Abstract
DPC4 (MADH4, SMAD4) encodes a nuclear transcription factor shown to be genetically inactivated in over one-half of conventional infiltrating ductal adenocarcinomas of the pancreas. Intraductal papillary mucinous neoplasms (IPMNs) of the pancreas have been suggested to be distinct neoplasms with a significantly less aggressive course than conventional ductal adenocarcinomas of the pancreas, but molecular comparisons of these tumor types have previously been impaired by technical difficulties. Recently, immunohistochemical labeling for the DPC4 gene product has been shown to be an extremely sensitive and specific marker for DPC4 gene alterations in pancreatic adenocarcinomas. Therefore, we analyzed the immunohistochemical expression of Dpc4 protein in 79 IPMNs using a previously characterized monoclonal antibody. Twenty-nine of the IPMNs also had an associated infiltrating adenocarcinoma available for analysis. The labeling patterns observed were compared to those we have previously reported for conventional ductal carcinomas. All 79 of the intraductal components of the IPMNs strongly expressed Dpc4 protein. In 77 of the 79 cases (97%), the labeling was diffusely positive, and in 2 of the 79 (3%) the labeling was focally positive. Dpc4 expression was seen in 28 (97%) of the associated 29 invasive cancers. The one infiltrating carcinoma that showed loss of Dpc4 expression was associated with an intraductal component which showed focal loss of Dpc4 expression. The strong and almost universal expression of Dpc4 in IPMNs contrasts sharply with the loss of Dpc4 expression seen in approximately 30% of in situ adenocarcinomas of the pancreas (so-called pancreatic intraepithelial neoplasms, grade 3; P < 0.001) and in 55% of pancreatic duct carcinomas (P < 0.0001). Differences in Dpc4 expression between IPMNs and ductal carcinomas suggest a fundamental genetic difference in tumorigenesis, which may relate to the significantly better clinical outcomes observed for IPMNs.
Intraductal papillary mucinous neoplasms (IPMNs) are distinct neoplasms of the pancreas, characterized by dilated pancreatic ducts and ductules that are lined by tall columnar mucin-producing neoplastic epithelial cells. 1-13 The neoplastic epithelium in IPMNs typically forms papillary or pseudopapillary projections into the lumens of the ducts. 14 IPMNs are classified as adenoma, borderline, or malignant tumors based on the degree of epithelial dysplasia present, with malignant IPMNs further subclassified into noninvasive or invasive tumors. 15 Little is known of the genetic alterations that occur in these tumors. However, Fujii et al studied 13 IPMNs using PCR-based microsatellite analysis to detect loss of heterozygosity (LOH) at a variety of different chromosomal sites and found frequent LOH at 6q (54%), 8p (31%), 9p(62%), 17p(38%), and 18q (38%), suggesting that targeted genetic inactivation may occur at these loci. 16
DPC4 (MADH4, SMAD4) is a tumor-suppressor gene on chromosome 18q which has been shown to mediate the downstream effects of TGF-β superfamily signaling, resulting in growth inhibition. 17,18 DPC4 is genetically inactivated in approximately 55% of pancreatic adenocarcinomas. 19-21 Inactivation of the DPC4 tumor-suppressor gene is relatively specific for pancreatic adenocarcinoma, although it has been shown to occur in a small percentage of primary carcinomas of the breast, ovary, colon, and biliary tract. 20-24 Wilentz et al have recently shown that immunohistochemical labeling for the Dpc4 protein is an extremely sensitive and specific marker for DPC4 genetic alterations, 25 thus providing a simple test for DPC4 gene status in archival material.
We analyzed a large series of IPMNs and their associated infiltrating carcinomas using this previously characterized antibody to the DPC4 gene product. By analyzing Dpc4 expression in this large series of IPMNs, we sought to determine the role of DPC4 in the genetic progression of this distinctive pancreatic neoplasm. The pattern of Dpc4 expression in IPMNs was also compared to that recently reported for in situ (pancreatic intraepithelial neoplasia (PanIN) grade 3) and infiltrating conventional ductal adenocarcinomas of the pancreas. 26
Materials and Methods
Specimen Selection
Pancreaticoduodenectomy (Whipple resections), distal pancreatectomy, and total pancreatectomy specimens from 79 patients with IPMNs were collected from the files of The Johns Hopkins Hospital (n = 36), Memorial Sloan-Kettering Cancer Center (n = 31), and Wayne State University (n = 12). Eleven pancreatectomy specimens included in the study had positive surgical resection margins. Pancreatectomy specimens included in the study were submitted in their entirety if no evidence of infiltrating carcinoma was found at the time of intraoperative consultation. If infiltrating carcinoma was found on intraoperative consultation, representative samples were submitted of the IPMN and associated infiltrating component. Clinical and pathological data were obtained from the patients’ medical records, The Johns Hopkins Hospital surgical pathology files, The Memorial Hospital for Cancer and Allied Diseases surgical pathology files, and The Detroit Medical Center surgical pathology files. Clinical and pathological variables included age, gender, race, tumor size, tumor location, grade of epithelial dysplasia, presence or absence of an invasive carcinoma, histological subtype of invasive carcinoma, presence of nodal or distant metastases, and survival. Pancreaticoduodenectomy specimens containing conventional infiltrating ductal adenocarcinomas and PanINs were collected, examined, and reported previously. 25,26
Grading of Dysplasia
Dysplasia in the slides labeled for Dpc4 expression was graded based on criteria established by the World Health Organization by three of the authors (R. H. H., N. V. A., and D. K.). 15 The intraductal component of each tumor was categorized into adenoma, borderline, or carcinoma in situ. Three cases were categorized as intraductal oncocytic papillary neoplasms (IOPN) and were included in the carcinoma in situ subset. Adenomas were those tumors with tall, columnar, mucin-containing epithelial cells showing slight or no atypia. The epithelium of adenomas was either papillary or nonpapillary. Borderline tumors were those with moderate epithelial atypia, having nonpapillary or papillary epithelium with moderate cytologic atypia including loss of polarity, nuclear crowding, nuclear enlargement, pseudo-stratification, and nuclear hyperchromatism. Papillary areas maintained identifiable stromal cores. Those designated as carcinoma in situ were those intraductal tumors with severe dysplastic epithelial changes. IOPNs were characterized by a papillary growth pattern, with the papillary structures lined by stratified oncocytic cells with pale pink cytoplasmic granules (finer than those seen in Paneth cells) and interspersed goblet cells. 27 Carcinomas contained papillary or micropapillary structures, with cribriform growth and budding of small clusters of epithelial cells into the lumen. Epithelial changes were seen as loss of polarity, loss of mucin content, cellular and nuclear pleomorphism, nuclear enlargement, and the presence of mitoses. If an invasive carcinoma was identified adjacent to the intraductal component of an IPMN or IOPN, it was separately categorized as either colloid (mucinous noncystic carcinomas) or tubular (resembling usual ductal adenocarcinomas) type. 15
Immunohistochemistry
The hematoxylin and eosin-stained slides from each of the 79 cases were screened by light microscopy for sections having IPMN, associated infiltrating carcinoma (if present), and adjacent normal pancreas. Unstained 5-μm sections were then cut from the paraffin block selected for each case and deparaffinized by routine techniques. Next, slides were treated with 1× sodium citrate buffer (diluted from 10× heat-induced epitope retrieval buffer, Ventana-Bio Tek Solutions, Tucson, AZ) before steaming for 20 minutes at 80°C. Slides were then cooled 5 minutes before incubating with a 1:100 dilution of monoclonal antibody to Dpc4 protein (clone B8, Santa Cruz Biotechnology, Santa Cruz, CA; 1:100 dilution) using the Bio Tek-Mate 1000 automated stainer (Ventana-Bio Tek Solutions). Finally, anti-Dpc4 antibody was detected by adding secondary antibody followed by avidin-biotin complex and 3,3′-diaminobenzidine chromagens. Sections were counterstained with hematoxylin.
Each immunolabeling procedure included an infiltrating pancreatic ductal adenocarcinoma previously shown to contain at least one wild-type DPC4 allele as a positive control, and a negative control pancreatic ductal adenocarcinoma with a known homozygous deletion of DPC4. 20,28
Immunohistochemical Evaluation
Immunohistochemical labeling of Dpc4 was evaluated by three of the authors (C. I.-D., R. H. H., and R. E. W.), with agreement in all cases examined. The immunolabeling pattern of each case was scored as positive, weakly positive, or negative. Tumors scored as positive showed strong, diffuse cytoplasmic labeling of the tumor epithelium, with scattered immunopositive nuclei also identified. Tumors scored as weakly positive showed only faint cytoplasmic labeling of tumor epithelium, with scattered positive nuclei also present. Tumors scored as negative showed no detectable cytoplasmic or nuclear Dpc4 protein. Positive and weakly positive cases were combined into a positive category for statistical analysis. The extent of immunolabeling was also categorized as diffuse (if the entire neoplasm labeled) or focal (if focal loss of expression was noted).
Normal pancreatic ducts, islets of Langerhans, pancreatic acini, lymphocytes, and stromal fibroblasts, all which had moderate expression of the DPC4 gene product, served as a positive internal control in each of the sections.
Statistical Analysis
Summary data are expressed as the mean ± SD unless otherwise indicated. When comparing differences between distributions, the χ 2 test was used with additional confirmation using the Fisher exact test for sample sizes less than 5. Probability values of 0.05 or less were considered significant.
Results
Clinicopathological Characteristics
Clinicopathological characteristics of available data of the 79 patients with an IPMN are shown in Table 1 ▶ . The mean age of the patients was 69.5 ± 8.4 years, including 40 males and 34 females; in 5 cases the gender was not given. Fifty-three cases (73%) were located in the head and/or body, 10 (14%) primarily in the tail, and 10 (14%) involved the entire length of the pancreatic duct (head, body, and tail). In 6 cases the location was not specified.
Table 1.
Clinicopathological Characteristics of Intraductal Papillary Mucinous Neoplasms
| Age (n = 74) | 69.5± 8.4 years |
| Gender (n = 74) | 40 Male (54%) |
| 34 Female (46%) | |
| Tumor Location (n = 73) | |
| Head/body | 53 (73%) |
| Tail | 10 (14%) |
| Head/body/tail | 10 (14%) |
| Tumor size (n = 70) | 4.5± 3.1 cm |
| Epithelial dysplasia (n = 79) | |
| Adenoma | 5 (6%) |
| Borderline | 18 (23%) |
| Carcinoma in situ | 53 (67%) |
| Oncocytic | 3 (4%) |
| Infiltrating carcinoma | 37 (47%) |
| Colloid type | 19/29 (66%)* |
| Tubular type | 10/29 (34%) |
| Lymph node status | |
| Positive | 13/37 (35%) |
| Negative | 24/37 (65%) |
| Postoperative survival (n = 67) | |
| Intraductal IPMNs (n = 34) | 32/34 (94%) |
| Infiltrating carcinomas (n = 33) | 25/33 (76%)† |
Complete clinicopathological data were not available for nine cases.
*Eight infiltrating carcinomas were not available for histologic review and Dpc4 labeling.
†P = 0.07 versus survival of those patients without invasion.
Of the 79 IPMNs, the intraductal components of 5 (6%) were classified as adenoma, 18 (23%) as borderline, and 53 (67%) as carcinoma in situ. Three 3 (4%) were classified as IOPN. Thirty-seven of the IPMNs were associated with an infiltrating carcinoma, of which 29 were available for Dpc4 labeling. Of these 29 infiltrating carcinomas, 28 (97%) were associated with an intraductal component showing carcinoma in situ and 1 (3%) was associated with an intraductal component with borderline epithelial dysplasia. The invasive component showed colloid features in 19 (66%) of the 29 cases and tubular features in 10 (34%) of the 29 cases. Lymph node metastases were identified in 13 (35%) of the 37 invasive carcinomas at the time of surgery, and 5 (16%) of 37 had metastases to distant sites.
Follow-up data were available for 35 of 37 patients with invasive IPMNs, and for 38 of the 42 patients with noninvasive IPMNs (mean/median follow-up, 28.7/19.0 months). Two patients with invasive IPMNs and four patients with noninvasive IPMNs died within 1 month of surgery of other causes, leaving postoperative survival data on 67 patients (33 patients with an invasive IPMN and 34 patients with a noninvasive IPMN). Among those 33 patients with invasive IPMNs, 8 of 33 (24%) had died, compared to only 2 of 34 (6%) patients with noninvasive IPMNs. Although a trend was observed, no significant differences in survival between these two groups were found (P = 0.07). All 8 of 8 patients (100%) who died with an invasive IPMN died of their disease, and 1 of these 8 had a positive surgical resection margin. The 2 patients who died with noninvasive IPMNs also died of their disease. One of these patients had an IPMN with borderline epithelial dysplasia which extended to the surgical resection margin, and the other had an IPMN with carcinoma in situ and no information available on this patient’s surgical resection margins.
Dpc4 Immunohistochemical Staining of the Intraductal Components of the IPMNs
The intraductal components of virtually all of the IPMNs labeled strongly for Dpc4. In 77 (97%) of the 79 IPMNs, the intraductal component of the neoplasm labeled diffusely positive (Figure 1, A and B) ▶ . In the remaining two IPMNs (3%) labeling of the intraductal component for Dpc4 was focally positive (Figure 2) ▶ . Dpc4 immunohistochemical staining was found in the intraductal component in all five adenomas, all of 18 borderline tumors, 51 of 53 carcinoma in situ, and all 3 IOPNs. The two IPMNs with focal labeling of the intraductal component for Dpc4 protein were both IPMNs with carcinoma in situ (Figure 2, A ▶ −C).
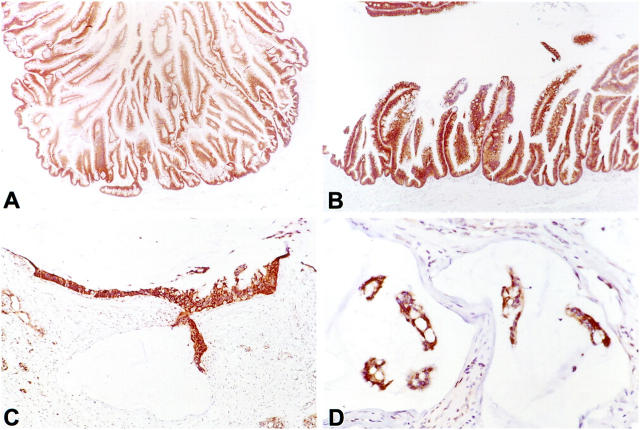
Figure 1.
Dpc4 protein immunolabeling in the intraductal component of an intraductal papillary mucinous neoplasm (IPMN) and its associated infiltrating carcinoma. A: Strong and uniform Dpc4 expression in an IPMN with carcinoma in situ. B: Higher power view of an IPMN with carcinoma in situ, showing intense cytoplasmic and nuclear labeling in the epithelium. C: Infiltrating colloid carcinoma with intense Dpc4 labeling. D: High power view of an infiltrating colloid carcinoma, demonstrating strong positive labeling of the neoplastic cells in a mucin pool.
Figure 2.
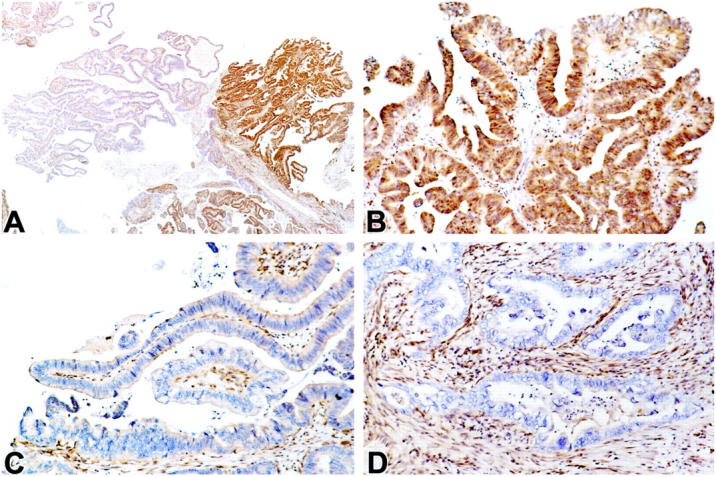
Heterogeneous Dpc4 protein immunolabeling in an IPMN with carcinoma in situ and an associated infiltrating tubular carcinoma. A: Low power view of an IPMN with carcinoma in situ, demonstrating focal intense expression (right side) and focal loss of expression (left side). B: Higher power view of the area expressing DPC4 in A. C: Higher power view of the area showing loss of DPC4 expression in A. D: High power view demonstrating loss of DPC4 expression in the infiltrating tubular carcinoma that arose in association with IPMN shown in A−C.
Dpc4 Staining of Infiltrating Carcinomas Associated with IPMNs
Twenty-nine of the IPMNs immunohistochemically analyzed for Dpc4 expression were associated with an invasive carcinoma, which was also available for labeling with the anti-Dpc4 antibody. Diffuse positive labeling of Dpc4 was seen in both the intraductal and infiltrating components in 27 of 29 (93%) of these IPMNs (Figure 1, C and D) ▶ . In the remaining two cases, the intraductal component of the IPMN had focal labeling for Dpc4, with clear-cut areas of the intraductal component showing loss of expression (Figure 2) ▶ . One of the two infiltrating carcinomas associated with these focally labeling IPMNs labeled positive, whereas the other infiltrating carcinoma was negative for Dpc4 protein expression (Figure 2D) ▶ . Thus, Dpc4 expression was identified in a total of 28 of the 29 infiltrating carcinomas (97%).
Dpc4 labeling of the invasive components of the IPMNs was also evaluated with respect to the morphology of the invasive component. All 19 infiltrating colloid carcinomas showed intense positive labeling for Dpc4 protein (100%), whereas 9 of the10 (90%) infiltrating tubular carcinomas showed positive labeling. In 4 of these 9 infiltrating tubular cancers, this labeling was weak. One infiltrating tubular carcinoma was negative for Dpc4 expression (Figure 2D) ▶ .
Relation of Dpc4 Immunohistochemical Staining to Clinicopathological Data
Clinical and pathological data of the 79 IPMN and 29 associated infiltrating carcinomas were collected and analyzed with respect to Dpc4 expression. Because of the almost universal expression of Dpc4 in the IPMNs, no significant differences in any clinical or pathological characteristic were identified with respect to Dpc4 protein expression.
Comparison of Dpc4 Expression in Intraductal Components of IPMNs to PanINs
We have previously reported studies of Dpc4 expression in conventional infiltrating ductal adenocarcinomas of the pancreas and in PanINs, the presumed precursor lesions to infiltrating ductal adenocarcinomas. 25,26 We therefore had a unique opportunity to compare the pattern of Dpc4 expression in IPMNs to the pattern of expression seen in the more aggressive conventional ductal adenocarcinomas. In a previous report, we examined Dpc4 protein expression in 188 PanINs from 40 pancreaticoduodenectomy specimens resected at The Johns Hopkins Hospital. 26 In this series, Dpc4 protein expression was found to be a marker of pancreatic neoplastic progression. 26 All low-grade PanINs (PanIN-1A, -1B, and -2) expressed Dpc4 protein, whereas 31% of the high-grade PanINs (PanIN-3, carcinoma in situ) did not. Although the overall frequency of Dpc4 labeling in the intraductal component of the IPMNs seen in the present study did not differ significantly from that seen in PanINs (79/79 IPMNs were positive for Dpc4 vs. 179/188 of PanINs; P = n.s.), there was a significant difference in Dpc4 expression when the IPMNs with carcinoma in situ (51/53) were compared to PanINs with carcinoma in situ (20/29; P < 0.001).
Comparison of Dpc4 Expression in Infiltrating Carcinomas Associated with IPMNs to Conventional Infiltrating Ductal Adenocarcinomas
We have previously reported our experience with Dpc4 protein expression in 39 infiltrating conventional ductal adenocarcinomas of the pancreas. 25 Dpc4 labeling was observed in 17 of 39 conventional infiltrating pancreatic ductal carcinomas (44%). By contrast, 28 of 29 (97%) infiltrating carcinomas associated with IPMNs labeled positively for Dpc4 (P < 0.0001).
Discussion
IPMNs are distinctive neoplasms of the pancreas characterized by dilatation of the pancreatic duct and/or its branches and by replacement of the duct epithelium by columnar, mucin-containing neoplastic epithelial cells, which form papillary or pseudopapillary structures. 4,14 Although several advances have been made in the understanding of the molecular genetics of conventional infiltrating pancreatic ductal adenocarcinomas, little is known of the genetic alterations that occur in IPMNs. Activating point mutations in the K-ras oncogene have been reported in 40 to 60% of IPMNs, HER-2neu overexpression in ∼75%, and the accumulation of the p53 gene product in 50%. 14,29-33 More recently, Fujii et al demonstrated frequent LOH at several chromosomal loci in IPMNs, with some IPMNs also showing genetic evidence of clonal progression. 16 The DPC4 tumor-suppressor gene on chromosome 18q appears to play an important role in the development of conventional infiltrating ductal carcinomas of the pancreas and LOH at 18q was observed in 38% of the IPMNs examined by Fujii et al. 16,17,20,21,34,35 Fujii et al, however, did not examine the remaining allele of the DPC4 gene, and functional DPC4 status could not, therefore, be determined in these tumors.
Recently, a sensitive and specific immunohistochemical stain for the DPC4 gene product has been developed. 25 We examined the expression of Dpc4 protein in 79 IPMNs, of which 29 had an associated invasive adenocarcinoma available for study. The intraductal components of the IPMNs uniformly and strongly expressed Dpc4 in 77 of the 79 (97%) cases. Focal expression of Dpc4 was seen in two of the 79 intraductal components of the IPMNs. Remarkably, Dpc4 protein expression was detectable in 28 of the 29 (97%) associated infiltrating carcinomas. This pattern of Dpc4 expression was significantly different from the pattern we have previous reported in high grade PanINs (P < 0.001) and in conventional infiltrating ductal adenocarcinomas (P < 0.0001). Approximately 70% of high grade PanINs and only 45% of infiltrating conventional adenocarcinomas show intact Dpc4 protein expression. 25,26
The almost universal immunodetection of Dpc4 in intraductal IPMNs and their associated infiltrating carcinomas suggests that genetic inactivation of DPC4 is an uncommon event in this distinctive neoplasm of the pancreas. Furthermore, in our previous series in which we examined Dpc4 expression in conventional ductal adenocarcinomas of the pancreas, there was a trend toward longer survival in the carcinomas which expressed Dpc4. 25 We can therefore hypothesize that the prolonged and indolent clinical course of IPMNs compared to conventional pancreatic duct carcinomas may be related in part to the persistant expression of Dpc4 in IPMNs. 4,5,10,11
The intraductal components of two IPMNs were particularly interesting in that they showed only focal positive labeling for Dpc4 with a portion of the intraductal IPMN showing clear loss of Dpc4 protein expression (Figure 2) ▶ . Similarly, LOH at chromosome 18q has been reported to be occasionally focal in IPMNs. 16 In one of these focally negative IPMNs, the associated infiltrating tubular carcinoma was also negative for Dpc4. This finding suggests that in a small percentage of IPMNs, Dpc4 inactivation may occur in the progression to infiltrating carcinoma, similar to the findings of Fujii et al, who also demonstrated clonal genetic progression in IPMNs. 16
Sixty-six percent of the infiltrating carcinomas in this study were colloid carcinomas and 33% were tubular carcinomas. All 19 colloid carcinomas showed strong and diffuse positive labeling for DPC4, whereas only 5 (50%) of 10 tubular carcinomas showed intense Dpc4 staining. Four (40%) of the 10 tubular carcinomas had weak labeling, and 1 (10%) had a loss of labeling. The finding of intense and uniform Dpc4 expression in these colloid carcinomas of the pancreas parallels observations made in the colorectum. Kern et al performed a detailed allelotype on a panel of colorectal carcinomas and found that mucinous carcinomas have less frequent allelic deletions of chromosome 18q than do conventional nonmucinous adenocarcinomas of the colorectum. 36
In summary, we report evidence that DPC4 inactivation, detected as loss of Dpc4 protein expression, is an infrequent event in IPMNs. This contrasts with the important role that DPC4 inactivation has been shown to play in the progression of conventional pancreatic ductal adenocarcinoma. Retention of Dpc4 expression in IPMNs may contribute to the indolent nature of these neoplasms, as compared to conventional ductal adenocarcinomas of the pancreas. However, further studies are warranted to determine the specific genetic events that are important in the progression of IPMNs. As these genes are characterized, they may aid in our understanding of the better prognosis associated with this distinct pancreatic neoplasm and, indirectly, help us to understand the course and consequences of the genetic progression model in pancreatic ductal neoplasia.
Footnotes
Address reprint requests to Ralph H. Hruban, M.D., Meyer 7–181, Department of Pathology, The Johns Hopkins Hospital, 600 N. Wolfe Street, Baltimore, MD 21287. E-mail: rhruban@jhmi.edu.
Supported by the National Institutes of Health Specialized Program in Research Excellence (SPORE) in gastrointestinal cancer grant CA62924.
References
- 1.Ohhashi K, Murakami Y, Takekoshi T: Four cases of “mucin producing” cancer of the pancreas on specific findings of the papilla of Vater. Prog Diagn Endosc 1982, 20:348–351 (abstr.)
- 2.Morohoshi T, Kanda M, Asanuma K, Klöppel G: Intraductal papillary neoplasms of the pancreas: a clinicopathologic study of six patients. Cancer 1989, 64:1329-1335 [DOI] [PubMed] [Google Scholar]
- 3.Furukawa T, Takahashi T, Kobari M, Matsuno S: The mucus-hypersecreting tumor of the pancreas. Cancer 1992, 70:1505-1513 [DOI] [PubMed] [Google Scholar]
- 4.Fukushima N, Mukai K, Kanai Y, Hasebe T, Shimada K, Ozaki H, Kinoshita T, Kosuge T: Intraductal papillary tumors and mucinous cystic tumors of the pancreas: clinicopathologic study of 38 cases. Hum Pathol 1997, 28:1010-1017 [DOI] [PubMed] [Google Scholar]
- 5.Fukushima N, Mukai K: Pancreatic neoplasms with abundant mucus production: emphasis on intraductal papillary-mucinous tumors and mucinous cystic tumors. Adv Anat Pathol 1999, 6:65-77 [DOI] [PubMed] [Google Scholar]
- 6.Conley CR, Scheithauer BW, Weiland LH, van Heerden JA: Diffuse intraductal papillary adenocarcinoma of the pancreas. Ann Surg 1987, 205:246-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loftus EV, Olivares-Pakzad BA, Batts KP, Adkins MC, Stephens DH, Sarr MG, DiMagno E: Intraductal papillary-mucinous tumors of the pancreas: clinicopathologic features, outcome and nomenclature. Gastroenterology 1996, 110:1909-1918 [DOI] [PubMed] [Google Scholar]
- 8.Nagai E, Ueki T, Chijiiwa K, Tanaka M, Tsuneyoshi M: Intraductal papillary mucinous neoplasms of the pancreas associated with so-called “mucinous ductal ectasia.” Histochemical and immunohistochemical analysis of 29 cases. Am J Surg Pathol 1995, 19:576-589 [DOI] [PubMed] [Google Scholar]
- 9.Ohta T, Nagakawa T, Akiyama T, Fukushima W, Ueno K, Miyazaki I, Suzuki M, Matsui O, Terada T, Nakanuma Y, Kanno M, Uogishi M, Sodani H: The “duct-ectatic” variant of mucinous cystic neoplasm of the pancreas: clinical and radiologic studies of seven cases. Am J Gastroenterol 1992, 87:300-304 [PubMed] [Google Scholar]
- 10.Kimura W, Makuuchi M, Kuroda A: Characteristics and treatment of mucin-producing tumor of the pancreas. Hepatogastroenterology 1998, 45:2001-2008 [PubMed] [Google Scholar]
- 11.Traverso LW, Peralta EA, Ryan JAJ, Kozarek RA: Intraductal neoplasms of the pancreas. Am J Surg 1998, 175:426-432 [DOI] [PubMed] [Google Scholar]
- 12.Azar C, Van de Stadt J, Rickaert F, Deviere M, Baize M, Klöppel G, Gelin M, Cremer M: Intraductal papillary mucinous tumours of the pancreas: clinical and therapeutic issues in 32 patients. Gut 1996, 39:457-464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paal E, Thompson LD, Przygodzki RM, Bratthauer GL, Heffess CS: A clinicopathologic and immunohistochemical study of 22 intraductal papillary mucinous neoplasms of the pancreas, with a review of the literature. Mod Pathol 1999, 12:518-528 [PubMed] [Google Scholar]
- 14.Z’graggen K, Rivera JA, Compton CC, Pins M, Werner J, Fernandez-del Castillo C, Rattner DW, Lewandrowski KB, Rustgi AK, Warshaw AL: Prevalence of activating K-ras mutations in the evolutionary stages of neoplasia in intraductal papillary mucinous tumors of the pancreas. Ann Surg 1997, 226:491–498 [DOI] [PMC free article] [PubMed]
- 15.Klöppel G, Solcia E, Longnecker DS, Capella C, Sobin LH: Histologic Typing of Tumours of the Exocrine Pancreas. 1996. Springer-Verlag, New York
- 16.Fujii H, Inagaki M, Kasai S, Miyokawa N, Tokusashi Y, Gabrielson E, Hruban RH: Genetic progression and heterogeneity in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol 1997, 151:1447-1454 [PMC free article] [PubMed] [Google Scholar]
- 17.Dai JL, Turnacioglu KK, Schutte M, Sugar AY, Kern SE: Dpc4 transcriptional activation and dysfunction in cancer cells. Cancer Res 1998, 58:4592-4597 [PubMed] [Google Scholar]
- 18.de Winter JP, Roelen BA, ten Dijke P, van der Burg B, van den Eijnden-van Raaij AJ: DPC4 (SMAD4) mediates transforming growth factor-beta1 (TGF-beta1) induced growth inhibition and transcriptional response in breast tumour cells. Oncogene 1997, 14:1891–1899 [DOI] [PubMed]
- 19.Moskaluk CA, Hruban RH, Schutte M, Lietman AS, Smyrk T, Fusaro L, Fusaro R, Lynch J, Yeo CJ, Jackson CE, Lynch HT, Kern SE: Genomic sequencing of DPC4 in the analysis of familial pancreatic carcinoma. Diagn Mol Pathol 1997, 6:85-90 [DOI] [PubMed] [Google Scholar]
- 20.Hahn SA, Schutte M, Hoque ATMS, Moskaluk CA, daCosta LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE: DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science 1996, 271:350–353 [DOI] [PubMed]
- 21.Schutte M, Hruban RH, Hedrick L, Cho KR, Nadasdy GM, Weinstein CL, Bova GS, Isaacs WB, Cairns P, Nawroz H, Sidransky D, Casero RA, Meltzer PS, Hahn SA, Kern SE: DPC4 gene in various tumor types. Cancer Res 1996, 56:2527-2530 [PubMed] [Google Scholar]
- 22.Hoque AT, Hahn SA, Schutte M, Kern SE: DPC4 gene mutation in colitis associated neoplasia. Gut 1997, 40:120-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hahn SA, Bartsch D, Schroers A, Galehdari H, Becker M, Ramaswamy A, Schwarte-Waldhoff I, Maschek H, Schmiegel W: Mutations of the DPC4/Smad4 gene in biliary tract carcinoma. Cancer Res 1998, 58:1124-1126 [PubMed] [Google Scholar]
- 24.Takagi Y, Kohmura H, Futamura M, Kida H, Tanemura H, Shimokawa K, Saji S: Somatic alterations of the DPC4 gene in human colorectal cancers in vivo. Gastroenterology 1996, 111:1369-1372 [DOI] [PubMed] [Google Scholar]
- 25.Wilentz RE, Su GH, Dai JL, Sparks AB, Argani P, Sohn TA, Yeo CJ, Kern SE, Hruban RH: Immunohistochemistry labeling for Dpc4 mirrors genetic status in pancreatic and peripancreatic adenocarcinomas: a new marker of DPC4 inactivation. Am J Pathol 2000, 156:37-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilentz RE, Iacobuzio-Donahue CA, Argani P, McCarthy DM, Parsons JL, Yeo CJ, Kern SE, Hruban RH: Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res 2000, 60:2002-2006 [PubMed] [Google Scholar]
- 27.Adsay NV, Adair CF, Heffess CS, Klimstra D: Intraductal oncocytic papillary neoplasms of the pancreas. Am J Surg Pathol 1996, 20:980-994 [DOI] [PubMed] [Google Scholar]
- 28.Hahn SA, Hoque ATMS, Moskaluk CA, daCosta LT, Schutte M, Rozenblum E, Seymour A, Weinstein CL, Yeo CJ, Hruban RH, Kern SE: Homozygous deletion map at 18q21.1 in pancreatic cancer. Cancer Res 1996, 56:490–494 [PubMed]
- 29.Kondo H, Sugano K, Fukayama N, Hosokawa K, Ohkura H, Ohtsu A, Mukai K, Yoshida S: Detection of K-ras gene mutations at codon 12 in the pancreatic juice of patients with intraductal papillary mucinous tumors of the pancreas. Cancer 1997, 79:900-905 [DOI] [PubMed] [Google Scholar]
- 30.Sessa F, Capella C, Klöppel K, Zamboni G, Scarpa A, Bonato MS: K-ras gene mutation in intraductal neoplasms of the pancreas. Lab Invest 1994, 70:135 (abstr. 788)
- 31.Satoh K, Shimosegawa T, Moriizumi S, Koizumi M, Toyota T: K-ras mutation and p53 protein accumulation in intraductal mucin-hypersecreting neoplasms of the pancreas. Pancreas 1996, 12:362-368 [DOI] [PubMed] [Google Scholar]
- 32.Tada M, Omata M, Ohto M: Ras gene mutations in intraductal papillary neoplasms of the pancreas: analysis in five cases. Cancer 1991, 67:634-637 [DOI] [PubMed] [Google Scholar]
- 33.Satoh K, Sasano H, Shimosegawa T, Koizumi M, Yamazuki T, Mochizuki F, Kobayashi N, Okano T, Toyota T, Sawai T: An immunohistochemical study of the c-erbB-2 oncogene product in intraductal mucin-hypersecreting neoplasms and in ductal cell carcinomas of the pancreas. Cancer 1993, 72:51-56 [DOI] [PubMed] [Google Scholar]
- 34.Dai JL, Schutte M, Bansal RK, Wilentz RE, Sugar AY, Kern SE: TGFβ responsiveness in DPC4/SMAD4-null cancer cells. Mol Carcinog 1999, 26:37-43 [DOI] [PubMed] [Google Scholar]
- 35.Schutte M: DPC4/SMAD4 gene alterations in human cancer, and their functional implications (in process citation). Ann Oncol 1999, 10(suppl 4):56-59 [PubMed] [Google Scholar]
- 36.Kern SE, Fearon ER, Tersmette KW, Enterline JP, Leppert M, Nakamura Y, White R, Vogelstein B, Hamilton SR: Allelic loss in colorectal carcinoma. JAMA 1989, 261:3099-3104 [DOI] [PubMed] [Google Scholar]