Abstract
PAX2 is a transcription factor belonging to the evolutionarily conserved paired box family and is required during development of the central nervous system and genitourinary axis. Mutations in the PAX2 gene cause a rare autosomal dominant renal-coloboma syndrome, characterized by optic nerve colobomas and renal hypoplasia. Recent analysis of a spontaneous PAX2 mutant mouse model (1Neu) revealed that the major cause of renal hypoplasia is reduced branching of the ureteric bud (UB) and fewer nephrons. We have observed that this abnormality is associated with a striking increase in the number of UB cells undergoing programmed cell death during nephrogenesis. To ascertain whether apoptosis is directly linked to the level of PAX2 expression, we have studied the role of PAX2 in cultured renal cells. We show that mIMCD-3 cells, a murine collecting duct cell line with high endogenous PAX2 expression, undergo apoptosis when transfected with anti-sense PAX2. In contrast, HEK293 cells expressing exogenous PAX2 are protected against apoptotic death induced by caspase-2. PAX2 has no effect on proliferation of embryonic kidney or in cultured kidney cells. Our observations imply a direct role for PAX2 in survival of ureteric bud cells.
Development of the mammalian metanephric kidney begins early in fetal life when the ureteric bud emerges from the Wolffian duct and grows outward in response to trophic signals from adjacent metanephric mesenchyme. 1 From its inception, the ureteric bud expresses PAX2, a gene belonging to the PAX family of transcription factors which share homologous DNA-binding paired domains and influence the patterns of tissue-specific development. As the ureteric bud penetrates naive mesenchyme, it continues to express PAX2 but responds to local signals and begins to undergo extensive branching as growth continues. 2,3 In turn, signals from the tip of each ureteric bud branch induce clusters of adjacent mesenchymal cells to adhere to one another and condense into discrete vesicular units, which will form the individual nephrons. As the induced mesenchymal cells undergo dramatic transformation into polarized epithelial cells lining a vesicular lumen, they also begin to express PAX2. At its distal end, each PAX2-positive vesicular structure fuses with the ureteric bud to establish continuity with the parent collecting system. At its proximal end, PAX2 is suppressed as each emerging tubule interacts with an ingrowing capillary to form the glomerular filtration apparatus. Renal PAX2 is down-regulated once nephrogenesis is complete and is not detected in mature kidney. 3,4
Without PAX2 function, the normal program of nephron development cannot proceed according to plan. Torres et al have shown that transgenic mice homozygous for a null PAX2 mutation are anephric and lack the caudal portion of the Wolffian duct, preventing outgrowth of the ureteric bud. 5 Even partial loss of PAX2 appears to effect normal kidney development; Sanyanusin et al have shown that humans who are heterozygous for PAX2 mutations have the autosomal dominant renal-coloboma syndrome consisting of ocular colobomas, renal hypoplasia, and progressive renal failure in childhood. 6,7 We recently examined fetal kidney morphology in a strain of mice (1Neu) transmitting a PAX2 mutation identical to that in some humans. 8,9 At an early stage (E15) of development, we noted that individual nephrons have normal morphology but are reduced in number, implying defective arborization of the ureteric bud. We also found a striking increase in apoptosis among cells of the developing collecting ducts derived from the ureteric bud. These observations prompted the hypothesis that PAX2 is critical for the survival of cells that form the branching fetal collecting duct and that PAX2 haploinsufficiency permits excessive apoptotic cell death, interfering with optimal branching morphogenesis.
Apoptosis, or programmed cell death, occurs in many tissues during normal vertebrate embryogenesis and is presumably essential for the continuous remodeling of emerging structures. 10 For example, in the rat optic nerve approximately 50% of newly produced oligodendrocytes undergo apoptosis during normal development. 11 Similar proportions of cells have been found to die by apoptosis during normal rat kidney development. 12,13 Unlike necrosis, in which the cell contents are spilled out, leading to inflammation, apoptosis is a self-programmed cell suicide in which the cell and its nucleus undergo specific morphological changes, including shrinkage and fragmentation. After fragmentation, the apoptotic cell is phagocytosed by adjacent cells and/or macrophages. The apoptotic process is rapid and dead cells are cleared within 1 to 2 hours. 14
An interesting aspect of apoptosis during embryogenesis is that selective control can be achieved by increasing the level of survival factors in specific tissues. For example, it was shown that metanephric mesenchymal cells undergo apoptosis during explant cultures of embryonic kidney; this apoptosis can be rescued by epithelial growth factor (EGF), protein kinase C activators, or signals from tissues with inductive activity, such as ureteric bud. 12 The ureteric bud itself and the collecting duct also undergo high levels of apoptosis during normal kidney development. 13 The mechanisms by which apoptosis is accomplished are gradually being elucidated, and these include the activation of a caspase cascade. 14 However, little is known about how the process is controlled in developing tissues and what determines the number of cells that ultimately survive.
In this report, we provide evidence for the hypothesis that PAX2 serves a critical anti-apoptotic function in developing murine kidney collecting duct. Heterozygosity for a null PAX2 mutation produces striking apoptosis of collecting duct cells in embryonic 1Neu mice without affecting markers of proliferation. In cultured murine collecting duct (mIMCD-3) cells, we show that inactivation of endogenous PAX2 expression greatly increases apoptotic cell death; conversely, PAX2 has no effect on proliferation of MDCK cells derived from mature collecting duct. We also demonstrate that cultured human embryonic kidney cells (HEK293) lacking endogenous PAX2 are relatively protected from caspase-2-induced apoptosis when transfected with a PAX2 expression vector.
Materials and Methods
Mouse Stocks and Embryo Preparation
All wild-type and PAX2 mutant (1Neu) mouse embryos were obtained from breeding colonies maintained in Neuherberg, Germany. Genomic DNA was extracted from embryonic yolk sacs and genotyped as described. 9 Heterozygous 1Neu mutant embryos and their wild-type littermates were fixed in paraformaldehyde and embedded in paraffin. Sagittal 5-μm sections of both kidneys from each mutant and wild-type embryo were prepared on Superfrost/Plus slides (Fisher, Fairtown, NJ).
Proliferating Cell Nuclear Antigen (PCNA) Immunostaining
Mouse sections were deparaffinized and hydrated. Endogenous peroxidase activity was quenched by incubating in methanol containing 0.3% H2O2. Slides were incubated in the presence or absence (control) of 2.5 μg/ml PCNA monoclonal antibody (Oncogene Research, Cambridge, MA) for 30 minutes, followed by two washes in phosphate buffered saline (PBS). The primary antibody was detected with Vectastain ABC Mouse IgG kit (Vector Laboratories, Burlingame, CA) as described by the manufacturer, followed by incubation with DAB substrate (Sigma, St. Louis, MO).
Analysis of Apoptosis on Embryo Sections
The in situ cell detection kit POD (Boehringer Mannheim, Mannheim, Germany) was used to detect apoptotic cells in heterozygous 1Neu mutant and wild-type kidneys by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay. Briefly, tissues were deparaffinized and treated with Proteinase K (30 μg/ml) in 1 mmol/L Tris, pH 7.4, for 20 minutes at 37°C. After washing with PBS, endogenous peroxidase was quenched in 0.3% H2O2 in methanol. Tissues were then incubated with labeling solution and terminal deoxytransferase (TdT) and detected by peroxidase 3,3′-diamino benzidine staining according to the manufacturer’s recommendations. Positive controls (sections treated with DNaseI before TdT reaction) and negative controls (no TdT enzyme) were included in each experiment. Each experiment was repeated three times.
Cell Lines, in Vitro Transfections, and Statistical Analyses
To examine the relationship of PAX2 to apoptosis, we used the following kidney cell lines: mIMCD-3 cells, widely used as a model of the murine collecting duct lineage 15 ; HEK293 cells and HEK293/tTA/hPAX2 cells, immortalized from human fetal kidney and stably transfected with a tetracycline-inhibitable PAX2 expression vector 16 ; COS-7 cells, an easily transfectable epithelial line lacking PAX2 expression originally, immortalized from monkey kidney 17 ; Madin-Darby canine kidney cells (MDCK) negative for PAX2 but expressing some features of the collecting duct 18 ; and stably transfected MDCK/tTA/hPAX2 cells expressing hPAX2 under a tetracycline-regulatable promoter as previously described. 16 All cell lines were plated in six-well dishes 24 hours before transient transfection experiments.
HEK293/tTA/hPAX2 cells and MDCK tTA/hPAX2 cells were grown in the presence or absence of 1 μg/ml of tetracycline (Sigma). Cells were transiently cotransfected using Fugene-6 according to the manufacturer’s recommendations (Boehringer Mannheim) with 2.5 μg of sense or anti-sense PAX2 construct or 0.5 to 1 μg of mutant (319 Cys to Gly) or wild-type caspase-2 expression vectors and 1.5 μg of RSV-β-galactosidase (β-Gal) or 1.0 μg of pTracer-SV40 (green fluorescent protein, GFP, Invitrogen, Carlsbad, CA) expression vectors per dish. Both wild-type and mutant (319 Cys to Gly) caspase-2 expression vectors were gifts from Dr. S. Kumar (Hanson Center for Cancer Research, Adelaide, Australia). Twenty-four hours after transfection, cells were fixed in 0.2% glutaraldehyde (Sigma) and 2% formaldehyde (Fisher) in PBS for 10 minutes, washed twice in PBS, and visualized by fluorescent microscopy (for GFP cotransfections) or, for β-Gal transfections, cells were stained with X-Gal substrate overnight and visualized by light microscopy as described by Kumar. 19 In each experiment, 3000–6000 β-Gal-positive cells were evaluated for morphology and classified as normal or apoptotic. In some experiments, the caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone, z-VAD-fmk (Calbiochem, La Jolla, CA), 20 or 50 μmol/L, was added at the time of transient transfections.
For proliferation assays, MDCK tTA/hPAX2 cells were grown in six-well dishes in the presence or absence of 1 μg/ml of tetracycline and 10% fetal calf serum in Dulbecco’s modified Eagle’s medium. Twenty-four hours before assay, medium was changed to Dulbecco’s modified Eagle’s medium containing 2% fetal calf serum, and cells were pulsed with [3H]-thymidine for 18 hours. Cells were then lysed and thymidine incorporation into TCA-precipitable material was measured by β-scintillation counting.
Statistical analysis was done using INSTAT-2 software (GraphPad Software Inc, San Diego, CA).
Plasmid Preparation
A sense human PAX2 expression vector was created by subcloning a 1.2-kb EcoRI-SpeI (542–1721 bp) cDNA fragment of the human PAX2 coding sequence. 3 The cDNA was cut out from a Bluescript vector using a PAX2 internal NcoI restriction site and the vector XbaI site. The NcoI site was converted into a Bgl-II restriction site by blunt-ending and ligation to a Bgl-II-adaptor. The resultant construct was subcloned into the Bgl-II-XbaI sites of pCMV5 expression vector (a gift of Dr. J. Pelletier, McGill University). An anti-sense PAX2 vector was constructed by subcloning a 2-kb EcoRI-XbaI fragment of the human PAX2 cDNA into XbaI-EcoRI sites of the pcDNA3.1 expression vector (Invitrogen, Carlsbad, CA). Transcription of both genes in HEK293 cells was detected by Northern blot analysis using a 2-kb EcoRI-SpeI hPAX2 cDNA probe.
Western Blot Analysis
mIMCD-3, COS-7, HEK293, HEK293 tTA/hPAX2, and MDCK tTA/PAX2 cells were isolated; nuclear protein extracts were prepared and resolved on 10% sodium dodecyl sulfate-polyacrylamide gels. Blots were probed with polyclonal anti-mPAX2 antibody (Zymed, San Francisco, CA) followed by the anti-rabbit IgG secondary antibody and detected with an enhanced chemiluminescence detection system (Amersham, Piscataway, NJ) as previously described. 16
Results
1Neu (+/−) Embryonic Mouse Kidneys Have Increased Apoptosis but No Change in Proliferation of Collecting Duct Cells
In a previous report, our detailed analysis of heterozygous 1Neu mice 15 to 16 embryonic days of age (E15–16) revealed that mutant kidneys are hypoplastic, averaging about 60% of the maximal cross-sectional area of wild-type littermates. 8 Individual E15 nephrons appeared to have normal morphology but the number of emerging nephrons was reduced, implying a decrease in the arborization of the ureteric bud/collecting ducts and, thus, in the rate of new nephron induction.
To ascertain whether renal hypoplasia in 1Neu heterozygotes is due to reduced cell proliferation versus increased apoptosis in the branching collecting ducts, we analyzed embryos from a timed mating between 1Neu heterozygous C57Bl/6 males and normal C57Bl/6 females. At E15, embryonic sections were immunostained with anti-PCNA antibody. This antigen (PCNA) is associated with rapidly dividing cells and has been used by others to identify zones of cell proliferation in developing organs. 20 In wild-type E15 kidneys, PCNA staining was intense in the collecting ducts and induced mesenchyme; no immunostaining was detectable when primary antibody was omitted (data not shown). We were unable to detect any difference in the pattern or intensity of anti-PCNA staining between mutant and wild-type E15 embryos (Figure 1, A and B) ▶ . In contrast, when E15 mutant kidneys were analyzed by TUNEL assay to detect apoptosis, we found a striking increase in the number of TUNEL-positive cells along the developing collecting ducts compared to wild-type littermates (Figure 1, C and D) ▶ . This effect was most striking in the medullary region.
Figure 1.
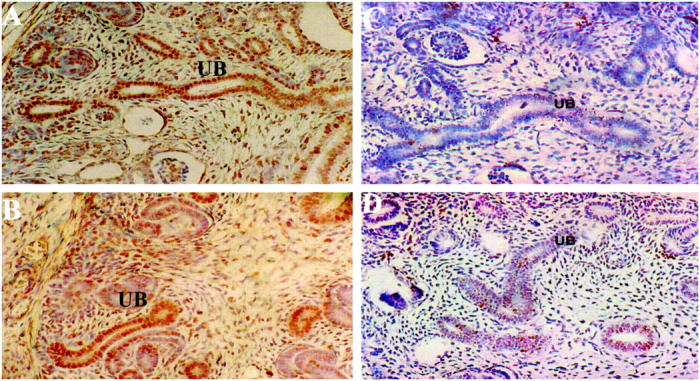
Analysis of cell proliferation and apoptosis in E15 fetal kidneys of wild-type and Pax21Neu +/− mice. PCNA immunostaining of wild-type (A) and 1Neu (B) fetal kidneys. UB, ureteric bud structures; original magnification, ×200. TUNEL staining of wild-type (C) and 1Neu (D) fetal kidneys. Note increased number of brown-stained nuclei in UB structures of the mutant embryo. Original magnification, ×200.
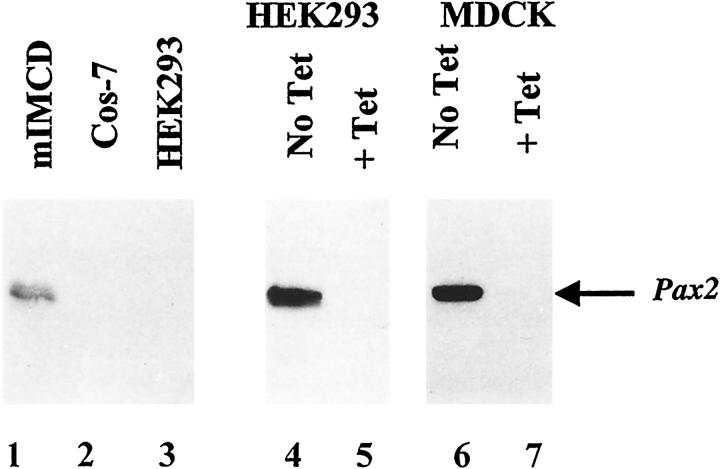
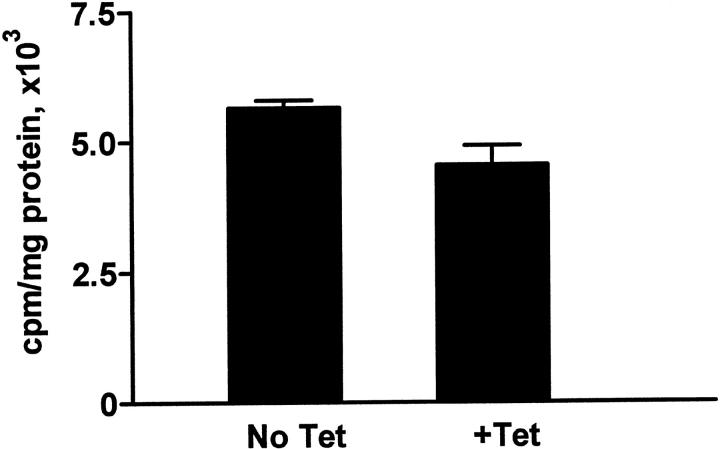
Several reports have noted an association between PAX2 expression and cell proliferation. To investigate whether PAX2 influences cell proliferation in cultured cells, MDCK cells were stably transfected with tetracycline-repressible PAX2 expression constructs (Figure 2 ▶ , lanes 6 and 7). MDCK cells, derived from mature canine collecting ducts, have no detectable endogenous PAX2 expression. We found no significant difference in [3H]-thymidine uptake by MDCK cells whether PAX2 was strongly expressed (no tetracycline) or fully suppressed (1 μg/ml tetracycline; Figure 3 ▶ ).
Figure 2.
Western immunoblotting with PAX2 antibody for various cell lines. Lanes 1–3: Endogenous expression of PAX2 protein in mIMCD-3, COS-7 and HEK293 kidney cell lines, respectively. Lanes 4 and 5: Expression of exogenous PAX2 protein in HEK293 tTA/PAX2 cells grown in the absence or the presence of 1 μg/ml of tetracycline. Lanes 6 and 7: Expression of exogenous PAX2 protein under regulation of the tetracycline-sensitive promoter in MDCKtTA/hPAX2 cells in the absence or presence of tetracycline.
Figure 3.
Analysis of proliferation in MDCK tTA/hPAX2 cells. Cells were grown in the absence or presence of 1 μg/ml of tetracycline and assayed for [3H]-thymidine incorporation as described in Materals and Methods. There is no significant difference between the rate of proliferation in cells with high (No Tet) or undetectable level (+Tet) of PAX2 protein expression. (P ≥ 0.5).
Apoptosis in Murine Inner Medullary Collecting Duct (mIMCD-3) Cells Transiently Transfected with Anti-Sense PAX2 cDNA
If collecting duct apoptosis in the 1Neu mouse is directly attributed to the level of PAX2 expression in collecting duct cells, then survival of collecting duct cells should be influenced by the level of PAX2 expression in vitro. We inactivated PAX2 mRNA in mIMCD-3 cells by transfecting them with an expression plasmid containing the full-length PAX2 cDNA in reverse orientation. mIMCD-3 cells are derived from the inner medullary collecting duct of normal mice and exhibit branching tubulogenesis when exposed to a variety of branching morphogens such as transforming growth factor-α (TGF-α) and hepatocyte growth factor (HGF). 21 In contrast to certain other renal cell lines (eg, HEK293 and MDCK), we showed by Western immunoblotting that IMCD-3 cells express PAX2 protein (Figure 2 ▶ , lane1) comparable to levels in developing kidney (data not shown).
To define the apoptotic phenotype of mIMCD-3 cells, we first cotransfected the cells with marker plasmids (β-Gal or GFP) and an expression vector containing caspase-2 or an inactive caspase-2 mutant containing a 319Cys-to-Gly substitution. Caspase-2 is a powerful initiator/effector of programmed cell death belonging to the family of mammalian interleukin-1β-converting enzyme (ICE)-related genes and has been shown to trigger apoptosis in NIH3T3 and other cells. 19 Twenty-four hours after transfection with caspase-2, about 40 to 45% of transfected mIMCD-3 cells showed evidence of apoptosis. These morphological changes included nuclear and cellular condensation, membrane blebbing and formation of apoptotic bodies in contrast to the flattened, spreading appearance of normal cells with intricate cytoplasmic extensions (Figure 4, A and D) ▶ . These apoptotic changes were noted in only 4 to 8% of mIMCD-3 cells transfected with the mutant caspase-2 or the empty pCDNA3.1 vector.
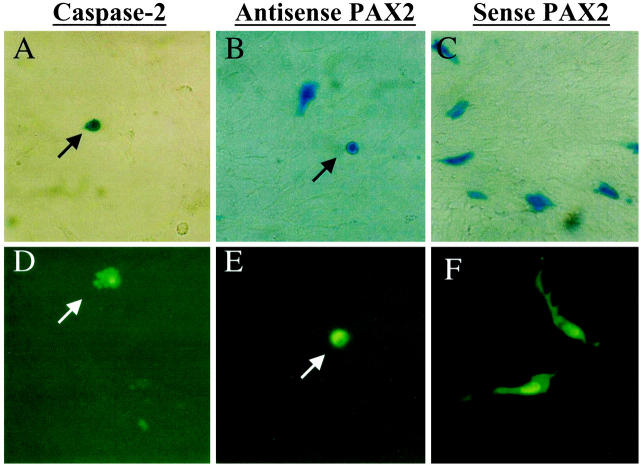
Figure 4.
Apoptosis in mIMCD-3 cells transfected with anti-sense PAX2 cDNA. Cells transfected with caspase-2 (A), anti-sense PAX2 (B), and sense PAX2 (C) vectors and β-Gal marker. Cells transfected with caspase-2 (D), anti-sense-PAX2 (E), and sense-PAX2 (F) and GFP marker. Apoptotic condensed cells (arrows) at early stage of apoptosis (A, B, and E) and vesicular apoptotic bodies (arrow, D) at late apoptosis stages are indicated.
To examine the effects of PAX2 inactivation on apoptosis, we attempted to stably transfect mIMCD-3 cells with an anti-sense PAX2 expression vector. However, despite extensive effort, we were unable to identify PAX2-deficient clones after selective growth in neomycin-containing medium. Consequently, we adopted the transient cotransfection strategy used in the caspase-2 experiments above. The anti-sense PAX2 construct, the corresponding sense PAX2 vector or an empty pCDNA3.1 plasmid were introduced into mIMCD-3 cells along with a marker plasmid. After 24 hours, apoptotic changes were consistently observed in 26.5 ± 0.9% (mean ± SEM) of cells transfected with anti-sense PAX2 vector (Figure 4, B and E ▶ , and Figure 5A ▶ ). This represents a 4.5-fold increase in apoptosis above baseline; only 7.0 ± 0.6 of IMCD-3 cells cotransfected with the sense PAX2 vector exhibited apoptotic features (Figure 5A) ▶ . By comparison, most cells transfected with the sense PAX2 construct retained a normal flattened appearance with graceful cytoplasmic extensions as above (Figure 4, C and F) ▶ . For each condition, we evaluated 3000–6000 transfected cells in five separate experiments. When the anti-sense PAX2 vector was introduced into cell lines (HEK293 and COS7) that lack endogenous PAX2 protein, no increase in the basal level of apoptosis (4–7%) was observed in either cell line. Thus, the pro-apoptotic effect of the anti-sense PAX2 cDNA is selective for cells with high endogenous PAX2 expression and does not represent nonspecific toxicity.
Figure 5.
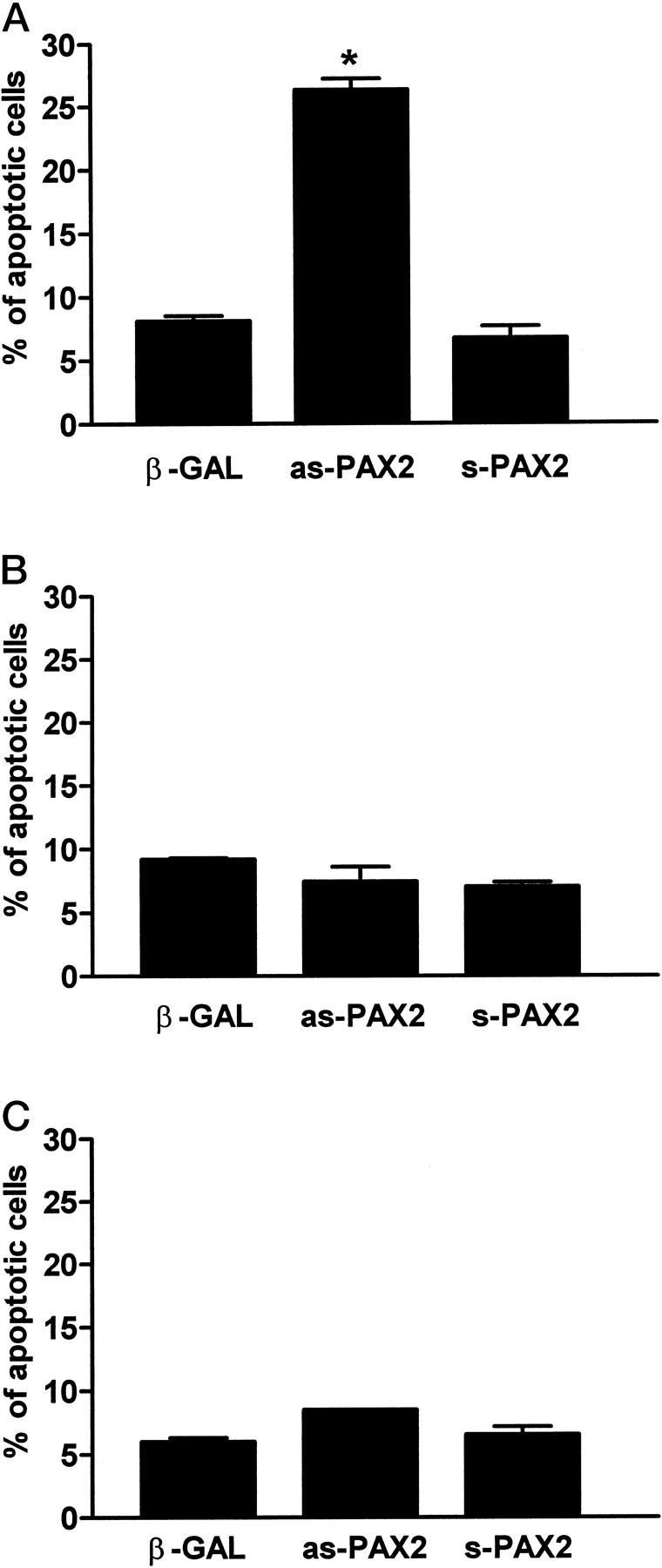
Anti-sense PAX2 causes increased apoptosis of cells expressing a high endogenous level of PAX2 protein. mIMCD-3 (A), COS-7 (B), and HEK293 (C) cells transfected with β-Gal marker alone, anti-sense-PAX2 and sense-PAX2 expression vectors as described in Materials and Methods. *, P ≤ 0.0001. Each condition represents the average of 4 or 5 separate experiments; in some cases the SE bar is not visible.
PAX2 Protects against Caspase-2-Induced Apoptosis in HEK293 Cells
On the basis of the experiments above, we reasoned that if inactivation of endogenous PAX2 expression increased apoptosis in mIMCD-3 cells, overexpression of PAX2 in cell lines lacking the endogenous transcription factor might protect cells against programmed cell death. To address this issue, we took advantage of an HEK293 cell system previously developed in our laboratory; these cells were stably transfected with a full-length PAX2 driven by a tetracycline-inhibitable promoter. 16 When cells were grown in the absence of tetracycline, PAX2 protein was strongly expressed; in the presence of tetracycline (1 μg/ml) PAX2 protein was undetectable (Figure 2 ▶ , lanes 4 and 5).
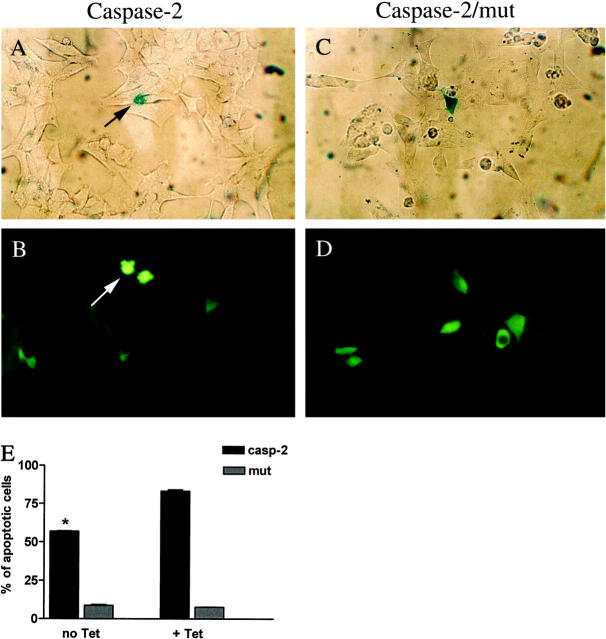
In HEK293 cells grown in the presence of tetracycline (PAX2 down-regulated), 83.1 ± 0.9% of caspase-2 transfected cells exhibited apoptotic morphological changes as opposed to basal levels of apoptosis (7.6 ± 0.3%) in cells transfected with the caspase-2 mutant. However, in the absence of tetracycline (PAX2-up-regulated), apoptosis occurred in only 56.9 ± 0.25% of transfected cells (Figure 6) ▶ . This represents protection for about 40% of these cells, which would have otherwise entered the apoptotic pathway.
Figure 6.
PAX2 overexpression protects HEK293 cells induced against apoptosis with caspase-2. A and B: HEK293 cells transfected with caspase-2 and either β-Gal marker (A) or GFP marker (B). C and D: HEK293 cells transfected with the mutant (319 Cys to Gly) form of caspase-2 and β-Gal (C) or GFP (D). Arrows show characteristic apoptotic cells at the late stage of programmed cell death. E: Graph showing the proportion of HEK293 cells undergoing apoptosis when PAX2 protein is present (no Tet) or when PAX2 expression is absent (+ Tet). *, P ≤ 0.0001.
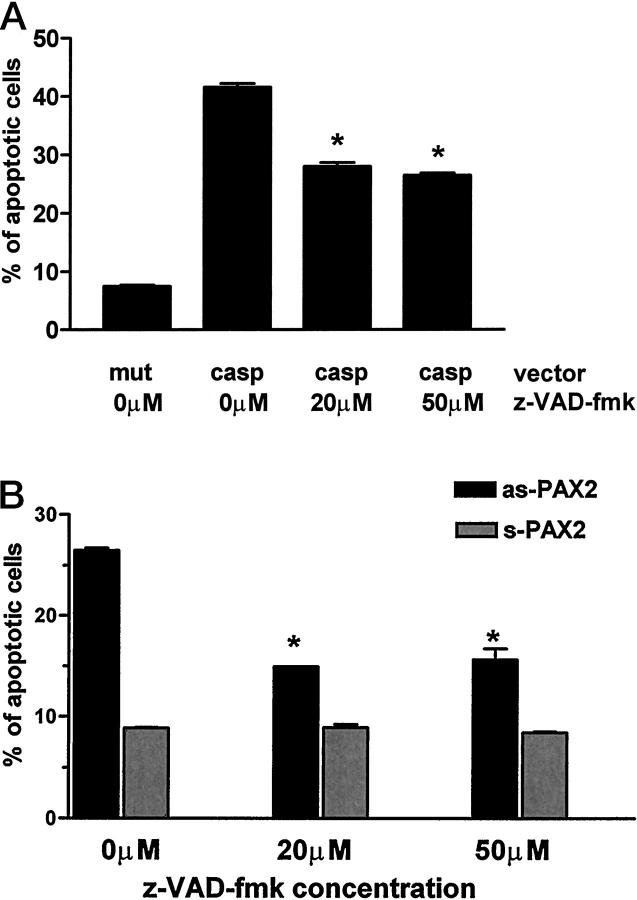
Caspase Inhibitors Rescue IMCD Cells Transfected with Anti-Sense PAX2
To determine whether PAX2 can intervene after the apoptotic pathway has been initiated, we determined whether a caspase inhibitor might rescue the transfected cells. z-VAD-fmk, an inhibitor of multiple caspases, 22,23 was added to the culture medium at the time of transfections. To establish the concentration of z-VAD-fmk effective in blocking the caspase cascade in mIMCD-3 cells, we first showed that 41.6 ± 0.64 of the mIMCD-3 cells transfected with caspase-2 exhibit apoptotic changes (Figure 7A) ▶ . Addition of z-VAD-fmk (20 or 50 μmol/L) to the culture medium rescued approximately 40% of the cells otherwise targeted by caspase-2 to undergo programmed cell death. The basal level of apoptosis (7.5 ± 0.3%) was unaffected by either dimethylsulfoxide (solvent for the caspase inhibitor) or by an inactive caspase-2 mutant (319 Cys-to-Gly). We then cotransfected IMCD-3 cells with the anti-sense-PAX2 expression construct and β-Gal marker plasmids in the presence or absence of z-VAD-fmk (20–50 μmol/L). As seen in Figure 7B ▶ , addition of z-VAD-fmk rescued 62 ± 0.25% of anti-sense PAX2-transfected IMCD-3 cells from apoptosis.
Figure 7.
Caspase inhibitor z-VAD-fmk rescues mIMCD-3 cells transfected with caspase-2 and anti-sense-Pax2 from apoptosis. A: Percentage of apoptosis in cells transfected with mutant or wild-type caspase-2 and treated with various concentrations of z-VAD-fmk. B: Percentage of apoptosis in cells transfected with sense-PAX2 or anti-sense-PAX2 and treated with various concentrations of z-VAD-fmk. *, P ≤ 0.0001. In some experiments, SE bars are not visible.
Discussion
Mutations of the PAX2 gene cause the autosomal dominant renal-coloboma syndrome (RCS) in humans (Online Mendelian Inheritance of Man #120330). This disease is associated with optic nerve colobomas, renal anomalies leading frequently to end stage renal failure, hearing loss, vesico-ureteral reflux, and some other, less common features. 6-8,24-27 The most common PAX2 mutation associated with RCS in humans (about 30% of cases) is a single basepair insertion (613+G) causing a frameshift within the DNA-binding PAX2 paired domain. 25 This same mutation has also arisen spontaneously in a mouse strain (1Neu) described by Favor et al. 9 Homozygous 1Neu mice die shortly after birth and have no detectable kidney tissue, making them unsatisfactory for investigation of the specific functions of PAX2 during renal development. On the other hand, heterozygous 1Neu mice, like their human counterparts, are viable despite moderate renal hypoplasia. PAX2 haploinsufficiency causes a marked decrease in PAX2 protein in developing kidney. Thus, the heterozygous 1Neu mouse is an ideal model of human RCS and affords the opportunity to investigate the consequences of reduced PAX2 expression during nephrogenesis.
In our previous study, we found that renal hypoplasia was evident among heterozygous 1Neu fetuses at early stages of kidney development. 8 The maximal cross-sectional area of mutant E15 kidneys was about 60% that of wild-type littermates, mimicking similar observations among humans with RCS. Histological analysis showed that hypoplasia is due to a defect in the extent of arborization of the collecting system and, thus, in the rate of new nephron induction. 8 This deficit in collecting system arborization was associated with a striking increase in TUNEL-positive apoptotic collecting duct cells. We inferred from these studies that high levels of PAX2 protein might be necessary for survival of collecting duct cells and optimal branching morphogenesis during renal development.
In this study, we confirmed the striking increase in TUNEL-positive apoptotic cells distributed throughout the trunk and branches of the collecting system of E15 1Neu (+/−) embryos. However, others have suggested that PAX2 could be involved in driving cell proliferation. When renal carcinoma cells expressing high levels of PAX2 were treated with anti-sense PAX2 oligonucleotides in vitro, cell number was dramatically reduced. 28 Gnarra et al inferred that this was due to an effect on cell proliferation, though apoptosis was not directly assessed. 28 Our observations both in vivo and in vitro indicate that the primary function of PAX2 is anti-apoptotic rather than mitogenic. PAX2 haploinsufficiency had no effect on cell proliferation in embryonic 1Neu mouse kidney as assessed by PCNA immunostaining (this study) or by BRDU staining 8 ; proliferation of MDCK cells (this study) and HEK293 cells 16 was unaltered by PAX2 expression in vitro. Thus, PAX2 haploinsufficiency appears to be associated with an increased susceptibility of fetal collecting duct cells to apoptosis, although surviving cells are able to respond to mitogenic signals normally.
To provide direct evidence for an effect of PAX2 on apoptotic pathways, we targeted endogenous PAX2 mRNA in mIMCD-3 cells, an established cell line derived from the inner medullary collecting ducts of mouse kidney. These cells were appropriate for the study, in that it was the collecting duct that underwent apoptosis in the fetal 1Neu mouse. We attribute our initial unsuccessful efforts to inactivate PAX2 in stably transfected mIMCD-3 cells to the hypothesis that PAX2 is critical for cell survival in vitro and that cells expressing the PAX2 anti-sense vector were eliminated during the selection process. As a practical alternative, we adopted the transient transfection approach used by other groups in the study of pro-apoptotic genes. 19,29 The efficiency of transient transfection of mIMCD-3 cells was approximately 2 to 4%; among these transfected cells, the background level of apoptosis was 7 to 8%. Morphological changes characteristic of apoptosis were present in a high percentage of cells transiently transfected with caspase-2, known to initiate the apoptotic caspase cascade. 19 Much of this apoptosis could be prevented with z-VAD-fmk. Similarly, transient transfection with anti-sense, but not sense PAX2 vector, resulted in a high percentage of apoptotic cells that also could be rescued by the caspase inhibitor.
In keeping with the above, we found that PAX2 expression was able to serve a pro-survival function in cells. The HEK293 human fetal kidney cell line lacks endogenous PAX2 expression and is susceptible to apoptosis induced by camptothecin and other pro-apoptotic drugs (E Torban, unpublished results). Our observation that PAX2 expression protects about 40% of HEK293 cells targeted to undergo apoptosis by caspase-2 transfection is comparable to the 60% protective effect of Bcl-2 on caspase-2-activated apoptosis described in other cell systems. 19
It is unclear how PAX2 influences the apoptotic cascade, but some general inferences can be drawn based on our observation that PAX2 has a protective effect on caspase-2-induced apoptosis. Caspase-2 is thought to be an “initiator” caspase. Certain pro-apoptotic signals cause Bcl-2-dependent release of caspase-2 from mitochondria in an inactive form, along with pro-caspase 9 and cytochrome C. 19,30,31 Once activated in the cytoplasm, caspase-2 activates other effector caspases such as caspase-3. 30 Because PAX2 promotes survival of cells transfected with caspase-2, its effects may lie within the effector limb of the apoptotic cascade. Interestingly, caspase-2 expression has been noted in the cortical layer of fetal kidney, during the same developmental window when PAX2 expression occurs. 19 It is conceivable that certain cells in the developing kidney are rendered susceptible to apoptosis by caspase-2 activation unless rescued by timely PAX2 expression.
Other investigators have reported an association between apoptosis and other members of the paired box gene family. For example, PAX3 mutant mice show increased apoptosis of the somitic mesoderm. 32 Moreover, PAX2, PAX5, and PAX8 all inhibit transcription of p53, a pivotal molecule regulating entry into the final common apoptotic pathway. 33,34 Mutation of eyeless, the Drosophila homologue of mammalian PAX6, leads to excessive apoptosis of photoreceptor precursors. 35 However, the consequent retinal degeneration can be prevented by overexpression of p35, a known baculoviral inhibitor of apoptosis. 36 In this study, we provide evidence for a direct anti-apoptotic effect of PAX2 on renal collecting duct cells. Thus, it is possible that there is a fundamental role for PAX genes in promoting cell survival by inhibiting the apoptotic pathway.
How could increased apoptosis in fetal ureteric bud lead to reduced arborization of the collecting duct system and renal hypoplasia? Our observations suggest a general concept of renal branching morphogenesis in which arborization is dependent on accumulation of sufficient cell mass in the ureteric bud trunk to allow the next branch-point in the tree to occur. By protecting collecting duct cells from apoptosis, PAX2 reduces the time required to accumulate sufficient cells to pass some threshold. Such a theory might invoke the existence of an inhibitor of branching, which forms a gradient along the trunk of the collecting system tree in between branch points. As the growing trunk extends beyond the influence of this inhibitory field, signals from nearby branching morphogens once again gain precedence and induce bifurcation. Interestingly, homozygous inactivation of the EGF receptor produces such renal hypoplasia and in vivo administration of EGF has been shown to protect against apoptosis in developing rat kidney. 12 Further experimentation is required to determine whether the effects of PAX2 on collecting duct cell apoptosis constitute the only mechanism by which it determines the extent of branching renal morphogenesis.
In conclusion, we show that renal hypoplasia in the PAX2 haploinsufficient 1Neu mouse is associated with a striking increase in apoptosis, providing direct in vitro evidence that PAX2 is a critical arbiter of apoptosis in renal cells. During development, expression of PAX genes may have a rate-limiting function in the selection of specific cell lineages for survival. Thus, in the developing kidney, PAX2 serves to enhance survival of selected cells and thereby optimizes branching morphogenesis. This hypothesis has implications for the pathogenesis of renal hypoplasia in the renal-coloboma syndrome and possibly for other pathologies (eg, polycystic kidney disease) in which PAX2 is inappropriately expressed in the postnatal period.
Acknowledgments
We acknowledge Jerry Pelletier and Sharad Kumar, the investigators who generously provided various molecular reagents for this work.
Footnotes
Address reprint requests to Dr. Paul Goodyer, McGill University Montreal Children’s Hospital, 2300 Tupper Street, Montreal, Quebec, Canada H3H 1P3. E-mail: Paul.Goodyer@muhc.mcgill.ca.
Supported by grants from the Medical Research Council of Canada (MT12954), the Cancer Society of New Zealand, the Health Research Council of New Zealand and the New Zealand Lottery Grants Board. E. T. was supported by Fonds de la recherche en santé du Québec-Formations de chercheurs et l’aide à la recherche (FCAR-FRSQ) and Lloyd Carr-Harris McGill Major Fellowship awards.
References
- 1.Saxen L: Organogenesis of the kidney. 1987. Cambridge University Press, Cambridge
- 2.Dressler GR, Deutsch U, Chowdhury K, Nornes OH, Gruss P: Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development 1990, 109:787-795 [DOI] [PubMed] [Google Scholar]
- 3.Eccles MR, Wallis LJ, Fidler AE, Spurr NK, Goodfellow PJ, Reeve AE: Expression of the Pax2 gene in human fetal kidney and Wilms’ tumor. Cell Growth Differ 1992, 3:279-289 [PubMed] [Google Scholar]
- 4.Dressler GR, Douglass EC: Pax2 is a DNA-binding protein expressed in embryonic kidney and Wilms’ tumor. Proc Natl Acad Sci USA 1992, 89:1179-1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres M, Gomez-Pardo E, Dressler G, Gruss P: Pax2 controls multiple steps of urogenital development. Development 1995, 121:4057-4065 [DOI] [PubMed] [Google Scholar]
- 6.Sanyanusin P, Schimmenti LA, McNoe LA, Ward TA, Pierpont MEM, Sullivan MJ, Dobyns WB, Eccles MR: Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nat Genet 1995, 9:358-364 [DOI] [PubMed] [Google Scholar]
- 7.Sanyanusin P, McNoe LA, Sullivan MJ, Weaver RG, Eccles MR: Mutation of the Pax2 in two siblings with renal-coloboma syndrome. Hum Mol Genet 1995, 4:2183-2184 [DOI] [PubMed] [Google Scholar]
- 8.Porteous S, Torban E, Cho N-P, Cunliffe HE, Chua L, McNoe LA, Ward TA, Souza C, Gus P, Giugliani R, Sato T, Yun K, Favor J, Sicotte M, Goodyer PR, Eccles MR: Primary renal hypoplasia in humans and mice with PAX2 mutations: evidence of increased apoptosis in fetal kidneys of PAX1Neu +/− mutant mice. Hum Mol Genet 2000, 9:1-11 [DOI] [PubMed] [Google Scholar]
- 9.Favor J, Sandulache R, Neuhauser-Klaus A, Pretsch W, Chatterjee B, Senft E, Wurst W, Blanquet V, Grimes P, Sporle R, Schughart K: The mouse PAX21Neu mutation is identical to a human PAX2 mutation in a family with renal-coloboma syndrome and results in developmental defects of brain, ear, eye, and kidney. Proc Natl Acad Sci USA 1996, 89:1179-1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobson MD, Weil M, Raff MC: Programmed cell death in animal development. Cell 1997, 88:347-354 [DOI] [PubMed] [Google Scholar]
- 11.Barres BA, Hart IK, Coles HSR, Burne JF, Voyvodic JJ, Richardson WO, Raff MC: Cell death and control of cell survival in the oligodendrocyte lineage. Cell 1992, 70:31-46 [DOI] [PubMed] [Google Scholar]
- 12.Coles HSR, Burne JF, Raff MC: Large scale normal cell death in the developing rat kidney and its reduction by epidermal growth factor. Development 1993, 118:777-784 [DOI] [PubMed] [Google Scholar]
- 13.Koseki C, Herzlinger D, Al-Awqati Q: Apoptosis in metanephric development. J Cell Biol 1992, 119:1327-1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steller H: Mechanisms and genes of cellular suicide. Science 1995, 267:1445-1449 [DOI] [PubMed] [Google Scholar]
- 15.Rauchman MI, Nigam SK, Delpire E, Gullans SR: An osmotically tolerant inner medullary collecting duct cell line from an SV40 transgenic mouse. Am J Physiol 1993, 265:F416-F424 [DOI] [PubMed] [Google Scholar]
- 16.Torban E, Goodyer PR: Effect of Pax2 expression in a human fetal kidney HEK293 cell line. Biochem Biophys Acta 1998, 1401:53-62 [DOI] [PubMed] [Google Scholar]
- 17.Gluzman Y: SV40 transformed simian cells support the replication early SV40 mutants. Cell 1981, 23:175-182 [DOI] [PubMed] [Google Scholar]
- 18.Gaush CR, Hard WL, Smith TF: Characterization of an established line of canine kidney cells (MDCK). Proc Soc Exp Biol Med 1966, 122:931-935 [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Kinoshita M, Noda M, Copeland NG, Jenkins NA: Induction of apoptosis by the mouse Nedd2 gene, which encodes a protein similar to the product of the Caenorhabditis elegans cell death gene ced-3 and mammalian IL-1β-converting enzyme. Genes Devel 1994, 8:1613-1626 [DOI] [PubMed] [Google Scholar]
- 20.Alpers CE, Seifert RA, Hudkins KL, Johnson RJ, Bowen-Pope DF: Developmental patterns of PDGF B-chain, PDGF-receptor, and alpha-actin expression in human glomerulogenesis. Kidney Int 1992, 42:390-399 [DOI] [PubMed] [Google Scholar]
- 21.Sakurai H, Nigram SK: In vitro branching tubulogenesis: implications for developmental and cystic disorders, nephron number, renal repair, and nephron engineering. Kidney Int 1998, 54:14-26 [DOI] [PubMed] [Google Scholar]
- 22.Qi XM, He H, Zhong H, Distelhorst CM: Baculovirus p35 and z-VAD-fmk inhibit thapsigargin-induced apoptosis in breast cancer cells. Oncogene 1997, 15:1207-1212 [DOI] [PubMed] [Google Scholar]
- 23.Hara H, Friedlander RM, Gagliardini V, Ayata C, Fink K, Huang Z, Shimizu-Sasamata M, Yuan J, Moscowitz MA: Inhibition of interleukin-1-β-converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc Natl Acad Sci USA 1997, 94:2007-2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schimmenti LA, Cunliffe HE, McNoe LA, Ward TA, French MM, Shim HH, Zhang YH, Proesmans W, Leys A, Byerly KA, Braddock SR, Masuno M, Imaizumi K, Devriendt K, Eccles MR: Further delineation of renal-coloboma syndrome in patients with remarkable variability of phenotype and identical Pax2 mutations. Am J Hum Genet 1997, 60:869-878 [PMC free article] [PubMed] [Google Scholar]
- 25.Eccles MR: The role of Pax2 in normal and abnormal development of urinary tract. Pediatr Nephrol 1998, 12:712-720 [DOI] [PubMed] [Google Scholar]
- 26.Narahara K, Baker E, Ito S, Yokoyama Y, Yu S, Hewitt D, Sutherland GR, Eccles MR, Richards RI: Localization of 10q breakpoint within the Pax2 gene in a patient with a de novo t(10;13) translocation and optic nerve coloboma-renal disease. J Med Genet 1997, 34:213-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cunliffe HE, McNoe LA, Ward TA, Devriendt K, Brunner HG, Eccles MR: The prevalence of Pax2 mutations in patients with isolated colobomas and colobomas associated with urogenital anomalies. J Med Genet 1998, 35:806-812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gnarra JR, Dressler GR: Expression of PAX2 in human renal carcinoma cells and growth inhibition by antisense oligonucleotides. Cancer Res 1995, 15:4092-4098 [PubMed] [Google Scholar]
- 29.Komarov PG, Komarova EA, Kondratov RV, Cristov-Tselkov K, Coon JS, Chernov MV, Gudkov AV: A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 1999, 285:1733-1737 [DOI] [PubMed] [Google Scholar]
- 30.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Brenner C, Larochette N, Prevost MC, Alzari PM, Kroemer G: Mitochondrial release of caspase-2 and -9 during the apoptotic process. J Exp Med 1999, 189:381-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thornberry N, Lazebnic Y: Caspases: enemies within. Science 1998, 281:1312-1316 [DOI] [PubMed] [Google Scholar]
- 32.Borycki AG, Li J, Emerson Jr CP, Epstein JA: Pax3 functions in cell survival and in Pax7 regulation. Development 1999, 126:1665–1674 [DOI] [PubMed]
- 33.Stuart ET, Haffner R, Oren M, Gruss P: Loss of p53 function through PAX-mediated transcriptional repression. EMBO J 1995, 14:5638-5645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine AJ: P53, the cellular gatekeeper for growth and division. Cell 1997, 88:323-331 [DOI] [PubMed] [Google Scholar]
- 35.Halder G, Callaerts P, Flister S, Walldorf U, Kloter U, Gehring WJ: Eyeless initiates the expression of both sine oculis and eyes absent during Drosophila compound eye development. Development 1998, 125:2181-2191 [DOI] [PubMed] [Google Scholar]
- 36.Davidson FF, Steller H: Blocking apoptosis prevents blindness in Drosophila retinal degeneration mutants. Nature 1998, 391:587-591 [DOI] [PubMed] [Google Scholar]