Abstract
Mice sensitized with Schistosoma mansoni eggs and IL-12 develop liver granulomas, on subsequent infection, which are smaller and less fibrotic than those in nonsensitized mice. The protective response is accompanied by a shift in the type-2 cytokine profile to one dominated by type-1 cytokines. The deviated response is associated with marked increases in inducible nitric oxide synthase (NOS-2) activity. Here, we demonstrate, by using NOS-2-deficient mice, that the anti-inflammatory and anti-fibrotic effects of the type-1 response are completely NOS-2-dependent. Strikingly, despite developing a polarized type-1 cytokine response that was similar in magnitude, the egg/IL-12-sensitized NOS-deficient mice developed granulomas 8 times larger than WT mice did. There was also no decrease in hepatic fibrosis in the sensitized mutant animals. Interferon-γ-deficient mice failed to exhibit the exacerbated inflammatory response, despite displaying a marked deficiency in nitric oxide production. However, immune deviation was unsuccessful in the latter animals, which suggested that the increase in inflammation in NOS-deficient mice resulted from a polarized but nitric oxide-deficient type-1 response. These results reveal a beneficial role for NOS-2 in the regulation of inflammation and suggest that the ultimate success of Th2-to-Th1 immune deviation strategies will rely on the efficient activation of NOS-2 expression in downstream effector cells.
Chronic inflammation mediated by Th2-type cytokines can lead to morbidity and mortality in allergy/asthma, 1 systemic autoimmune disease, 2 and helminthic infection. 3 Therefore, major research goals in recent years have been to understand the immunological mechanisms controlling Th2 response development and to design effective immunotherapies to treat or prevent such reactions. A commonly used model to explore Th2-dependent immunopathology is the murine model of schistosomiasis. Disease following Schistosoma mansoni infection develops primarily as a consequence of chronic granulomatous inflammation in the liver. Eggs laid by adult parasites are trapped in the liver, a process that leads to marked inflammation, tissue eosinophilia, collagen deposition, and, ultimately, extensive hepatic fibrosis. The cytokine cascade induced by schistosome eggs is characterized by increased production of several type-2-associated cytokines including interleukin (IL)-4, IL-5, IL-10, and IL-13. Cytokine depletion and knockout experiments have been especially useful for dissecting the specific contributions of type-2 cytokines to the pathogenesis of schistosomiasis. 3-5 We have shown that granulomatous inflammation and hepatic fibrosis is markedly reduced in infected mice when the type-2 cytokine pattern is converted to a more dominant type-1 response. 6,7 This effect was achieved by sensitizing mice to egg antigens in the presence of IL-12, a potent Th1-inducing adjuvant.
The Th2-to-Th1 deviated immune response in egg/IL-12-sensitized and infected mice is characterized by a marked increase in interferon (IFN)-γ, IL-12, and tumor necrosis factor (TNF)-α mRNA expression in the granulomatous livers. Cytokine ablation experiments demonstrated that all three type-1-associated cytokines were required for the maintenance of the Th1 response and, most importantly, for the reduction in granuloma size and hepatic fibrosis. 8 Recent studies suggest that inducible nitric oxide synthase (NOS-2) may be an important regulator of IL-12-induced responses. 9 Up-regulation of NOS-2 by IL-12 can induce immune suppression and reduce the efficacy of IL-12. 10 NOS-2 can also suppress Th1 cell development, perhaps through its potent antiproliferative effect on T cells. 11 Thus, iNO is not only a potent cytotoxic and antimicrobial agent, 12 but also exhibits significant immunoregulatory activity.
Because IL-12 promotes the differentiation of Th1 cells, and IFN-γ and TNF-α up-regulate NOS-2 expression, 13 we hypothesized that production of iNO is up-regulated in egg/IL-12-sensitized mice and that this may limit the Th2-suppressing activity of IL-12 and, consequently, its anti-pathology effect in schistosomiasis. Moreover, given its suspected role in disease progression in schistosomiasis, 14,15 NOS-2 might also exhibit tissue-damaging activity in egg/IL-12 sensitized mice, which could also limit the efficacy of this anti-pathology vaccine. Therefore, an improved anti-pathology effect might be expected in the absence of NOS-2, since the antiproliferative effects of iNO on Th1 cells would be eliminated, as well as its potentially tissue destructive and pro-inflammatory activities. To test this hypothesis, we sensitized WT and NOS-2-deficient mice with schistosome eggs and IL-12 and subsequently infected the animals with S. mansoni cercariae. The effects on liver pathology, antigen-specific proliferation of lymphocytes, and cytokine production were examined in detail.
These experiments demonstrated that relatively normal type-1 and type-2 polarization occurred in the absence of NOS-2 in egg/IL-12 sensitized and unsensitized animals, respectively. This was confirmed both in vitro, in lymphocyte cultures re-stimulated with parasite antigen, and in vivo within the granulomatous tissues. Surprisingly, however, despite developing the predicted Th cell cytokine response, the egg/IL-12-sensitized NOS-2-deficient mice not only failed to down-regulate egg-induced inflammation and fibrosis, but displayed a marked exacerbation in the response. These data demonstrate that although normal or possibly improved Th2-to-Th1 immune deviation occurred in the egg/IL-12-sensitized NOS-2-deficient mice, the downstream anti-inflammatory and antifibrotic effects of the egg-specific type-1 response were completely eliminated in the absence of iNO.
Materials and Methods
Mice, Parasites, and Antigen Preparations
Female 42-day-old C57BL/6, C57BL/6Ai-[KO] NOS-2, and C57BL/6Ai-[KO] IFN-γ mice were obtained from Taconic Farms (Germantown, MD). All mice were housed under specific-pathogen-free conditions in a National Institutes of Health animal facility approved by the American Association for the Accreditation of Laboratory Animal Care. Cercariae of a Puerto Rican strain of S. mansoni (Biomedical Research Institute, Rockville, MD) were obtained from infected Biomphalaria glabrata snails (Biomedical Research Institute). Soluble egg antigen (SEA) and soluble worm antigen preparations (SWAP) were from homogenized S. mansoni eggs and adult parasites as previously described. 7
Immunizations and Infections
S. mansoni eggs were extracted from the livers of infected mice (Biomedical Research Institute) and enriched for mature eggs. Infection and sensitization of mice with eggs and rIL-12 has been previously described. 7 Briefly, groups of 10 NOS-2-deficient, IFN-γ-deficient and wild-type (WT) controls were injected i.p. with 5000 eggs on three occasions separated by 2-week intervals. Animals were also injected i.p. with rIL-12 (0.25 μg/dose) on 5 consecutive days beginning on the day of each immunization. Naïve mice and egg/IL-12 presensitized mice were infected 2 to 4 weeks after the last IL-12 injection by percutaneous exposure of tail skin for 40 minutes in water containing 25 cercariae. All mice were sacrificed 8 weeks after infection. At the time of sacrifice, liver tissue was taken for histology and RNA extraction. The draining lymph nodes and spleens were used to prepare cell suspensions for in vitro culture. We noted no mortalities in any group up to the point of sacrifice.
Histopathology and Fibrosis Measurement
Approximately half of the liver was removed and fixed in Bouin-Hollande solution. Histological sections were processed and stained with Giemsa (Histo-Path of America, Clinton, MD). The diameter and cell composition of granulomas (30/mouse) surrounding single eggs were measured by using an ocular micrometer, and the volume of each granuloma was calculated assuming a spherical shape. Only granulomas around eggs containing a mature miracidium were measured. Egg viability was assessed microscopically in the same liver sections. The collagen content of the liver samples, determined as hydroxyproline, was analyzed as described previously. 3 Additionally, collagen deposition in tissue sections was evaluated after staining with picrosirius red (Histo-Path of America). Serum alanine aminotransferase/aspartate aminotransferase levels were also monitored at the time of sacrifice, and although levels of both enzymes increased after infection, there was no difference detected between the WT egg/IL-12-sensitized or infected control mice (data not shown).
Lymphocyte Culture and Cytokine Detection
Spleen and mesenteric lymph nodes were removed aseptically and single cell suspensions were prepared. Cells were plated in 24-well tissue culture plates at a final concentration of 4 × 10 6 cells/ml (spleen) or 3 × 10 6 cells/ml (lymph nodes) in RPMI 1640 supplemented with 10% fetal calf serum, 2 mmol/L glutamine, 1 mmol/L sodium pyruvate, 50 μmol/L 2-mercaptoethanol antibiotic-antimycotic solution (all Life Technologies, Gaithersburg, MD). Cultures were incubated at 37°C in a humidified atmosphere of 5% CO2. Cells were stimulated with SEA (20 μg/ml), SWAP (50 μg/ml), Concanavalin A (1 μg/ml), or medium alone. Supernatant fluids were harvested at 72 hours and assayed for cytokine production. IFN-γ and IL-5 were measured by two-site enzyme-linked immunosorbent assay (ELISA) as previously described. 7 IL-4 (Genzyme Diagnostics, Cambridge, MA) and IL-13 (R & D Systems, Minneapolis, MN) were detected by two-site ELISA according to the manufacturer protocol. Cytokine levels were calculated with standard curves constructed using recombinant murine cytokines.
T Cell Proliferation
Spleen cells were cultured in 96-well microtiter plates at a concentration of 5 × 10 5 cells per well. Cells were stimulated in vitro with SEA (20 μg/ml), SWAP (50 μg/ml), Concanavalin A (1 μg/ml), or medium alone. After 48 hours cells were pulsed with 37 kBq (Methyl-3H) thymidine (ICN, Costa Mesa, CA) and harvested after an additional 24 hours of incubation to determine incorporated (3H) thymidine. Incorporated radioactivity was measured on a β-counter (1450 MicroBeta-TriLux, EG&G Wallac, Gaithersburg, MD).
Nitrite Analysis
The concentration of nitrite in supernatants of spleen cells stimulated in vitro as described above was determined spectrophotometrically by using the Griess reagent. Supernatants were collected after 72 hour, mixed 1/1 with Griess reagent, and absorbance measured at 543 nm using a SpectraMax 190 (Molecular Devices, Sunnyvale, CA). The nitrite concentration was determined using sodium nitrite as standard.
Reverse Transcriptase-Polymerase Chain Reaction (PCR) Detection of mRNAs
Relative quantities of mRNA for IFN-γ, IL-5, IL-13, hypoxanthine-phosphoribosyltransferase (HPRT), and NOS-2, expressed in inflammatory tissue, were determined by RT-PCR as previously described. 16 The sequence of primers and probes has been published previously. 16,17 The amplified DNA was analyzed by electrophoresis, Southern blotting, and hybridization with non-radioactive cytokine specific probes. The chemiluminescent signals were quantified using a ScanJet IIP (Hewlett-Packard, Palo Alto, CA). The amount of PCR product was determined by comparing the ratio of cytokine-specific signal density to that of HPRT-specific signal density for individual samples. Arbitrary densitometric units for individual samples were subsequently multiplied by a factor of 100 and compared with those for control mice (uninfected mice tissue).
Immunohistochemistry
Liver samples were quick frozen in O.C.T. compound (Miles Inc., Elkhart, IN) and stored at −75°C. Sequentially cut cryostat tissue sections (8 μm) were fixed with acetone and stored at −75°C. For CD11b staining, slides were rehydrated in wash solution (100 mmol/L Tris/HCl, pH 7.6, 0.15 mol/L NaCl, 0.05% Tween 20) for 10 minutes at room termperature. Protein block was added (10 mmol/L Tris/HCl pH 7.6, 0.15 mol/L NaCl, 3% Rad Free, Schleicher & Schuell) and incubated for 30 minutes at room temperature. Slides were washed 3× and incubated for 30 minutes with 10 μg/ml anti-CD11b-FITC Ab (Caltag, Burlingame, CA) at room temperature. Slides were washed 3× and mounted with Vectashield me-dium (Vector Laboratories, Burlingame, CA). Negative controls included matched isotype Ab (Pharmingen, San Diego, CA).
Statistics
Hepatic fibrosis (adjusted for egg number) decreases with increasing intensity of infection (worm pairs) in the infection experiments. These variables were, therefore, compared by analysis of covariance, using the log of total liver eggs as the covariate and the log of hydroxyproline per egg. All other variables were compared by Student’s t-test. In all cases, results were considered significant for P < 0.05.
Results
NOS-2 Expression Is Increased in Antigen/IL-12-Sensitized and Challenged Mice
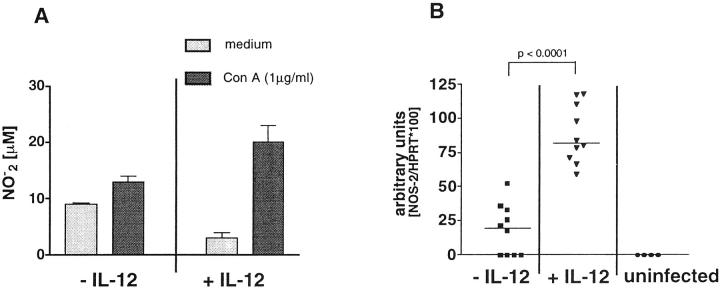
Splenocytes from unsensitized and egg/IL-12-sensitized and infected mice were stimulated in vitro with mitogen and culture supernatants were assayed for levels of nitrite, as a marker of NOS-2 activity. Sensitizing animals in the presence of IL-12 increased the accumulation of Con A-induced nitrite after challenge (Figure 1A) ▶ . Inflammatory tissues were also isolated and analyzed by semiquantitative RT-PCR for expression of NOS-2 mRNA. The results clearly demonstrate significantly increased levels of NOS-2 mRNA in the livers of the infected egg/IL-12-treated mice (Figure 1B) ▶ . NOS-2 mRNA was undetectable in the uninfected controls and only a very low level was detected in the infected non-IL-12-treated group.
Figure 1.
Production of iNO and expression of NOS-2 mRNA is increased in mice sensitized with schistosome eggs and IL-12. C57BL/6 mice were sensitized with schistosome eggs and IL-12, infected with S. mansoni, and sacrificed 8 weeks later. Spleen cells were pooled and restimulated in vitro with Concanavalin A (1 μg/ml) or left unstimulated. Supernatants were collected after 72h and analyzed for nitrite content (A). Liver tissues (B) were taken from all mice including naïve controls and total RNA was isolated. RNA was analyzed for NOS-2 mRNA expression by semiquantitative PCR. Nitrite accumulation is shown as the average concentration (in μmol/L) ± SD values per group (A). NOS-2 mRNA levels are expressed as arbitrary O. D. units, and each dot represents the result for a single mouse (B). The bar shows the group medians.
Egg/IL-12-Sensitized NOS-2-Deficient Mice Fail to Control Egg-Induced Inflammation
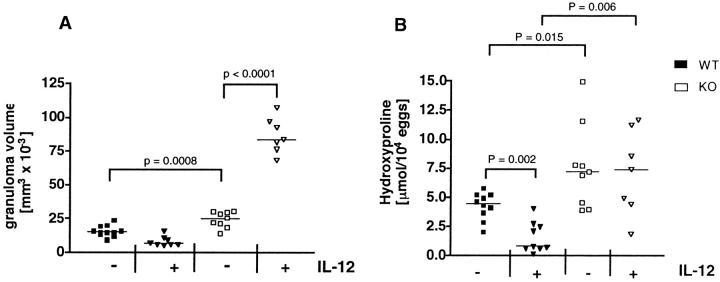
Although iNO is an important by-product of type-1 responses, it has also been shown to antagonize their development, which may result from its potent anti-proliferative effect on T cells. 11 We hypothesized that the anti-pathology effects of IL-12 might be improved in the absence of NOS-2, since the Th1-dampening effects of iNO would be eliminated as well as its suspected downstream pro-inflammatory activity. 11,14,15,18 To test this hypothesis, we sensitized WT and NOS-2-deficient mice with schistosome eggs and IL-12 and subsequently infected the animals with S. mansoni cercariae. To determine whether NOS-2 was affecting granuloma formation, the livers of egg-sensitized and infected mice were examined histologically. Tissue sections were Giemsa-stained and the size of granulomas assessed micro- scopically. Unexpectedly, the control nonsensitized NOS-2-deficient mice showed a small but neverthelesssignificant increase in granuloma size when compared with the control WT group (Figure 2A) ▶ . This suggested that the small amount of iNO detected in infected WT control mice (Figure 1B) ▶ was sufficient to mediate a partial anti-inflammatory effect. The anti-inflammatory effect was even more obvious in the egg/IL-12-sensitized WT group, in which NOS-2 levels increased (Figure 1B) ▶ and granuloma size decreased (Figure 2A) ▶ . Much more striking, however, was the more than eightfold difference in granuloma volume observed between the egg/IL-12-sensitized WT and NOS-2-deficient mice. Indeed, the mutant animals showed a marked exacerbation of egg-induced inflammation when sensitized with eggs and IL-12.
Figure 2.
Sensitizing mice with eggs/IL-12 fails to control egg-induced granuloma formation and hepatic fibrosis in infected NOS-2-KO mice. Granuloma size was measured in liver tissue as described in Material and Methods. Data in A represent measurements from individual mice and the bar denotes the group median (n = 7–10). Statistical significance is indicated in the figures. Fibrosis was quantified in granulomatous liver tissue by analysis of hydroxyproline content, expressed as μmol/L hydroxyproline per 10,000 eggs, calculated after subtracting the mean hydroxyproline content of uninfected livers (B). Data points represent the mean of duplicate measurements and the bar indicates the group median (n = 7–10). Statistical significance between experimental groups was determined by analysis of covariance (P < 0.05). Similar results were obtained in a second experiment.
In the Absence of NOS-2, Hepatic Fibrosis Is Increased and Completely Unaffected by Egg/IL-12 Presensitization
Because the egg/IL-12-sensitized NOS-2-deficient mice were unable to down-regulate the egg-induced inflammatory response (Figure 2A) ▶ , we examined whether there was a similar affect on fibrosis in these animals. Fibrosis was quantified in the granulomatous livers by analysis of hydroxyproline. 3 As expected, fibrosis was significantly increased in the livers of infected WT mice and reduced by prior egg/IL-12-sensitization (Figure 2B) ▶ . In agreement with their increased inflammatory response, hepatic hydroxyproline levels were significantly increased in NOS-2-deficient mice when compared with the WT non-IL-12-treated control group. Interestingly, unlike the WT animals, there was no reduction in fibrosis in the livers of the egg/IL-12-sensitized mutant mice. These observations were confirmed in tissue sections stained with the collagen specific stain, picrosirius red. Here, the contrast between egg/IL-12-sensitized WT (Figure 3B) ▶ and NOS-2-deficient mice (Figure 3D) ▶ is immediately obvious. A large rim of collagen surrounds the lesion in the mutant mouse, but is markedly reduced in the small granulomas formed in the infected WT animals.
Figure 3.
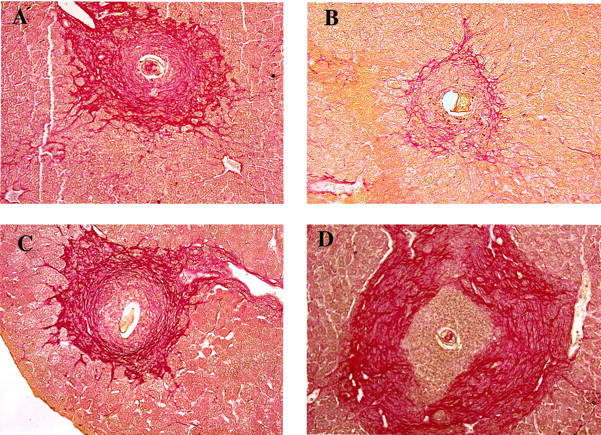
Collagen staining in granulomatous liver tissues shows large areas of collagen deposition in egg/IL-12 sensitized NOS-2-KO mice, but not in WT controls. Liver sections were stained with the collagen-specific stain picrosirius red. Hepatic granuloma from an infected WT (A), egg/IL-12 sensitized and infected WT (B), infected NOS-2-KO (C), and egg/IL-12 sensitized and infected NOS-2-KO mouse (D) are shown. Representative granulomas were photographed at a magnification of ×200.
WT and NOS-2-Deficient Mice Develop a Similar Cytokine Expression Profile in Vitro and in Vivo
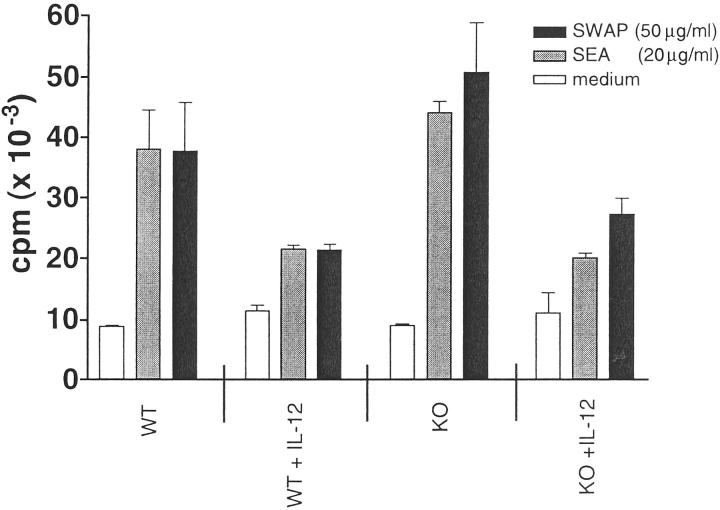
Surprisingly, the in vitro proliferative response of splenocytes to parasite antigens was mostly unaffected by the absence of NOS-2. Both WT and NOS-2-deficient mice displayed a similar response to SEA or SWAP stimulation (Figure 4) ▶ . There was a trend toward increased proliferative responses in the non-IL-12-treated NOS-2-deficient cultures, although this was not significant in all experiments. However, there was a consistent reduction in proliferation observed in the IL-12-treated groups and this was of similar magnitude in both WT and NOS-2-deficient cultures. These results suggested that factors other than iNO were likely responsible for the decreased proliferative responses observed in IL-12-treated mice. Related studies have suggested that IFN-γ 19 and/or IL-10 20 could be involved.
Figure 4.
The proliferative response of splenocytes to parasite antigens is unaffected by the absence of NOS-2. Splenocytes from infected egg/IL-12-sensitized and nonsensitized NOS-2-KO and WT mice were pooled, plated in 96 well plates at 5 × 10 5 cells/well, and restimulated with medium alone, soluble egg antigen (SEA, 20 μg/ml) or soluble worm antigen (SWAP, 50 μg/ml). After 48 hours, cells were pulsed with 37 kBq (Methyl-3H) thymidine, harvested after an additional 24 hours and counted. Bars represent the mean ± SD of three individual wells. Similar results were obtained in several repeat experiments.
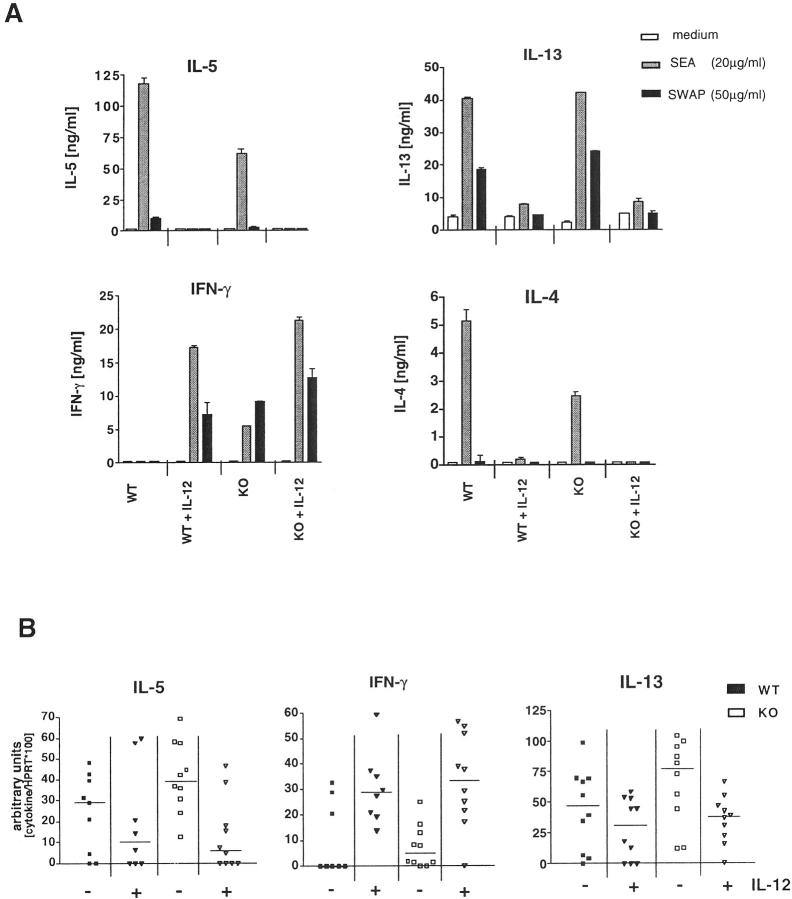
To determine whether NOS-2 activation was affecting the cytokine-producing profile of egg/IL-12-sensitized mice, spleen cells were isolated and analyzed by ELISA (Figure 5A) ▶ and ELISPOT assay (data not shown) for antigen specific cytokine responses. Granulomatous liver tissues were also analyzed by quantitative RT-PCR for IL-13, IFN-γ and IL-5 mRNA (Figure 5B) ▶ .
Figure 5.
NOS-2-deficiency does not significantly affect the establishment of a type-1 dominant response in egg/IL-12-sensitized mice. Single cell suspensions of splenocytes, pooled from groups of egg/IL-12-sensitized or nonsensitized infected NOS-2-KO or WT mice were plated in 24-well plates (4 × 10 6 cells/well) and restimulated with medium alone, SEA (20 μg/ml) or SWAP (50 μg/ml). Supernatants were collected after 72 hours and production of IFN-γ, IL-4, IL-5, and IL-13 were measured by ELISA. Each bar in A represents the results obtained with pooled cells from 7 to 10 mice (similar results were obtained in a second assay). In addition, liver biopsies were taken from all mice at the time of sacrifice. Total RNA was isolated and examined for IL-5, IL-13, and IFN-γ mRNA by semiquantitative RT-PCR. Each data point represents the result for an individual mouse and the bar indicates the group median. B shows the results of two separate experiments with 5 mice randomly chosen for analysis from each study.
In vitro restimulated lymphocytes (data not shown) and splenocytes from egg/IL-12-sensitized NOS-2-deficient mice displayed an overall pattern that was similar to WT mice. Infected WT controls displayed high levels of IL-4, IL-5, and IL-13, and virtually no IFN-γ, whereas egg/IL-12 sensitized mice developed a dominant IFN-γ response (Figure 5A) ▶ . Interestingly, the NOS-2-deficient control group displayed reduced levels of IL-4 and IL-5 when compared with WT mice. There was also a detectable IFN-γ response in these mice, suggesting that the non-IL-12-treated NOS-2-deficient animals were developing a less polarized Th2 response than their WT counterparts. The egg/IL-12-sensitized NOS-2-deficient mice also displayed a more marked IFN-γ response compared to WT animals, although this was only a modest increase in most experiments. Both groups of infected WT and NOS-2-deficient mice displayed marked reductions in IL-4, IL-5, and IL-13 when sensitized with eggs and IL-12, and there was no evidence for a more complete ablation in the NOS-2-deficient mice. The data suggest that NOS-2 deficiency does not dramatically affect schistosome egg-induced Th2 or Th1-type cytokine polarization. In fact, the data indicate that a slightly improved Th1-polarized response is achieved in the egg/IL-12-sensitized NOS-2-deficient mice. There was also no change in the frequencies of type-1 or type-2 cytokine producing cells as determined by ELISPOT analysis (data not shown).
Finally, lung and liver tissues were assayed by semiquantitative RT-PCR for IL-5, IL-13, and IFN-γ mRNA. High levels of IL-5 and IL-13 and low levels of IFN-γ mRNA in the tissues of the WT and NOS-2-deficient control groups confirmed that a dominant type-2 response was established in these mice (Figure 5B) ▶ . IL-5 and IL-13 levels decreased and IFN-γ mRNA increased to the same extent in both groups of egg/IL-12-sensitized mice, further demonstrating that NOS-2 plays little or no regulatory role in the development of these responses.
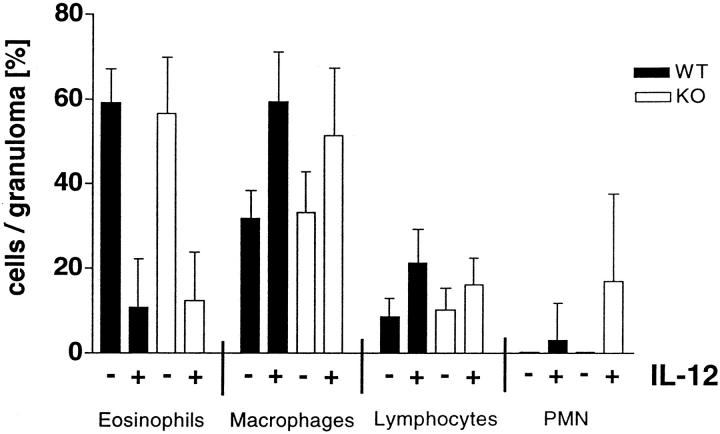
The Cellular Composition of Egg-Induced Granulomas Is Not Affected by NOS-2 Deficiency, Though the Total Number of Macrophages Increases Dramatically in the Egg/IL-12-Sensitized Mutant Mice
A detailed analysis of the cellular composition of hepatic granulomas revealed no major differences between the NOS-2-deficient and WT control groups (Figure 6) ▶ . There were however, marked changes resulting from egg/IL-12 sensitization, although these changes were similar in both WT and NOS-2-deficient mice. The lesions in both control groups were composed of between 40 to 75% eosinophils and moderate numbers of macrophages and lymphocytes. Polymorphonuclear (PMN) cells were absent. In contrast, granulomas in egg/IL-12-sensitized mice were composed of a larger population of macrophages (50–60%) and far fewer eosinophils (approximately 10%). There was also a slight increase in the lymphocyte population and foci of PMNs were visible in the lesions of the egg/IL-12-treated mice. These findings demonstrate that tissue eosinophilia, an important marker of Th2-mediated inflammation, was regulated normally in the livers of IL-12-treated WT and NOS-2-deficient mice.
Figure 6.
Analysis of the cellular composition of hepatic granulomas revealed no major differences between NOS-2-deficient and WT control mice. The cellular composition of hepatic granulomas was evaluated by microscopic analysis of Giemsa-stained liver sections (10 granulomas/liver section). The bars represent the mean cell number ± SD per granuloma in each group (n = 7–10 mice).
The general architecture of the granulomas in the NOS-2 control and egg/IL-12-sensitized mice was also quite different, as seen in Figure 3, C and D ▶ , and Figure 7, A and B ▶ . Most obvious was the increase in macrophages, which appeared to be more centrally localized in the egg/IL-12-sensitized group (Figure 7B) ▶ . Macrophages were identified by immunohistochemistry using CD11b (Mac-1) as a marker. As shown in Figure 7, a ▶ population of CD11b+ cells was predominantly at the periphery of granulomas in the control (Th2 dominant lesion) NOS-2-deficient mice (Figure 7A) ▶ , which was similar to findings in infected WT controls (data not shown). In contrast, the Th1-dominant lesions observed in the egg/IL-12-sensitized NOS-2 mice exhibited a large population of CD11b+ cells, which extended to the deposited eggs. As demonstrated in Figure 3B ▶ , egg/IL-12-sensitization reduced granuloma size dramatically in WT mice and therefore, the number of detectable CD11b+ cells was small and their location in the granulomas was more variable (data not shown). Together, these observations suggest that the dramatic increase in granuloma size in the egg/IL-12-treated NOS-2-deficient mice (Figure 2A) ▶ is mostly attributable to a marked expansion in the number of tissue macrophages.
Figure 7.
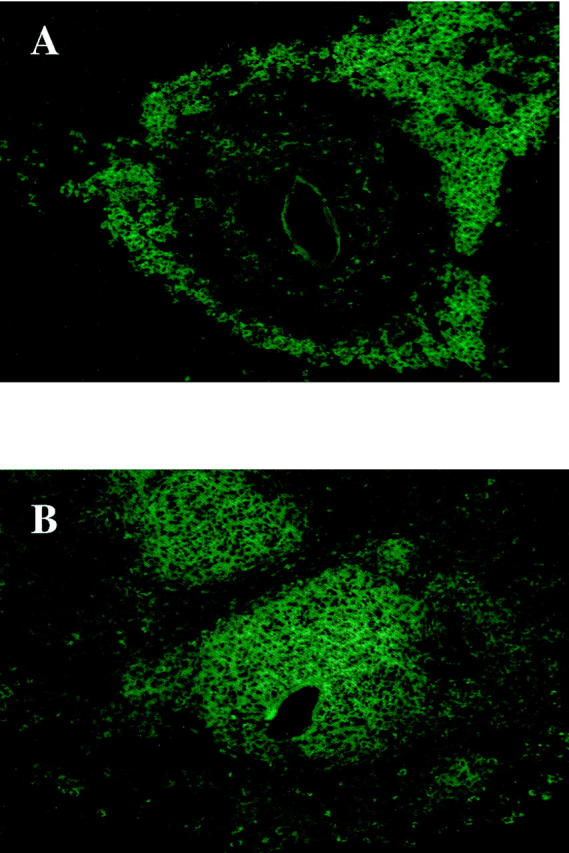
CD11b+ cells were found at the periphery of the lesions in infected NOS-2-KO mice but were more centrally localized in the egg/IL-12-sensitized animals. Cryosections (8 μm) of livers were stained with an antibody specific for CD11b, a macrophage marker, as described in Materials and Methods. Hepatic granulomas from an infected non-sensitized NOS-2-KO (A) and egg/IL-12-sensitized NOS-2-KO (B) mice are shown. Representative granulomas were photographed at a magnification of ×400.
IL-12-Treated IFN-γ-Deficient Mice Fail to Develop an Exacerbated Inflammatory Response, Despite Exhibiting a Marked NOS-2 Deficiency
The previous findings suggested that iNO functions as a potent anti-inflammatory mediator in the granulomatous livers of infected egg/IL-12-sensitized mice (Figure 2A) ▶ . Both ELISA and RT-PCR results confirmed that a type-1 response was established in the IL-12-treated NOS-2-deficient animals (Figure 5) ▶ . This suggested that type-1 rather than type-2 cytokines were likely responsible for the exacerbated inflammatory response observed in these animals. To determine whether this was indeed a type-1-driven effect, the granulomatous response of infected egg/IL-12-sensitized IFN-γ-deficient mice was also evaluated. These mice, unlike infected WT controls, failed to up-regulate NOS-2 expression significantly after egg/IL-12 sensitization (Figure 8A) ▶ . Nevertheless, despite exhibiting a marked deficiency in NOS-2, they did not develop the exacerbated inflammatory response observed in the IL-12-treated NOS-2-deficient animals (Figure 8B) ▶ . These mice, however, were incapable of undergoing successful immune deviation (Figures 8C and 8D) ▶ , which suggests that a polarized but nitric-oxide-deficient type-1 response is required to generate the exacerbated granulomatous response. As expected, fibrosis was tightly regulated by the relative dominance of type-2 versus type-1 cytokine expression (Figure 8E) ▶ .
Figure 8.
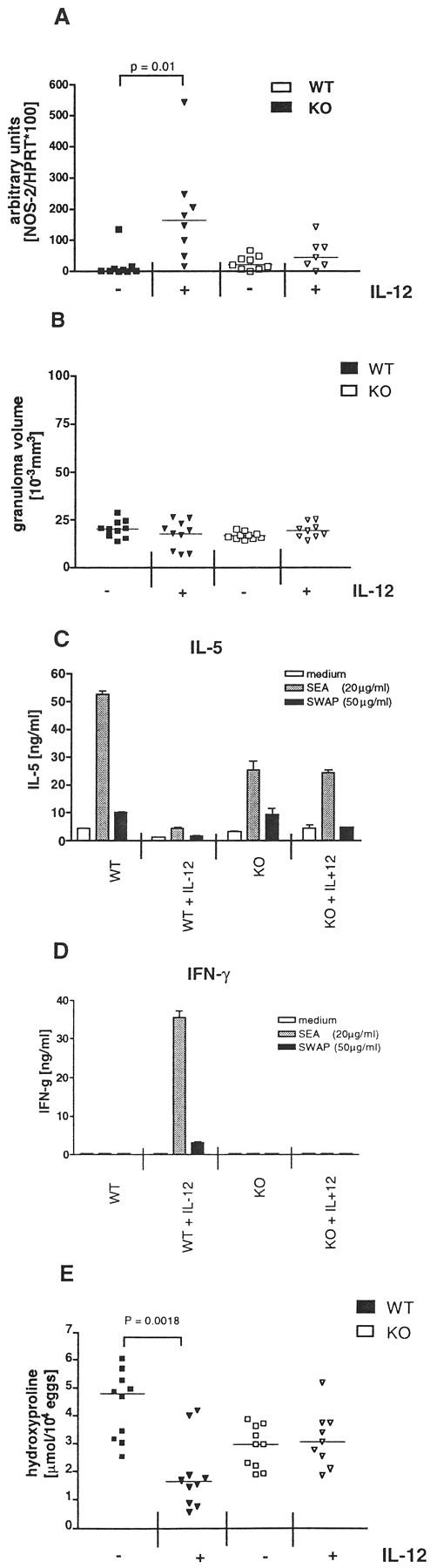
Egg/IL-12-sensitized IFN-γ-deficient mice fail to develop an exacerbated inflammatory response, despite exhibiting a marked NOS-2 deficiency. WT C57BL/6 and C57BL/6Ai-[KO] IFN-γ mice were sensitized and infected as described previously. A: Expression of NOS-2 mRNA in inflammatory liver tissue was analyzed by semiquantitative RT-PCR. Each data point represents the result of an individual mouse and the bar denotes the group median (n = 9–10). B: Granuloma size was measured in the livers of infected mice. C and D: Production of IL-5 (C) and IFN-γ (D) by in vitro restimulated splenocytes was measured in cell supernatants by ELISA. Each bar in C and D represents the results of pooled samples from all mice in an experimental group. E: Fibrosis was quantified in granulomatous liver tissue by analysis of hydroxyproline content. Data points in E show the mean of duplicate measurements and the bar indicates the group median. Statistical significance between experimental groups was determined by analysis of covariance (P < 0.05). Similar results were produced in a second experiment.
Discussion
Severe disease caused by schistosomiasis results from the chronic granulomatous response induced by eggs trapped in the liver. The chronic inflammation and resultant fibrosis ultimately lead to the development of portal-systemic shunts, bleeding, and death in some individuals. The magnitude of the egg-induced inflammatory response, as well as the mechanism leading to severe hepatic fibrosis, appears to be regulated by the relative dominance of type-1 versus type-2 cytokines. 4,5,21 In this disease, type-2 cytokines exhibit significant pro-inflammatory and pro-fibrotic activity, and type-1 cytokines induce the opposite effect. 7,21,22 The results from the current study, however, demonstrate that NOS-2 is an equally important regulatory factor controlling egg-induced inflammation, particularly in mice in which the type-2 response is deviated to type-1. Although NOS-2-deficient mice responded well to IL-12 and developed a highly polarized egg-specific type-1 response after infection, there was a marked exacerbation of granulomatous inflammation. There was also a failure to down-modulate hepatic fibrosis in the egg/IL-12-sensitized NOS-2-deficient animals. These data demonstrate that although normal or possibly improved Th2-to-Th1 immune deviation occurred in egg/IL-12-sensitized NOS-2-deficient mice, the downstream anti-inflammatory and anti-fibrotic effects of the egg-specific type-1 response were eliminated in the absence of NOS-2.
The effects of iNO on the immune response are diverse. iNO exhibits cytostatic and cytotoxic activity for many intracellular pathogens, 12 induces apoptosis in selected target cells, 23 and can regulate immune responses by controlling the proliferation of IFN-γ-producing cells. 11 Thus, the failure to control egg-induced inflammation in the egg/IL-12-sensitized NOS-2-deficient mice may have resulted from alterations in the Th1/Th2 cytokine balance. Nevertheless, the data confirmed that Th2 cytokines were decreased by IL-12 to nearly the same extent in both WT and NOS-2-deficient animals. There was also a tendency for a greater increase in IFN-γ production in the mutant mice, suggesting that normal or possibly improved type-1 polarization was achieved in the absence of NOS-2. This was confirmed by ELISA and ELISPOT assays of draining lymph node and splenocyte cultures and by semiquantitative RT-PCR analysis of the granulomatous tissues (Figure 5) ▶ . The marked decrease in tissue eosinophilia also supported this conclusion (Figure 6) ▶ . Thus, these data indicate that NOS-2 activation is not essential for Th1 response development in vivo, that type-1 response polarization by IL-12 is not dramatically improved by the absence of iNO, 10 and that relatively normal type-2 responses are generated in the absence of added IL-12.
Production of iNO by activated macrophages is induced by several proinflammatory cytokines, including IFN-γ 24 and TNF-α. 25 Excessive or continuous release of iNO can induce tissue damage and may contribute to the pathology seen in septic shock 26 and several infectious 27,28 and autoimmune diseases. 29-32 These observations indicate that in addition to its antimicrobial activity, iNO may exhibit tissue-destroying and pathogenic activity during immune responses. Interestingly, however, a recent paper investigating the role of NO in acute murine schistosomiasis suggested that iNO plays an important host protective role during infection with S. mansoni. 15 In that study, iNO was inhibited by treating mice with aminoguanidine, a selective inhibitor of NOS-2. WT mice treated with aminoguanidine exhibited severe cachexia, reduced hepatosplenomegaly, and exacerbated liver pathology. The development of large areas of coagulative necrosis, smaller granulomas, and increased numbers of apoptotic cells characterized liver pathology in the aminoguanidine-treated mice. From these observations, they concluded that iNO was necessary to limit hepatocyte damage when the liver is first exposed to eggs. Thus, blocking NO production had a profound deleterious effect.
Surprisingly however, our infected NOS-2-deficient animals failed to generate the same type of destructive liver pathology reported in the study by Brunet et al. 15 Although our animals were examined on day 56 postinfection, rather than day 47, there was no sign of cachexia in either the WT or NOS-deficient mice. WT mice weighed 23.62 ± 0.2 g (n = 10) and NOS-deficient mice weighed 27.51 ± 1.3 g (n = 9) at the time of sacrifice. There was also no evidence of reduced hepatomegaly or appearance of coagulative necrosis in the NOS-2-deficient animals. Liver weights were 1.57 ± 0.09 (n = 10) in WT and 1.74 ± 0.11 (n = 10) in NOS-2 KO mice, and the difference was not significant. A TUNEL assay was also performed on liver sections to determine whether there was an increase in apoptotic cells in the NOS-2-deficient granulomatous tissues, but no difference between WT and NOS-2-deficient mice was detected (data not shown). In the study by Brunet et al, the aminoguanidine treated WT mice also generated granulomas that were, on average, 32% smaller than the nontreated controls. 15 However, we noted an opposite phenotype, since granuloma size significantly increased rather than decreased in the infected NOS-2-deficient versus WT mice (Figure 2A) ▶ . Thus, there was no indication that NOS-2 was exhibiting the host protective activity described by Brunet et al. 15 We have no specific explanation for the differences between these studies, but it is possible that the complete ablation of NOS-2 in the knockout versus aminoguanidine-treated mice is a contributing factor. Moreover, although aminoguanidine is relatively nontoxic in uninfected mice, continued treatment in already compromised infected mice could generate undesirable toxic effects. Although minor but nevertheless significant effects on pathology were detected in the NOS-2-deficient versus WT mice, our data suggest only a limited role for NOS-2 during the natural course of infection with S. mansoni. Such observations are probably not that unexpected, given the low levels of NOS-2 mRNA detected in livers of infected type-2 dominant WT mice (Figure 1B) ▶ .
However, a much more dramatic role for NOS-2 was observed in mice sensitized with eggs and IL-12. The data strongly suggest that iNO plays a critical anti-inflammatory and anti-fibrotic role in egg/IL-12-sensitized and infected mice. Indeed, more than an eightfold difference in granuloma volume was observed between the egg/IL-12-sensitized WT and NOS-2-deficient mice (Figure 2A) ▶ . Thus, unlike WT animals where fibrosis and, to a lesser extent, granuloma size decrease as a result of Th2-to-Th1 immune deviation, 7 (Figure 2A) ▶ , sensitized NOS-2-deficient animals manifest an uncontrolled and exacerbated type-1-associated inflammatory response. These data were somewhat surprising, in that the study by Brunet et al 15 suggested that prolonged NO production might in fact be deleterious for the infected host. In that study, type-2 response deficient IL-4−/− C57BL/6 mice developed severe morbidity and succumbed during the acute phase of infection, and the authors hypothesized that iNO was contributing to the mortality of the animals. Unfortunately, that hypothesis was impossible to investigate in greater detail because their aminoguanidine-treated iNO inhibited IL-4-deficient mice died even earlier than the nontreated animals. Though it is difficult to directly compare findings from IL-4−/− mice with those from Th1-polarized egg/IL-12-senstized WT mice, our data suggest that increased NO production is not necessarily tissue-destroying or lethal for the infected host. In our study, granulomatous inflammation increased in the Th1-polarized egg/IL-12-sensitized NOS-2-deficient mice (Figure 2A) ▶ , whereas an opposite effect was observed in the aminoguanidine-treated Th2-deficient IL-4−/− mice. 15 Thus, our data suggest that the more important function of iNO during a type-1-dominant response is to serve as an anti-inflammatory rather than hepatotoxic mediator.
Similar observations were recently reported by Hogaboam et al in an experimental purified protein derivative model of pulmonary granulomatous inflammation. 33,34 In that model, mice injected intravenously with purified protein derivative-coated sepharose beads developed significantly larger lung lesions 34 and more collagen deposition 33 when treated with the NOS inhibitor L-NAME (NG-nitro-L-arginine-methyl ester). The growth in granuloma size was primarily associated with an increase in polymorphonuclear cells in the lesion, and the authors hypothesized that NO was regulating granuloma development indirectly by altering the chemokine- and cytokine-producing profile in the lung. Our data in the schistosomiasis model are mostly consistent with these observations, although the exacerbated lesions in the livers of egg/IL-12-sensitized type-1-polarized NOS-2-deficient mice were composed of a large population of macrophages, rather than PMNs. The paper by Hogaboam et al also noted increased IL-4 and IL-10 and reduced levels of IL-12 and IFN-γ in the lungs of L-NAME-treated mice. 34 However, we failed to detect any major differences in the cytokine producing profiles in the livers of our egg/IL-12-sensitized S. mansoni-infected groups. Although a preliminary screen of a panel of chemokines also failed to reveal differences (data not shown), the significant accumulation of macrophages in the egg/IL-12-sensitized NOS-deficient lesions suggests that chemokine regulation may be a possible explanation. The fact that lesion formation was not exacerbated in the egg/IL-12-sensitized IFN-γ-deficient mice (Figure 8) ▶ strongly suggests that the growth in granuloma size is mediated by a NOS-2-deficient but polarized type-1 response. Thus, we are particularly interested in examining chemokines that are regulated by type-1 cytokines in this model. We also speculate that the NOS-2 deficiency could influence the balance between pro- and anti-inflammatory macrophages and therefore affect the macrophage-mediated pathological response. 35,36 Regardless of the exact mechanism, our data, when combined with the findings of Hogaboam et al, suggest a major role for NO in the regulation of type-1-mediated inflammation and clarify the roles of NOS-2 in murine schistosomiasis. The use of NOS-2-deficient mice rather than NOS-inhibiting compounds 15 also confirmed that the alterations in granuloma formation were mediated by the inducible NOS-2 isoform.
Previous studies demonstrated that the type-2-associated cytokines IL-4 and IL-13 are the critical mediators of egg-induced inflammation and fibrosis in infected WT mice. 4,5,21 However, the data presented here suggest that significant inflammation and fibrosis can also accompany highly polarized type-1 immune responses, if there is an additional deficiency in NOS-2 expression. Interestingly, however, IL-13 expression was not completely ablated in the egg/IL-12-sensitized NOS-2-deficient mice (Figure 5B) ▶ . Therefore, fibrosis may still be regulated by the presence of IL-13. Nevertheless, given the fact that IL-4/IL-13 expression decreased, it seems much less likely that these cytokines were directly contributing to the marked increase in granulomatous inflammation observed in the sensitized mutant animals. Regardless of the exact mechanism, these data demonstrate that the downstream anti-inflammatory and antifibrotic effects of the egg-specific type-1 response are highly NOS-2-dependent. Immune deviation strategies have been proposed for other Th2-mediated diseases including allergy and asthma. 7,37-40 The results presented here suggest that the ultimate success of these strategies will rely not only on the successful establishment of a type-1-dominant response, but also on the simultaneous and efficient activation of NOS-2 expression in downstream effector populations. Indeed, it is intriguing to speculate that the previously reported inability of Th1 cells to modulate Th2-mediated inflammation 38,39 may be due entirely to the inefficient activation of NOS-2 at sites of inflammation.
Acknowledgments
We thank Dr. Fred Lewis and his colleagues at the Biomedical Research Institute for providing the parasite materials used in this study and Dr. Joe Sypek for providing recombinant murine IL-12. We also thank David Stephany for help with fluorescence-activated cell sorting, Dr. Matthew Park for helping to analyze chemokine expression, and Drs. Monica Chiaramonte, Karl Hoffmann, Richard Krause, Manuel Modolell, Stephanie James, and Alan Sher for many helpful discussions and for critically reading the manuscript.
Footnotes
Address reprint requests to Thomas A. Wynn, Laboratory of Parasitic Diseases, NIH/NIAID, Bldg. 7, Room 318, Bethesda, MD 20892. E-mail: twynn@atlas.niaid.nih.gov.
M. H. is supported by a Feodor-Lynen-Fellowship of the Alexander v. Humboldt Foundation, Germany.
References
- 1.Durham SR: The inflammatory nature of allergic disease. Clin Exp Allergy 1998, 28(suppl 6):20-24 [DOI] [PubMed] [Google Scholar]
- 2.Richaud-Patin Y, Alcocer-Varela J, Llorente L: High levels of TH2 cytokine gene expression in systemic lupus erythematosus. Rev Invest Clin 1995, 47:267-272 [PubMed] [Google Scholar]
- 3.Cheever A, Williams M, Wynn TA, Finkelman F, Seder R, Cox T, Hieny S, Caspar P, Sher A: Anti-IL-4 treatment of: Schistosoma mansoni-infected mice inhibits development of T cells and non-B, non-T cells expressing Th2 cytokines while decreasing egg-induced hepatic fibrosis. J Immunol 1994, 153:753-759 [PubMed] [Google Scholar]
- 4.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA: An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory respone. J Clin Invest 1999, 104:777-785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiaramonte MG, Schopf LR, Neben TY, Cheever AW, Donaldson DD, Wynn TA: IL-13 is a key regulatory cytokine for Th2 cell-mediated pulmonary granuloma formation and IgE responses induced by Schistosoma mansoni eggs. J Immunol 1999, 162:920-930 [PubMed] [Google Scholar]
- 6.Wynn TA, Eltoum I, Oswald I, Cheever A, Sher A: Endogenous interleukin 12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. J Exp Med 1994, 179:1551-1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wynn TA, Cheever A, Jankovic D, Poindexter R, Caspar P, Lewis F, Sher A: An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature 1995, 376:594-596 [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann KF, Caspar P, Cheever AW, Wynn TA: IFN-gamma, IL-12, and TNF-alpha are required to maintain reduced liver pathology in mice vaccinated with Schistosoma mansoni eggs and IL-12. J Immunol 1998, 161:4201-4210 [PubMed] [Google Scholar]
- 9.Huang FP, Feng GJ, Lindop G, Stott DI, Liew FY: The role of interleukin 12 and nitric oxide in the development of spontaneous autoimmune disease in MRLMP-lprlpr mice. J Exp Med 1996, 183:1447-1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koblish HK, Hunter CA, Wysocka M, Trinchieri G, Lee WM: Immune suppression by recombinant interleukin (rIL)-12 involves interferon gamma induction of nitric oxide synthase 2 (iNOS) activity: inhibitors of NO generation reveal the extent of rIL-12 vaccine adjuvant effect. J Exp Med 1998, 188:1603-1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei XQ, Charles IG, Smith A, Ure J, Feng GJ, Huand FP, Xu D, Muller W, Moncada S, Liew FY: Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 1995, 375:408-411 [DOI] [PubMed] [Google Scholar]
- 12.Bogdan C: Of microbes, macrophages and nitric oxide. Behring Inst Mitt 1997, 99:58-72 [PubMed] [Google Scholar]
- 13.MacMicking J, Xie QW, Nathan C: Nitric oxide and macrophage function. Annu Rev Immunol 1997, 15:323-350 [DOI] [PubMed] [Google Scholar]
- 14.Brunet LR, Finkelman FD, Cheever AW, Kopf MA, Pearce EJ: IL-4 protects against TNF-alpha-mediated cachexia and death during acute schistosomiasis. J Immunol 1997, 159:777-785 [PubMed] [Google Scholar]
- 15.Brunet LR, Beall M, Dunne DW, Pearce EJ: Nitric oxide and the Th2 response combine to prevent severe hepatic damage during schistosoma mansoni infection (in process citation). J Immunol 1999, 163:4976-4984 [PubMed] [Google Scholar]
- 16.Wynn T, Eltoum I, Cheever A, Lewis F, Gause W, Sher A: Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J Immunol 1993, 151:1430-1440 [PubMed] [Google Scholar]
- 17.McCafferty DM, Mudgett JS, Swain MG, Kubes P: Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology 1997, 112:1022-1027 [DOI] [PubMed] [Google Scholar]
- 18.Pearce EJ, La Flamme A, Sabin E, Brunet LR: The initiation and function of Th2 responses during infection with Schistosoma mansoni. Adv Exp Med Biol 1998, 452:67-73 [DOI] [PubMed] [Google Scholar]
- 19.Gajewski TF, Joyce J, Fitch FW: Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol 1989, 143:15–22 [PubMed]
- 20.Hoffmann KF, James SL, Cheever AW, Wynn TA: Studies with double cytokine-deficient mice reveal that highly polarized Th1- and Th2-type cytokine and antibody responses contribute equally to vaccine-induced immunity to Schistosoma mansoni. J Immunol 1999, 163:927-938 [PubMed] [Google Scholar]
- 21.Kaplan MH, Whitfield JR, Boros DL, Grusby MJ: Th2 cells are required for the Schistosoma mansoni egg-induced granulomatous response. J Immunol 1998, 160:1850-1856 [PubMed] [Google Scholar]
- 22.Wynn TA, Cheever AW: Cytokine regulation of granuloma formation in schistosomiasis. Curr Opin Immunol 1995, 7:505-511 [DOI] [PubMed] [Google Scholar]
- 23.Kim YM, Bombeck CA, Billiar TR: Nitric oxide as a bifunctional regulator of apoptosis. Circ Res 1999, 84:253-256 [DOI] [PubMed] [Google Scholar]
- 24.Stuehr DJ, Marletta MA: Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J Immunol 1987, 139:518-525 [PubMed] [Google Scholar]
- 25.Gazzinelli R, Eltoum I, Wynn T, Sher A: Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol 1993, 151:3672-3681 [PubMed] [Google Scholar]
- 26.Kirkeboen KA, Strand OA: The role of nitric oxide in sepsis: an overview. Acta Anaesthesiol Scand 1999, 43:275-288 [DOI] [PubMed] [Google Scholar]
- 27.Khan IA, Schwartzman JD, Matsuura T, Kasper LH: A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc Natl Acad Sci USA 1997, 94:13955-13960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman GL, Colston JT, Zabalgoitia M, Chandrasekar B: Contractile depression and expression of proinflammatory cytokines and iNOS in viral myocarditis. Am J Physiol 1998, 274:H249–H58. [DOI] [PubMed]
- 29.McCartney-Francis N, Allen JB, Mizel DE, Albina JE, Xie QW, Nathan CF, Wahl SM: Suppression of arthritis by an inhibitor of nitric oxide synthase. J Exp Med 1993, 178:749-754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinberg JB, Granger DL, Pisetsky DS, Seldin MF, Misukonis MA, Mason SN, Pippen AM, Ruiz P, Wood ER, Gilkeson GS: The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-L- arginine. J Exp Med 1994, 179:651-660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santiago E, Perez-Mediavilla LA, Lopez-Moratalla N: The role of nitric oxide in the pathogenesis of multiple sclerosis. J Physiol Biochem 1998, 54:229-237 [PubMed] [Google Scholar]
- 32.Ding M, Zhang M, Wong JL, Rogers NE, Ignarro LJ, Voskuhl RR: Antisense knockdown of inducible nitric oxide synthase inhibits induction of experimental autoimmune encephalomyelitis in SJL/J mice. J Immunol 1998, 160:2560-2564 [PubMed] [Google Scholar]
- 33.Hogaboam CM, Gallinat CS, Bone-Larson C, Chensue SW, Lukacs NW, Strieter RM, Kunkel SL: Collagen deposition in a non-fibrotic lung granuloma model after nitric oxide inhibition. Am J Pathol 1998, 153:1861-1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogaboam CM, Chensue SW, Steinhauser ML, Huffnagle GB, Lukacs NW, Strieter RM, Kunkel SL: Alteration of the cytokine phenotype in an experimental lung granuloma model by inhibiting nitric oxide. J Immunol 1997, 159:5585-5593 [PubMed] [Google Scholar]
- 35.Munder M, Eichmann K, Modolell M: Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol 1998, 160:5347-5354 [PubMed] [Google Scholar]
- 36.Goerdt S, Orfanos CE: Other functions, other genes: alternative activation of antigen-presenting cells. Immunity 1999, 10:137-142 [DOI] [PubMed] [Google Scholar]
- 37.Kline JN, Waldschmidt TJ, Businga TR, Lemish JE, Weinstock JV, Thorne PS, Krieg AM: Modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. J Immunol 1998, 160:2555-2559 [PubMed] [Google Scholar]
- 38.Hansen G, Berry G, DeKruyff RH, Umetsu DT: Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J Clin Invest 1999, 103:175-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Randolph DA, Stephens R, Carruthers CJ, Chaplin DD: Cooperation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation (in process citation). J Clin Invest 1999, 104:1021-1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohn L, Homer RJ, Niu N, Bottomly K: T helper 1 cells and interferon gamma regulate allergic airway inflammation and mucus production. J Exp Med 1999, 190:1309-1318 [DOI] [PMC free article] [PubMed] [Google Scholar]