Abstract
Photodynamic therapy (PDT) generates free radicals through the absorption of light by photosensitizers. PDT shows promise in the treatment of intimal hyperplasia, which contributes to restenosis, by completely eradicating cells in the vessel wall. This study investigates the mechanisms of PDT-induced cell death. PDT, using the photosensitizer chloroaluminum-sulfonated phthalocyanine (1 mg/kg) and laser light (λ = 675 nm) 100 J/cm2 was administered to rat carotid arteries after balloon injury-induced intimal hyperplasia. Apoptosis was determined by cell morphology with light microscopy and transmission electron microscopy, DNA cleavage by terminal dUTP nick-end labeling staining, and nucleosomal fragmentation (ladder pattern) by DNA agarose gel electrophoresis. Four hours after PDT, apoptosis was observed in vascular cells, as evidenced by terminal dUTP nick-end labeling staining and transmission electron microscopy. Within 24 hours no cells were present in the neointima and media. Immunofluorescence using an α-smooth muscle cell actin antibody confirmed the disappearance of all neointimal and medial cells within 24 hours. No inflammatory cell infiltrate was observed during this time frame. Apoptosis was sharply confined to the PDT treatment field. These data demonstrate that vascular PDT induces apoptosis as a mechanism of rapid, complete, and precise cell eradication in the artery wall. These findings and the lack of inflammatory reaction provide the basis for understanding and developing PDT for a successful clinical application in the treatment of hyperplastic conditions such as restenosis.
Diseases of the vascular system are the leading cause of death and disability in the Western world. Although interventions for arterial occlusive disease, such as angioplasty and bypass grafting, are initially successful in the majority of cases, the vascular response to injury and ensuing restenosis limits their long-term efficacy. 1 Several factors contribute to this process: constrictive remodeling, intimal hyperplasia (IH), and elastic recoil. IH results from migration and subsequent proliferation of smooth muscle cells into the subintima, with unrestrained deposition of extracellular matrix. 2,3 The failure to develop a clinically effective pharmacological approach to inhibit IH has spawned novel experimental strategies including using antibodies against growth factors, genetic modulation of the cell cycle, and γ irradiation. 4-7 All have led to a decrease in smooth muscle cell proliferation and inhibition of experimental IH, and the last is currently being tested in clinical trials with promising results.
Another approach to inhibit IH is photodynamic therapy (PDT), a process dependent on the tissue uptake of a photosensitizing dye and subsequent irradiation of the site with visible or infrared light of an appropriate wavelength that is absorbed by the photosensitizer. PDT generates reactive free radicals and oxygen intermediates with very short half-lives that exert their effect locally by nonspecifically altering proteins and other membrane constituents, ultimately leading to cellular damage and cell death. 8,9 PDT is effective in preventing experimental IH, 10,11 and a clinical trial is currently underway to assess its safety and efficacy. 12
The major, consistent result of vascular PDT in experimental models is the complete eradication of cells in the vessel wall within 24 hours, without inducing an inflammatory response. The acellular artery is rapidly recovered with endothelial cells and the structural integrity of the vascular wall is maintained without a delayed intimal hyperplastic response. 13 Mechanisms regulating these PDT-induced processes have not yet been fully elucidated. Particularly, the reason for the absence of an inflammatory reaction, despite complete cell eradication, remains unclear. A possible explanation may be that vascular PDT induces cell death by apoptosis, a morphologically defined form of programmed cell death. Because apoptosis does not induce an inflammatory response, it is currently considered a favorable way of eliminating cells. 14 Whether a cell dies by apoptosis or necrosis in response to PDT depends on the type of photosensitizer, the cellular localization and concentration of the photosensitizer, the PDT dose, and cell type. 15-17 This study was undertaken to determine whether and to what extent apoptosis was the mechanism for vascular PDT-induced cell eradication.
Materials and Methods
Induction of IH
Male Sprague-Dawley rats (Charles River Breeding Laboratories, Wilmington, MA) were anesthetized using ketamine (75 mg/kg)/xylazine (5 mg/kg)/atropine (40 μg/kg) i.p. Common carotid artery balloon injury was performed using a 2F Fogarty balloon catheter (Baxter Health Care Corp., Edwards Div., Irvine, CA) as previously described. 10 Animal care was in compliance with “Principles of Laboratory Animal Care” and the “Guide for the Care and Use of Laboratory Animals” (National Institutes of Health publication No. 80–23, revised 1985) and approved by the institutional animal care committee.
Photodynamic Therapy
Seven days after balloon injury, rats were anesthetized and chloroaluminum-sulfonated phthalocyanine (1 mg/kg; Novartis, Basel, Switzerland) was injected intravenously. The carotid artery was dissected and optically isolated with a mylar reflector to achieve uniform artery irradiation. Twenty minutes after injection of the chloroaluminum-sulfonated phthalocyanine, a 1-cm segment of the artery was irradiated with an argon pumped dye laser (λ = 675 nm, irradiance 100 mW/cm2, fluence 100 J/cm2, Coherent Innova I 100 and Coherent CR 599; Coherent, Palo Alto, CA).
Harvest of Arteries
Control non-PDT-treated animals (n = 4) received saline and sham carotid exposure before pentobarbital overdose. PDT-treated animals were sacrificed at 2 hours (n = 4), 4 hours (n = 8), 6 hours (n = 4), 10 hours (n = 4), and 24 hours (n = 4) after PDT. Harvested arteries were flushed with saline, and perfusion-fixed in situ with 10% buffered formalin for light microscopy, with 1.5% glutaraldehyde in cacodylate buffer for transmission electron microscopy (TEM) (time, 4 hours), or with saline only for DNA extraction (time, 24 hours).
Histology
Cross-sections were prepared from half of each treated arterial segment and longitudinal sections of the other half to visualize the irradiated/nonirradiated interface. Sections (4 μm) were stained with hematoxylin and eosin for cell morphology.
Transmission Electron Microscopy
To identify ultrastructural cell morphology, TEM was performed on arteries 4 hours after PDT or sham treatment. Fixed specimens were postfixed in 2% OsO4, dehydrated in graded alcohol, and flat embedded in Epon 812 (Electron Microscopy Sciences, Fort Washington, PA). TEM sections (100 nm) were cut on an ultramicrotome (Reichert-Jung Ultracut, Vienna, Austria), stained with uranyl acetate and lead citrate, and examined with an electron microscope (CM 10; Philips, Eindhoven, The Netherlands).
Terminal dUTP Nick-End Labeling (TUNEL) Staining
TUNEL staining was used to identify apoptotic cells using a standard fluorescein kit (Apoptag apoptosis detection kit; Oncor, Purchase, NY) in deparaffinized sections counterstained with propidium iodide. In the TUNEL-stained specimens, the ratio of apoptotic cells (fluorescein-positive) to the total number of cells (propidium iodide-positive) in the neointima, media, and adventitia was determined. Four ×400 microscopic fields per slide were counted and four slides per animal (n = 4 rats) were analyzed. Means of the fields were obtained for each slide and the means of the four slides per animal were compared to those of the other animals.
Immunofluorescence
Four-micrometer thick sections were deparaffinized with xylene and rehydrated with graded methanol. Smooth muscle cells were detected using a monoclonal anti-α smooth muscle actin fluorescein isothiocyanate-conjugated antibody (Clone 1A4; Sigma Chemical Co., St. Louis, MO) used at 1:2,000 dilution in phosphate-buffered saline/10% fetal bovine serum (1 hour at 37°C in a humidified chamber). Nuclei were stained with propidium iodide at 0.33 μg/ml (Oncor). The distribution of immunopositive cells was determined using a confocal microscope (Bio-Rad MRC-1024/2P multiphoton microscope; Bio-Rad, Hercules, CA). Digital images were captured using an excitation of 494 nm and 488 nm, emission at 518 nm and 525 to 550 nm for fluorescein isothiocyanate and propidium iodide, respectively.
Analysis of Internucleosomal DNA Fragmentation: DNA Laddering
PDT was administered at 14 days instead of 7 days after balloon injury to increase the cell number and allow analysis of the DNA in the studied artery segment. Two (8-mm in length) arteries per sample were used for DNA isolation with the QIAamp Tissue Kit (Quiagen, Santa Clara, CA). The eluted DNA was separated using standard 1.5% agarose (Bio-Rad) gel electrophoresis at 10 V/cm. DNA was visualized by staining with ethidium bromide (0.5 μg/ml) and photographed under UV illumination.
Statistical Evaluation
All values were expressed as mean ± SD. The statistical significance of TUNEL-positive cell count ratio was determined using the two-tailed unpaired Student’s t-test. Differences were considered significant at P < 0.05.
Results
PDT-Induced Cytotoxicity
Balloon injury to the common carotid artery resulted in the development of IH at 7 days. PDT treatment led to a complete eradication of cells in the vessel wall by 24 hours, without inflammation (Figure 1) ▶ . Four hours after PDT the nuclear morphology of some cells in the media and in the neointima (Figure 1B ▶ , arrows) suggested apoptosis. The majority of the neointimal cells had died by 6 hour leaving empty areas in the matrix (Figure 1C ▶ , arrow). Twenty-four hours after PDT, no cells were present in the neointima and media and only an occasional cell could be seen in the adventitia.
Figure 1.
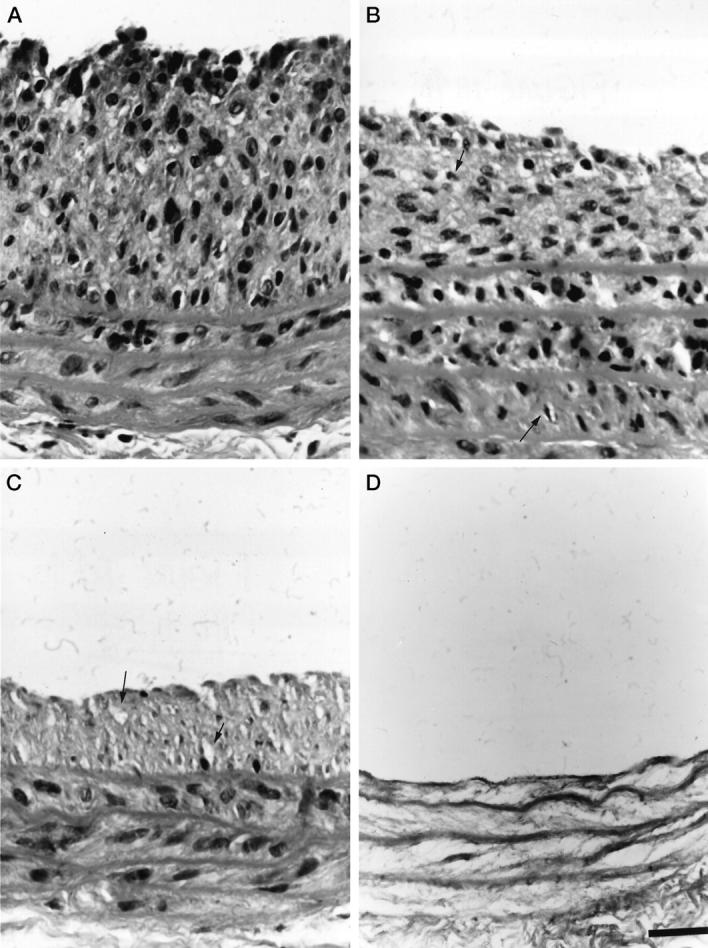
Composite photomicrographs depicting histological cross sections of balloon-injured rat common carotid arteries non-PDT treated (A), and 4 hours (B), 6 hours (C), and 24 hours (D) after PDT. Note the decline of the neointimal hyperplastic lesion (IH) with a complete eradication at 24 hours. Small, fragmented nuclei suggesting cells undergoing apoptosis (arrows) are evident 4 hours after PDT (B). Empty compartments indicate void left by cells (arrows) 6 hours after PDT (C). All cells disappear between 6 and 24 hours after PDT. Stained with hematoxylin and eosin. Scale bar, 25 μm.
Electron Microscopy
Morphological criteria were used to identify apoptotic cells by TEM. Intimal hyperplastic cells in control arteries displayed normal nuclear morphology and abundant cytoplasmic rough endoplasmic reticulum (Figure 2A) ▶ . Four hours after PDT treatment, cells developed morphological features characteristic of apoptosis: chromatin compaction into uniform electron-dense masses with nuclear margination, nuclear fragmentation, cellular shrinkage, cell membrane vacuolization and blebbing, and the increase in electron-density of the cytosol (Figure 2B) ▶ .
Figure 2.
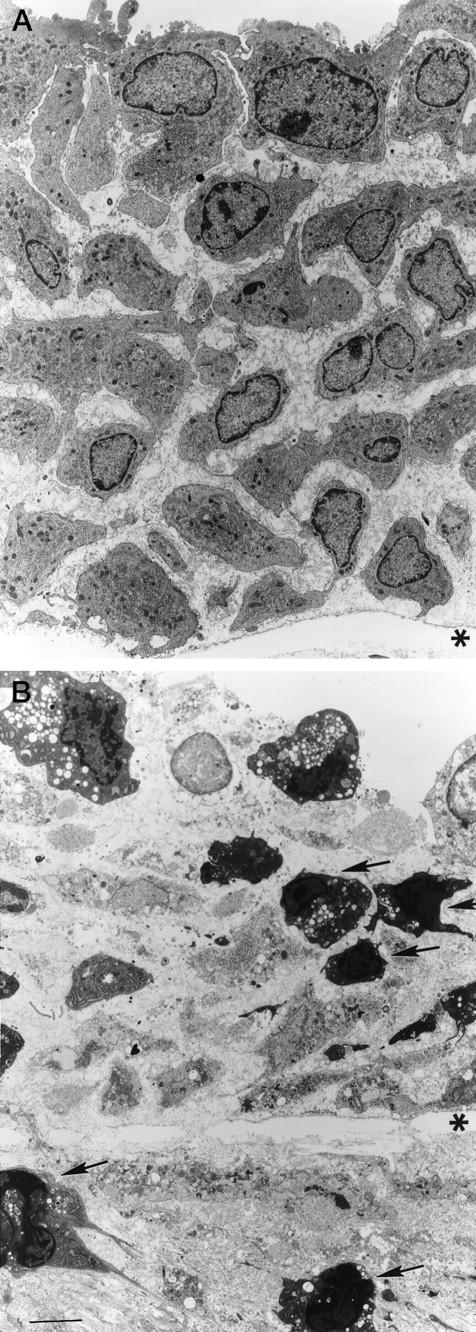
Transmission electron micrographs of the rat common carotid artery 7 days after the induction of IH. Control animal (A): cells with abundant rough endoplasmic reticulum, and oval nuclei. Four hours after PDT (B): ultrastructural features of apoptosis, including nuclear and cytoplasmic condensation, membrane blebbing, cell fragmentation, and apoptotic bodies are noted (arrows). *, internal elastic lamina. Scale bar, 6 μm.
TUNEL and Immunofluorescence
TUNEL staining was performed to detect internal and end-strand breaks, which often occur in the early stages of apoptosis. In longitudinal sections, 4 hours after PDT, TUNEL-positive cells were only present in the PDT-treated parts of the artery (Figure 3A ▶ , arrow). TUNEL-positive cells appeared in a time-dependent manner, progressing from the intima through the layers of the arterial wall.
Figure 3.
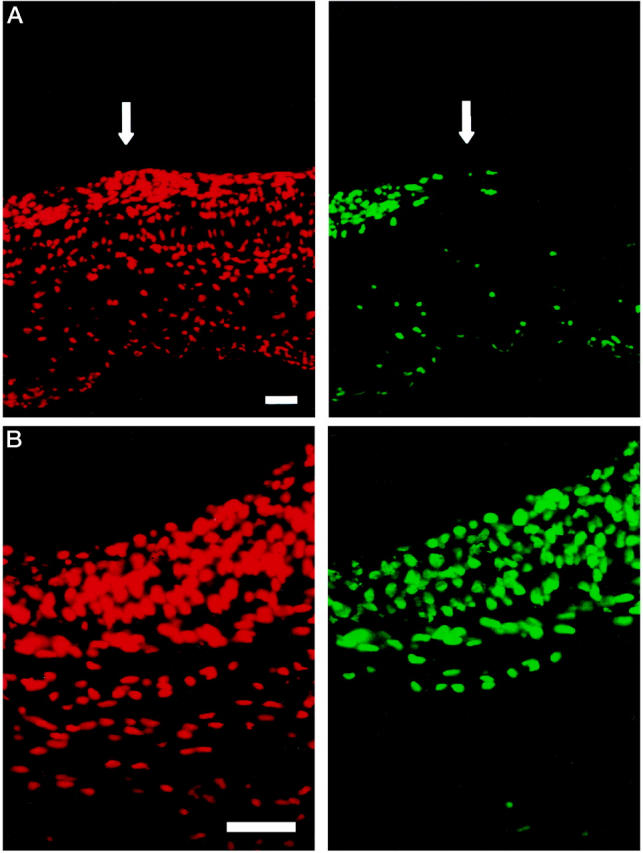
Fluorescent photomicrographs of a longitudinal section (A) and a cross section (B) of a balloon-injured rat common carotid artery 4 hours after PDT. Note the sharp demarcation line at the interface of the PDT-treated (left) and nontreated part (right) of the artery (arrow) in the longitudinal section. Note complete apoptosis of the neointima and beginning apoptosis of the media 4 hours after PDT (B). TUNEL staining, scale bars, 10 μm. The propidium iodide counterstain (red cells) was visualized using a filter of 450 to 490 nm for excitation and of 515 to 565 nm for emission. The corresponding apoptotic cells (green) were visualized using 564 nm for excitation and a long-pass filter of >590 nm for emission.
In the neointima, the percentage of apoptotic cells increased significantly from 10% ± 4.3 in controls to 44% ± 14.1 (P < 0.001) within 2 hours after PDT, followed by a further increase up to 98% ± 1 (P < 0.0001) at 4 hours and 100% at 10 hours. The absolute numbers of cells present in the neointima began to decrease 4 hours after PDT with complete cell loss at 24 hours.
In the media, the kinetics of apoptosis induction and eradication of apoptotic cells followed a similar pattern as in the neointima, but with a time delay. Two hours after PDT there was no increase of apoptotic cells (7.7% ± 1.6 in controls, 7.4% ± 2.8 in PDT-treated arteries). Thereafter, the number of apoptotic cells increased to 52% ± 8.1 (P < 0.001) at 4 hours and at 10 hours there was both a decline in medial cell number and simultaneously all of the remaining cells were apoptotic. There were no medial cells left at 24 hours.
The adventitia of control vessels had a relatively high percentage of apoptotic cells (46% ± 19.8) because of surgical manipulation after dissection of the arteries for balloon injury. This increased in the first hours after PDT treatment (56.7% ± 31.2), to a maximum of 73.7% ± 20.8 (P < 0.05) at 4 hours and remaining relatively constant thereafter. The absolute cell numbers in the adventitia decreased within 10 hours of treatment. A rare cell was still present 24 hours after the treatment.
Immunofluorescence staining for α-smooth muscle cell actin revealed numerous positive cells in media of the control artery (Figure 4A) ▶ . Immunopositive cells were still present at 4 and 6 hours after PDT, but by 24 hours no α-smooth-positive cells remained in PDT-treated arteries (Figure 4D) ▶ .
Figure 4.
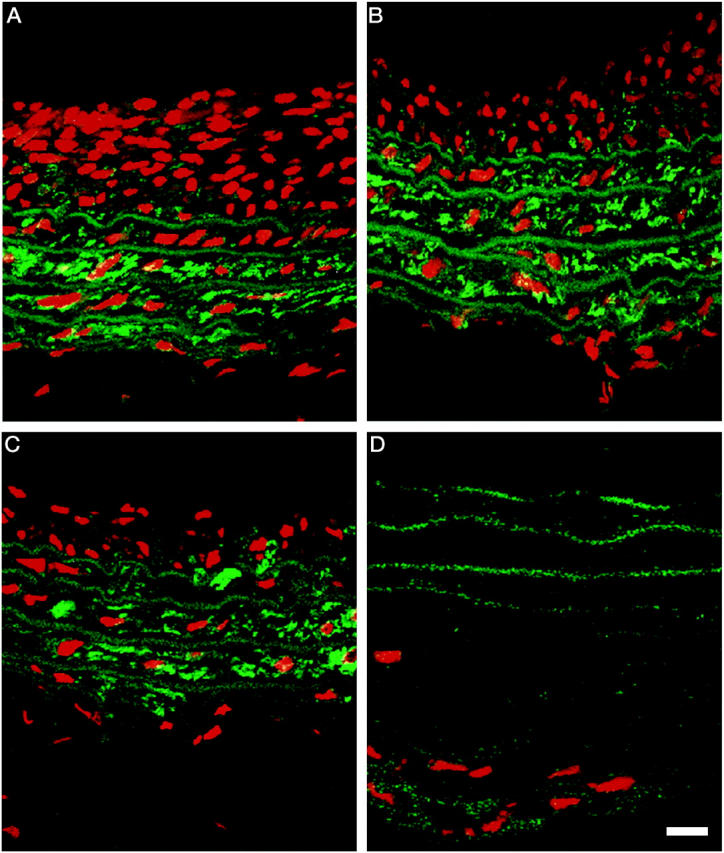
Fluorescent micrographs of control and PDT-treated arteries. Seven days after balloon-injury, animals were either sham treated or administered PDT. Arteries were harvested from controls (A) and PDT-treated animals at 4 (B), 6 (C), and 24 hours (D). Smooth muscle cells were detected using a monoclonal anti-α smooth muscle actin fluorescein isothiocyanate-conjugated antibody and the distribution of immunopositive cells was determined using a confocal microscope. Images were captured using an excitation of 494 nm and emission at 518 nm. Scale bar, 5μm. The propidium iodide counterstain (red cells) was visualized using a filter of 450 to 490 nm for excitation and of 515 to 565 nm for emission.
DNA Ladder
To detect nucleosomal DNA fragmentation which produces a DNA ladder, a characteristic biochemical hallmark of apoptosis, DNA was isolated from arteries 4 hours after PDT, a time when apoptotic cells were observed by TUNEL staining and TEM. A distinct DNA ladder was present only from the DNA isolated from PDT-treated hyperplastic arteries, but was absent in the controls (Figure 5) ▶ .
Figure 5.
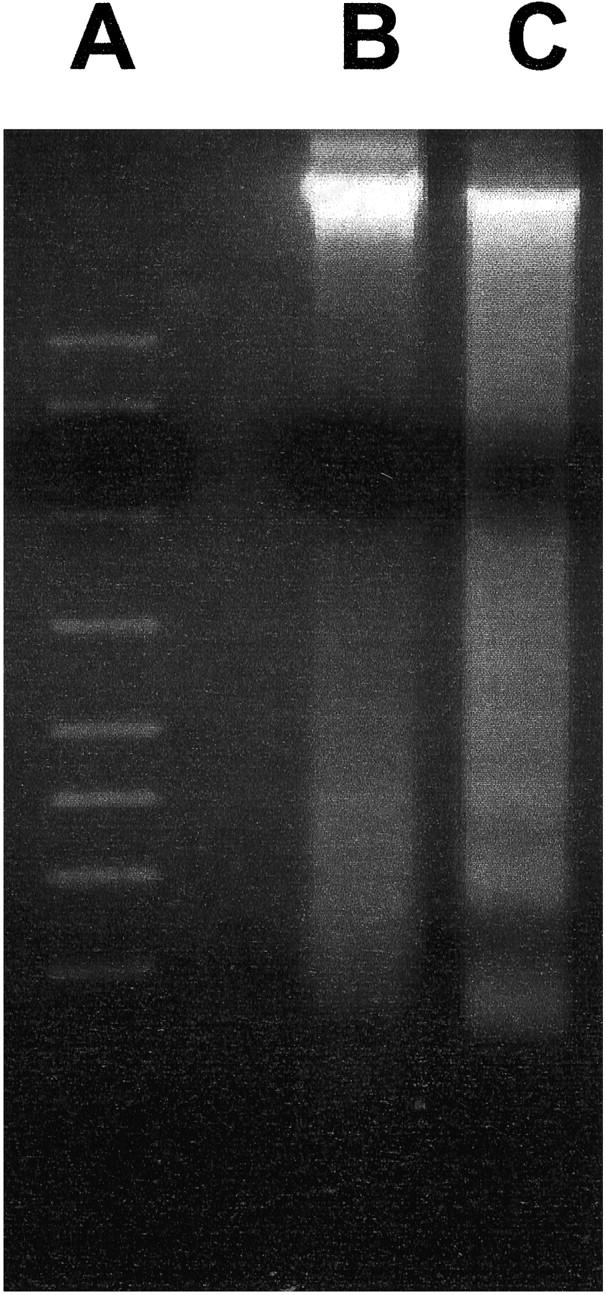
Agarose gel electrophoresis of DNA extracted from the rat common carotid artery after balloon injury. A: Standards; B: untreated vessel; C: 4 hours after PDT. Note the distinctive ladder pattern of fragmented DNA after PDT.
Discussion
The present study investigated the contribution of apoptosis to the eradication of vascular cells after PDT, using parameters known to successfully inhibit experimental hyperplasia. PDT has been used to inhibit IH in multiple experimental models 10,11,13 and a recent clinical study. 12 The mechanism by which PDT induces this cytotoxicity in vivo remained to be elucidated. There is in vitro evidence that PDT can cause apoptosis in vascular smooth muscle cells 18. but only one study so far provided evidence for apoptosis in the microvasculature in vivo. 19 Indeed, depending on the cell type, photosensitizer, and dosimetry, PDT can either induce apoptosis or necrosis. 16,17
One of the characteristics of apoptosis, potentially beneficial for clinical applications, is the lack of ensuing inflammation. 14,20 Inflammatory infiltrates are undesirable in blood vessels because they participate in neointimal proliferation, thrombus formation, loss of vascular wall integrity, and the development of aneurysms. Apoptosis is accompanied by fragmentation of the cell into membrane-bound apoptotic bodies, which generally undergo phagocytosis by nearby cells without associated inflammation. In fact, it has been demonstrated that the induction of apoptosis in IH might be beneficial in preventing excessive cellular proliferation and increased lumen narrowing. 21 In this study, PDT resulted in cell eradication within 24 hours without evidence of inflammation, suggesting that the mechanism of cytotoxicity is apoptosis. In addition, PDT is known to inactivate growth factors and chemoattractants, also explaining the observed lack of inflammation. 22-24
The main finding in this study is that PDT of balloon injury-induced hyperplastic arteries causes significant apoptosis in vivo. Because arterial balloon injury in itself results in a rapid onset of cell death, including apoptosis, 25 a time point of 7 days after injury was chosen to segregate the effects of PDT from the acute effects of the balloon injury. The conclusion that PDT induces apoptosis in vivo was based on different assays: TEM determination of ultrastructural features characteristic of apoptosis, immunofluorescence TUNEL labeling, and the presence of standard length nucleosomal DNA bp fragments characteristic of apoptotic cells (ladder formation). Indeed, TUNEL staining alone can be misleading because of low concentrations of broken DNA strands in necrotic cells, but the characteristic nuclear condensation and margination of apoptosis was also observed. 20,26
This experimental model was not suitable to investigate the effects of PDT on the adventitia because of the high number of TUNEL-positive cells in control arteries, as the result of surgical injury. In the neointima, however, apoptosis appeared more rapidly than in the medial cells. It is likely that photosensitizer concentration gradients over the arterial wall after its systemic administration play a role in the kinetics of apoptosis in the vessel wall. 27 Indeed, higher drug concentrations in the luminal cells may lead to higher effective PDT dosimetry and a more rapid initiation of apoptosis than in the cells that are located further from the lumen. Although the photosensitizer concentrations may have resulted in a gradient of effective PDT dose, the light concentration at these wavelengths (>650 nm) has not been shown to be significantly attenuated until it has passed through millimeters of tissue. 28 Irrespective of a possible variation in the PDT dose, it resulted in sufficient PDT dosimetry to induce apoptosis homogeneously throughout the vascular wall within 24 hours. Dosimetry also affects the mode of cell death. As has been shown previously, chloroaluminum-sulfonated phthalocyanine localizes throughout the cytoplasm and catalyzes both lysosomal and mitochondrial photodamage. 16 Higher light doses yield progressively more membrane photodamage and inhibit the apoptotic response. 16
It is conceivable that PDT induces apoptosis via release of lysosomal enzymes which in turn activate caspases or apoptosis-associated target proteins, like poly(ADP-ribose) polymerase. Alternatively, mitochondrial damage can trigger apoptotic cell death mechanisms by the release of cytochrome C into the cytoplasm, and proteolytic activation of the caspase cascade. The pathways leading to apoptosis in vascular cells will be the subject of future experiments.
After PDT treatment of the vessel wall, specialized phagocytic cells, eg, macrophages, were not observed. This lack of inflammation supports the argument for apoptosis. However, the fate of the apoptotic bodies or cellular debris produced remains unsolved. Apoptotic cells can be phagocytosed by viable neighboring cells, without the need for specialized scavenging cells. 14 Herein, it is unlikely that the debris of apoptotic cells would be phagocytized by adjacent cells because these cells were also undergoing apoptosis. A better explanation might be that most of the cell debris in the neointima was washed out by the blood and debris in the media remained in the vessel wall. Indeed, a faint background TUNEL staining could be detected throughout the media 24 hours after PDT, suggesting the presence of cellular debris dispersed into the matrix. By electron microscopy there was still evidence of cellular debris in the media 16 weeks after PDT, suggestive of slow elution of PDT-damaged cell constituents. 13 The results of the immunofluorescence staining for α-smooth muscle actin confirmed the disappearance of cells and smooth muscle cell-specific α-actin by 24 hours. Before PDT, only cells in the media and not the neointima express α-smooth muscle actin. 29
In this apoptosis study, no long-term investigation was undertaken because the treated artery became acellular by 24 hours and the natural history of the experimental effects of vascular PDT have been described elsewhere. 10,13 In arteries treated with PDT, the media remains acellular except for the occasional cell up to 16 weeks after PDT. Re-endothelialization of the lumen starts after 2 weeks and is complete by 4 weeks, as shown by scanning electron microscopy and TEM. 13
The finding that the mechanism of cellular eradication is the induction of extensive apoptosis has some important implications. Gene therapy, which has attracted interest as a method to inhibit restenosis, has been found to induce apoptosis. 30 This suggests that apoptosis may be a more important mechanism of inhibiting IH than is presently recognized. Compared to gene therapy, where effectiveness is somewhat limited because of low transfection rate and loss of gene expression throughout time, 31 PDT is unique in its ability to induce extensive apoptosis of a vessel wall. With the well-documented acute and long-term experimental effects of PDT on the vessel wall (no structural deterioration of the matrix and no aneurysm formation, repopulation of the adventitia, and endothelial recovering of the luminal surface), PDT, at the proper dosimetry, has no reported untoward effects on the vessel wall. Furthermore, examining longitudinal sections through the artery at the border between PDT and non-PDT-treated portions of the artery revealed a very sharp demarcation for the presence of apoptotic cells, which were only identified in the PDT-treated portion of the artery. Because the half-life of PDT-generated free radicals is very short, in the order of μs, 8 they cannot migrate further than ∼0.1 μm. Thus, the application of PDT results in very sharply demarcated, localized effects, which are strictly limited to the field of light activation of the photosensitizer (Figure 3A) ▶ . Despite multiple other considerations necessary for the proper delivery of PDT to human atherosclerotic arteries, this form of precision delivery makes PDT very well suited for local artery therapy application to an angioplasty-injured artery for the inhibition of restenosis.
Footnotes
Address reprint requests to Glenn M. LaMuraglia, M.D., Massachusetts General Hospital, ACC 464, Boston, MA 02114.
Supported in part by ONR Contract N00014-94-I-0927. J. S. is a recipient of the United States Department of Energy Fellowship Grant (FG02-91-ER-61228). J. H. is a recipient of Deutsche Forschungsgemeinschaft Fellowship Grant (HE 2926/1-1).
References
- 1.Bauters C, Meurice T, Hamon M, McFadded E, LaBlanche JM, Bertrand ME: Mechanisms and prevention of restenosis; from experimental models to clinical practice. Cardiovasc Res 1996, 31:835-846 [PubMed] [Google Scholar]
- 2.Davies MG, Hagen PO: Pathobiology of intimal hyperplasia. Br J Surg 1994, 81:1254-1269 [DOI] [PubMed] [Google Scholar]
- 3.Clowes AW, Reidy MA, Clowes MM: Mechanism of stenosis after arterial injury. Lab Invest 1983, 49:208-215 [PubMed] [Google Scholar]
- 4.Hart CE, Kraiss LW, Vergel S, Gilbertson D, Kenagy R, Kirkman T, Crandall DL, Tickle S, Finney H, Yarranton G, Clowes AW: PDGF beta receptor blockade inhibits intimal hyperplasia in the baboon. Circulation 1999, 99:564-569 [DOI] [PubMed] [Google Scholar]
- 5.DeYoung MB, Dichek DA: Gene therapy for restenosis: are we ready? Circ Res 1998, 82:306-313 [DOI] [PubMed] [Google Scholar]
- 6.Waksman R, Rodriguez JC, Robinson KA, Cipolla GD, Crocker IR, Scott NA, King SB III, Wilcox JN: Effect of intravascular irradiation on cell proliferation, apoptosis, and vascular remodeling after balloon overstretch injury of porcine coronary arteries. Circulation 1997, 96:1944–1962 [DOI] [PubMed]
- 7.Teirstein PS, Massullo V, Jani S, Popma JJ, Russo RJ, Schatz RA, Guarneri EM, Steuterman S, Sirkin K, Cloutier DA, Leon MB, Tripuraneni P: Three-year clinical and angiographic follow-up after intracoronary radiation: results of a randomized clinical trial. Circulation 2000, 101:360-365 [DOI] [PubMed] [Google Scholar]
- 8.Hendersen BW, Dougherty TJ: How does photodynamic therapy work? Photochem Photobiol 1991, 55:145-157 [DOI] [PubMed] [Google Scholar]
- 9.Oleinick NL, Evans HH: The photobiology of photodynamic therapy: cellular targets and mechanisms. Radiat Res 1998, 150:S146-S156 [PubMed] [Google Scholar]
- 10.Ortu P, LaMuraglia GM, Roberts GW, Flotte TJ, Hasan T: Photodynamic therapy of arteries: a novel approach for treatment of experimental intimal hyperplasia. Circulation 1992, 85:1189-1196 [DOI] [PubMed] [Google Scholar]
- 11.Hsiang YN, Houston G, Crespo T, To E, Sobeh M, Bower R: Preventing intimal hyperplasia with photodynamic therapy using an intravascular probe. Ann Vasc Surg 1995, 9:80-86 [DOI] [PubMed] [Google Scholar]
- 12.Jenkins MP, Buonaccorsi GA, Raphael M, Nyamekye I, McEwan JR, Bown SG, Bishop CC: Clinical study of adjuvant photodynamic therapy to reduce restenosis following femoral angioplasty. Br J Surg 1999, 86:1258-1263 [DOI] [PubMed] [Google Scholar]
- 13.LaMuraglia GM, ChandraSekar NR, Flotte TJ, Abbott WM, Michaud N, Hasan T: Photodynamic therapy inhibition of experimental intimal hyperplasia: acute and chronic effects. J Vasc Surg 1994, 19:321-331 [DOI] [PubMed] [Google Scholar]
- 14.Wyllie AH: Apoptosis: an overview. Br Med Bull 1997, 53:451-465 [DOI] [PubMed] [Google Scholar]
- 15.Godar DE: Light and death: photons and apoptosis. J Invest Dermatol Symp Proc 1999, 4:17-23 [DOI] [PubMed] [Google Scholar]
- 16.Luo Y, Kessel D: Initiation of apoptosis versus necrosis by photodynamic therapy with chloroaluminum phthalocyanine. Photochem Photobiol 1997, 66:479-483 [DOI] [PubMed] [Google Scholar]
- 17.Ochsner M: Photophysical and photobiological processes in the photodynamic therapy of tumours. J Photochem Photobiol B 1997, 39:1-18 [DOI] [PubMed] [Google Scholar]
- 18.Heckenkamp J, Leszczynski D, Schiereck J, Kung J, LaMauraglia GM: Different effects of photodynamic therapy and gamma irradiation on vascular smooth muscle cells and matrix: implications for inhibiting restenosis. Arterioscler Thromb Vasc Biol 1999, 19:2154-2161 [DOI] [PubMed] [Google Scholar]
- 19.Engbrecht BW, Menon C, Kachur AV, Hahn SM, Fraker DL: Photofrin-mediated photodynamic therapy induces vascular occlusion and apoptosis in a human sarcoma xenograft model. Cancer Res 1999, 59:4334-4342 [PubMed] [Google Scholar]
- 20.Majno G, Joris I: Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol 1995, 146:3-15 [PMC free article] [PubMed] [Google Scholar]
- 21.Bochaton-Piallat ML, Gabbiani F, Redard M, Desmouliere A, Gabbiani G: Apoptosis participates in cellularity regulation during rat aortic intimal thickening. Am J Pathol 1998, 146:1059-1064 [PMC free article] [PubMed] [Google Scholar]
- 22.Statius van Eps RG, Mark LL, Schiereck J, LaMuraglia GM: Photodynamic therapy inhibits the injury-induced fibrotic response of vascular smooth muscle cells. Eur J Vasc Endovasc Surg 1999, 18:417–423 [DOI] [PubMed]
- 23.Statius van Eps RG, LaMuraglia GM: Photodynamic therapy inhibits transforming growth factor b activity associated with vascular smooth muscle cell injury. J Vasc Surg 1997, 25:1044–1053 [DOI] [PubMed]
- 24.Statius van Eps RG, Adili F, LaMuraglia GM: Photodynamic therapy inactivates cell-associated basic fibroblast growth factor: a silent way of vascular smooth muscle cell eradication. Cardiovasc Res 1997, 35:334–340 [DOI] [PubMed]
- 25.Perlman H, Maillard L, Krasinski, Wals K: Evidence for the rapid onset of apoptosis in medial smooth muscle cells after balloon injury. Circulation 1997, 95:981–987 [DOI] [PubMed]
- 26.Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R: In situ detection of fragmented DNA fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology 1995, 21:1465-1468 [DOI] [PubMed] [Google Scholar]
- 27.LaMuraglia GM, Ortu P, Flotte TJ, Roberts WG, Schomaker KT: Chloaluminum sulfonated phtalocyanine partitioning in normal and intimal hyperplastic artery in the rat: implications for photodynamic therapy. Am J Pathol 1993, 142:1898-1905 [PMC free article] [PubMed] [Google Scholar]
- 28.Cheong W, Prah SA, Welch AJ: A review of the optical properties of biological tissues. J Quantum Electron 1990, 26:2166-2185 [Google Scholar]
- 29.Gabbiani G, Kocher O, Bloom WS, Vandekerckhove J, Weber K: Actin expression in smooth muscle cells of rat aortic intimal thickening, human atheromatous plaque, and cultured rat aortic media. J Clin Invest 1984, 73:148-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.George SJ, Lloyd CT, Angelini GD, Newby AC, Baker AH: Inhibition of late vein graft neointima formation in human and porcine models by adenovirus-mediated overexpression of tissue inhibitor of metalloproteinase-3. Circulation 2000, 101:296-304 [DOI] [PubMed] [Google Scholar]
- 31.Steg PG, Tahlil O, Aubailly N, Caillaud JM, Dedieu JF, Berthelot K, Le Roux A, Feldman L, Perricaudet M, Denefle P, Branellec D: Reduction of restenosis after angioplasty in an atheromatous rabbit model by suicide gene therapy. Circulation 1997, 96:408-411 [DOI] [PubMed] [Google Scholar]


