Abstract
To determine whether stretch-induced activation of p53 is necessary for the up-regulation of the local renin-angiotensin system and angiotensin II (Ang II)-induced apoptosis, ventricular myocytes were infected with an adenoviral vector carrying mutated p53, Adp53m, before 12 hours of stretch. Noninfected myocytes and myocytes infected with AdLacZ served as controls. Stretching of Adp53m-infected myocytes prevented stimulation of p53 function that conditioned the expression of p53-dependent genes; quantity of angiotensinogen (Aogen), AT1, and Bax decreased, whereas Bcl-2 increased. Ang II generation was not enhanced by stretch. Conversely, stretch produced opposite changes in noninfected and AdLacZ-infected myocytes: Aogen increased twofold, AT1 increased 2.1-fold, Bax increased 2.5-fold, and Ang II increased 2.4-fold. These responses were coupled with 4.5-fold up-regulation of wild-type p53. Stretch elicited comparable adaptations in p53-independent genes, in the presence or absence of mutated p53; renin increased threefold, angiotensin-converting enzyme increased ninefold, and AT2 increased 1.7-fold. Infection with Adp53m inhibited myocyte apoptosis after stretch. Conversely, stretch increased apoptosis by 6.2-fold in myocytes with elevated endogenous wild-type p53. Thus, a competitor of p53 function interfered with both stretch-induced Ang II formation and apoptosis, indicating that p53 is a major modulator of myocyte renin-angiotensin system and cell survival after mechanical deformation.
The tumor suppressor gene, p53, has been implicated in the modulation of cardiac myocyte apoptosis in vitro 1-3 and in vivo. 4 However, not all results are in agreement; forced re-entry of myocytes into the cell cycle by infection of cells with E2F-1 leads to the stimulation of the endogenous cell death pathway, independently from p53. 5 Conversely, hypoxia 1 or disruption of the vacuolar proton-ATPase system 3,6 initiates myocyte apoptosis in which p53 seems to play a significant role. 3 p53 is a transcriptional activator of the proapoptotic gene product Bax 7 and a p53-negative response element has been identified in the promoter region of the antiapoptotic gene product Bcl-2. 8 However, in nonmyocytes, increases in p53 quantity and activity and decreases in Bcl-2:Bax protein ratios enhance the susceptibility of cells to apoptotic death signals. In adult ventricular myocytes, p53 has been reported to up-regulate the cellular renin-angiotensin system (RAS), resulting in the synthesis of the octapeptide angiotensin II (Ang II) in vitro 2,9 and in vivo. 10 Ang II, in turn, activates p53, creating a positive feed-back loop in which hormone production and p53 function are intimately related in myocytes. 2,9,10 Indirect evidence favoring this possibility, has been obtained by applying physical forces to myocytes in vitro, 2 or by adding IGF-1 to this preparation. 9 In the first case, stretching was associated with release of Ang II, up-regulation of p53 and p53-inducible genes, enhanced cellular RAS, and myocyte apoptosis. In the second case, IGF-1 inhibited p53 via the formation of Mdm2-p53 protein complexes, decreasing Ang II synthesis and cell death. A more causal relationship between the local RAS and p53 was obtained by infecting myocytes with an adenovirus carrying wild-type p53; p53 activated the cellular RAS, decreased the Bcl-2:Bax ratio and produced extensive apoptosis. 11 Despite these findings, the postulated effects of sarcomere stretching on p53, p53-dependent genes, myocyte RAS, and apoptosis 2,9 remain to be unequivocally clarified. This is a relevant issue because acute changes in diastolic loading and sarcomere elongation occur immediately after myocardial infarction, resulting in wall thinning and chamber dilation. 12 Myocyte apoptosis is a critical event in acute ventricular remodeling, 13,14 and the decrease in Bcl-2 and increase in Bax in the spared myocytes of the postinfarcted heart suggest involvement of p53 in this setting. 13 Moreover, increases in end-diastolic pressure and sarcomere stretching characterize ventricular dysfunction and failure of different origin. 12 To obtain direct, functional evidence for the role of p53 in stretch-mediated Ang II generation and apoptotic signaling, adult ventricular myocytes were infected with a recombinant adenovirus expressing a dominant-negative form of p53 before stretching. Results indicate that induction of p53 by mechanical deformation of myocytes for a period of 12 hours is causally related to both phenomena. Infection with dominant-negative p53 was induced before mechanical deformation to avoid activation of endogenous wild-type p53 with stretch in the absence of its mutated inhibitory form.
Materials and Methods
Myocyte Cultures
Myocytes were isolated from 3-month-old Sprague-Dawley rats. 2,9,11,14 From each left ventricle, 6.0 × 10 6 myocytes were obtained. Cells were plated at a density of 2 × 10 4 per cm 2 in a device with a silicon substrate coated with 0.5 μg/cm 2 laminin. 2,9 The entire study included 44 animals and a total of ∼300 stretch devices.
Myocyte Infection
Cells were infected with a replication-deficient adenovirus, containing human mutated p53, Adp53m, under the control of the human cytomegalovirus immediate early gene promoter. 15,16 p53mutations consisted of substitution of cysteine in position 135 with serine, and proline in position 72 with arginine. 15 A second vector, expressing β-galactosidase, AdLacZ, was used as control. Myocytes were infected 2 hours after plating and maintained in serum-free medium (SFM) for 12 hours; infection was terminated by removing the medium and adding fresh SFM. This phase lasted 36 hours. Myocytes were infected for 48 hours with 100, 300, 500, and 1,000 pfu/cell of AdLacZ; infection efficiency was 60 ± 9% (n = 3), 87 ± 5% (n = 5), 88 ± 3% (n = 5), and 91 ± 3% (n = 5), respectively. In all studies, infection with 300 pfu/cell was used.
Myocyte Stretch
After 48 hours of infection, a 20% strain was applied to a stretch device for 12 hours. 2,9 In each preparation, sarcomere length was measured in 150 myocytes before and after stretch. To assess whether stretch injured cells, ethidium monoazide bromide (EMB; Molecular Probes, Eugene, OR), 5 μg/ml, was used. EMB binds to nucleic acids only in cells with membrane breakage. 17 After incubation with EMB and exposure to light, cells were fixed in 1% paraformaldehyde. EMB-labeled cells were measured by confocal microscopy.
Gel Retardation Assay
Two synthetic oligonucleotides, 5′-AGCCTCTGTACAGAGTAGCC-TGGGAATAGATCCATCTTC-3′ and 5′-GAAGATGGATCTATTCCCAGGCTACTCTGTAC-AGAGGCT-3′, corresponding to two half-sites of the p53 motif in the rat angiotensinogen (Aogen) promoter were used. Two synthetic oligonucleotides, 5′-GCTGAGCTTGGATCTGGAAGGCGACACTGGG-3′ and 5′-CCCAGTGTCGCCTTCCAGATCCAAGCTCAGC-3′, corresponding to two half-sites of the p53 motif in the rat AT1 promoter were used. Two synthetic oligonucleotides, 5′-AGCTTGCTCACAAGTTAGAGACAAGCCTGGGCGTGGCTATATTGA-3′ and 5′-AGCTTCAATATAGCCCACGCCCAGGCTTGTCTCTAACTTGTGAGCA-3′, corresponding to two half-sites of the p53 motif in the human bax promoter were used. Two additional sites of the p53 motif are located in the 3′-region of the perfect p53 element at 0 and 6 bp where they partially overlap. 7 Nuclear extracts were obtained by incubating myocytes in hypotonic buffer and in high-salt buffer 2,4,9,10 ; 30 μg of proteins were diluted in binding buffer with 1 to 2 μl of labeled probe. Nuclear extracts were exposed to p53 antibodies consisting of 0.5 μg pAb 240 and DO-1 (Santa Cruz, Santa Cruz, CA), or 0.5 μg pAb 246 (Ab-4, Calbiochem, San Diego, CA). Controls included unlabeled Aogen, AT1, and bax probes as competitors and unlabeled mutated Aogen (5′-AGCCTCTATAAAGAGTAGCCTGGGAATAGATCCATCTTC-3′), AT1 (5′-GCTGAGATTAGATCTGGAAGGCGACACTGGG3′), and bax (5′-AAGTTAGAGATAATGCTGGGCGAG-3′) as noncompetitors.
Western Blot of p53, Bax, Bcl-2, Aogen, Renin, ACE, and AT1 and AT2 Receptors
Myocytes were lysed in the presence of protease inhibitors. Samples were incubated on ice, centrifuged, and 50 μg proteins were separated by 8 to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were transferred to nitrocellulose and exposed to mouse monoclonal anti-human p53 (pAb 240, Santa Cruz), rabbit polyclonal anti-human Bax (P19, Santa Cruz), rabbit polyclonal anti-human Bcl-2 (ΔC21, Santa Cruz), mouse anti-rat Aogen (Swant, Bellinzona, Switzerland), mouse anti-rat renin (Swant), mouse anti-rat ACE (gift from Dr. David E. Dostal), rabbit polyclonal anti-human AT1 (306, Santa Cruz), and goat polyclonal anti-human AT2 (C-18, Santa Cruz) antibodies. Bound antibodies were identified by horseradish peroxidase-conjugated anti-mouse, anti-rabbit, anti-goat IgG, or anti-mouse IgM and recognized by a peroxidase chemiluminescent detection reagent. 2,4,9,10
Immunoprecipitation and Western Blot of Bax and Bcl-2
Bcl-2 Bound to Bax
Two hundred μg of proteins were incubated with 3 μg of rabbit polyclonal anti-human Bax antibody (P19, Santa Cruz) and 250 μl of HNTG buffer (20 mmol/L Hepes, pH 7.5, 150 mmol/L NaCl, 0.1% Triton X-100, 10% glycerol), containing protease inhibitors at 4°C overnight. Fifty μl of protein A-agarose was added. Proteins were separated by 12% polyacrylamide gel electrophoresis. Proteins were transferred to nitrocellulose membranes and exposed to rabbit polyclonal anti-human Bcl-2 (ΔC21, Santa Cruz) at a concentration of 1 μg/ml Tris-buffered saline/Tween 20 (TBST).
Bax Bound to Bcl-2
An identical procedure was followed, with the exception that rabbit polyclonal anti-human Bcl-2 antibody (ΔC21, Santa Cruz) was used to immunoprecipitate myocyte lysates 9,10 and that membranes were exposed to rabbit polyclonal anti-human Bcl-2 and rabbit polyclonal anti-human Bax.
p53 Localization
Cells were fixed in methanol and acetone (2:1) at −20°C and were incubated with two p53 antibodies: DO-1 (Santa Cruz) which recognizes human p53 and pAb 246 (Ab-4; Calbiochem, San Diego, CA) which binds to rodent wild-type p53. The fraction of p53-positive myocyte nuclei was evaluated by confocal microscopy; 4 500 cells were examined in each case.
Terminal-Deoxynucleotidil Transferase Assay Assay
Cultures were incubated with 50 μl of solution, containing 5 U of TdT, 2.5 mmol/L CoCl2, and 0.5 nmol/L biotin-16-dUTP. Myocytes were stained with 5 μg/ml of fluorescein isothiocyanate-extravidin in 4× standard saline citrate buffer. Cells were treated with α-sarcomeric actin antibody and with rhodamine-labeled anti-mouse IgG. Nuclei were labeled by propidium iodide (PI). 2,4,9,18
In Situ Ligation of Hairpin Oligonucleotide
Myocytes were stained with 50 mmol/L Tris/HCl, pH 7.8, 10 mmol/L MgCl2, 10 mmol/L dithiothreitol, 1 mmol/L ATP, 15% polyethylene glycol 8,000, 1 U/μl T4 ligase, and 35 ng/μl hairpin probe with single-base 3′ overhang. 19 Cells were exposed to fluorescein isothiocyanate-extravidin in bicarbonate buffer and stained for confocal microscopy. 2,18
Ang II Amount
Ang II in conditioned medium was measured by enzyme-linked immunosorbent assay (Peninsula, Belmont, CA). Conditioned medium was treated with 10% trifluoroacetic acid and centrifuged. Supernatant was dried, residue dissolved in 0.1% trifluoroacetic acid, and purified in a C-18 Sep-Pak column. Ang II fraction was eluted with 30% acetonitrile in 5 ml of 0.1% trifluoroacetic acid, dried, and dissolved in 0.25 ml of TBST. Samples, 50 μl, were analyzed in a microtiter plate using Ang II antibody (1:32,000) and a tracer, biotinylated Ang II. Color reaction was developed with tetramethyl-benzidine. Absorbance was recorded at 450 nm and concentration calculated from standard curves. 9,10
Data Analysis
Results are presented as mean ± SD. Autoradiograms were assessed by an image analyzer. Significance between two measurements, P < 0.05, was determined by Student’s t-test. Significance among several preparations was established by the Bonferroni method. 20
Results
Adp53m
pAb 240 p53 antibody recognized both wild-type and mutated p53; small amounts of p53 were detectable in control AdLacZ-infected myocytes. In Adp53m-infected cells, p53 increased from 24 to 48 hours when 100 and 300 pfu/cell were added. In comparison with AdLacZ-infected cells for 48 hours, infection of myocytes with 100 and 300 pfu/cell of Adp53m resulted in a 71-fold (P < 0.001) and 107-fold (P < 0.001) increase in p53 (data not shown). The 1.5-fold difference in p53 quantity between these doses of Adp53m was significant (P < 0.001).
Stretch and p53 DNA Binding
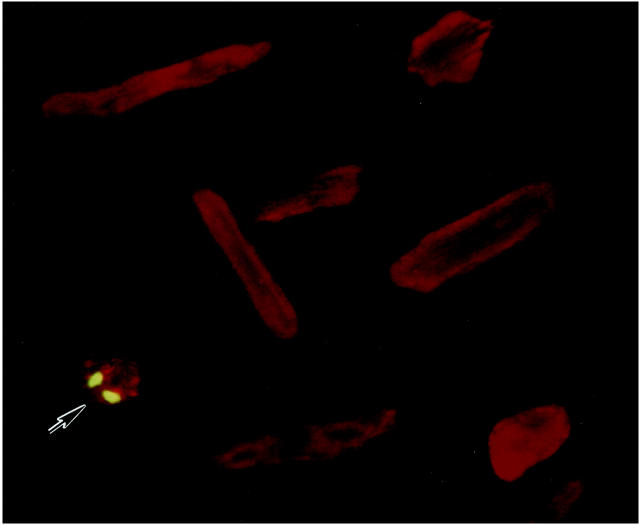
Stretching of noninfected and infected myocytes produced a 14% (P < 0.001) increase in sarcomere length, from 1.85 ± 0.02 μm (n = 90) to 2.11 ± 0.03 μm (n = 90). Sarcomere length was constant among experiments. 2,9 Cell membrane was occasionally disrupted. EMB-positive myocytes were 0.4 to 0.6% in cultures of noninfected and infected cells and in the absence and presence of stretch; the 420 molecular weight molecule was mostly detected in the nucleus (Figure 1) ▶ . The minimal level of myocyte damage indicates that the utilization of 300 pfu/cell of Adp53m as an infection protocol did not alter cell viability. Similar values have been obtained with mechanical deformation only. 2 Additionally, this high dose was used because the medium added to the stretch devices was 5 ml. This quantity of medium was necessary for the large size of the devices; when more than 1 ml of medium has to be used, the diffusion factor is decreased and larger titers of virus have to be applied. 21
Figure 1.
EMB labeling of a stretched (S) Adp53m-infected myocyte. Green fluorescence reflects EMB-positive nuclei; myocyte cytoplasm shows red fluorescence because of α-sarcomeric actin staining. Several EMB-negative myocytes are illustrated. Confocal microscopy: ×300. n = 4 in all cases. Noninfected myocytes: NS = 0.59 ± 0.10%; S = 0.48 ± 0.16%. AdLacZ-infected myocytes: NS = 0.51 ± 0.19%; S = 0.60 ± 0.15%. Adp53m-infected myocytes: NS = 0.45 ± 0.12%; S = 0.56 ± 0.20%.
The consensus site for p53 consists of a 10-bp element, 5′-PuPuPuC(A/T)(A/T)GPyPyPy-3′, and two copies, separated by 0 to 13 bp, are required for high-affinity binding. 22-24 C and G in position 4 and 7 are critical for DNA binding. 25 Aogen promoter contains 20 putative motifs for p53. Two copies of the 10-bp p53 motif, separated by an 11-nucleotide spacer, were selected. They correspond to the rat Aogen sequence from −76 to −46, located 15 bp 5′ from the TATA box. The first half site is perfect and the second has four mismatched nucleotides. C and G are in position 4 and 7: 5′-TCTGTACAGA-3′ and 5′-ATAGATCCAT-3′. Thus, a 36-bp oligonucleotide was radiolabeled and used in a gel shift assay. In comparison with nonstretched Adp53m-infected cells, p53 binding increased markedly with stretch (Figure 2A) ▶ . The p53-shifted complex was also stronger in stretched than in nonstretched noninfected myocytes.
Figure 2.
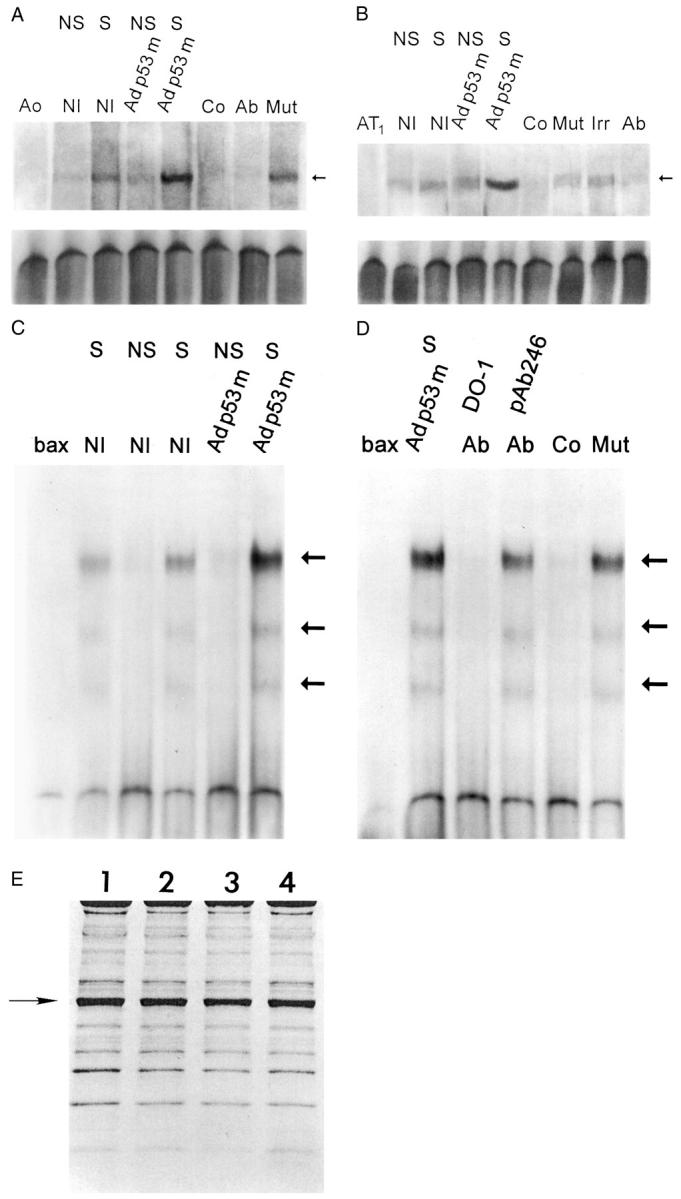
p53 binding to the promoter of Aogen (A), AT1 receptor (B), and bax (C and D). Nuclear extracts were obtained from NS and S noninfected (NI), and Adp53m-infected myocytes. Arrows in A, B, C, and D indicate the position of the p53-shifted bands. p53 bands were subjected to competition with excess of unlabeled self-oligonucleotides (Co) and with p53 antibodies (Ab); pAb 240 was used for Aogen and AT1 assays whereas DO-1 and pAb 246 were used for bax assay. Addition of an irrelevant antibody (Irr) or mutated form of Aogen, AT1, or bax (Mut) did not interfere with p53 binding. Ao, Aogen probe; AT1, AT1 probe; bax, bax probe (n = 3 in all cases). OD (A) in noninfected myocytes: NS = 1.50 ± 0.27; S = 3.76 ± 0.48; Adp53m-infected myocytes: NS = 2.02 ± 0.36; S = 10.3 ± 1.5. OD (B) in noninfected myocytes: NS = 2.15 ± 0.56; S = 4.00 ± 0.82; Adp53m-infected myocytes: NS = 4.23 ± 0.91; S = 19.9 ± 1.7. OD (C and D) in noninfected myocytes: NS = 0.28 ± 0.15; S = 3.3 ± 0.61; Adp53m-infected myocytes: NS = 0.32 ± 0.14; S = 11.59 ± 1.3. E: Pattern of proteins corresponding to nuclear preparations for mobility shift assay is shown by Coomassie blue. OD of the actin band (arrow) was significantly reduced and was consistent throughout, confirming that the level of cytoplasmic contamination was similar in the nuclear preparations: 1 = noninfected NS myocytes; 2 = noninfected S myocytes; 3 = Adp53m-infected NS myocytes; 4 = Adp53m-infected S myocytes.
The promoter of AT1 contains 24 putative motifs for p53. Two copies of the 10-bp element of the p53 site, separated by a 5-nucleotide spacer, were selected as a probe. They correspond to the rat AT1 sequence, from −197 to −173, located 140 bp 5′ from the TATA box. The first half-site has two mismatched nucleotides and the second three. In the first copy, C and G are in position 4 and 7: 5′-GAGCTTGGAT−3′ and 5′-AGGCGACACT-3′. Thus, a 31-bp oligonucleotide was labeled and used. In comparison with nonstretched Adp53m-infected myocytes, the p53 complex was enhanced after stretch (Figure 2B) ▶ . Similarly, p53 complex in stretched noninfected myocytes was slightly higher than in nonstretched myocytes.
The promoter of bax contains two copies of the 10-bp element of the p53 motif which are separated by a single nucleotide: 5′-TCACAAGTTA-3′ and 5′-AGACAAGCCT−3′. They correspond to the human bax sequence, from −484 bp to −465 bp, located 70 bp 5′ from the TATA box. The first half-site has three mismatched nucleotides and the second is perfect. Two additional sites for p53 are present in the 3′ region of the perfect p53 motif at 0 and 6 bp: 5′-GGGCGTGGGC−3′ and 5′-GGGCTATATT−3′. They have seven of 10 and eight of 10 matches with the consensus sequence of p53. 7 Thus, a 46-bp oligonucleotide was labeled and used.
p53 binding to the bax promoter was evaluated to confirm that Adp53m functions as a dominant-negative despite the fact that the 135 mutation was located in the DNA-binding domain. A similar behavior of p53 binding to the well-known sequences present in the bax promoter would support the observations with Aogen and AT1 receptor probes. Figure 2C ▶ documents that stretching of myocytes increased p53 binding to bax under control condition and after infection of myocytes with Adp53m. To confirm the specificity of the assay, nuclear extracts from Adp53m-infected myocytes were exposed to the anti-human p53 antibody DO-1 because the adenoviral vector carried a human mutated form of p53; this interaction opposed the appearance of a p53-shifted complex. Conversely, the addition of the anti-rat p53 antibody pAb 246 did not interfere with the p53 bands. The slight decrease in optical density of the bands most likely reflects the semiquantitative nature of this assay and not an actual difference in DNA binding. A similar phenomenon was noted when nuclear extracts were treated with an unlabeled mutated form of bax that left the p53 bands essentially intact (Figure 2D) ▶ . Nuclear proteins from noninfected myocytes were not exposed to DO-1 antibody, because DO-1 does not react with rat p53. Consistency in protein loading, lack of protein degradation, and uniformity in the relative purity of nuclear extracts are shown in Figure 2E ▶ .
Increased binding of mutated p53 to the Aogen, AT1, and bax promoter with stretch (Figure 2) ▶ suggests that translocation to the nucleus of this inactive p53 form may require phosphorylation, in a manner comparable to wild-type p53. This possibility is consistent with the higher fraction of myocyte nuclei labeled by mutated p53 after sarcomere elongation. These results, obtained by immunolabeling and confocal microscopy, are described below.
Stretch and p53
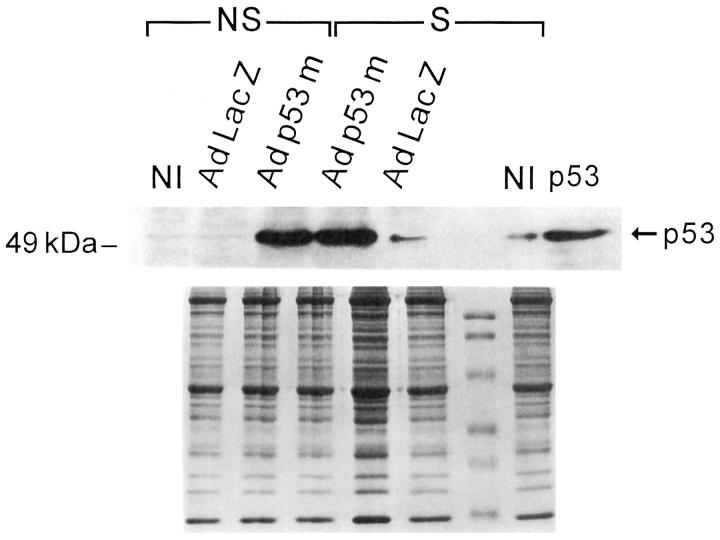
To establish whether translocation of wild-type p53 was impaired in myocytes overexpressing Adp53m, the effects of 12 hours of stretch on the nuclear localization of mutated and wild-type p53 was assessed by confocal microscopy in Adp53m-infected myocytes and compared with the distribution of wild-type p53 in AdLacZ-infected cells. Endogenous wild-type p53, detected by anti-rat p53 pAb 246 antibody, was seen in 1.3 ± 1.0% (n = 6) of nuclei of nonstretched myocytes infected with AdLacZ (Figure 3, A–C) ▶ and in 20 ± 7% (n = 6) of nuclei of the same cells after stretch (Figure 3, D–F) ▶ . This latter change was consistent with activation and translocation to the nucleus of functioning p53. Mutated p53 was not detectable in the nucleus of nonstretched Adp53m-infected myocytes by anti-human p53 DO-1 antibody (Figure 3, G–I) ▶ , but was observed with the same antibody in 83 ± 5% (n = 6) of nuclei of Adp53m-infected cells after stretch (Figure 3, J–L) ▶ . In Adp53m-infected myocytes, endogenous wild-type p53, identified by anti-rat p53 pAb 246 antibody, was found in 0.4 ± 0.2% (n = 6) of nuclei of nonstretched and in 5.4 ± 1.1% (n = 6) of nuclei of stretched cells. The presence of mutated p53 in the majority of myocyte nuclei after stretch suggests that activation of the mutated protein occurred with mechanical deformation. This contention was supported by the characteristics of DNA binding shown in Figure 2 ▶ . Therefore, it is possible that the mutated p53 may have acted as a dominant-negative by competing with wild-type p53 for the translocation to the myocyte nucleus, and, subsequently, at the level of the nucleus, for the binding to specific DNA sequences. Interaction between mutated p53 and target DNA did not result in transactivation of p53-regulated genes (see Figures 5, A and B, and 6 ▶ ▶ , A and B). Infection with Adp53m reduced by 75% (5% versus 20%) the translocation of endogenous wild-type p53 to the nucleus as a result of stretching of sarcomeres. The impact of 12 hours of stretch on p53 quantity was measured by Western blot in Adp53m-infected cells and in control noninfected and AdLacZ-infected myocytes. Stretch increased p53 protein 4.3-fold (P < 0.001) and 4.8-fold (P < 0.001) in noninfected and AdLacZ-infected myocytes (Figure 4) ▶ . In contrast, the high p53 level in nonstretched Adp53m-infected cells remained constant after stretch (+15%, NS). There was no apparent difference in the electrophoretic mobility of wild-type and mutated p53 proteins.
Figure 3.
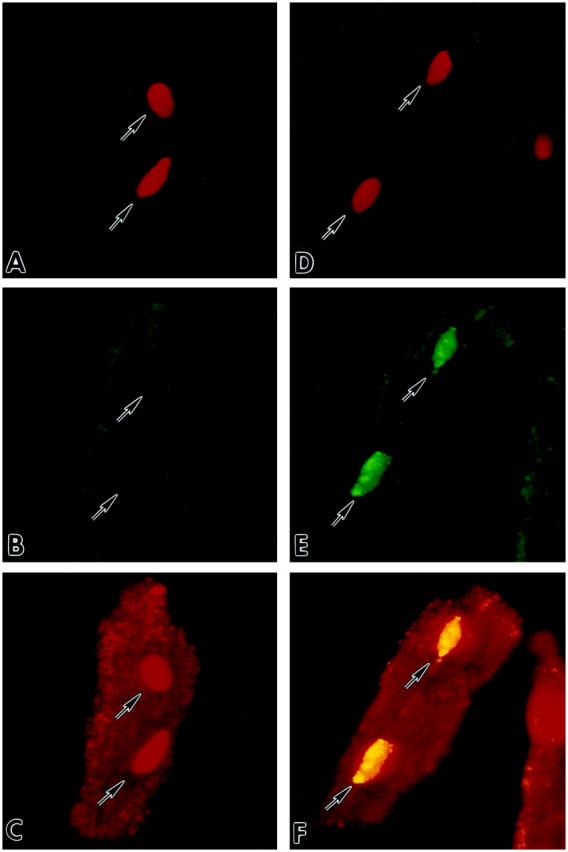
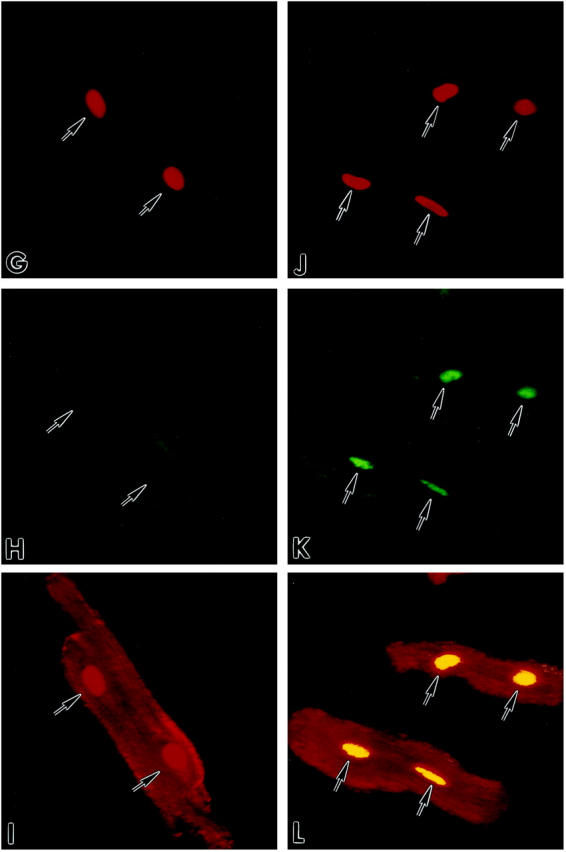
Detection by confocal microscopy of p53 in cultures of AdLacZ- (A–F) and Adp53m- (G–L) infected myocytes before (A–C; G–I) and after (D–F; J–L) stretch. Red fluorescence reflects PI staining of nuclei (A, D, G, J); green fluorescence in B and E corresponds to endogenous wild-type p53 which was detected with anti-rat p53 pAb 246 antibody; p53 labeling was present in the cytoplasm of B and E, and in the nuclei of E only. Green fluorescence in H and K corresponds to Adp53m which was detected with anti-human p53 DO-1 antibody; p53 labeling was present in the cytoplasm of H and K, and in the nuclei of K only. Laser power was set to 90% for images in B and E, and 10% for images in H and K. Red fluorescence depicts α-sarcomeric actin staining of the myocyte cytoplasm in C and F of AdLacZ-infected myocytes, and in I and L of Adp53m-infected myocytes. Red fluorescence of nuclei in C and I illustrates PI labeling alone, and yellow fluorescence of nuclei in F and L illustrates the combination of PI and p53 stainings. Arrows point to unlabeled and labeled nuclei. Confocal microscopy: A–F, ×800; G–L, ×600.
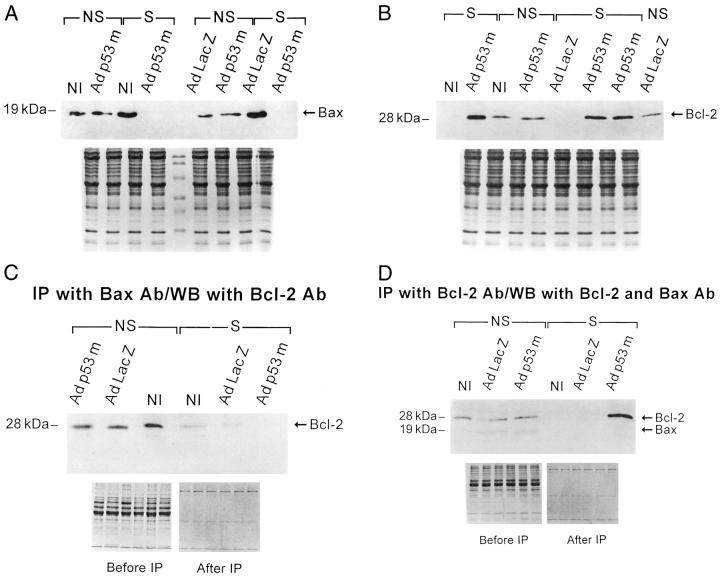
Figure 5.
Western blot of Bax (A) and Bcl-2 (B) in NS- and S-noninfected (NI), and AdLacZ- and Adp53m-infected myocytes (n = 6 in all cases). A: OD in noninfected myocytes: NS = 0.88 ± 0.12; S = 2.04 ± 0.21; AdLacZ-infected myocytes: NS = 0.82 ± 0.20; S = 2.22 ± 0.72; Adp53m-infected myocytes: NS = 0.93 ± 0.13; S = 0.03 ± 0.02. B: OD in noninfected myocytes: NS = 0.66 ± 0.16; S = 0.04 ± 0.02; AdLacZ-infected myocytes: NS = 0.71 ± 0.13; S = 0.03 ± 0.02; Adp53m-infected myocytes: NS = 0.64 ± 0.10; S = 1.25 ± 0.25. C: Immunoprecipitation (IP) with Bax antibody (Ab) and Western blot (WB) with Bcl-2 antibody of myocyte lysates obtained from NS- and S-noninfected (NI), and AdLacZ- and Adp53m-infected myocytes (n = 6 in all cases). OD of Bcl-2 bound to Bax in noninfected myocytes: NS = 5.2 ± 1.0; S = 1.1 ± 0.3; AdLacZ-infected myocytes: NS = 4.9 ± 1.2; S = 0.9 ± 0.2; Adp53m-infected myocytes: NS = 5.3 ± 1.5; S = 0.03 ± 0.02. D: IP with Bcl-2 Ab and WB with Bax and Bcl-2 Ab of myocyte lysates obtained from NS- and S-noninfected (NI), and AdLacZ- and Adp53m-infected myocytes (n = 6 in all cases). OD of the total Bcl-2 in noninfected myocytes: NS = 3.6 ± 0.7; S = 0.02 ± 0.02; AdLacZ-infected myocytes: NS = 3.3 ± 0.6; S = 0.02 ± 0.01; Adp53m-infected myocytes: NS = 3.9 ± 1.1; S = 11.5 ± 3.0. OD of Bax bound to Bcl-2 in noninfected myocytes: NS = 1.6 ± 0.4; S = 0.03 ± 0.01; AdLacZ-infected myocytes: NS = 1.8 ± 0.5; S = 0.02 ± 0.01; Adp53m-infected myocytes: NS = 1.5 ± 0.5; S = 0.02 ± 0.02.
Figure 6.
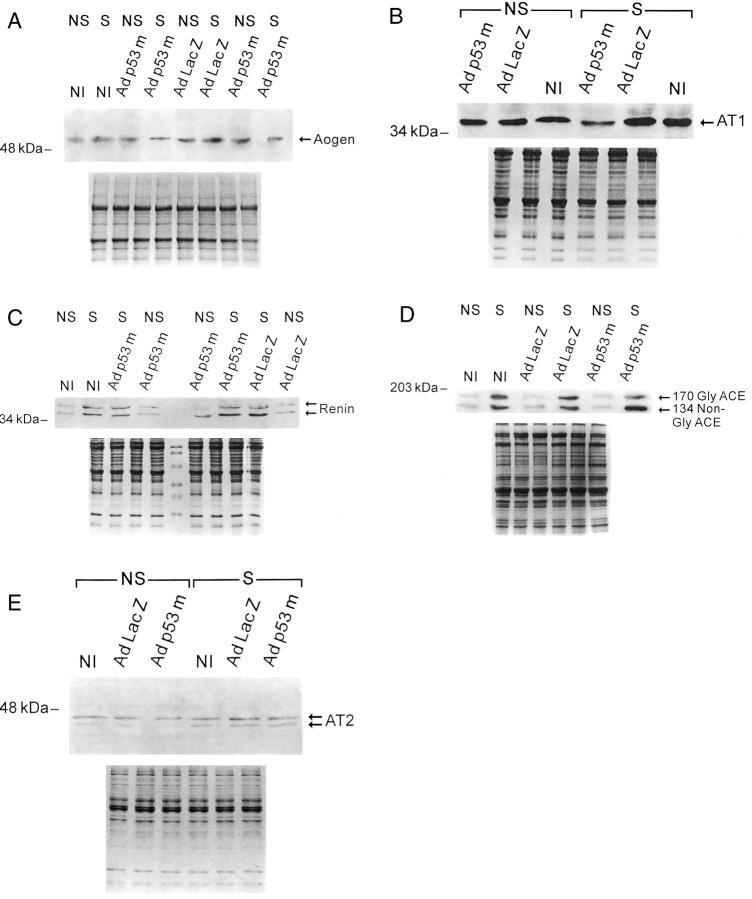
Western blot of Aogen (A), AT1 (B), renin (C), glycosylated (Gly) and nonglycosylated (non-Gly) ACE (D), and AT2 (E) in NS and S noninfected (NI), and AdLacZ- and Adp53m-infected myocytes (n = 6 in all cases). A: OD in noninfected myocytes: NS = 0.65 ± 0.14; S = 1.27 ± 0.17; AdLacZ-infected myocytes: NS = 0.62 ± 0.15; S = 1.29 ± 0.18; Adp53m-infected myocytes: NS = 0.69 ± 0.15; S = 0.38 ± 0.08. B: OD in noninfected myocytes: NS = 0.47 ± 0.10; S = 0.91 ± 0.14; AdLacZ-infected myocytes: NS = 0.52 ± 0.13; S = 1.14 ± 0.16; Adp53m-infected myocytes: NS = 0.35 ± 0.08; S = 0.14 ± 0.07. C: OD in noninfected myocytes: NS = 0.56 ± 0.13; S = 1.73 ± 0.36; AdLacZ-infected myocytes: NS = 0.63 ± 0.22; S = 2.02 ± 0.35; Adp53m-infected myocytes: NS = 0.66 ± 0.30; S = 2.18 ± 0.39. D: OD of Gly and non-Gly together in noninfected myocytes: NS = 0.60 ± 0.14; S = 5.41 ± 0.99; AdLacZ-infected myocytes: NS = 0.66 ± 0.20; S = 5.98 ± 0.64; Adp53m-infected myocytes: NS = 0.55 ± 0.20; S = 5.16 ± 1.45. E: OD in noninfected myocytes: NS = 0.78 ± 0.16; S = 1.36 ± 0.24; AdLacZ-infected myocytes: NS = 0.75 ± 0.25; S = 1.26 ± 0.23; Adp53m-infected myocytes: NS = 0.77 ± 0.28; S = 1.29 ± 0.32.
Figure 4.
Western blot of p53 in NS- and S-noninfected (NI), and AdLacZ- and Adp53m-infected myocytes (n = 6 in all cases). OD in noninfected myocytes: NS = 0.018 ± 0.008; S = 0.077 ± 0.026; AdLacZ-infected myocytes: NS = 0.020 ± 0.009; S = 0.096 ± 0.019; Adp53m-infected myocytes: NS = 3.95 ± 0.46; S = 4.54 ± 0.57. The p53 lane corresponds to p53-purified protein from Sf9 cells.
Stretch and p53-Dependent Genes, Bax, and Bcl-2
Bax and Bcl-2 were measured in nonstretched and stretched, noninfected and AdLacZ- and Adp53m-infected myocytes. Stretch for 12 hours increased Bax in noninfected and AdLacZ-infected myocytes 2.3-fold (P < 0.001) and 2.7-fold (P < 0.001), respectively (Figure 5A) ▶ . This difference was not significant, but a larger variability was noted in AdLacZ-infected myocytes. Infection with Adp53m not only prevented stretch-mediated up-regulation of Bax in myocytes, but also reduced its quantity by 97% (P < 0.001). Adp53m had an opposite effect on Bcl-2 (Figure 5B) ▶ . Bcl-2 increased 2.0-fold (P < 0.001) in stretched Adp53m-infected myocytes, whereas it decreased 94% (P < 0.001) and 96% (P < 0.001) in stretched noninfected and AdLacZ-infected myocytes. The changes in Bax and Bcl-2 in stretched Adp53m-infected myocytes were consistent with the localization of mutated p53 in 83% of nuclei. This may have resulted in inhibition of wild-type p53 function below baseline.
As stated, stretch essentially abolished the expression of Bcl-2 and more than doubled Bax quantity in control-noninfected and -infected myocytes. Conversely, stretch of Adp53m-infected myocytes resulted in opposite changes in the amount of Bcl-2 and Bax in the cells. To confirm these findings and evaluate the relationship between these two antiapoptotic and proapoptotic proteins, the interaction of Bax with Bcl-2 was examined by immunoprecipitation. Myocyte lysates were incubated with Bax antibody and immunoprecipitated proteins were then exposed to Bcl-2 antibody to obtain the fraction of Bcl-2 bound to Bax (Figure 5C) ▶ . The decrease of Bax in stretched Adp53m-infected myocytes (Figure 5A) ▶ resulted in an almost undetectable amount of Bcl-2 linked to Bax. The higher levels of Bax in stretched noninfected and AdLacZ-infected myocytes (Figure 5A) ▶ were coupled with minimal quantities of Bcl-2 associated protein, reflecting the down-regulation of Bcl-2 in these cells with stretch (Figure 5, A and B) ▶ . Moreover, total Bcl-2 and the fraction of Bax linked to Bcl-2 were identified by immunoprecipitation of myocyte lysates with Bcl-2 antibody and subsequent exposure of the blots to Bax and Bcl-2 antibodies (Figure 5D) ▶ . The large quantity of total Bcl-2 in stretched Adp53m-infected myocytes was not coupled with Bax because this latter protein almost disappeared in these cells after stretch. On the other hand, the lack of Bax associated with Bcl-2 in noninfected and AdLacZ-infected myocytes was because of severe reduction of Bcl-2 in these cells after stretch.
Stretch and Local RAS
Aogen and AT1 are p53-regulated genes. 2,9-11 Stretch increased Aogen by twofold (P < 0.001) in noninfected and AdLacZ-infected myocytes. However, Aogen decreased 45% (P < 0.01) in stretched Adp53m-infectedmyocytes (Figure 6A) ▶ . Similarly, AT1 increased 90% (P < 0.001) and 120% (P < 0.001) in stretched noninfected and AdLacZ-infected myocytes, but decreased 60% (P < 0.01) in stretched Adp53m-infected cells (Figure 6B) ▶ . Infection with Adp53m had no effect on the p53-independent genes, renin, ACE, and AT2 receptor in myocytes. Stretch increased renin 198% (P < 0.001), 220% (P < 0.001) and 232% (P < 0.001) in noninfected, and in AdLacZ- and Adp53m-infected myocytes (Figure 6C) ▶ . ACE increased a ninefold average (P < 0.001) with stretch in the three groups of myocytes (Figure 6D) ▶ . AT2 was also enhanced by stretch, 68 to 74% (P < 0.05-P < 0.005), in all cells (Figure 6E) ▶ .
Stretch and Ang II Formation
Ang II was measured by enzyme-linked immunosorbent assay in conditioned medium of nonstretched and stretched AdLacZ- and Adp53m-infected myocytes. Ang II increased 2.4-fold (P < 0.001) in stretched AdLacZ-infected myocytes, from a baseline value of 454 ± 93 pg/hour/10 6 cells (n = 5) to a value of 1,104 ± 115 pg/hour/10 6 cells (n = 5) at 12 hours after stretch. Conversely, the 10% increase noted with stretch in Adp53m-infected cells was not statistically significant: nonstretched, 493 ± 88 pg/hour/10 6 cells, n = 4; stretched, 540 ± 122 pg/hour/10 6 cells, n = 7; P = 0.98.
Stretch and Apoptosis
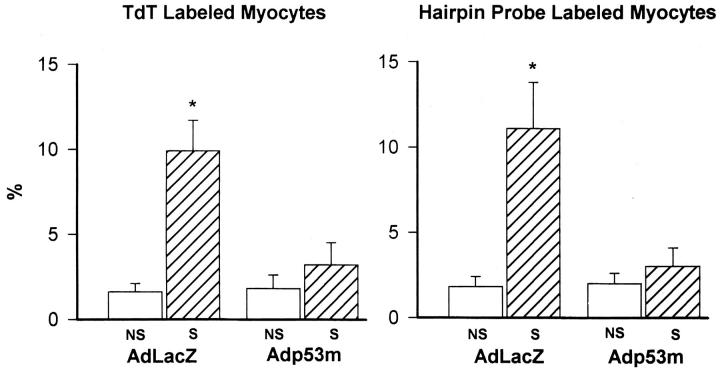
Myocyte apoptosis was measured by TdT assay 14 and in situ ligation of a hairpin probe with single-base 3′ overhang. 19 This latter method identifies DNA damage which is present only with apoptosis. 2,18,19,26 Labeled nuclei were assessed by confocal microscopy that allowed detection of structural features of apoptosis (Figure 7) ▶ . With both techniques, apoptosis increased an average 6.2-fold (P < 0.001) in stretched AdLacZ-infected myocytes (Figure 8) ▶ . However, the 63% increase in cell death in stretched Adp53m-infected myocytes was not significant (P = 0.67–0.61). Apoptosis in stretched Adp53m-infected myocytes was 70% (P < 0.001) lower than in stretched AdLacZ-infected myocytes.
Figure 7.
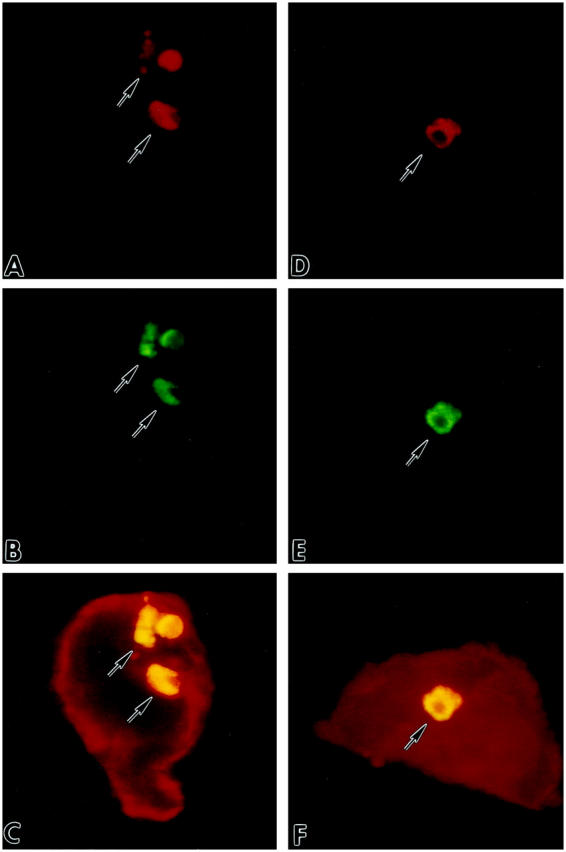
A and D illustrate nuclei by red fluorescence of PI; B and E show by green fluorescence TdT (B) and hairpin (E) labeling of apoptotic nuclei; myocyte cytoplasm is depicted by red fluorescence of α-sarcomeric actin staining in C and F. Yellow fluorescence represents the combination of PI and TdT (C), and PI and hairpin (F) labelings. Confocal microscopy, ×1,200.
Figure 8.
Effects of stretch for 12 hours on myocyte apoptosis. Results are presented as mean ± SD; n = 6 in all cases. *Indicates a significant difference, P < 0.05, from NS myocytes.
Discussion
We have previously reported correlative evidence for a functional association between myocyte stretch and p53 activation, Ang II production, and apoptosis. 2,9-11 In the current study, we present definitive proof that p53 is an integral component of this pathway and an essential co-factor in the stimulation of pro-apoptotic genes and induction of cell death by stretch. Overexpression of a dominant-negative p53 mutant in adult ventricular myocytes specifically inhibited stretch-mediated up-regulation of Aogen, AT1 receptor, and Bax. Other genes, including renin, ACE, and AT2 receptor, that lack p53 regulatory sites, were activated by stretch in the presence of mutated p53. These results show that Adp53m interfered selectively with p53-dependent gene expression, blocking the functions of endogenous wild-type p53, reducing Ang II generation and myocyte apoptosis after mechanical deformation.
Adp53m and p53 Transcriptional Activity
The p53 mutant used in this study lacks transcriptional activation function because of the cysteine to serine substitution at amino acid position 135. 15,16,27 DNA binding to p53 consensus sites is retained, and the mutated p53 acts as a dominant-negative by competing with the wild-type protein for binding to target genes. The p53 motif consists of 10 nucleotides 22 and high-affinity binding requires two copies of this sequence. 23,25 They were previously found in the promoter of bax, 7 and here in the promoter of Aogen and AT1 receptor genes. Sarcomere elongation in noninfected myocytes was characterized by enhanced p53 binding to the promoter of Aogen, AT1 receptor, and bax that resulted in equivalent increases in the expression of Aogen, AT1 receptor, and Bax proteins. In contrast, opposite findings were obtained in stretched Adp53m-infected myocytes; increases in p53-shifted complexes were not coupled with corresponding accumulations of Aogen, AT1 receptor, and Bax proteins in the cells.
The system used, which depressed p53 function after sarcomere elongation, 2,9 inhibited stretch-induced up-regulation of Bax and down-regulation of Bcl-2. Moreover, expression of Aogen and AT1 receptor was markedly attenuated after stretch, documenting that p53 plays a relevant role in the modulation of these two components of the cellular RAS. Decreased availability of Aogen may reduce Ang II production. 28 Additionally, ligand binding to AT1 receptors is critical for initiation of myocyte growth 29,30 and apoptosis, 2,10,11 and reduction in Ang II binding sites may affect cell hypertrophy and cell death. Ang II formation, myocyte apoptosis, and reactive myocyte growth are enhanced in pathological states. 30-32 Lack of interference with p53 transactivation 2,9 and potentiation of p53 activity 11 increase Ang II-mediated myocyte death 2,9,11 and possibly myocyte growth.
Observations indicate that mechanical deformation is necessary for p53 translocation to the nucleus and activation of p53-dependent genes and local RAS in myocytes. However, we have previously shown that infection with an adenoviral vector containing wild-type human p53 led to similar responses in myocytes in the absence of sarcomere stretching. 11 These results suggest that high level of expression of wild-type human p53 by viral infection was characterized by translocation and activation of exogenous p53 at the level of the nucleus, independently from the application of physical forces. Such a notion is consistent with enhanced p53 DNA binding to the promoter of bax, Aogen, and AT1 receptor, and increased transcription of Aogen and AT1 receptor mRNAs. 11 Additionally, up-regulation of p53 binding activity was accompanied by a supershift for bax, Aogen, and AT1 when the anti-human p53 DO-1 antibody was used. 11 This excludes activation of endogenous p53 in myocytes as a secondary event promoted by infection of wild-type human p53. However, these findings do not prove unequivocally that p53 translocation to the nucleus is required for the induction of apoptotic cell death.
Adp53m and Myocyte RAS
Myocytes possess the various components of RAS 29,33,34 and generate Ang II. 2,9-11,29 Conditions of overload in vivo up-regulate this local system 34 and Ang II blockade improves the outcome of ventricular dysfunction and failure of ischemic and nonischemic origin. 31,32,35-37 We have identified previously that Aogen and AT1 belong to the group of p53-inducible genes. 9-11 However, myocyte stretching was used and this in vitro manipulation activates multiple factors 38,39 that could have stimulated RAS independently from p53. Conversely, overexpression of p53 in myocytes, in the absence of stretch, typically showed enhanced p53 binding to the promoter of Aogen and AT1 receptor, increased expression of these two genes, and synthesis and secretion of Ang II. 11 The current investigation strengthens these findings by documenting two additional points: 1) inhibition of p53 function after stretch, by previous infection of myocytes with Adp53m, decreased below baseline the expression of Aogen and AT1 receptor; and 2) down-regulation of Aogen prevented the increase in Ang II generation in mechanically loaded myocytes, despite an increase in renin and ACE proteins. Aogen played a key role in the synthesis of the peptide; availability of Aogen as a substrate catalyzed by renin seemed to be the limiting factor in Ang II formation.
Stretching increased the quantity of Aogen, renin, ACE, and AT1 and AT2 receptors in noninfected and AdLacZ-infected myocytes, and this adaptation of the entire RAS was associated with a nearly 2.5-fold increase in the generation of Ang II. Similar increases in renin, ACE, and AT2 receptor were noted in stretched Adp53m-infected myocytes, demonstrating that p53 dominant-negative had no influence on p53-independent genes. Enhanced expression of renin and ACE in stretched Adp53m-infected myocytes may have compensated for the attenuation of Aogen, contributing to maintain cellular Ang II to levels comparable to those in nonstretched AdLacZ-infected myocytes. Additionally, this may suggest a greater conversion of Aogen in Ang I. The reduction in AT1 receptors below controls in stretched cells expressing dominant-negative p53 may be accounted for by impaired receptor cycling mediated by p53 with time.
AT2 receptors have not been previously identified in adult myocytes. AT2 receptors are numerous in neonatal cardiac myocytes, but their number decreases rapidly postnatally and adult ventricular myocytes have been claimed to possess exclusively AT1. 40,41 The possibility was raised that AT2 is re-expressed in the overloaded heart in both myocytes 42 and fibroblasts, 43 but the myocyte localization remained unclear. In this study, AT2 was detected in nonstretched myocytes and its expression increased with the mechanical stimulus. The functional significance of AT2 receptor in differentiated myocytes has not been defined. They oppose the growth-promoting effect of AT1 receptors in neonatal myocytes, 44 but a similar response was not observed in adult myocytes. 30 In aortic smooth muscle cells, AT2 receptors inhibit cell growth and trigger apoptosis, by interfering with the AT1 effector pathway. 45,46
Adp53m and Myocyte Apoptosis
The proapoptotic effects of p53 have been shown in numerous systems, 47-49 including myocyte stretching, 2 hypoxia of transformed cells, 50 and DNA damage. 51 p53 up-regulates Bax and down-regulates Bcl-2, increasing the susceptibility of cells to undergo apoptosis. 7 However, changes in Bax and/or Bcl-2 alone are not sufficient to trigger cell death. 7 Overexpression of wild-type p53 in myocytes increases Bax and decreases Bcl-2, but cell death is conditioned by the synthesis and secretion of Ang II. 11 Interference with ligand binding to surface AT1 receptors abrogates myocyte apoptosis. 2,9,11 Infection of myocytes with p53 dominant-negative impacts at least three steps in the stretch-induced apoptotic pathway: first, it increases the Bcl-2:Bax protein ratio; second, it inhibits the formation of Ang II; and third, it decreases surface AT1 receptor. Several pathways of apoptosis have also been described that are not regulated by p53. 48,52 The activity of p53 is highly dose-, cell type-, and cell context-dependent: p53 overexpression does not by itself induce apoptosis in vascular smooth muscle cells 53,54 ; overexpression of p53-dependent p21 protects skeletal muscle from apoptosis 55 ; and activation of p53 by γ-radiation may induce apoptosis or cell cycle withdrawal. 51
Infection of stretched myocytes with Adp53m reduced the formation of Ang II and AT1 receptor, both responsible for the initiation of events resulting in DNA fragmentation; DNA damage was characterized by staggered ends with single-base 3′ overhang. 19,26 DNase I activation is critically influenced by elevated intracellular Ca2+, proposed here to be mediated by AT1 receptor stimulation and downstream translocation of protein kinase C ε and δ. 56 Contrary to what has been found in smooth muscle cells, 57 AT2 plays no role in this model of myocyte apoptosis. The death promoting effects of Ang II on myocytes are inhibited by AT1 blockers in vitro 2,11,56 and in vivo. 31,32
Footnotes
Address reprint requests to Annarose Leri, M.D., Department of Medicine, Vosburgh Pavilion-Room 302, New York Medical College, Valhalla, NY 10595. E-mail: annarose_leri@nymc.edu.
Supported by National Institutes of Health Grants HL-38132, HL-39902, HL-43023, and AG-15756.
References
- 1.Long X, Boluyt MO, Hipolito ML, Lundberg MS, Zheng JS, O’Neill L, Cirielli C, Lakatta EG, Crow MT: p53 and the hypoxia-induced apoptosis of cultured neonatal rat cardiac myocytes. J Clin Invest 1997, 99:2635-2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leri A, Claudio PP, Li Q, Wang X, Reiss K, Wang S, Malhotra A, Kajstura J, Anversa P: Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin-angiotensin system and decreases the Bcl-2-to-Bax protein ratio in the cell. J Clin Invest 1998, 101:1326-1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long X, Crow MT, Sollott SJ, O’Neill L, Menees DS, de Lourdes Hipolito M, Boluyt MO, Asai T, Lakatta EG: Enhanced expression of p53 and apoptosis induced by blockade of the vacuolar proton ATPase in cardiomyocytes. J Clin Invest 1998, 101:1453-1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leri A, Li Y, Malhotra A, Li Q, Stiegler P, Claudio PP, Giordano A, Kajstura J, Hintze TH, Anversa P: Pacing-induced heart failure in dogs enhances the expression of p53 and p53-dependent genes in ventricular myocytes. Circulation 1998, 97:194-203 [DOI] [PubMed] [Google Scholar]
- 5.Agah R, Kirshenbaum LA, Abdellatif M, Truong LD, Chakraborty S, Michael LH, Schneider MD: Adenoviral delivery of E2F-1 directs cell cycle reentry and p53-independent apoptosis in postmitotic adult myocardium in vivo. J Clin Invest 1997, 100:2722-2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karwatowska-Prokopczuk E, Nordberg JA, Li HL, Engler RL, Gottlieb RA: Effect of vacuolar proton ATPase on pHi, Ca2+, and apoptosis in neonatal cardiomyocytes during metabolic inhibition/recovery. Circ Res 1998, 82:1139-1144 [DOI] [PubMed] [Google Scholar]
- 7.Miyashita T, Reed J: Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995, 80:293-299 [DOI] [PubMed] [Google Scholar]
- 8.Miyashita T, Harigai M, Hanada M, Reed JC: Identification of a p53-dependent negative response element in the bcl-2 gene. Cancer Res 1994, 54:3131-3135 [PubMed] [Google Scholar]
- 9.Leri A, Liu Y, Claudio PP, Kajstura J, Wang X, Wang S, Kang P, Malhotra A, Anversa P: Insulin-like growth factor-1 induces Mdm2 and down-regulates p53, attenuating the myocyte renin-angiotensin system and stretch-mediated apoptosis. Am J Pathol 1999, 154:567-580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leri A, Liu Y, Wang X, Kajstura J, Malhotra A, Meggs LG, Anversa P: Overexpression of insulin-like growth factor-1 attenuates the myocyte renin-angiotensin system in transgenic mice. Circ Res 1999, 84:752-762 [DOI] [PubMed] [Google Scholar]
- 11.Pierzchalski P, Reiss K, Cheng W, Cirielli C, Kajstura J, Nitahara JA, Rizk M, Capogrossi MC, Anversa P: p53 induces myocyte apoptosis via the activation of the renin-angiotensin system. Exp Cell Res 1997, 234:57-65 [DOI] [PubMed] [Google Scholar]
- 12.Pfeffer MA, Braunwald E: Ventricular remodeling after myocardial infarction. Circulation 1990, 81:1161-1172 [DOI] [PubMed] [Google Scholar]
- 13.Cheng W, Li B, Kajstura J, Li P, Wolin MS, Sonnenblick EH, Hintze TH, Olivetti G, Anversa P: Stretch induced programmed myocyte cell death. J Clin Invest, 1995, 96:2247–2259 [DOI] [PMC free article] [PubMed]
- 14.Cheng W, Kajstura J, Nitahara JA, Li B, Reiss K, Liu Y, Clark WA, Krajewski S, Reed JC, Olivetti G, Anversa P: Programmed myocyte cell death affects the viable myocardium after infarction in rats. Exp Cell Res 1996, 226:316-327 [DOI] [PubMed] [Google Scholar]
- 15.Bacchetti S, Graham FL: Inhibition of cell proliferation by an adenovirus vector expressing the human wild type p53 protein. Int J Oncol 1993, 3:781-788 [DOI] [PubMed] [Google Scholar]
- 16.Webster KA, Discher DJ, Kaiser S, Hernandez O, Sato B, Bishopric NH: Hypoxia-activated apoptosis of cardiac myocytes requires reoxygenation or a pH shift and is independent of p53. J Clin Invest 1999, 104:239-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riedy MC, Muirhead KA, Jensen CP, Stewart CC: Use of a photolabeling technique to identify nonviable cells in fixed homologous or heterologous cell populations. Cytometry 1991, 12:133-139 [DOI] [PubMed] [Google Scholar]
- 18.Li B, Setoguchi M, Wang X, Andreoli AM, Leri A, Malhotra A, Kajstura J, Anversa P: Insulin-like growth factor-1 attenuates the detrimental impact of nonocclusive coronary artery constriction on the heart. Circ Res 1999, 84:1007-1019 [DOI] [PubMed] [Google Scholar]
- 19.Didenko VV, Tunstead JR, Hornsby PJ: Biotin-labeled hairpin oligonucleotides: probes to detect double-strand breaks in DNA in apoptotic cells. Am J Pathol 1998, 152:897-902 [PMC free article] [PubMed] [Google Scholar]
- 20.Wallenstein S, Zucker CL, Fleiss JL: Some statistical methods useful in circulation research. Circ Res 1980, 47:1-9 [DOI] [PubMed] [Google Scholar]
- 21.Donahue JK, Kikkawa K, Johns DC, Marban E, Lawrence JH: Ultrarapid, highly efficient viral gene transfer to the heart. Proc Natl Acad Sci USA 1997, 94:4664-4668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Deiry WS, Kern SE, Pietenpol JS, Kinzler KW, Vogelstein B: Definition of a consensus binding site for p53. Nat Genet 1992, 1:45-49 [DOI] [PubMed] [Google Scholar]
- 23.Zauberman A, Lupo A, Oren M: Identification of p53 target genes through immune selection of genomic DNA: the cyclin G gene contains two distinct p53 binding sites. Oncogene 1995, 10:2361-2366 [PubMed] [Google Scholar]
- 24.Schäfer H, Trauzold A, Sebens T, Deppert W, Folsch UR, Schmidt WE: The proliferation-associated early response gene p22/PRG1 is a novel p53 target gene. Oncogene 1998, 16:2479-2487 [DOI] [PubMed] [Google Scholar]
- 25.Seol DW, Chen Q, Smith ML, Zarnegar R: Regulation of the c-met proto-oncogene promoter by p53. J Biol Chem 1999, 274:3565-3572 [DOI] [PubMed] [Google Scholar]
- 26.Didenko W, Hornsby PJ: Presence of double-strand breaks with single-base 3′ overhangs in cells undergoing apoptosis but not necrosis. J Cell Biol 1996, 135:1369-1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buchman VL, Chumakov PM, Ninkina NN, Samarina OP, Georgiev GP: A variation in the structure of the protein-coding region of the human p53 gene. Gene 1988, 70:245-252 [DOI] [PubMed] [Google Scholar]
- 28.Baker KM, Booz GW, Dostal DE: Cardiac actions of angiotensin II: role of an intracardiac renin-angiotensin system. Annu Rev Physiol 1992, 54:227-241 [DOI] [PubMed] [Google Scholar]
- 29.Sadoshima J, Xu J, Slayter HS, Izumo S: Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell 1993, 75:977-984 [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Leri A, Li B, Wang X, Cheng W, Kajstura J, Anversa P: Angiotensin II stimulation in vitro induces hypertrophy of normal and postinfarcted ventricular myocytes. Circ Res 1998, 82:1145-1159 [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Bing OHL, Long X, Robinson KG, Lakatta EG: Increased cardiomyocyte apoptosis during the transition of heart failure in the spontaneously hypertensive rat. Am J Physiol 1997, 272:H2313-H2319 [DOI] [PubMed] [Google Scholar]
- 32.Tanimura M, Sharov VG, Shimoyama H, Mishima T, Levine TB, Goldstein S, Sabbah HN: Effects of AT1-receptor blockade on progression of left ventricular dysfunction in dogs with heart failure. Am J Physiol 1999, 276:H1385-H1392 [DOI] [PubMed] [Google Scholar]
- 33.Dostal DE, Rothblum KN, Chernin MI, Cooper GR, Baker KM: Intra-cardiac detection of angiotensinogen and renin: a localized renin-angiotensin system in neonatal rat heart. Am J Physiol 1992, 263:C838-C850 [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Dostal DE, Reiss K, Cheng W, Kajstura J, Li P, Huang H, Sonnenblick EH, Meggs LG, Baker KM, Anversa P: Identification and activation of autocrine renin-angiotensin system in adult ventricular myocytes. Am J Physiol 1995, 269:H1791-H1802 [DOI] [PubMed] [Google Scholar]
- 35.Goussev A, Sharov VG, Shimoyama H, Tanimura M, Lesch M, Goldstein S, Sabbah HN: Effects of ACE inhibition on cardiomyocyte apoptosis in dogs with heart failure. Am J Physiol 1998, 275:H626-H631 [DOI] [PubMed] [Google Scholar]
- 36.SOLVD Investigators: Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 1992, 327:685–691 [DOI] [PubMed]
- 37.CONSENSUS Trial Study Group: Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Study Group. N Engl J Med 1987, 316:1429–1435 [DOI] [PubMed]
- 38.Sadoshima J, Takahashi T, Jahn L, Izumo S: Roles of mechano-sensitive ion channels, cytoskeleton, and contractile activity in stretch-induced immediate-early gene expression and hypertrophy of cardiac myocytes. Proc Natl Acad Sci USA 1992, 89:9905-9909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sadoshima J, Izumo S: Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J 1993, 12:1681-1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsubara H, Inada M: Molecular insights into angiotensin II type 1 and type 2 receptors: expression, signaling and physiological function and clinical application of its antagonists. Endocr J 1998, 45:137-150 [DOI] [PubMed] [Google Scholar]
- 41.Meggs LG, Coupet J, Huang H, Cheng W, Li P, Capasso JM, Homcy CJ, Anversa P: Regulation of angiotensin II receptors on ventricular myocytes after myocardial infarction in rats. Circ Res 1993, 72:1149-1162 [DOI] [PubMed] [Google Scholar]
- 42.Lopez JJ, Lorell BH, Ingelfinger JR, Weinberg EO, Schunkert H, Diamant D, Tang SS: Distribution and function of cardiac angiotensin AT1- and AT2- receptor subtypes in hypertrophied rat hearts. Am J Physiol 1994, 267:H844-H852 [DOI] [PubMed] [Google Scholar]
- 43.Tsutsumi Y, Matsubara H, Okhubo N, Mori Y, Nozawa Y, Murasawa S, Kijima K, Maruyama K, Masaki H, Moriguchi Y, Shibasaki Y, Kamihata H, Inada M, Iwasaka T: Angiotensin II type 2 receptor is upregulated in human heart with interstitial fibrosis, and cardiac fibroblasts are the major cell type for its expression. Circ Res 1998, 83:1035-1046 [DOI] [PubMed] [Google Scholar]
- 44.Booz GW, Baker KM: Role of type 1 and type 2 angiotensin receptors in angiotensin II-induced cardiomyocyte hypertrophy. Hypertension 1996, 28:635-640 [DOI] [PubMed] [Google Scholar]
- 45.Horiuchi M, Hayashida W, Akishita M, Tamura K, Daviet L, Lethonen JY, Dzau VJ: Stimulation of different subtypes of angiotensin II receptors, AT1 and AT2 receptors, regulates STAT activation by negative crosstalk. Circ Res 1999, 84:876-882 [DOI] [PubMed] [Google Scholar]
- 46.Horiuchi M, Akisita M, Dzau VJ: Recent progress in angiotensin type 2 receptor research in the cardiovascular system. Hypertension 1999, 33:613-621 [DOI] [PubMed] [Google Scholar]
- 47.Ko LJ, Prives C: p53: puzzle and paradigm. Genes Dev 1996, 10:1054-1072 [DOI] [PubMed] [Google Scholar]
- 48.Bellamy COC: p53 and apoptosis. Br Med Bull 1997, 53:522-538 [DOI] [PubMed] [Google Scholar]
- 49.Canman CE, Kastan MB: Role of p53 in apoptosis. Adv Pharmacol 1997, 41:429-460 [DOI] [PubMed] [Google Scholar]
- 50.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ: Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumors. Nature 1996, 379:88-91 [DOI] [PubMed] [Google Scholar]
- 51.Agarwal ML, Taylor WR, Chernov MV, Chernova OB, Stark GR: The p53 network. J Biol Chem 1998, 273:1-4 [DOI] [PubMed] [Google Scholar]
- 52.Steller H: Mechanisms and genes of cellular suicide. Science 1995, 267:1445-1449 [DOI] [PubMed] [Google Scholar]
- 53.Bennet MR, Evan GI, Schwartz SM: Apoptosis of rat vascular smooth muscle cells is regulated by p53-dependent and -independent pathways. Circ Res 1995, 77:266-273 [DOI] [PubMed] [Google Scholar]
- 54.Bennet MR, Littlewood TD, Schwartz SM, Weissberg PL: Increased sensitivity of human vascular smooth muscle cells from atherosclerotic plaques to p53-mediated apoptosis. Circ Res 1997, 81:591-599 [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Guo K, Wills KN, Walsh K: Rb functions to inhibit apoptosis during myocyte differentiation. Cancer Res 1997, 57:351-354 [PubMed] [Google Scholar]
- 56.Kajstura J, Cigola E, Malhotra A, Li P, Cheng W, Meggs LG, Anversa P: Angiotensin II induces apoptosis of adult ventricular myocytes in vitro. J Mol Cell Cardiol 1997, 29:859-870 [DOI] [PubMed] [Google Scholar]
- 57.Horiuchi M, Hayashida W, Kambe T, Yamada T, Dzau VJ: Angiotensin type 2 receptors dephosphorylates Bcl-2 by activating mitogen-activated protein kinase phosphatase-1 and induces apoptosis. J Biol Chem 1997, 272:19022-19026 [DOI] [PubMed] [Google Scholar]