Abstract
β-catenin is a protein involved in cell-cell adhesion and proliferation. In neoplastic diseases, defects in the regulation of the cellular β-catenin content and cytoplasmic accumulation of the protein contribute to the uncontrolled cell proliferation and migration. Whether β-catenin plays a role in the controlled proliferative and migratory responses to injury, eg, of vascular endothelial cells during neovascularization after myocardial infarction (MI), is not known. In the present study, we examined the localization of β-catenin in the infarcted rat heart at different time points after MI. Cytoplasmic β-catenin was observed in the endothelial cells of the newly formed and pre-existing blood vessels in the infarct area in the first week after MI, but not in the uninjured parts of the heart and not at later time points. Adenomatous polyposis coli (APC) protein was also detected; interaction of APC with β-catenin has been reported to be critical in epithelial tube formation in vitro. Moreover, the expression of dishevelled-1, an upstream regulatory molecule of the cellular β-catenin content, was observed in vascular endothelial cells in the infarct area. These findings suggest a role for the β-catenin-APC complex in the proliferation and migration of vascular endothelial cells during neovascularization of the infarct area.
β-catenin is an intracellular protein which can interact with different cellular targets. It was originally described as a component of adherens junctions. Recently, however, it has also been identified as an activator of gene expression. In cell adhesion, it functions in a complex of proteins that links cadherins, a family of transmembrane cell-cell adhesion receptors, to the actin cytoskeleton. 1-3 Modulation of gene expression by β-catenin is mediated through its interaction with transcription factors of the T-cell factor/lymphoid enhancer factor (TCF/LEF) family in the nucleus. 4-7
β-catenin has recently received considerable attention in cancer research. 8,9 Elevated levels of β-catenin and a translocation of this protein to the cytoplasm have been associated with the development of colon carcinoma as well as a rapidly increasing number of other neoplastic diseases. 4,6,10 These elevated levels of β-catenin can be caused by mutations in the protein itself, but are more frequently the result of mutations in the adenomatous polyposis coli (APC) protein which regulates the degradation of β-catenin. 11 Mice lacking the β-catenin gene show lethal organizational defects early during development, 12 underscoring the crucial role for this protein in embryogenesis. This is further supported by studies which report a role for β-catenin during normal development. 13-15
Taken together, the above-mentioned studies clearly define a role for β-catenin in the proliferative and migratory responses of cells during embryogenesis and in neoplastic disease. In contrast, little is known about a potential role for β-catenin in normal, controlled cell proliferation and migration during repair processes after injury, eg, of vascular endothelial cells during neovascularization of the infarct area after myocardial infarction (MI) in adult animals. 16,17 In vitro studies, however, have identified the cadherin-catenin complex as a crucial component of endothelial cell-cell junctions, which has to be dissociated and reorganized during angiogenesis. 18 In several reports, a decrease of membrane-bound and a concomitant increase in cytoplasmic β-catenin during the migration of human umbilical vein endothelial cells 19,20 and epithelial cells 21 has been described, underscoring the importance of the dissociation of the cadherin-catenin complex during angiogenesis.
The aim of the present study was to investigate the potential role of β-catenin in the neovascularization which occurs in vivo during infarct healing after MI. To this end, infarcted rat hearts were obtained at different time points (2 to 21 days) after MI and the β-catenin contents of the vascular endothelial cells around the infarct area and in the uninjured myocardium were compared. Because β-catenin-APC interactions have been shown to be critical for epithelial tubule formation in vitro, 22 we have performed immunohistochemical staining for APC as well. Moreover, β-catenin recently has been identified as a second messenger molecule in the Wnt polarity signal transduction pathway. 15,23 In this pathway, genes from the dishevelled family are known to regulate the degradation of β-catenin by controlling its phosphorylation by glycogen synthase kinase-3β. 5 Therefore we have studied the expression of the three murine dishevelled genes 24-26 in the infarcted rat heart by in situ hybridization.
Materials and Methods
Animals and Surgery
Adult male Wistar rats (250 to 300 g; Winkelmann, Borchen, Germany) were used in this study. They were housed in groups of 3 to 4 rats with free access to food and tap water. MI was induced as described previously. 27
In Situ Hybridization for Dishevelled-Homologues
The expression of the three dishevelled genes was studied by in situ hybridization. These experiments were performed on paraffin sections (4 μm) from formalin-fixed infarcted rat hearts. The in situ hybridizations were performed as described previously. 28 Briefly, radiolabeled riboprobes were transcribed from polymerase chain reaction products of dvl-1, dvl-2, and dvl-3, obtained by amplification of reverse-transcribed RNA isolated from rat heart as previously described 29 (primer sets: dvl-1: upper CAGGGCACTGACAGCCAC, lower CAGTAGATGCACTGTCTGGAGG; dvl-2: upper AAGAGCGTTTTGCAGCGG, lower GACACAAGCCAGGAGACAAC; dvl-3: upper CCCCTTTCTGTGCTGACAAC, lower GCTCAATCCGGGAGACCTT), and cloned into a pGEM-T cloning vector (Promega, Madison, WI). The identity and orientation of the polymerase chain reaction products were verified by sequencing. RNA transcription was performed using an RNA labeling kit (Amersham, Little Chalfond, UK) in the presence of [35S]-UTP. The sections were hybridized overnight at 55°C with the radiolabeled probes. After washing the sections, unbound probe was digested with RNase (20 μg/ml; Promega), the sections were dehydrated, dried, dipped in photographic emulsion (Kodak NTB2; Technorama, Zürich, Switzerland) and exposed for 1 to 2 weeks in the dark at 4°C. After development, the sections were briefly stained with hematoxylin.
Immunohistochemistry
Immunohistochemistry was performed according to routine procedures. Paraffin sections were mounted on aminopropyltriethoxysilane-coated slides. A monoclonal antibody for β-catenin was obtained from Transduction Labs (Lexington, KY). After blocking the endogenous peroxidase, sections were boiled twice for 5 minutes in 10 mmol/L citrate buffer (pH 6.0) and incubated with the primary antibody in a 1:500 dilution overnight at room temperature. Immunohistochemistry for APC was performed using the N-15 monoclonal antibody obtained from Santa Cruz Biotechnology (Santa Cruz, CA) in a 1:2,000 dilution and incubating for 1 hour at room temperature. Sections were pretreated with 1 mg/ml of pepsin (Boehringer, Mannheim, Germany). Biotinylated multilink swine-anti-goat, -mouse, and -rabbit secondary antibody (dilution 1:100; DAKO, Glostrup, Denmark) and the Vectastain ABC kit (Vector, Burlingame CA) were used according to the manufacturers’ instructions to visualize the binding of both primary antibodies. Sections were briefly counterstained with hematoxylin and mounted with Entellan (Merck, Darmstad, Germany). The identity of vascular smooth muscle cells and myofibroblasts was determined using an antibody against α-smooth muscle actin (dilution 1:500; DAKO).
Confocal Microscopy
Confocal microscopy was performed using a Bio-Rad MRC600 confocal scanning laser microscope (Bio-Rad, Hempel Hemstead, UK) as previously described. 30 Immunohistochemistry for β-catenin was performed as described above with fluorescein isothiocyanate-labeled rat-anti-mouse (dilution 1:100; DAKO) as secondary antibody. The nuclei were counterstained using propidium iodide.
BrdU Incorporation
BrdU (Serva, Heidelberg, Germany) was continuously infused using osmotic minipumps (Alzet model 2002; Alza Corp., Palo Alto, CA) implanted subcutaneously between the shoulder blades. BrdU was dissolved in 0.9% NaCl (10 mg/ml) and infused at a rate of 240 ng/kg/min. The pumps were implanted at the time of MI induction and animals were sacrificed 4 and 7 days after MI. The cumulative labeling of BrdU in the infarct area was determined using a monoclonal antibody directed against BrdU according to previously published methods. 31
Results
Time Course of the β-Catenin Expression in Newly Formed Vessels around the Infarct Area
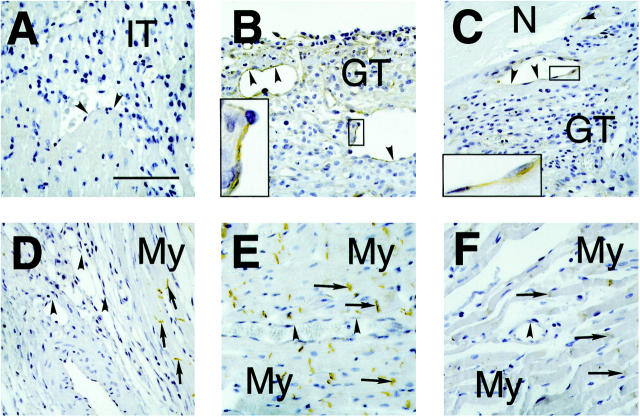
The expression of β-catenin in the infarcted part of the left ventricle of the rat at different time points after MI is shown in Figure 1 ▶ . Two days after infarction, no staining for β-catenin was observed around the area of infarction (Figure 1A) ▶ . In this phase of the wound healing no neovascularization or formation of granulation tissue has yet occurred in the infarct area. At 4 days after MI, however, many small blood vessels have emerged in the border zone around the area of infarction. Interestingly, the cytoplasm of the vascular endothelial cells stained positively for β-catenin (Figure 1B) ▶ . This staining was not observed in vascular endothelial cells in the noninjured myocardium (Figure 1E) ▶ and in vascular endothelial cells of sham-operated animals (not shown). β-catenin staining of vascular endothelial cells was also observed 7 days after infarction (Figure 1C) ▶ . In contrast, the myofibroblasts present in the granulation tissue around the area of infarction did not show any β-catenin staining, and vascular endothelial cells in noninfarcted parts did not show cytoplasmic β-catenin staining (Figure 1F) ▶ . At 14 (not shown), 21 (Figure 1D) ▶ , and 90 days (not shown) after MI, most of the vascular endothelial cells in the areas of newly formed blood vessels around the infarct did not show β-catenin staining. Again, the myofibroblasts did not show any staining for β-catenin. The intercalated disks between the uninjured cardiomyocytes were positive for β-catenin (Figure 1, D–F) ▶ at all time points as previously reported. 32
Figure 1.
Time course of cytoplasmic β-catenin localization in small blood vessels in the area of infarction in sections of infarcted rat hearts. Arrowheads indicate the vascular endothelial cell layer whereas arrows indicate the intercalated disks of cardiomyocytes. A: Two days after MI neovascularization has not yet started. The small vessels in the infarct area do not show β-catenin staining, probably because these vessels are remnants of the vasculature present before infarction. The border zone of the infarct consists of inflammatory tissue (IT). Scale bar, 100 μm. B: Four days after MI many newly formed small vessels were present in the border zone of the infarct area. In the cytoplasm of the endothelial cells β-catenin staining was observed (see high-power magnification in inset). In contrast, the granulation tissue (GT), which is formed in the border zone of the infarct, did not show detectable β-catenin staining. C: Seven days after MI, the cytoplasm of the vascular endothelial cells of small vessels in the infarct area was still positively staining for β-catenin (inset shows high-power magnification). The granulation tissue again was negative. D: Twenty-one days after MI, no β-catenin staining could be detected in most of the vascular endothelial cells of the small vessels. The intercalated disks of the cardiomyocytes (My) stained positively for β-catenin. E: A small vessel in a noninfarcted area of the heart, 4 days after MI, is shown. No β-catenin staining of the cytoplasm of the vascular endothelial cells could be observed. The intercalated disks of the myocytes were positively staining for β-catenin. F: Small vessel in a noninfarcted part of the heart, 7 days after MI. Again no positive staining of vascular endothelial cells was observed.
β-Catenin Expression in Larger, Muscular Arteries in the Infarct Area
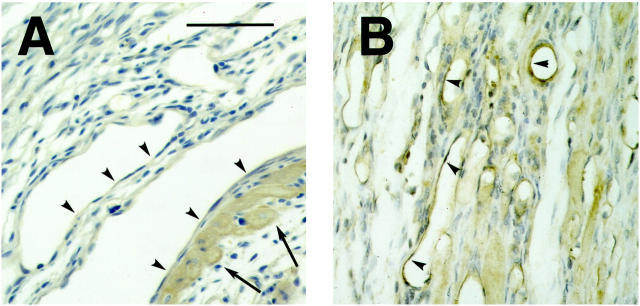
Cytoplasmic β-catenin staining of the vascular endothelium was not confined to the newly formed small vessels around the infarct area, but could also be observed in larger arteries in the injured parts of the heart (Figure 2A) ▶ . These arteries most likely were already present in the area before the infarction, because a fully developed media containing vascular smooth muscle cells (Figure 2B) ▶ and a layer of surviving cardiomyocytes around these arteries was observed around them. In contrast, arteries of similar diameter in the uninjured parts of the heart (Figure 2, D and E) ▶ or in sham-operated animals (not shown) did not show endothelial β-catenin staining.
Figure 2.
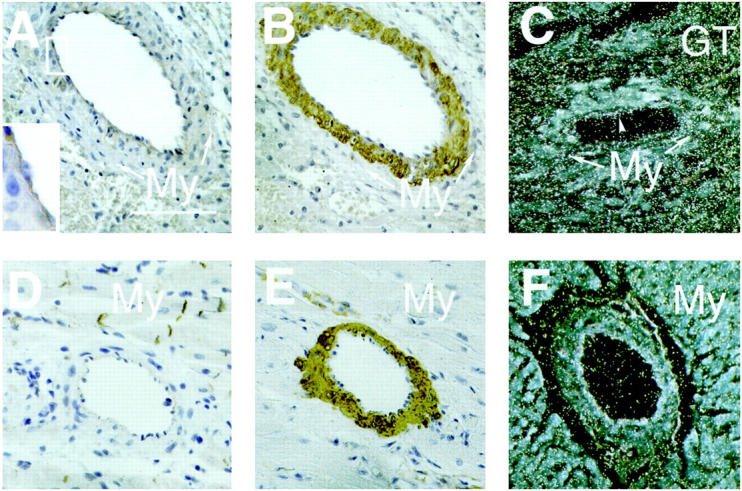
A: β-catenin staining of endothelial cells of a larger, pre-existing artery in the area of infarction, 4 days after MI. Around the artery a thin layer of surviving cardiomyocytes (My) was present, confirming that the artery was present before infarction. Scale bar, 100 μm. B: Immunohistochemistry for α-smooth muscle actin (ASMA) shows staining of the vascular smooth muscle cells in the media of the same artery, but not of the endothelial cell layer or the cardiomyocytes. C: Dark-field image of an in situ hybridization with a dishevelled-1 (dvl-1) probe of a section of rat heart, 4 days after MI. The photographic grains are represented as white spots. Note that grains are localized over the vascular endothelium (arrowhead), whereas the amount of grains over the cardiomyocytes (My) is lower. A high density of grains was observed over the granulation tissue (GT) in the border zone of the infarct. D and E: Muscular arteries in the noninfarcted parts of the myocardium did not show endothelial β-catenin staining, but the vascular smooth muscle cells stained positively for (ASMA). F: No dvl-1 expression was observed in muscular arteries in the noninfarcted areas of the heart.
Because β-catenin is known to act as a second messenger in the Wnt-frizzled-dishevelled signal transduction pathway, we performed in situ hybridization for the three known murine dishevelled (dvl) homologues. As shown in Figure 2C ▶ , silver grains were observed over the endothelial cells of the larger arteries in the infarct area when the dvl-1 probe was used. In contrast, labeling above background levels could be not be detected in these cells using the dvl-2 or the dvl-3 probe (not shown). Muscular arteries in the uninfarcted parts of the heart did not show signal above background levels with the dvl-1 probe (Figure 2F) ▶ . Abundant labeling with the dvl-1 probe was also observed in the granulation tissue around the infarct area, which is rich in (myo)fibroblasts and monocytes (Figure 2C) ▶ . This abundant labeling obscured the unambiguous detection of dvl-1 signal in endothelial cells of the small, newly formed vessels, although in the dvl-1 autoradiograms the density of silver grains in areas with many newly formed blood vessels was above background levels.
Confocal Microscopy of β-Catenin
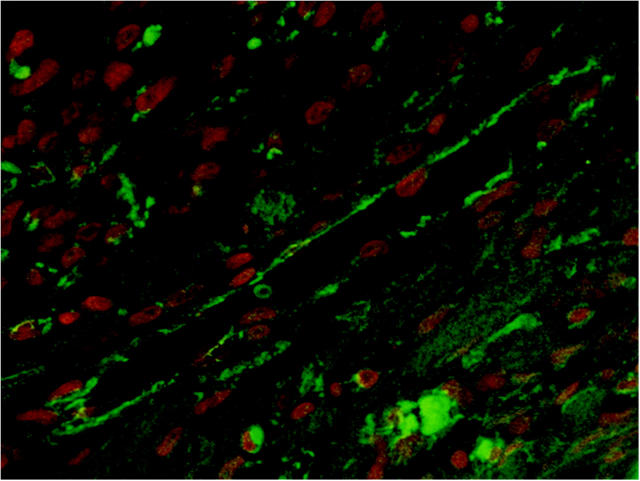
To determine the intracellular localization of β-catenin in more detail, confocal microscopy was performed on sections of infarcted rat heart 4 and 7 days after MI. As shown in Figure 3 ▶ , immunofluorescence was observed in the cytoplasm of the vascular endothelial cells around the infarct area but not in the nuclei, as observed with immunohistochemistry.
Figure 3.
Confocal microscopy of a small vessel in the infarct area of a rat heart, 7 days after MI. β-Catenin staining is depicted in green and the nuclear counterstain (propidium iodide) is depicted in red. Note that the β-catenin staining is present in the cytoplasm of the vascular endothelial cells, whereas β-catenin staining in the nuclei could not be detected.
Localization of APC Protein in the Infarcted Rat Heart
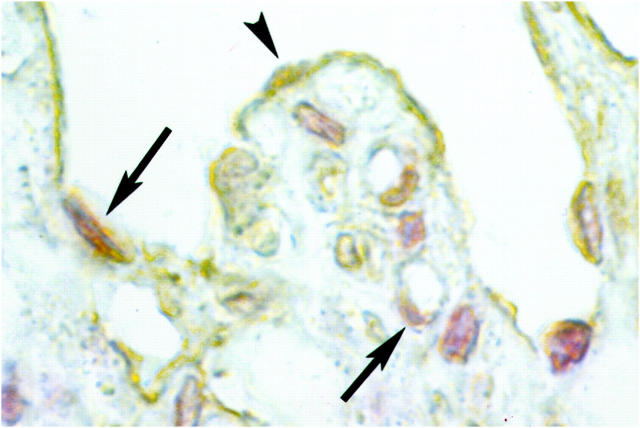
The expression of the APC protein, involved in the degradation of β-catenin, was studied in infarcted rat tissue after MI. Seven days after MI, some staining for APC protein was observed in the endothelial cells of the newly formed vessels around the infarct area (Figure 4A) ▶ . A similar pattern of staining, although of considerably higher intensity, was observed 21 days after MI (Figure 4B) ▶ , and positive vascular endothelial cells could still be detected at 90 days after MI (not shown). Surviving cardiomyocytes close to the area of infarction showed a diffuse staining for APC protein (Figure 4A) ▶ , which was not observed in cardiomyocytes in the uninjured parts of the heart. APC staining was also observed in vascular smooth muscle cells at all time points studied.
Figure 4.
Localization of the APC protein in vascular endothelium during infarct healing in the rat. APC staining was observed in the endothelial cells of newly formed vessels around the infarct area at 7 days after MI (A). In contrast to the β-catenin staining, APC staining was still observed in these cells 21 days after MI (B).
Double-Staining for β-Catenin and BrdU
Because cytosolic β-catenin accumulation has been associated with cell proliferation in a number of neoplastic diseases, we performed immunohistochemical double-staining for β-catenin and BrdU, a nucleotide analogue which is incorporated in the DNA during cell proliferation. As shown in Figure 5 ▶ , positive nuclei were observed in some, but not all of the vascular endothelial cells that stained positive for β-catenin. Because BrdU incorporation was not a generalized phenomenon in β-catenin-positive vascular endothelial cells, this suggests that the cytoplasmic β-catenin in these cells is not a direct inducer of cell proliferation.
Figure 5.
High-power magnification of immunohistochemistry for BrdU and β-catenin in a small vessel and capillaries in the infarct area of a rat heart, 4 days after MI. BrdU-positive nuclei of proliferating cells are depicted in red, and β-catenin is shown in brown. Some of the vascular endothelial cells that contain β-catenin in their cytoplasm have a BrdU-positive nucleus (arrows), but β-catenin-positive cells without nuclear BrdU incorporation were also observed (arrowhead).
Discussion
The aim of the present study was to disclose a potential link between the presence of β-catenin in the cytoplasm of the vascular endothelial cells and their proliferation and migration during the process of neovascularization in infarct healing. To this end, we have studied the localization of β-catenin, its regulatory protein APC, and the expression of the dishevelled genes, located upstream in the Wnt-dishevelled-β-catenin signal transduction pathway, in infarcted rat hearts at different time points. Because the neovascularization which takes place after MI consists of angiogenesis as well as the remodeling of pre-existing arteries, 16 processes which are both dependent on endothelial cell proliferation and migration, 17 this model seems to be very suitable to study a link between β-catenin expression and the proliferation and migration of endothelial cells in vivo.
β-catenin was observed in the cytoplasm of the endothelial cells of newly formed vessels around the area of infarction 4 and 7 days after MI. In contrast, vascular endothelial cells in the noninfarcted part of the heart showed no detectable β-catenin levels in their cytoplasm. Because remodeling of existing capillaries as well as de novo generation of capillaries take place in the first week after MI, 17,33,34 the time frame of the appearance of cytoplasmic β-catenin matches with that of the neovascularization. Based on in vitro data in which the appearance of β-catenin in the cytoplasm was observed during human umbilical vein endothelial cell migration, 19,20 our observations suggest a similar translocation phenomenon in endothelial cell proliferation and migration in vivo. These observations are also in accordance with in vitro studies in which the vascular endothelium (VE)-cadherin-catenin complex was found to be essential for de novo capillary formation 35 and in which vascular endothelial growth factor-induced phosphorylation of the cadherin-catenin complex was found to be essential for the loosening of endothelial cell-cell contacts, a first step in angiogenesis. 36 Moreover, in a recent study by Carmeliet et al 37 it was shown that the VE-cadherin/β-catenin complex is essential for endothelial cell remodeling and maturation during gestation in mice, and that targeted disruption of the intracellular part of the VE-cadherin gene leads to abnormal vascular development, underscoring the importance of these molecules in the formation and remodeling of blood vessels. Moreover, β-catenin translocation to the cytoplasm was observed in endothelial cells in culture during their remodeling in response to shear stress, suggesting that this is likely to be a normal phenomenon during rearrangement of endothelial cells.
An abundant presence of APC protein was observed in vascular endothelium and myocytes around the infarct area as well as in vascular smooth muscle cells. In a recent study by Pollack et al, 22 a functional interaction between β-catenin and APC has been shown to be necessary for tubule formation of Madin Darby canine kidney cells in vitro. Moreover, there is increasing evidence that APC itself is involved in the formation of stable membrane extrusions during active cell migration. 38 It is tempting to speculate that the co-expression of β-catenin and APC in the area of neovascularization around the infarct area promotes vascular endothelial cell proliferation and migration in both angiogenesis and remodeling of pre-existing vessels. On the other hand, the rise in APC protein content which coincides with the decrease in β-catenin content of the vascular endothelial cells may suggest that the APC protein contributes to the β-catenin degradation in these cells. The role for APC in vascular smooth muscle cells and cardiomyocytes, however, is unclear.
Recently, β-catenin has been identified as a second messenger in the signal transduction cascade of Wnt proteins, which are involved in control of tissue polarity. 15,23 In the original description of this signal transduction system in Drosophila, the dishevelled protein was shown to act directly downstream of frizzled, now recognized as the receptor for proteins from the Wnt family. 39 In the mean time, three mammalian dishevelled homologues have been identified in the mouse. 24-26 Using these mouse sequences, we have designed primer sets which allowed polymerase chain reaction-amplification of three fragments from rat DNA. Partial sequencing of these fragments revealed homologies of >90% with the published mouse sequences, confirming the identity of these fragments as rat homologues of mouse dishevelled 1–3. The three polymerase chain reaction fragments were subsequently used for in situ hybridization. In sections of infarcted rat hearts, 7 days after MI, expression of dvl-1 was detected in vascular endothelial cells of the larger vessels around the infarct area, but not in uninfarcted areas of the heart. Areas rich in newly formed capillaries around the infarct area also showed signal for dvl-1 above background levels, but because the resolution of the radioactive in situ technique is not high enough to allow precise cellular localization, the interpretation of these data is difficult. This problem is further aggravated by the high levels of dvl-1 expression in the myofibroblasts, which are abundant around the area of infarction. 28,33 However, from the data obtained with the larger arteries we can conclude that a key upstream component of the Wnt-frizzled-dishevelled tissue polarity cascade is present in the vascular endothelial cells during neovascularization of the infarct area, which may suggest a role for this pathway in the control of proliferation and/or migration of these cells. This concept is supported by a recent study of Duplàa et al 40 in which a relation between the level of expression of FrzA, a scavenger for Wnt proteins which is structurally related to frizzled receptors, and endothelial cell proliferation in vitro was demonstrated. Moreover, recently the presence of a Wnt/frizzled signal transduction system which, when activated, increases the cytoplasmic β-catenin content has been described in vascular endothelial cells in primary culture, 41 providing additional evidence that Wnt/frizzled activation may be involved in the β-catenin translocation observed in the present study.
In conclusion, the present study shows the presence of cytoplasmic β-catenin and APC protein in vascular endothelial cells during neovascularization after MI. Because β-catenin is known to be a key regulator of both cell proliferation and migration, a role for this protein in angiogenesis and vascular remodeling can be anticipated. This is further substantiated by data from in vitro studies in which translocation of β-catenin to the cytoplasm has been linked to angiogenesis. Moreover, the expression of dvl-1 in these cells suggests an involvement of the Wnt-dishevelled-β-catenin polarity cascade in this process. These findings underscore that β-catenin is not only a crucial factor during development and in neoplastic disease, but can also play a role in the vascular endothelial cell proliferation and migration during neovascularization after MI.
Acknowledgments
We thank Dr. J. L. V. Broers, Dept. of Molecular Cell Biology and Genetics, Maastricht University, The Netherlands, for his assistance with the confocal microscopy.
Footnotes
Address reprint requests to W. M. Blankesteijn Ph.D., Dept. of Pharmacology, CARIM, Universiteit Maastricht, P.O. Box 616, 6200 MD Maastricht, The Netherlands. E-mail: wm.blankesteijn@farmaco.unimaas.nl.
Supported by the Dutch Society for Scientific Research (NWO, Grant 902-18-287).
References
- 1.Takeichi M: Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem 1990, 59:237-252 [DOI] [PubMed] [Google Scholar]
- 2.Geiger B, Ayalon O: Cadherins. Annu Rev Cell Biol 1992, 8:307-332 [DOI] [PubMed] [Google Scholar]
- 3.Gumbiner BM: Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 1996, 84:345-357 [DOI] [PubMed] [Google Scholar]
- 4.Shapiro L: The multi-talented β-catenin makes its first appearance. Structure 1997, 5:1265-1268 [DOI] [PubMed] [Google Scholar]
- 5.Dale TC: Signal transduction by the Wnt family of ligands. Biochem J 1998, 329:209-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korinek V, Barker N, Morin PJ, Van Wichen D, De Weger R, Kinzler KW, Vogelstein B, Clevers H: Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 1997, 275:1784-1787 [DOI] [PubMed] [Google Scholar]
- 7.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW: Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 1997, 275:1787-1790 [DOI] [PubMed] [Google Scholar]
- 8.Peifer M: β-Catenin as oncogene: the smoking gun. Science 1997, 275:1752-1753 [DOI] [PubMed] [Google Scholar]
- 9.Gumbiner BM: Carcinogenesis: a balance between β-catenin and APC. Curr Biol 1997, 7:R443-R446 [DOI] [PubMed] [Google Scholar]
- 10.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P: Stabilization of β-catenin by genetic defects in melanoma cell lines. Science 1997, 275:1790-1792 [DOI] [PubMed] [Google Scholar]
- 11.Kitaeva MN, Grogan L, Williams JP, Dimond E, Nakahara K, Hausner P, DeNobile JW, Soballe PW, Kirsch IR: Mutations in β-catenin are uncommon in colorectal cancer occurring in occasional replication error-positive tumors. Cancer Res 1997, 57:4478-4481 [PubMed] [Google Scholar]
- 12.Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R: Lack of β-catenin affects mouse development at gastrulation. Development 1995, 121:3529-3537 [DOI] [PubMed] [Google Scholar]
- 13.Resnik E: β-catenin-one player, two games. Nat Genet 1997, 16:9-11 [DOI] [PubMed] [Google Scholar]
- 14.Takeichi M: Cadherin cell adhesion receptors as a morphogenetic regulator. Science 1991, 251:1451-1455 [DOI] [PubMed] [Google Scholar]
- 15.Miller JR, Moon RT: Signal transduction through β-catenin and specification of cell fate during embryogenesis. Genes Dev 1996, 10:2527-2539 [DOI] [PubMed] [Google Scholar]
- 16.Battegay EJ: Angiogenesis: mechanistic insights, neovascular diseases, and therapeutic prospects. Mol Med 1995, 73:333-346 [DOI] [PubMed] [Google Scholar]
- 17.Fan T-PD, Jaggar R, Bicknell R: Controlling the vasculature: angiogenesis, antiangiogenesis and vascular targeting of gene therapy. Trends Pharmacol Sci 1995, 16:57-66 [DOI] [PubMed] [Google Scholar]
- 18.Schnittler H-J: Structural and functional aspects of intercellular junctions in vascular endothelium. Basic Res Cardiol 1998, 93(suppl. 3):30-39 [DOI] [PubMed] [Google Scholar]
- 19.Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E: The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, β-catenin, and α-catenin with vascular endothelial cadherin (VE-cadherin). J Cell Biol 1995, 129:203-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMahon GA, Garfinkel S, Prudovsky I, Hu X, Maciag T: Intracellular precursor interleukin (IL)-1α, but not mature IL-1α, is able to regulate human endothelial cell migration in vitro. J Biol Chem 1997, 272:28202-28205 [DOI] [PubMed] [Google Scholar]
- 21.Muller T, Choidas A, Reichmann E, Ullrich A: Phosphorylation and free pool of β-catenin are regulated by tyrosine kinases and tyrosine phosphatases during epithelial cell migration. J Biol Chem 1999, 274:10173-10183 [DOI] [PubMed] [Google Scholar]
- 22.Pollack AL, Barth AIM, Altschuler Y, Nelson WJ, Mostov KE: Dynamics of β-catenin interactions with APC protein regulate epithelial tubulogenesis. J Cell Biol 1997, 137:1651-1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willert K, Nusse R: Beta-catenin: a key mediator of Wnt signaling. Curr Opin Genet Dev 1998, 8:95-102 [DOI] [PubMed] [Google Scholar]
- 24.Sussman DJ, Klingensmith J, Salinas P, Adams PS, Nusse R, Perrimon N: Isolation and characterization of a mouse homolog of the Drosophila segment polarity gene Dishevelled. Dev Biol 1994, 166:73-86 [DOI] [PubMed] [Google Scholar]
- 25.Klingensmith J, Yang Y, Axelrod JD, Beier DR, Perrimon N, Sussman DJ: Conservation of dishevelled structure and function between flies and mice: isolation and characterization of Dvl2. Mech Dev 1996, 58:15-26 [DOI] [PubMed] [Google Scholar]
- 26.Tsang M, Lijam N, Beirer DR, Wynshaw-Boris A, Sussman DJ: Isolation and characterization of mouse Dishevelled-3. Dev Dyn 1996, 207, 253–262 [DOI] [PubMed]
- 27.Passier RCJJ, Smits JFM, Verluyten MJA, Studer R, Drexler H, Daemen MJAP: Activation of angiotensin-converting-enzyme in the infarct zone following myocardial infarction. Am J Physiol 1995, 269:H1268-H1276 [DOI] [PubMed] [Google Scholar]
- 28.Blankesteijn WM, Essers-Janssen YPG, Verluyten MJA, Daemen MJAP, Smits JFM: A homologue of Drosophila tissue polarity gene frizzled is expressed in migrating myofibroblasts in the infarcted rat heart. Nat Med 1997, 3:541-544 [DOI] [PubMed] [Google Scholar]
- 29.Blankesteijn WM, Essers-Janssen YPG, Ulrich MMW, Smits JFM: Increased expression of a homologue of Drosophila tissue polarity gene ‘frizzled’ in left ventricular hypertrophy in the rat, as determined by subtractive hybridization. J Mol Cell Cardiol 1996, 28:1187-1191 [DOI] [PubMed] [Google Scholar]
- 30.Broers JLV, Machiels BM, Van Eys GJJM, Kuijpers HJH, Manders EMM, Van Driel R, Ramaekers FCS: Dynamics of the nuclear lamina as monitored by GFP-tagged A-type lamins. J Cell Sci 1999, 112:3463-3475 [DOI] [PubMed] [Google Scholar]
- 31.Van Kleef EM, Smits JF, Schwartz SM, Daemen MJ: Doxazosin blocks the angiotensin II-induced smooth muscle cell DNA synthesis in the media, but not in the neointima of the rat carotid artery after balloon injury. Cardiovasc Res 1996, 31:324-330 [DOI] [PubMed] [Google Scholar]
- 32.Kurth T, Scwhartz H, Schneider S, Hausen P: Fine structural immunohistochemistry of catenins in amphibian and mammalian muscle. Cell Tissue Res 1996, 286:1-12 [DOI] [PubMed] [Google Scholar]
- 33.Cleutjens JPM, Blankesteijn WM, Daemen MJAP, Smits JFM: The infarcted myocardium: simply dead tissue or a lively target for therapeutic interventions. Cardiovasc Res 1999, 44:232-241 [DOI] [PubMed] [Google Scholar]
- 34.Nelissen-Vrancken HJMG, Debets JJM, Snoeckx LHEH, Daemen MJAP, Smits JFM: Time-related normalization of maximal coronary flow in isolated perfused hearts of rats with myocardial infarction. Circulation 1996, 93:349-355 [DOI] [PubMed] [Google Scholar]
- 35.Cai J, Jiang WG, Mansel RE: Inhibition of the expression of VE-cadherin-catenin complex by gamma linolenic acid in human vascular endothelial cells, and its impact on angiogenesis. Biochem Biophys Res Comm 1999, 258:113-118 [DOI] [PubMed] [Google Scholar]
- 36.Esser S, Lampugnani MG, Corada M, Dejana E, Risau W: Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci 1998, 111:1853-1865 [DOI] [PubMed] [Google Scholar]
- 37.Carmeliet P, Lampugnani M-G, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellillo A, Mattot V, Nuyens D, Lutgens E, Clotman F, De Ruiter M, Gittenberger-de Groot AC, Poelmann R, Lupu F, Herbert J-M, Collen D, Dejana E: Targeted deficiency of cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 1999, 98:147–157 [DOI] [PubMed]
- 38.Barth AIM, Nathke IS, Nelson WJ: Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol 1997, 9:683-690 [DOI] [PubMed] [Google Scholar]
- 39.Krasnow RE, Wong LL, Adler PN: Dishevelled is a component of the frizzled signaling pathway in Drosophila. Development 1995, 121:4095-4102 [DOI] [PubMed] [Google Scholar]
- 40.Duplaa C, Jaspard B, Moreau C, D’Amore PA: Identification and cloning of a secreted protein related to the cystein-rich domain of frizzled: evidence for a role in endothelial cell growth control. Circ Res 1999, 84:1433-1445 [DOI] [PubMed] [Google Scholar]
- 41.Wright M, Aikawa M, Szeto W, Papkoff J: Identification of a Wnt-responsive signal transduction pathway in primary endothelial cells. Biochem Biophys Res Comm 1999, 263:384-388 [DOI] [PubMed] [Google Scholar]