Abstract
In a previous report, we described the effects of cyclin D1 expression in epithelial tissues of transgenic mice. To study the involvement of D-type cyclins (D1, D2, and D3) in epithelial growth and differentiation and their putative role as oncogenes in skin, transgenic mice were developed which carry cyclin D2 or D3 genes driven by a keratin 5 promoter. As expected, both transgenic lines showed expression of these proteins in most of the squamous tissues analyzed. Epidermal proliferation increased in transgenic animals and basal cell hyperplasia was observed. All of the animals also had a minor thickening of the epidermis. The pattern of expression of keratin 1 and keratin 5 indicated that epidermal differentiation was not affected. Transgenic K5D2 mice developed mild thymic hyperplasia that reversed at 4 months of age. On the other hand, high expression of cyclin D3 in the thymus did not produce hyperplasia. This model provides in vivo evidence of the action of cyclin D2 and cyclin D3 as mediators of proliferation in squamous epithelial cells. A direct comparison among the three D-type cyclin transgenic mice suggests that cyclin D1 and cyclin D2 have similar roles in epithelial thymus cells. However, overexpression of each D-type cyclin produces a distinct phenotype in thymic epithelial cells.
The cyclins are a family of key cell-cycle regulators that function by association with and activation of cyclin-dependent kinases (CDKs) at specific points of the cell cycle to phosphorylate various proteins that are important during cell-cycle progression. 1 Three D-type cyclins (D1, D2, and D3) are expressed in the G1 phase of the cell cycle and depending on cell lineage, various combinations of D-type cyclins are induced by mitogens. 1,2 D-type cyclins form complexes with and activate CDK4 and CDK6 during the G1 phase of the cell cycle. 3 A key substrate for G1 cyclin/CDK complexes is the retinoblastoma protein, pRb. Phosphorylation of pRb, a tumor suppressor gene product, has been attributed to cyclin/CDK complexes and implicated in the regulation of proliferation in keratinocytes and other cell types. 4,5 Thus, phosphorylation of pRb blocks its ability to suppress the activity of S phase promoting transcription factors such as E2F. 4,6 Reconstitution of D-type cyclin/kinase complexes in baculovirus showed that all possible complexes are capable of phosphorylating pRb in vitro. 7,8 These results suggest that the fundamental role of D-type cyclins is to integrate extracellular signals with the cell-cycle machinery. 2 Initially, several reports assigned redundant roles to the three members of D-type cyclins, but in the last few years, it has become evident that each member plays a specific role and is differentially expressed in various tissues. 2 Recently, CDK-independent functions of D-type cyclins were also described. For example, ligand independent activation of estrogen receptors by cyclin D1 and inhibition of androgen receptors by binding of cyclin D1 or cyclin D3 was reported. 9-12
Cyclin D1 and cyclin D2 seem to contribute to the neoplastic phenotypes in human and mouse tumors. Indeed, the cyclin D1 gene was originally cloned as an oncogene termed PRAD1, that was activated by chromosomal translocations present in parathyroid adenoma. 13 Cyclin D2 overexpression or amplification was also described in several tumors. In fact, cyclin D2 accumulation in the cytoplasm of gastric carcinoma cells seems to play a role in cancer progression. 14 Also, the overexpression of cyclin D2 in carcinoma in situ identified it as a candidate gene in male germ-cell malignancies. 15,16 Cyclin D2 is also overexpressed in chronic B-cell malignancies. 17 Although, fewer reports suggest that cyclin D3 plays a role in tumorigenesis, cyclin D3 overexpression was associated with increased expression of p27Kip1 in a subset of aggressive B-cell lymphomas. 18 In addition, coordinated elevation of cyclin D3 and cyclin D1 was observed in the breast cell line MCF-7. 19 These data suggest that normal progression through the G1 phase requires distinct sets of D-type cyclins in different tissues. For example, G1/S transition in hematopoietic cells and inhibition of granulocyte differentiation are regulated by cyclin D2 and D3 whereas cyclin D1 seems to be dispensable in these cell types. 20,21 In contrast cyclin D3, but not D1 or D2, was up-regulated on induction of HL-60 leukemia cells to differentiation and has been shown to accumulate at high levels in a wide range of quiescent cell types. 22
The function of D-type cyclins has also been studied in D-type cyclin-deficient mice. Cyclin D1 knockout mice presented symptoms of neurological impairment as well as deficient development of retina and mammary glands. 23,24 Cyclin D2-deficient mice, showed alterations in gonadal cell proliferation. 25 At present, generation of cyclin D3-deficient mice has not been reported. Taken together, these data suggest tissue-specific functions of D-type cyclins.
The murine skin model is a valuable system for studying epidermal proliferation, precancerous changes, and tumor progression in vivo. 26 The use of this model allowed us to detect expression of the three D-type cyclins in normal, hyperplastic and neoplastic epidermis in vivo. 27,28 Cyclin D1 and cyclin D2 are expressed at the mid-G1 phase and form complexes with CDK4/6, a process that is dependent on the relative abundance of these cyclins. Cyclin D3 also forms complexes with CDK4/6; however, complex formation requires an additional regulatory event other than the simple relative abundance of these proteins. 29 Another interesting difference is that during premalignant tumor progression in chemically-induced mouse carcinogenesis, cyclin D1 and cyclin D2 are overexpressed and form complexes with CDK4/6, whereas cyclin D3 only forms complexes with CDK4/6 in hyperproliferative epidermis (hyperplastic skin). 27
We have previously reported the generation of a transgenic mouse that expresses human cyclin D1 in squamous epithelial tissues, resulting in moderate epidermal and severe thymic hyperplasia. 30,31 To complete the study of the role of D-type cyclins in squamous epithelial tissues, we generated transgenic mice that expressed either cyclin D2 or cyclin D3, driven by the regulatory sequence of bovine keratin 5 (K5D2 and K5D3). We determined that overexpression of either cyclin D2 or cyclin D3 results in hyperproliferative epidermal hyperplasia. However, there was a clear difference in the thymic phenotypes. Thymic hyperplasia in the cyclin D2 transgenic mice regressed spontaneously in older mice, in contrast to the hyperplasia in the cyclin D1 transgenic mice which was progressive and fatal. 30 At the other end of the spectrum are the cyclin D3 mice, which did not develop thymic hyperplasia. Thus, whereas overexpression of cyclin D1, D2, and D3 produces a similar epidermal phenotype, each D-type cyclin transgene induces a unique thymic phenotype.
Materials and Methods
Generation of Transgenic Mice
An EcoRV/XbaI fragment containing the mouse cyclin D2 or cyclin D3 cDNA was excised from the plasmid pBluescript II and introduced into the polylinker of the vector pBK5 which contained the 5.2-kb bovine keratin 5 (K5) regulatory sequences, β-globin intron 2 and the 3′ polyadenylylation sequences. These constructs were designated as pK5D2 and pK5D3. The transgenes were excised from the plasmid vector by digestion with BssHII, separated by low-melting-point agarose electrophoresis and purified using a Geneclean II Kit (BIO101, Vista, CA). These transgenes were microinjected into the C57BL/6xSJL hybrid strains, which took place in the National Institute of Child Health and Human Development National Transgenic Mouse Development Facility (NTMDF) at the University of Alabama, Birmingham. Transgenic mice were crossed for two generations with the SSIN strain to generate 75% SSIN background mice.
Transgenes DNA-Specific Polymerase Chain Reaction (PCR)
Genomic DNA was extracted from mouse tail clips and used for PCR detection of the transgenes. We used an upstream primer (5′TTCAGGGTGTTGTTTAGAATGG3′) and a downstream primer (5′CAATAAGAATATTTCCACGCCA3′) specific for the β-globin intron 2 sequence. With this process, we screened the entire transgenic mouse lines. The DNA amplification renders a 450-bp PCR product. PCR was performed by denaturation at 95°C for 1 minute, followed by 32 cycles of amplification as follows: denaturation at 95°C for 30 seconds, annealing at 55°C for 40 seconds, and extension at 72°C for 45 seconds, with a final extension at 72°C for 10 minutes.
Cyclin D2 and Cyclin D3 Immunohistochemical Staining
Immunohistochemical staining of formalin-fixed paraffin-embedded tissues was performed with polyclonal mouse cyclin D2 (M20) or cyclin D3 (C16) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Epithelial cell proliferation was measured by intraperitoneal injection of BrdU (60 μg/g; Sigma Chemical Co., St. Louis, MO) 30 minutes before the mice were killed. BrdU incorporation was detected by immunohistochemical staining of paraffin-embedded sections with mouse anti-BrdU monoclonal antibody (Becton-Dickinson Immunocytometry System; Becton-Dickinson, San Jose, CA). The reaction was visualized with a biotin-conjugated anti-mouse antibody (Vector Laboratories, Inc., Burlingame, CA) and avidin-biotin-peroxidase kit (Vectastain Elite, Vector Laboratories, Inc.) with diaminobenzidine as chromogen.
Western Blotting Analysis, Immunoprecipitation, and Kinase Assay
Mouse dorsal skins were treated with a depilatory agent for 1 minute and then washed. After mice were sacrificed, the epidermal tissue was scraped off with a razor blade, placed into homogenization buffer (50 mmol/L HEPES, pH 7.5, 150 mmol/L NaCl, 2.5 mmol/L EGTA, 1 mmol/L ethylenediaminetetraacetic acid, 0.1% Tween-20, 1 mmol/L dithiothreitol, 0.1 mmol/L phenyl methyl sulfonyl fluoride, 10 mmol/L β-glycerophosphate, 0.2 mmol/L sodium vanadate, and 2 mmol/L NaF) and homogenization was achieved with a manual homogenizer. The epidermal homogenate was centrifuged at 11,000 × g to collect the supernatant which was used directly for Western blotting analysis or stored at −70°C. Thymic proteins were extracted by using the same buffer and conditions as stated above. The protein concentration in each skin or thymus lysate was measured with the Bio-Rad protein assay system (Bio-Rad Laboratories, Richmond, CA). Protein lysates (25 μg from each sample) were electrophoresed through 12% acrylamide gels and electrophoretically transferred onto nitrocellulose membranes. After being blocked with 5% nonfat powdered milk in Dulbecco’s phosphate-buffered saline (Sigma Chemical Co.), the membranes were incubated with 1 μg/ml of specific antibodies. The following antibodies were used: polyclonal antibodies against cyclin D2 (M-20), cyclin D3 (C-16), pRb (C15), p107 (C18), and p130 (C20) (Santa Cruz Biotechnology, Inc.). pRb (G3-245) (Pharmingen, San Diego, CA.) was used for Western blot analysis of thymus proteins. Horseradish peroxidase-conjugated secondary antibody (Amersham Corp., Arlington Heights, IL), followed by enhanced chemiluminescence (ECL detection kit; Amersham Corp.) were used for immunoblotting detection. Bio-image analysis was used to quantitate the expression levels of those proteins.
To study cyclin D/CDK complex formations and kinase activities, we used polyclonal anti-CDK4 and anti-CDK2 antibodies conjugated with protein A-Sepharose beads (Life Technologies Inc., Grand Island, NY) to immunoprecipitate fresh protein lysates for 1 hour at 4°C with constant rotation. After washing three times with extraction buffer, Western blot analysis was performed as described above with polyclonal antibody described previously. To study the kinase activities of CDK4 and CDK2, protein lysates were obtained as described above, but the homogenate was frozen on powdered dry-ice, thawed in ice water, incubated on ice for 15 minutes and centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant was collected and used for a kinase assay. Eight hundred micrograms of protein lysate were immunoprecipitated with antibodies against CDK4 or CDK2. Thirty μl of precoated antibody beads (Life Technologies Inc.) was incubated with the lysate for 1 hour at 4°C. The beads were washed twice with homogenization buffer and twice with kinase buffer (50 mmol/L HEPES, pH 7.5, and 10 mmol/L MgCl2). Then, 30 μl of kinase buffer, 0.5 μg of pRb substrate (Santa Cruz Biotechnology, Inc.), 5 μCi [γ-32P]-ATP (6,000 Ci/mmol), 2.5 mmol/L EGTA, 1 mmol/L dithiothreitol, 20 μmol/L ATP, 10 mmol/L β-glycerophosphate, 0.2 mmol/L sodium vanadate, and 2 mmol/L NaF) was added to the bead pellet and incubated for 30 minutes at 30°C. Sodium dodecyl sample buffer was added, and each sample was boiled for 5 minutes and electrophoresed through a 10% acrylamide gel.
Flow Cytometry
Thymocytes were obtained by pressing thymic tissue through a nylon mesh. Thymocytes were stained with anti-CD4-coupled phycoerythrin and anti-CD8-coupled fluorescein isothiocyanate and analyzed by two-color immunofluorescence with a Coulter Elite Flow cytometer as previously described. 30
Results
Generation of Cyclin D2 and Cyclin D3 Transgenic Mice
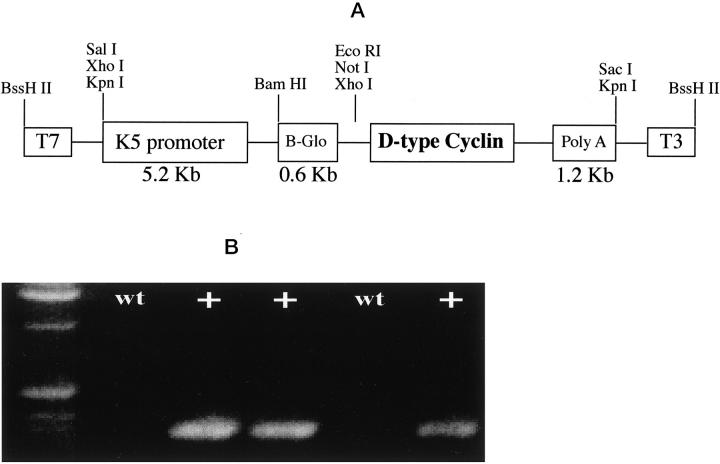
The construct used to generate transgenic mice is depicted in Figure 1A ▶ . The expression of D-type cyclins was targeted to stratified epithelia by the 5′-regulatory fragment of the bovine K5 gene. The K5-cyclin D2 and K5-cyclin D3 vectors were made by subcloning the mouse cyclin D2 or cyclin D3 cDNA into a vector containing a 5.2-kb fragment of the bovine K5 promoter, the rabbit β-globin intron 2, and the SV40 polyadenylation signal. As reported, this fragment drives expression of a reporter gene in basal cells of squamous stratified epithelia, where K5 is normally expressed. 32,33 All of the transgenic mice were generated in the C57BL/6xSJL genetic background. Three mice with cyclin D3-positive and five mice with cyclin D2-positive integration were identified by PCR analysis (Figure 1B) ▶ . Based on those results, the integration-positive mice were selected as founders and crossed with SSIN inbred mice. A second screening to verify transgene expression was performed by Western blot analysis of epidermal preparations with cyclin D2 or cyclin D3 antibodies as described. 29 These results permitted the selection of two mice of each D-type cyclin transgene as founders of high expression lines (2101 and 2102 of K5D2, 2201 and 2203 of K5D3 mice).
Figure 1.
pK5-Transgene construct and screening techniques. A: Diagram of the K5D-type cyclin construct. B: PCR amplification of DNA extracted from mouse tails. β-globin sequence was amplified resulting in a 450-bp product.
Expression of D-Type Cyclins and pRb Family Proteins in Epidermis
To quantify the level of cyclin D2 and cyclin D3 protein expression, we isolated the epidermis of transgenic and normal siblings for immunoblot analysis. The cyclin D3 protein is expressed at high levels in both K5D3 lines compared to their normal siblings (fourfold in 2201 and 5.5-fold in 2203 transgenic lines) (Figure 2) ▶ . Increased levels of cyclin D2 protein were observed in both K5D2 lines, although the level of expression was barely twofold higher than the level observed in their normal siblings (Figure 2) ▶ . It is worth mentioning that the protein level of cyclin D3 decreases in K5D2 mice and the cyclin D2 protein level decreases in K5D3 animals. The effect was most notable in K5D3 animals (lines 2201 and 2203) where the level of cyclin D2 expression was half compared to the wild-type animals (Figure 2) ▶ .
Figure 2.
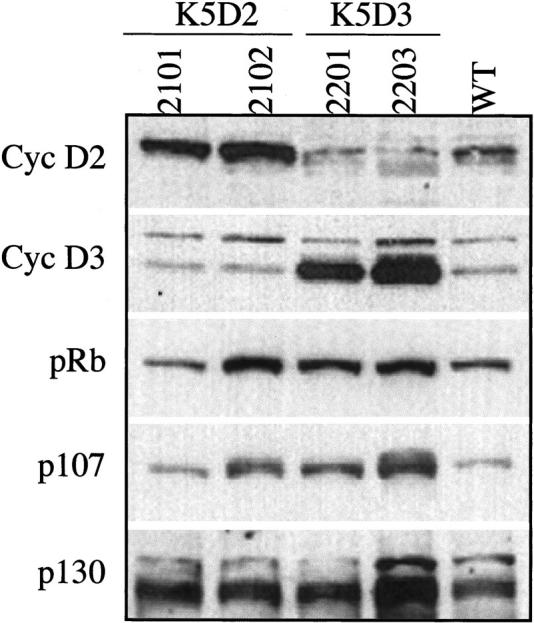
Western Blot analysis of cyclin D2, cyclin D3, and pRb family expression in epidermis of transgenic mice. Protein lysates of epidermis were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted to a nitrocellulose membrane. Primary antibodies against cyclin D2, cyclin D3, pRb, p107, and p130 were used for immunoblot analysis. The levels of each protein were quantified with a densitometer.
The pRb tumor suppressor is a negative regulator that acts in the G1 phase of the cell cycle 5,34 and the related p130 and p107 proteins may have similar functions. Therefore, we analyzed expression of pRb family protein in epidermal lysates from transgenic and normal sibling mice (Figure 2) ▶ . Both pRb and p107 protein expression were clearly increased in 2102-K5D2 and both K5D3 transgenic lines. The transgenic line 2203-K5D3 showed a strong induction of p107 as well as changes in mobility consistent with phosphorylation. Similar phosphorylation was also found in the transgenic line 2102-K5D2. No apparent change in phosphorylation of pRb was observed. Increased p130 protein was detected in both K5D2 and K5D3 transgenic lines, whereas the 2203-K5D3 line again showed a stronger induction of this protein (Figure 2) ▶ . These data are consistent with the stronger expression of cyclin D22102 or cyclin D32203 in these transgenic animals and with our previously reported data of elevated levels of p107 and pRb protein in mouse epidermal tissue after proliferative induction by TPA. 29
Development of Epidermal Hyperproliferation and Hyperplasia in Transgenic Mice
The newborn cyclin D2 and cyclin D3 transgenic mice did not demonstrate any obvious developmental abnormalities and there was no difference in size compared to wild-type littermates. Cytokeratin 5 is normally expressed in the basal cell layer of the epidermis. Consistent with this, immunohistochemical staining showed high expression of cyclin D2 or cyclin D3 in the epidermis of the respective transgenic mice (Figure 3C ▶ and Figure 4C ▶ ). Because the presence of cyclin D2 and D3 expressing cells is difficult to detect in wild-type epidermis the high level of expression detected in the K5D2 and K5D3 lines confirms the results obtained by Western blot and PCR analysis (Figures 1 and 2) ▶ ▶ .
Figure 3.
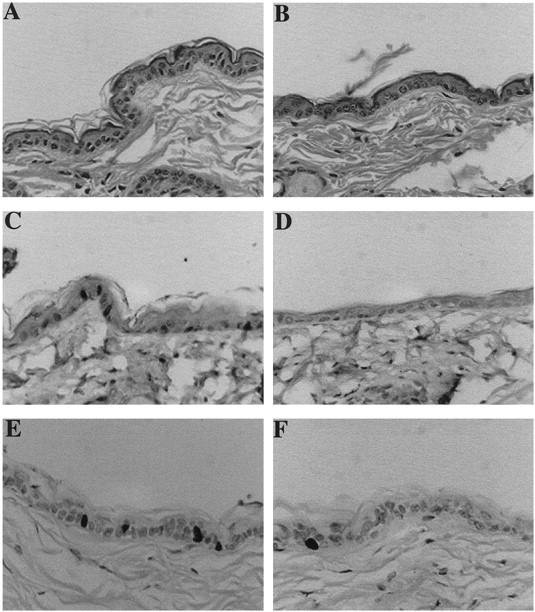
Skin phenotype of K5D2 transgenic mice. Representative paraffin-sections of skin from K5D2 transgenic mice (A) and normal siblings (B) were stained with hematoxylin and eosin. Expression of cyclin D2 in transgenic (C) and wild-type skin (D) was determined with specific antibodies. BrdU incorporation of paraffin sections of transgenic (E) and normal (F) skin was detected with mouse monoclonal anti-BrdU antibody. Antibody binding was detected by secondary antibody conjugated with horseradish peroxidase, and the reaction was developed with diaminobenzidine. Original magnification, ×200.
Figure 4.
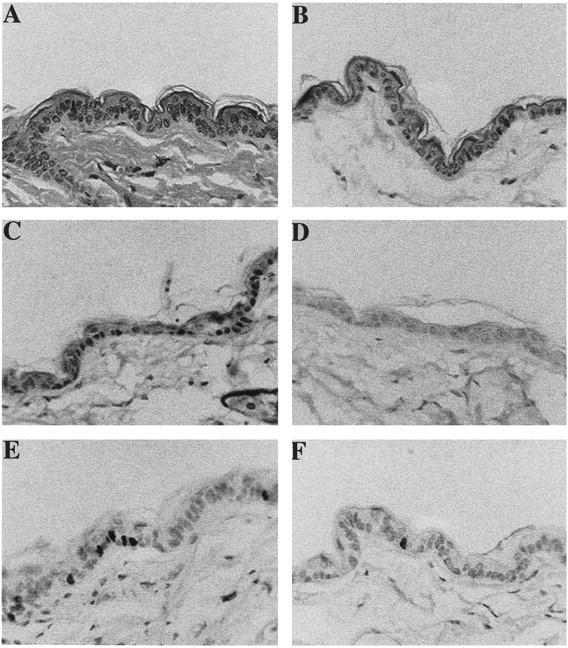
Skin phenotype of K5D3 transgenic mice. Representative paraffin-sections of skin from K5D3 transgenic mice (A) and normal siblings (B) were stained with hematoxylin and eosin. Expression of cyclin D3 in transgenic (C) and wild-type skin (D) was determined with specific antibodies. BrdU incorporation of paraffin sections of transgenic (E) and normal (F) skin was detected with mouse monoclonal anti-BrdU antibody. Antibody binding was detected by secondary antibody conjugated with horseradish peroxidase, and the reaction was developed with diaminobenzidine. Original magnification, ×200.
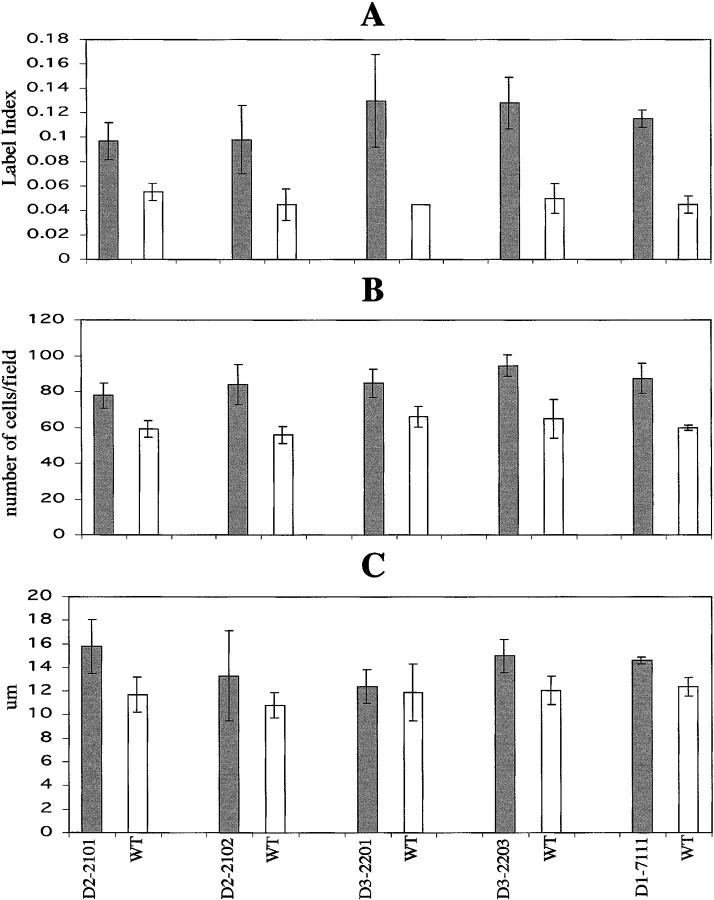
Epidermal thickness was similar in the K5D2 and K5D3 lines, both of which contain an increased number of nucleated cells compared to nontransgenic littermates (Figures 3A, 4A, and 5C) ▶ ▶ ▶ . However, no gross phenotype was evident in the hair follicles, which showed normal distribution and morphology. The increased number of nucleated cells in K5D2 and K5D3 mice is similar to that of previously described K5D1-7111 transgenic mice 30 (Figure 5B) ▶ . However, direct comparison with the earlier report was not possible because of the different genetic backgrounds. For this reason, these parameters where re-evaluated after crossing the K5D1 transgene into the same genetic background as the K5D2 and K5D3 mice. Expression of each cyclin D transgene in the same genetic background results in a significant increase in the number of nucleated cells of the interfollicular epidermis compared to normal siblings (2101, P = 0.001; 2102, P < 0.008; 2201, P < 0.0003; 2203, P < 0.02; 7111, P < 0.01, Mann-Whitney) (Figures 3, 4, and 5) ▶ ▶ ▶ . Consistent with the epidermal hyperplasia, hyperproliferation of the interfollicular epithelia was observed by BrdU incorporation as a marker of cells in S phase (Figures 3E, 4E, and 5A) ▶ ▶ ▶ . Compared to nontransgenic littermates, there was a 1.7- and 2.2-fold increase respectively in the number of BrdU-positive cells in K5D2 transgenic lines 2101 and 2102. The K5D3 transgenic lines, 2201 and 2203, also showed a 2.9- and 2.5-fold increase in BrdU-positive cells and the K5D1-7111 line showed a mild increase of 2.5-fold (2101, P = 0.02; 2102, P = 0.008; 2201, P = 0.001; 2203, P = 0.05; 7111, P = 0.0001, Mann-Whitney) (Figure 5A) ▶ . To complete the description of interfollicular epidermis, we determined the thickness of the epidermis in transgenic and wild-type animals (Figure 5C) ▶ . Each of the transgenic lines with the exception of K5D3-2201 demonstrated an increase in epidermal thickness. A direct comparison with K5D1-7111 showed that, with the exception of the K5D3-2201 line, the increase of any of the three D-type cyclins produced a similar phenotype in mouse epidermis. Table 1 ▶ summarizes the changes in the three principal parameters (BrdU label Index, number of nucleated cells and epidermal thickness) that define the epidermal phenotype of cyclin D1, D2, and D3 transgenic lines. To study whether D-type cyclin expression affects the normal pattern of epidermal differentiation, immunohistochemical staining was performed to detect K1, which is expressed only in terminally differentiated cells, and K5, which is expressed in basal epithelial cells. The expression pattern of these keratins was comparable to nontransgenic controls (data not shown). However, the transgenic epithelia have a more densely packed basal and suprabasal cell compartment (basal cell hyperplasia) reflecting an expanded proliferative compartment.
Figure 5.
Quantification of epidermal proliferation in transgenic and normal sibling mice. A: The bars indicate the labeling index or the percentage of BrdU incorporation in basal cells from interfollicular epithelia. B: Basal cell hyperplasia. The bars indicate the number of nucleated cells in 200-μm interfollicular epithelia. C: The bars indicate the thickness of whole mouse skin in μm. Gray bars, transgenic mice; white bars, normal siblings.
Table 1.
Summary of Epidermis Parameters in Transgenic Mice
| Transgenic lines | Thickness* | Labeling index* | Nucleate cells* |
|---|---|---|---|
| K5D2-2101 | 35 | 70 | 31 |
| K5D2-2102 | 23 | 123 | 54 |
| K5D3-2201 | 6 | 191 | 28 |
| K5D3-2203 | 23 | 153 | 46 |
| K5D1-7111 | 17 | 155 | 48 |
*Percentage of increase in transgenic mice compared to normal sibling.
Development of Thymic Hyperplasia in K5D2 Mice
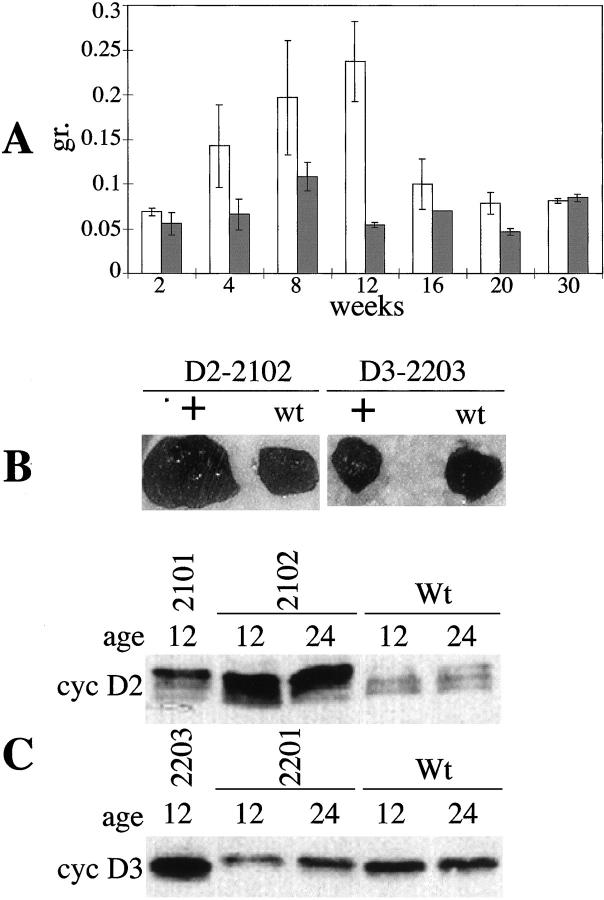
Three of the five founders of K5D2 transgenic animals died at 5 months of age. Autopsy revealed a severe hyperplasia of the thymus. Two of these founders died before transgenic lines could be established. The remaining founder, 2102, was crossed with SSIN inbred mice and a transgenic line was established. To study the time frame of development of hyperplasia, K5D2-2102 mice were sacrificed at intervals from 2 to 30 weeks of age. The maximum thymus weight of the normal siblings reached 0.11g at 8 weeks as previously reported. 30 The maximum thymus weight in the cyclin D2 transgenic mice was 0.24 g at 12 weeks of age which represents an increase of 4.5-fold compared to wild-type animals (Figure 6, A and B) ▶ . After this time point, the thymic weight declines and at 20 weeks both the transgenic and wild-type thymi are similar in size (Figure 6A) ▶ . To determine cyclin D2 expression levels, thymic extracts were analyzed by Western blot. Consistent with the thymic hyperplastic phenotype, the K5D2-2102 thymi expressed five times more cyclin D2 than the wild-type animals at 12 and 24 weeks of age (Figure 6C) ▶ . On the other hand, the K5D2-2101 line did not develop thymic hyperplasia and the level of protein expression was only twofold greater than the wild type. Notably, cyclin D2 protein expression did not decrease at 24 weeks of age in the 2102 line when the thymic involution had occurred (Figure 6C ▶ , lines 2 and 3).
Figure 6.
Development of thymic hyperplasia in K5D2 mice. A: Time frame of the development of thymic hyperplasia. Thymus from K5D2 and wild-type mice were harvested and weighed from line 2102 transgenic mice and normal siblings at different ages from 2 to 30 weeks. White bars, transgenic mice; gray bars, normal siblings. B: Thymus of K5D2-2102, K5D3-2203 and normal sibling animals at 11 weeks of age. C: Western blot analysis of cyclin D2 and cyclin D3 expression from protein lysates of thymus of K5D2 (2101 and 2102), K5D3 (2203 and 2201) and normal sibling mice (Wt) at 12 and 24 weeks of age.
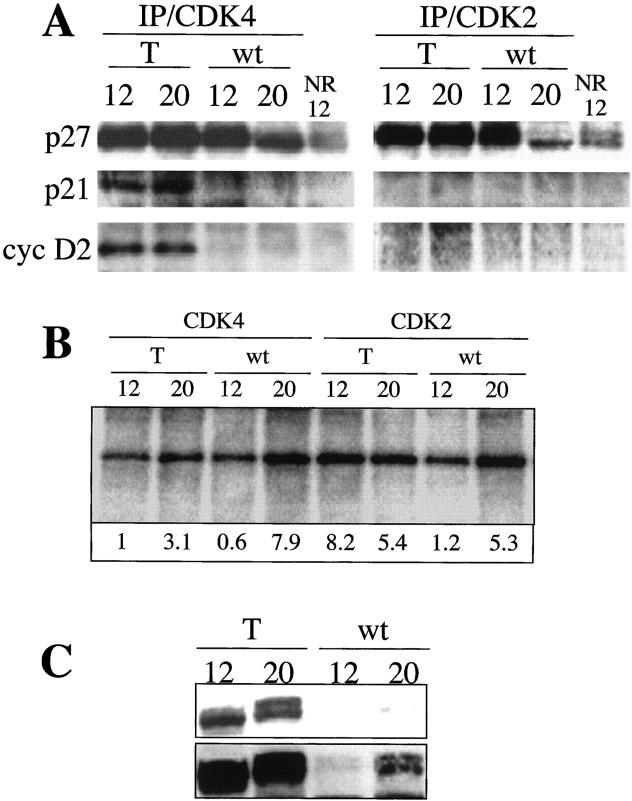
To study if cyclin D2 overexpression resulted in changes in the composition of CDK/cyclin/CDK inhibitor complexes before or after thymic regression, we analyzed complexes formation by immunoprecipitation with an antibody against CDK4 or CDK2 and detection of cyclin D2 or CKIs by Western blot. Figure 7 ▶ shows a similar level of association between CDK4 and cyclin D2 at 12 and 20 weeks of age in transgenic mice. Although CDK4/cyclin D2 levels were barely detectable in the wild-type thymus, these complexes were apparent when the Western blot membranes were overexposed (data not shown). The p27Kip1 inhibitor complexed with CDK4 in both transgenic and wild-type mice, although there was a slight decrease in the level of CDK4-associated p27Kip1 in 20-week-old wild-type thymi (Figure 7A) ▶ . Interestingly, p21Cip1 was clearly associated with CDK4 in transgenic, but not in wild-type mice (Figure 7A) ▶ . As expected cyclin D2 did not co-precipitate with CDK2, whereas high levels of p27Kip1 were associated with CDK2 in transgenic and wild-type thymi. Furthermore, there was a decrease in the level of p27Kip1 associated with CDK2 in 20-week wild-type thymi (Figure 7A) ▶ . These results suggest that neither p27Kip1 nor p21Cip1 are involved in the regression of thymi hyperplasia by blocking CDK4 or CDK2 activities at 20 weeks. p16Ink4a and p15Ink4b inhibitors were not detected in CDK4 complexes (data not shown). We also analyzed the kinase activity of CDK4 and CDK2 complexes in vitro using pRb as a substrate. Figure 7B ▶ shows an increase in CDK4 kinase activity at 20 weeks in transgenic and wild-type thymi. A similar pattern was observed for CDK2 activity in wild-type thymi. However, the kinase activity in cyclin D2 transgenic thymi was greater at 12 than at 20 weeks. The elevated kinase activity in young thymi could be related to the observed hyperplasia. However, we cannot rule out the possibility that the different cell populations that compose the thymus could mask the CDK activities of the epithelial component (which overexpress cyclin D2). In addition, pRb protein levels and phosphorylation states were determined in thymus extracts of transgenic and wild-type mice. Figure 7C ▶ shows that the pRb level is elevated in transgenic mice compared with normal siblings. Also, a slower migrating band consistent with hyperphosphorylation is observed in transgenic mice at 20 weeks. Overexposure of the membrane showed that pRb protein levels also increased in wild-type mice at 20 weeks of age, although the protein level was still inferior compared to transgenic mice (Figure 7C) ▶ . Altogether, these results indicate that CDK4 and CDK2 kinase activities are not reduced during the thymic size regression in transgenic mice and neither p27Kip2 nor p21Cip1 seemed to be involved in this event.
Figure 7.
CDK complex formation and kinase activities in K5D2 thymus. A: Fresh protein lysates of K5D1-2102 transgenic mice thymus at 12 and 20 weeks of age were immunoprecipitated with polyclonal anti-CDK4 (IP/CDK4) and anti-CDK2 (IP/CDK2) antibodies and immunoblotted with polyclonal antibody for p27Kip1, p21Cip1, and cyclin D2. The control was immunoprecipitated with normal rabbit serum with lysates obtained from 2102 transgenic thymus at 12 weeks of age (NR/12). B: CDK4 and CDK2 kinase activities of K5D2 transgenic (T) and normal sibling animals (wt). Fresh protein lysates of thymus were immunoprecipitated as below in an in vitro kinase assay, with pRb as a substrate, was performed. pRb phosphorylation levels were determined by measuring 32P incorporation and Bio-image analysis was used to quantitate the kinase activities. C: Western blot analysis of pRb expression in thymus of transgenic and wild-type mice. Protein lysates of thymus were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted to a nitrocellulose membrane. Primary antibody against pRb was used for immunoblot analysis. T, transgenic; wt, wild type, NR, normal rabbit serum.
Unlike K5D2, none of the K5D3 transgenic lines developed thymic hyperplasia although line 2203 showed a 3.5-fold increased level of cyclin D3 (Figure 6C) ▶ . Consistent with these data none of the three K5D3 founders developed thymus hyperplasia as was established for cyclin D1 30 and cyclin D2.
To determine whether expression of the cyclin D2 transgene in thymic epithelium altered T cell development, we analyzed the distribution of thymocyte subsets in normal and in transgenic mice at 12 weeks of age, when the thymus was hyperplastic. Flow cytometry determination showed that each major thymocyte subset, defined by CD4 and CD8 expression, is present in 2102 transgenic mice at 12 weeks of age (thymic hyperplasia) (data not shown).
Discussion
D-type cyclins function as regulatory subunits of cyclin-dependent kinases and are rate-limiting factors of the G1 progression. Depending on cell lineage, various combinations of cyclins D1, D2, and D3 are induced by mitogens during G1 and their continued synthesis throughout the cycle depends on persistent growth factor stimulation. 2 Redundant roles of D-type cyclins have been suggested in several reports, but, the highly conserved sequences of cyclin D proteins (∼94% between human and mouse D-type cyclins) suggest that each family member has important and unique roles in cell-cycle regulation that constrain evolutionary divergence. Indeed, individual D-type cyclins have a greater similarity between mouse and human species (94% identity) than among themselves in the same species (cyclin D1/cyclin D2, 64%; cyclin D1/cyclin D3, 49%; cyclin D2/cyclin D3, 64%). 35-37 The three D-type cyclins are differentially expressed in normal mouse epidermis,. 27,29 Cyclin D1 protein expression and cyclinD1-CDK4/6 complex formation are strongly regulated during the G1 phase after 12-0-tetradecanoylphorbol-13-acetate (TPA) stimulation of keratinocytes. 29 On the other hand, cyclin D2 and cyclin D3 protein expression seem to be constitutive and only minor changes in their protein levels were observed after TPA stimulation. Cyclin D1 and cyclin D2 overexpression or amplification have been described in several human and mouse tumors and were considered as candidates for oncogenes. 27,38-40
To study the role of each D-type cyclin in normal proliferation and tumorigenesis in mouse skin, we have developed two new transgenic mice that express cyclin D2 or cyclin D3 in squamous epithelial tissues. Previously, we described the generation of a transgenic mouse that expressed human cyclin D1 under the regulatory sequence of keratin 5 (K5D1) directing expression to basal cells in squamous epithelial tissues. 30 The principal phenotypic characteristics of the cyclin D1 transgenic animals were mild epidermal hyperplasia and severe thymic hyperplasia in three integration events of different genetic backgrounds. 30 The cyclin D2 and cyclin D3 transgenic mice express the respective transgene under the same keratin 5 promoter. The K5D2 and K5D3 showed a similar epidermal phenotype including an increased number of nucleated cells, epidermal thickness, and elevated labeling index. BrdU incorporation showed that in S phase, cells were present not only in basal but also in the suprabasal layers. The epidermal cells are more densely packed than in normal siblings, however, no differences in the cell size were detected. Comparison with K5D1 transgenic mice on the same genetic background reveals a similar phenotype. The more substantial increase in skin thickness, that was previously shown in the K5D1 animals, is attributed to the different background in the earlier study. 30 The cyclin D2 and cyclin D3 transgenic lines showed detectable cyclin D2 or D3 protein in basal cells consistent with the K5D2 and K5D3 epidermal phenotype. However, whereas cyclin D2 was observed in 40% of basal cells in the interfollicular epidermis, cyclin D3 was observed in almost all of the basal cells (Figures 3C and 4C) ▶ ▶ . Furthermore, there was only a twofold increase in cyclin D2 in Western blot whereas, cyclin D3 expression was elevated fourfold to fivefold. Differences in the stability and/or degradation between these two cyclins may be responsible for those results.
The pattern of expression of keratin 1 (expressed in terminally differentiated cells) and keratin 5 (expressed in basal epithelial cells) indicated that epidermal differentiation was not affected and hyperproliferation was compensated by terminal differentiation. Thus, transgenic animals have a more densely packed basal cell compartment and suprabasal proliferative cells resulting in mild acanthosis (increase in the thickness of the nucleated layers of the epithelia) without hyperkeratosis. No evidence of spontaneous skin tumor development was found until 10 months of age in either K5D2 or K5D3 animals. We find it noteworthy that overexpression of cyclin D1 and cyclin D2, but not cyclin D3, was detected in premalignant lesions after 7,12-dimethylbenz[a]antracene (DMBA)/TPA applications (two-stage carcinogenesis protocol). 27 In this sense, cyclin D1 expression is necessary but not sufficient for development of skin tumors 41,42 because D1 knockout mice have a reduced number of papillomas whereas K5D1 mice did not have an increased number of skin tumors. We also performed carcinogenesis experiments using the two-stage protocol to test whether overexpression of cyclin D2 or cyclin D3 increased the susceptibility to chemical carcinogenesis. Overexpression of cyclin D2 did not seem to increase sensitivity to spontaneous or carcinogen-induced skin tumors (Rodriguez-Puebla M, Conti CJ, unpublished results). However, as in the case of cyclin D1, cyclin D2 expression may be necessary to promote skin tumor development.
Another unexpected result is the down-regulation of cyclin D2 in K5-D3 mice and down-regulation of cyclin D3 in K5-D2 mice (Figure 2) ▶ . These results are not specific for these two D-type cyclins because K5-D1 mice also show a reduced protein level of cyclin D3. 42 We can hypothesize that overexpression of one of the D-type cyclins is compensated by reducing the level of expression of another member of the family. It is possible that the transcription factors involved in the regulation of D-type cyclin genes are responsible for these regulatory loops. Also noteworthy is the finding that various transcription factor binding sites were found in mouse cyclin D3 gene, that includes GATA, NF-αB, ATF, E2F, Aprf, TCE, GAGA, TRE/Ap1, Sp1, and Ap2. 43 Because Sp1 and E2F binding sites were found in the cyclin D1 gene, 44 these transcription factors could participate in this regulatory circuit.
Expression levels of the pRb family of proteins, pRb and p107, were clearly elevated in K5D3 and in K5D2–2102 transgenic lines. The K5D3–2203 line showed a stronger induction of p107 and changes in mobility consistent with phosphorylation. This is consistent with the greater level of cyclin D3 expression in this transgenic line. Similarly, the K5D3-2203 transgenic mice showed stronger induction of p130 other cyclin D2 and D3 lines showed a mild increase of this protein. Consistent with these results, we previously found an increase in p107 and pRb protein levels in mouse epidermal tissue after proliferative induction by TPA. 29 pRb and p107 gene regulation has been suggested to be mediated by binding of E2F-1 to E2F sites. 45 We therefore believe that induction of p107 and pRb in transgenic animals may be mediated by E2F.
One of the more relevant differences between the three transgenic mice overexpressing D-type cyclins was the development of thymic hyperplasia in cyclin D1 30 and cyclin D2, but not in cyclin D3 transgenic mice. The fact that cyclin D2 expression was only double in the K5D2-2101 line whereas it increased by fivefold in the K5D2-2102 line may explain why the K5D2-2101 line did not develop thymic hyperplasia. Mechanistic studies of CDK4 and CDK2 complex formations in the thymus of K5D2-2102 did not show relevant differences at 12 and 20 weeks of age. We did in vitro immunoprecipitation with thymic lysates of the transgenic and wild-type mice. To determine whether cyclin D2 overexpression resulted in changes in the composition of CDK/cyclin/CKI complexes, we analyzed CDK4 and CDK2 complex formations and kinase activities. We determined that neither p21Cip1 nor p27Kip1 are involved in the thymic regression at 20 weeks of age in transgenic mice. The kinase activities of CDK4 and CDK2 did not change during the thymic size regression. However, elevated CDK2 kinase activity at 12 weeks of age may be responsible for the hyperplastic phenotype in cyclin D2 transgenic thymus. Several reports have described that kinase activation of CDK2 occurs when increased levels of CDK4/cyclin complexes bind to the CKIs and release CDK2. A similar mechanism could be involved in the development of the hyperplastic thymus, where an increase of cyclin D2 protein levels bind some inhibitor in a binary or ternary complex with CDK4, although CKIs other than p21Cip1 and p27Kip1 would be responsible for this event. Interestingly, pRb protein levels were also increased in transgenic mouse thymus compared with normal sibling mice, as was shown in epidermal cells. In addition, the level of pRb phosphorylation increased at 20 weeks in the thymus of K5D2 animals. These data clearly show that in the older thymus (20 weeks) the pRb protein seems to be relatively inactive rather than the active form. However, we cannot rule out the possibility that the presence of cell populations in the thymus other than epithelial cells mask subtle differences in complex formations and also influence the pRb state observed at 12 and 20 weeks of age.
Interestingly the K5D3-2203 transgenic line expressed the transgene at a 3.5-fold higher level than nontransgenic, but did not develop thymic hyperplasia. This is consistent with the fact that none of the K5D3 founders developed thymic hyperplasia whereas three K5D1 30 and three K5D2 founders developed this phenotype. An interesting possibility is that cyclin D3 has a distinct role in thymic epithelial cells compared to the other D-type cyclins. In this regard, cyclin D3 has been reported to be induced in differentiation in other systems. For example, cyclin D3 is strongly up-regulated on induction of HL-60 leukemia cells to differentiate and it accumulates to high levels in a wide range of quiescent cells in mouse and human tissues. 22 In addition, myoblast induction of cyclin D3 expression is closely coupled with withdrawal from the cell cycle and differentiation. 46 In contrast, cyclin D1 and cyclin D2 have been established to play a role in proliferation and this function may be responsible for the hyperplastic thymus phenotype.
Each major subset of T cell, defined by CD4 and CD8 expression, is present in K5D2-2102 transgenic line at 12 weeks of age (thymic hyperplasia). These results show that despite the altered architecture, the thymic environment is able to generate mature T cells. Furthermore, these results confirm the histological diagnosis of hyperplasia and rule out the possibility of thymic lymphoma because, in this case, a single predominant phenotype should be present whereas thymus from K5D2-2102 transgenic line contain each of the major T cell subsets.
A relevant difference between thymic hyperplasia of K5D1-7108 30 and K5D2-2102 transgenic mice was that, in the latter, the increasing size of the thymus stops at 12 weeks of age and at 20 weeks, the size was similar to normal siblings (Figure 6A) ▶ . The possibility that the transgene expression was turned-off after 12 weeks was ruled-out because the expression levels determined by Western blot analysis at 24 weeks of age was similar to 12-week-old mice (Figure 6C) ▶ . Again the results suggest that a different mechanism of action exists between cyclin D1 and cyclin D2. Although both transgenic mice overexpressing these cyclins show thymic hyperplasia, their histological characteristics are different and in one case the hyperplasia reverts with age whereas, in the other, it progresses and causes the death of the animal.
In summary, the mammalian D-type cyclins seem to have redundant functions in some systems, but clear differences have also been reported. Cyclin D1 and cyclin D2 are considered as proto-oncogenes based on genetic aberrations in human and animal malignancies. 47 In contrast, cyclin D3 has not been firmly implicated in oncogenesis. Despite considerable similarities, unique phenotypes were reported for cyclin D1 and cyclin D2 knockout mice, 23-25 whereas effects of cyclin D3 knockouts are unknown. Our results, using two new transgenic models, suggest that in some tissues (epidermis) D-type cyclins may have similar functions, whereas in others (thymus) the biological roles of the individual D-type cyclins are not fully redundant.
Acknowledgments
We thank April Weiss for help with the mouse experiments; the Science Park animal facility personnel; Dr. Irma B. Gimenez-Conti and The Science Park histology service for assistance with the immunohistochemical staining; Cassie Smith Bigbee for technical assistance; Melissa Bracher for secretarial assistance; and Sharon Stockman for editing the paper. We also thank Dr. Heather Poetschke for help and advice with flow cytometry studies.
Footnotes
Address reprint requests to Marcelo L. Rodriguez-Puebla, The University of Texas, M. D. Anderson Cancer Center, Science Park-Research Division, Smithville, TX 78957. E-mail: mlrodri@odin.mdacc.tmc.edu.
Supported by Department of Human Services Grants CA 42157 and CA 57596; an M. D. Anderson Cancer Center institutional grant (CA 16672) for the animal facility; and a center grant (P30-E507784-01) for the histology service
References
- 1.Sherr CJ: Mammalian G1 cyclins. Cell 1993, 73:1059-1065 [DOI] [PubMed] [Google Scholar]
- 2.Sherr CJ: D-type cyclins. Trends Biochem Sci 1995, 20:187-190 [DOI] [PubMed] [Google Scholar]
- 3.Sherr CJ: G1 phase progression: cyclin on cue. Cell 1994, 79:551-555 [DOI] [PubMed] [Google Scholar]
- 4.Munger K, Pieternpol JA, Pittelkow MR, Holt JT, Moses HL: Transforming growth factor B1 regulation of c-myc expression, pRb phosphorylation, and cell cycle progression in keratinocytes. Cell Growth Differ 1992, 3:291-298 [PubMed] [Google Scholar]
- 5.Nevins J, Chellappan S, Mudryj M, Hiebert S, Devoto S, Horowitz J, Hunter T, Pines J: E2F transcription factor is a target for the RB protein and the cyclin A protein. Cold Spring Harb Symp Quant Biol 1991, 56:157-162 [DOI] [PubMed] [Google Scholar]
- 6.Nevins JR: E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science 1992, 258:424-429 [DOI] [PubMed] [Google Scholar]
- 7.Kato J-Y, Matsuoka M, Strom DK, Sherr CJ: Regulation of cyclin D-dependent kinase 4 (cdk4) by cdk4-activating kinase. Mol Cell Biol 1994, 14:2713-2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyerson M, Harlow E: Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol 1994, 14:2077-2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knudsen K, Cavenee W, Arden K: D-type cyclins complex bind with the androgen receptor and inhibits its transcriptional transactivation ability. Cancer Res 1999, 59:2297-2301 [PubMed] [Google Scholar]
- 10.Zwijsen R, Wientjens E, Klompmaker R, van der Sman J, Bernards R, Michalides R: CDK-independent activation of estrogen receptor by cyclin D1. Cell 1997, 88:405-415 [DOI] [PubMed] [Google Scholar]
- 11.McHannon C, Suthiphongchai T, DiRenzo J, Ewen M: P/CAF associates with cyclin D1 and potentiates its activation of the estrogen receptor. Proc Natl Acad Sci USA 1999, 96:5382-5387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neuman E, Ladha M, Lin N, Upton T, Miller S, DiRenzo J, Pestell R, Hinds P, Dowdy S, Brown M, Ewen M: Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol Cell Biol 1997, 17:5338-5347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold A, Kim H, Gaz R, Eddy R, Fukushima Y, Byers M, Shows T, Kronenberg H: Molecular cloning and chromosomal mapping of DNA rearranged with the parathyroid hormone gene in a parathyroid adenoma. J Clin Invest 1989, 83:2034-2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasogawa Y, Takamo Y, Okayasu I, Kakita A: The 5D4 antibody (anti-cyclin D1/D2) related antigen: cytoplasmic staining is correlated to the progression of gastric cancer. Pathol Int 1998, 48:717-722 [DOI] [PubMed] [Google Scholar]
- 15.Murty V, Chaganti R: A genetic perspective of male germ cell tumors. Semin Oncol 1998, 25:133-144 [PubMed] [Google Scholar]
- 16.Houldsworth J, Reuter V, Bosl G, Chaganti R: Aberrant expression of cyclin D2 in an early event in human male germ cell tumorigenesis. Cell Growth Differ 1997, 8:292-299 [PubMed] [Google Scholar]
- 17.Delmer A, Ajchenbaum-Cymbalista F, Tang R, Ramond S, Faussat A, Marie J, Zittoun R: Overexpression of cyclin D2 in chronic B-cell malignancies. Blood 1995, 85:2870-2876 [PubMed] [Google Scholar]
- 18.Sanchez-Beato M, Camacho F, Martinez-Montero J, Saez A, Villuendas R, Sanchez-Verde L, Garcia J, Piris M: Anomalous high p27/Kip1 expression in a subset of aggressive B-cell lymphomas is associated with cyclin D3 overexpression. p27/Kip1-cyclin D3 colocalization in tumor cells. Blood 1999, 94:765–772 [PubMed]
- 19.Russell A, Thompson M, Hendley J, True L, Armes J, Germain D: Cyclin d1 and D3 associate with the SCF complex and are coordinately elevated in breast cancer. Oncogene 1999, 18:1983-1991 [DOI] [PubMed] [Google Scholar]
- 20.Ando K, Ajchenbaum-Cymbalista F, Griffin J: Regulation of G1/S transition by cyclin D2 and D3 in hematopoietic cells. Proc Natl Acad Sci USA 1993, 90:9571-9575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato J-Y, Sherr C: Inhibition of granulocyte differentiation by G1 cyclins D2 and D3 but not D1. Proc Natl Acad Sci USA 1993, 90:11513-11517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartkova J, Lukas J, Strauss M, Bartek J: Cyclin D3: requirement for G1/S transition anf high abundance in quiescent tissues suggest a dual role in proliferation and differentiation. Oncogene 1998, 17:1027-1037 [DOI] [PubMed] [Google Scholar]
- 23.Sicinski P, Donaher J, Parker S, Li T, Fazeli A, Gardener H, Haslam S, Bronson R, Elledge S, Weinberg R: Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 1995, 82:621-630 [DOI] [PubMed] [Google Scholar]
- 24.Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C: Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev 1995, 9:2364-2372 [DOI] [PubMed] [Google Scholar]
- 25.Sicinski P, Donaher J, Geneg Y, Parker S, Garder H, Park M, Robker R, Richards J, McGinnis L, Biggers J, Epping J, Bronson R, Elledege S, Weinberg R: Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 1996, 384:470-474 [DOI] [PubMed] [Google Scholar]
- 26.Klein-Szanto AJP: Morphological evaluation of tumor promoter effects on mammalian skin. Slaga TJ eds. Mechanisms of Tumor Promotion, Tumor Promotion and Skin Carcinogenesis. 1984, :pp 42-72 CRC Press, Boca Raton [Google Scholar]
- 27.Rodriguez-Puebla ML, LaCava M, Gimenez-Conti IB, Jonhson DG, Conti CJ: Deregulated expression of cell-cycle proteins during premalignant progression in SENCAR mouse skin. Oncogene 1998, 17:2251-2258 [DOI] [PubMed] [Google Scholar]
- 28.Zhang S-Y, Liu S-C, Goodrow T, Morris R, Klein-Szanto AJP: Increased expression of G1 cyclins and cyclin-dependent kinases during tumor progression of chemically induced mouse skin neoplasms. Mol Carcinog 1997, 18:142-152 [PubMed] [Google Scholar]
- 29.Rodriguez-Puebla ML, Robles AI, Johnson DG, LaCava M, Conti CJ: Synchronized proliferation induced by TPA treatment of mouse skin: an in vivo model for cell cycle regulation. Cell Growth Differ 1998, 9:31-39 [PubMed] [Google Scholar]
- 30.Robles AI, Larcher F, Whalin RB, Murillas R, Richie E, Gimenez-Conti IB, Jorcano JL, Conti CJ: Expression of cyclin D1 in epithelial tissues of transgenic mice results in epidermal hyperproliferation and severe thymic hyperplasia. PNAS 1996, 93:7634-7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klug DB, Crouch E, Carter C, Coghlan L, Conti CJ, Richie ER: Transgenic expression of cyclin D1 in thymic epithelial precursors promotes epithelial and T cell development. J Immunol 2000, 164:1881-1888 [DOI] [PubMed] [Google Scholar]
- 32.Ramirez A, Bravo A, Jorcano J, Vidal M: Sequences 5′ of the bovine keratin 5 gene direct tissue- and cell-type-specific expression of a lacZ gene in the adult and during development. Differentiation 1994, 58:53-64 [DOI] [PubMed] [Google Scholar]
- 33.Murillas R, Larcher F, Conti C, Santos M, Ullrich A, Jorcano J: Expression of a dominant negative mutant of epidermal growth factor receptor in the epidermis of transgenic mice elicits striking alterations in hair follicles development and skin structure. EMBO J 1995, 14:5216-5223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinberg R: Tumor suppressor genes. Science 1991, 254:1138-1146 [DOI] [PubMed] [Google Scholar]
- 35.Motokura T, Keyomarsi K, Kronenberg H, Arnold A: Cloning and characterization of human cyclin D3, a cDNA closely related in sequence to the PRAD1/cyclin D1 proto-oncogene. J Biol Chem 1992, 267:20412-20415 [PubMed] [Google Scholar]
- 36.Kiyokawa H, Busquets C, Powell C, Ngo L, Rifkind R, Marks P: Cloning of a D-type cyclin from murine erythroleukemia cells. Proc Natl Acad Sci USA 1992, 89:2444-2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsushine H, Roussel M, Ashmun R, Sherr C: Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell 1991, 65:701-713 [DOI] [PubMed] [Google Scholar]
- 38.Robles AI, Conti CJ: Early overexpression of cyclin D1 protein in mouse skin carcinogenesis. Carcinogenesis 1995, 16:781-786 [DOI] [PubMed] [Google Scholar]
- 39.Bianchi AB, Fischer SM, Robles AI, Rinchik EM, Conti CJ: Overexpression of cyclin D1 in mouse skin carcinogenesis. Oncogene 1993, 8:1127-1133 [PubMed] [Google Scholar]
- 40.Sgambato A, Han E, Zhang Y, Moon R, Santella R, Weinstein I: Deregulated expression of cyclin D1 and other cell cycle-related genes in carcinogen-induced rat mammary tumors. Carcinogenesis 1995, 16:2193-2198 [DOI] [PubMed] [Google Scholar]
- 41.Robles A, Rodriguez-Puebla M, Glick A, Trempus C, Hansen L, Sicinski P, Tennant R, Weinberg R, Yuspa S, Conti C: Reduced skin tumor development in cyclin D1 deficient mice highlights the oncogenic ras pathway in vivo. Genes Dev 1998, 12:2469-2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez-Puebla ML, LaCava M, Conti C: Cyclin D1 overexpression in mouse epidermis increases cyclin-dependent kinase activity and cell proliferation in vivo but does not affect skin tumor development. Cell Growth Differ 1999, 10:467-472 [PubMed] [Google Scholar]
- 43.Wang A, Sicinski P, Weinberg R, Zhang Y, Ravid K: Characterization of the mouse cyclin D3 gene: exon/intron organization and promoter activity. Genomics 1996, 3:156-163 [DOI] [PubMed] [Google Scholar]
- 44.Motokura T, Arnold A: PRAD1/cyclin D1 proto-oncogene: genomic organization 5′ DNA sequence of a tumor-specific rearrangement breakpoint. Genes Chromosom Cancer 1993, 7:89-95 [DOI] [PubMed] [Google Scholar]
- 45.Shan B, Chang C-Y, Jones D, Lee W-H: The transcription factor E2F-1 mediates the autoregulation of Rb gene expression. Mol Cell Biol 1994, 14:299-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiess M, Gill R, Hamel P: Expression of the positive regulator of cell cycle progression, cyclin D3, is reduced during differentiation of myoblasts into quiescent myotubes. Oncogene 1995, 10:159-166 [PubMed] [Google Scholar]
- 47.Hall M, Peters G: Genetic alterations of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human cancer. Adv Cancer Res 1996, 68:67-108 [DOI] [PubMed] [Google Scholar]