new treatments for patients with proliferative retinopathies.
Footnotes
Address reprint requests to Peter A. Campochiaro, M.D., Maumenee 719, The Johns Hopkins University School of Medicine, 600 N. Wolfe Street, Baltimore, MD 21287-9277. E-mail: pcampo@jhmi.edu.
Supported by PHS grants EY05951, EY12609, EY10017, EY09769, and core grant P30EY1765 from the National Eye Institute, a Juvenile Diabetes Foundation fellowship grant (to N. O.), a Lew R. Wasserman Merit Award (to P. A. C.), a career development award (to D. J. Z.), and an unrestricted grant from Research to Prevent Blindness, Inc., the Rebecca P. Moon, Charles M. Moon, Jr., and Dr. P. Thomas Manchester Research Fund, a grant from the Association for Retinopathy of Prematurity and Related Diseases, a grant from Mrs. Harry J. Duffey, and a grant from Dr. and Mrs. William Lake. P. A. C. is the George S. and Dolores Dore Professor of Ophthalmology.
M. S. S. and N. O. contributed equally to this manuscript.
Dr. Seo’s current address: Chonnam National University Medical School and Hospital, Kwangju, Korea.
References
- 1.Ross R, Glomset JA, Kariya B, Harker L: A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci USA 1974, 71:1207-1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heldin CH, Westermark B: Platelet-derived growth factor: mechanism of action and possible in vivo function. Cell Reg 1990, 8:555-566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pierce GF, Mustoe TA, Altrock BW, Deuel TF, Thomason A: Role of platelet-derived growth factor in wound healing. J Cell Biochem 1991, 45:319-326 [DOI] [PubMed] [Google Scholar]
- 4.Mudhar HS, Pollock RA, Wang C, Stiles CD, Richardson WD: PDGF and its receptors in the developing rodent retina and optic nerve. Development 1993, 118:539-552 [DOI] [PubMed] [Google Scholar]
- 5.Campochiaro PA, Hackett SF, Vinores SA, Freund J, Csaky C, La Rochelle W, Henderer J, Johnson M, Rodriguez IR, Friedman Z, Derevjanik N, Dooner J: Platelet-derived growth factor is an autocrine growth stimulator in retinal pigmented epithelial cells. J Cell Sci 1994, 107:2459-2469 [DOI] [PubMed] [Google Scholar]
- 6.Lindahl P, Johansson BR, Leveen P, Betsholtz C: Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 1997, 277:242-245 [DOI] [PubMed] [Google Scholar]
- 7.Benjamin LE, Hemo I, Keshet E: A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B. Development 1998, 125:1591-1598 [DOI] [PubMed] [Google Scholar]
- 8.Cowley M, Conway BP, Campochiaro PA, Kaiser D, Gaskin H: Clinical risk factors for proliferative vitreoretinopathy. Arch Ophthalmol 1989, 107:1147-1151 [DOI] [PubMed] [Google Scholar]
- 9.Vinores SA, Henderer JD, Mahlow J, Chiu C, Derevjanik NL, LaRochelle W, Csaky C, Campochiaro PA: Isoforms of platelet-derived growth factor and its receptors in epiretinal membranes: immunolocalization to retinal pigmented epithelial cells. Exp Eye Res 1995, 60:607-619 [DOI] [PubMed] [Google Scholar]
- 10.Campochiaro PA: Pathogenic mechanisms in proliferative vitreoretinopathy. Arch Ophthalmol 1997, 115:237-241 [DOI] [PubMed] [Google Scholar]
- 11.Mercola M, Wang C, Kelley J, Brownlee C, Jackson-Grusby L, Stiles C, Bowen-Pope D: Selective expression of PDGF and its receptor during early mouse embryogenesis. Devel Biol 1990, 138:114-122 [DOI] [PubMed] [Google Scholar]
- 12.Leveen P, Pekny M, Gerbre-Medhin S, Swolin B, Larsson E, Betsholtz C: Mice deficient for PDGF-B show renal, cardiovascular, and hematological abnormalities. Genes Dev 1994, 8:1875-1887 [DOI] [PubMed] [Google Scholar]
- 13.Soriano P: Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev 1994, 8:1888-1896 [DOI] [PubMed] [Google Scholar]
- 14.Schatteman GC, Motley ST, Effmann EL, Bowen-Pope DF: Platelet-derived growth factor receptor alpha subunit deleted patch mouse exhibits severe cardiovascular dysmorphogenesis. Teratology 1995, 51:351-366 [DOI] [PubMed] [Google Scholar]
- 15.Smits A, Kato M, Westermark B, Nister M, Heldin CH, Funa K: Neurotrophic activity of platelet-derived growth factor (PDGF): rat neuronal cells possess functional PDGF beta-type receptors and respond to PDGF. Proc Natl Acad Sci USA 1991, 88:8159-8163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart CE, Bowen-Pope DF: Platelet-derived growth factor receptor: current views of the two subunit model. J Invest Dermatol 1990, 94:535-575 [DOI] [PubMed] [Google Scholar]
- 17.Barres BA, Raff MC: Control of oligodendrocyte number in the developing rat optic nerve. Neuron 1994, 12:935-942 [DOI] [PubMed] [Google Scholar]
- 18.Battegay EJ, Rupp J, Iruela-Arispe L, Sage EH, Pech M: PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J Cell Biol 1994, 125:917-928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada H, Yamada E, Ando A, Seo M-S, Esumi N, Okamoto N, Vinores M, LaRochelle W, Zack DJ, Campochiaro PA: Platelet-derived growth factor-A-induced retinal gliosis protects against ischemic retinopathy. Am J Pathol 2000, 156:477-487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zack DJ, Bennett J, Wang Y, Davenport C, Klaunberg B, Gearhart J, Nathans J: Unusual topography of bovine rhodopsin promoter-lac z fusion gene expression in transgenic mouse retinas. Neuron 1991, 6:187-199 [DOI] [PubMed] [Google Scholar]
- 21.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 22.LaRochelle WJ, Robbins KC, Aaronson SA: Immunochemical localizations of the epitope for a monoclonal antibody that neutralizes human platelet-derived growth factor mitogenic activity. Mol Cell Biol 1989, 9:3538-3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinores SA, Youssri AI, Luna JD, Chen Y-S, Bhargave S, Vinores MA, Schoenfeld C-L, Peng B, Chan C-C, LaRochelle W, Green WR, Campochiaro PA: Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathol 1997, 12:99-109 [PubMed] [Google Scholar]
- 24.Ozaki H, Okamoto N, Ortega S, Chang M, Ozaki K, Sadda S, Vinores MA, Derevjanik N, Zack DJ, Basilico C, Campochiaro PA: Basic fibroblast growth factor is neither necessary nor sufficient for the development of retinal neovascularization. Am J Pathol 1998, 153:757-765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reneker LW, Overbeek PA: Lens-specific expression of PDGF-A in transgenic mice results in retinal astrocytic hamartomas. Invest Ophthalmol Vis Sci 1996, 37:2455-2466 [PubMed] [Google Scholar]
- 26.Hirschi KK, Rohovsky SA, Beck LH, Smith SR, D’Amore PA: Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res 1999, 84:298-305 [DOI] [PubMed] [Google Scholar]
- 27.Sahagun G, Moore SA, Fabry Z, Shelper RL, Hart MN: Purification of murine endothelial cell cultures by flow cytometry using fluorescein-labeled Griffonia simplicifolia agglutinin. Amer J Pathol 1989, 134:1227-1232 [PMC free article] [PubMed] [Google Scholar]
- 28.Schulte BA, Spicer SS: Histochemical evaluation of mouse and rat kidneys with lectin-horseradish peroxidase conjugates. Amer J Anat 1983, 168:345-362 [DOI] [PubMed] [Google Scholar]
- 29.Vinores SA, Derevjanik NL, Ozaki H, Okamoto N, Campochiaro PA: Cellular mechanisms of blood-retinal barrier dysfunction in macular edema. Doc Ophthalmol 1999, 97:217-228 [DOI] [PubMed] [Google Scholar]
- 30.Schimmenti LA, Yan HC, Madri JA, Albelda SM: Platelet endothelial cell adhesion molecule, PECAM-1, modulates cell migration. J Cell Physiol 1992, 153:417-428 [DOI] [PubMed] [Google Scholar]
- 31.Romer LH, McLean NV, Yan HC, Daise M, Sun J, DeLisser HM: INF-gamma and TNF-alpha induce redistribution of PECAM-1 (CD 31) on human endothelial cells. J Immunol 1995, 154:6582-6592 [PubMed] [Google Scholar]
- 32.Schlingemann RD, Rietveld FJ, Kwaspen F, van de Kerkhof PC, de Waal RM, Ruiter DJ: Differential expression of markers for endothelial cells, pericytes, and basal lamina in the microvasculature of tumors and granulation tissue. Am J Pathol 1991, 138:1335-1347 [PMC free article] [PubMed] [Google Scholar]
- 33.Smith LEH, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA: Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 1994, 35:101-111 [PubMed] [Google Scholar]
- 34.Okamoto N, Tobe T, Hackett SF, Ozaki H, Vinores MA, LaRochelle W, Zack DJ, Campochiaro PA: Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol 1997, 151:281-291 [PMC free article] [PubMed] [Google Scholar]
- 35.Hirschi KK, Rohovsky SA, D’Amore PA: PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle cell fate. J Cell Biol 1998, 141:805-814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Algvere P, Kock E: Experimental fibroplasia in the rabbit vitreous: retinal detachment induced by autologous fibroblasts. Graefes Arch Clin Exp Ophtalmol 1976, 199:215-222 [DOI] [PubMed] [Google Scholar]
- 37.Cleary PE, Ryan SJ: Method of production and natural history of experimental posterior penetrating eye injury in the rhesus monkey. Am J Ophthalmol 1979, 88:212-220 [DOI] [PubMed] [Google Scholar]
- 38.Fastenberg DM, Diddie KR, Sorgente N, Ryan SJ: A comparison of different cellular inocula in an experimental model of massive periretinal proliferation. Am J Ophthalmol 1982, 93:559-564 [DOI] [PubMed] [Google Scholar]
- 39.Chandler DB, Rozakis G, de Juan E, Machemer R: The effect of tiamcinolone acetonide on a refined experimental model of proliferative vitreoretinopathy. Am J Ophthalmol 1985, 99:686-690 [DOI] [PubMed] [Google Scholar]
- 40.Campochiaro PA, Gaskin HC, Vinores SA: Retinal cryopexy stimulates traction retinal detachment formation in the presence of an ocular wound. Arch Ophthalmol 1987, 105:1567-1570 [DOI] [PubMed] [Google Scholar]
- 41.Sen HA, Robertson TJ, Conway BP, Jaccoma EH: The role of breakdown of the blood-retinal barrier in cell-injection models of proliferative vitreoretinopathy. Arch Ophthalmol 1988, 106:1685-1687 [DOI] [PubMed] [Google Scholar]
- 42.Rubsamen PE, Davis PA, Hernandez E, O’Grady GE, Cousins SW: Prevention of experimental proliferative vitreoretinopathy with a biodegradable intravitreal implant for the sustained release of fluorouracil. Arch Ophthalmol 1994, 112:407-413 [DOI] [PubMed] [Google Scholar]