Abstract
Reactive nitrogen species may play a mechanistic role in neurodegenerative diseases by posttranslationally altering normal brain proteins. In support of this hypothesis, we demonstrate that an anti-3-nitrotyrosine polyclonal antibody stains all of the major hallmark lesions of synucleinopathies including Lewy bodies, Lewy neurites and neuraxonal spheroids in dementia with Lewy bodies, the Lewy body variant of Alzheimer’s disease, and neurodegeneration with brain iron accumulation type 1, as well as glial and neuronal cytoplasmic inclusions in multiple system atrophy. This antibody predominantly recognized nitrated α-synuclein when compared to other in vitro nitrated constituents of these pathological lesions, such as neurofilament subunits and microtubules. Collectively, these findings imply that α-synuclein is nitrated in pathological lesions. The widespread presence of nitrated α-synuclein in diverse intracellular inclusions suggests that oxidation/nitration is involved in the onset and/or progression of neurodegenerative diseases.
Oxidative injury has been implicated in the pathogenesis of numerous neurodegenerative diseases including Alzheimer’s disease, 1 Parkinson’s disease, 2,3 dementia with Lewy bodies (DLB), 4 amyotrophic lateral sclerosis, 5 and Huntington’s disease. 6 Oxidative injury occurs when an imbalance is created by the production of reactive species that escape or overwhelm the compensatory anti-oxidant capacity of a cell. Both reactive oxygen and nitrogen species are produced in vivo and may act synergistically to form nitrating agents that can modify proteins as well as lipids and thiol and aldehyde moieties in other biomolecules. 7,8 More specifically, tyrosine residues or free tyrosine can be modified by peroxynitrite, a compound generated by the reaction of superoxide radical and nitric oxide, to generate 3-nitrotyrosine (3-NT). The formation of the peroxynitrite-CO2 adduct or the presence of other catalysts (redox active metal, metalloproteins) increases the reactivity of peroxynitrite. 9,10 Further, in the presence of myeloperoxidase or eosinophil peroxidase, hydrogen peroxide can oxidize nitrite to another biologically active nitrating agent, 11,12 which also generates 3-NT. Nitrated tyrosine residues have been detected in Lewy bodies (LBs) of Parkinson’s disease brains 13 and in neurofibrillary tangles of Alzheimer’s disease brains, 14,15 but no studies have examined these or additional hallmark lesions of other neurodegenerative disorders and the molecular target(s) of nitration in these lesions have yet to be identified.
α-Synuclein (α-syn) is a 140-amino acid long highly conserved protein that is abundant in neurons, particularly in presynaptic terminals. 16,17 Two mutations in the α-syn gene have been shown to be pathogenic for familial Parkinson’s disease in rare kindreds, 18-20 and it has been demonstrated that α-syn is the major component of LBs and Lewy neurites (LNs) in Parkinson’s disease, DLB, and the LB variant of Alzheimer’s disease (LBVAD). 21-27 More recently, α-syn has been recognized to be a major component of the glial (GCIs) and neuronal cytoplasmic inclusions in multiple system atrophy (MSA) brains 28-34 as well as of the LB-like inclusions, neuraxonal spheroids, and LNs in neurodegeneration with brain iron accumulation type 1 (NBIA1; previously known as Hallervorden-Spatz disease). 33,35,36 Thus, neurodegenerative disorders characterized neuropathologically by α-syn lesions now are referred to as synucleinopathies.
Here, we report that the majority of α-syn inclusions in DLB, LBVAD, MSA, and NBIA1 contain nitrated proteins. Further, we also demonstrate that α-syn, nitrated in vitro, is recognized by the rabbit anti-3-NT polyclonal antibody (3-NT pAb) in Western blots thereby implicating α-syn as a plausible target for nitrative modification in these inclusions. Thus, we infer from these data that oxidative/nitrative modifications of α-syn are involved in mechanisms underlying neurodegenerative synucleinopathies.
Materials and Methods
In Vitro Nitration and Western Blot Analysis
To assess the relative specificity of the 3-NT pAb for proteins previously detected in synucleinopathy lesions, we performed Western blot analyses with this antibody on purified proteins after in vitro nitration. Recombinant human α-syn was expressed and purified from bacteria as previously described. 37 Recombinant mouse low molecular weight neurofilament (NF) protein (NFL) were expressed in Escherichia coli BL21 (DE3) using a mouse NFL cDNA cloned into the pET-23d expression vector (Novagen, Inc. Madison, WI) after which transformed bacteria were selected and maintained in Luria-Bertani medium (10 g/ml bacto-tryptone, 5 g/ml bacto-yeast extract, 10 g/ml NaCl) or Terrific Broth (12 g/ml bacto-tryptone, 24 g/ml bacto-yeast extract, 0.4% gycerol, 17 mmol/L KH2PO4, 72 mmol/L K2PO4) containing 100 μg/ml ampicillin. Bacteria were grown to an OD600 of 0.6 and the expression of the recombinant protein was induced with 0.5 mmol/L of isopropyl-β-d-thiogalactopyranoside for 2 hours. To recover bacterially expressed NFL, cells were pelleted, resuspended into lysis buffer (25% sucrose, 1 mmol/L ethylenediaminetetraacetic acid, 50 mmol/L Tris, pH 8.0, 2 mg/ml lysozyme, and a cocktail of protease inhibitors) and incubated on ice for 30 minutes. Ten mmol/L of MgCl2, 1 mmol/L MnCl2, 10 μg/ml DNase 1 and 10 μg/ml RNase A were added to the homogenate, which was incubated on ice for another 30 minutes. Two ml of detergent buffer (0.2 mol/L NaCl, 1% deoxycholic acid, 1% Nonidet P-40, 20 mmol/L Tris, pH 7.5, 2 mmol/L ethylenediaminetetraacetic acid) per ml of lysis buffer were added and, after vigorous mixing, the insoluble material was sedimented at 5,000 × g for 30 minutes. The supernatant was discarded and the pellet was repeatedly washed with buffer containing 0.5% Triton and 1 mmol/L ethylenediaminetetraacetic acid to generate a highly compact pellet which was resuspended in 8 mol/L urea, 1% β-mercaptoethanol, 10 mmol/L NaPO4, pH 7.0, for subsequent purification of NFL using hydroxylapatite (Bio-Rad Laboratories, Richmond, CA). 38 Medium (NFM) and high (NFH) molecular weight NF proteins were purified from bovine spinal cords as previously described. 39 Tubulin proteins recovered from phosphocellulose purified bovine microtubules were purchased from Cytoskeleton, Inc., Denver, CO. All of these proteins were nitrated with a 10-fold molar excess of peroxynitrite as previously described, 40 and then the relative specificity of the 3-NT pAb for each of these proteins was assessed by Western blot methods reported earlier. 33,37,40
Tissue Collection and Processing
The harvesting, fixation, and further processing of the brain tissue specimens were conducted as previously described. 22,41 Briefly, tissue blocks were removed at autopsy from the cingulate cortex and mesencephalon of six DLB and five LBVAD brains, the cerebellar white matter from seven MSA brains, and the insular cortex and globus pallidus from one NBIA1 brain (see Table 1 ▶ ). The diagnostic assessment of all cases was performed in concordance with published guidelines. 42-44 Samples from these brains were fixed by immersion in 70% ethanol with 150 mmol/L NaCl for 24 to 36 hours, and paraffin embedded according to a previously described schedule. 45 These blocks were then cut into multiple, near serial, 6-μm sections for immunohistochemical staining.
Table 1.
Case Demographics
| Case no. | Age | Sex | Postmortem interval | Diagnosis |
|---|---|---|---|---|
| 1 | 55 | M | 7 | MSA |
| 2 | 67 | F | 5 | MSA |
| 3 | 60 | F | 10.5 | MSA |
| 4 | 64 | F | 17 | MSA |
| 5 | 72 | F | 14 | MSA |
| 6 | 43 | M | 16.5 | MSA |
| 7 | 79 | M | 16 | MSA |
| 8 | 58 | M | 20.5 | DLB |
| 9 | 83 | F | 14.5 | DLB |
| 10 | 76 | M | 13.5 | DLB |
| 11 | 74 | M | 15.5 | DLB |
| 13 | 63 | F | 22 | DLB |
| 14 | 79 | M | 12 | DLB |
| 12 | 65 | M | 9.5 | LBVAD |
| 15 | 73 | M | 12 | LBVAD |
| 16 | 79 | M | 20.5 | LBVAD |
| 17 | 82 | F | 8 | LBVAD |
| 18 | 77 | M | 10.5 | LBVAD |
| 19 | 29 | M | 6 | NBIA1 |
Antibodies and Immunohistochemistry
The presence of 3-NT modified substrates in human tissue was detected by immunohistochemistry using the 3-NT pAb (generously provided by Joseph S. Beckman; previously characterized by Beckman et al 46 and Ye et al 47 ), and the avidin-biotin complex (ABC) system (Vectastain ABC Elite Kit, Vector Laboratories, Burlingame, CA) with the chromagen 3,3′-diaminobenzidine as previously described. 48 Briefly, sections were heated at 60°C for 60 minutes, deparaffinized and hydrated through graded ethanols, rinsed in 0.1 mol/L phosphate-buffered saline (PBS), pH 7.1, and endogenous peroxidases were neutralized with 5% H2O2 in methanol for 20 minutes. Sections were then washed and blocked in PBS containing 10% goat serum and 1% bovine serum albumin for 30 minutes at 37°C. 3-NT pAb was diluted 1:400 in PBS containing 10% goat serum and 1% bovine serum albumin, and incubated at 37°C for 90 minutes. Slides were washed and incubated with biotinylated goat anti-rabbit antibody at 37°C for 40 minutes. Slides were washed and incubated with ABC solution at 37°C for 30 minutes. Bound antibody complexes were visualized with 3,3′-diaminobenzidine followed by a brief wash in distilled water. The sections were then lightly counterstained with hematoxylin, dehydrated, and coverslipped. Negative controls for the 3-NT pAb included preabsorption of this antibody with a 10-fold excess of purified 3-NT and pre-incubation of tissue sections with 100 mmol/L dithionite as described previously. 48
Quantitation of GCIs and LBs
Consecutive 6-μm sections were taken from each of the MSA (cerebellum) or DLB/LBVAD (cingulate cortex) brains and immunostained with either the 3-NT pAb as described above, or a mouse anti-α-syn-specific monoclonal antibody, Syn 202, 49 to count the total number of GCIs or LBs in a given field. Immunostaining with the Syn 202 was performed as described previously. 45,50 Briefly, the sections were deparaffinized, hydrated through graded ethanols, treated with 5% H2O2 in methanol, and blocked in 2% donor horse serum in 0.1 mol/L Tris (Tris/donor horse serum) for 5 minutes. Primary incubation was performed with Syn 202 diluted 1:1,500 in Tris/DHS overnight at 4°C. After washing, sections were sequentially incubated with biotinylated secondary antibodies for 1 hour and avidin-horseradish peroxidase complex (Vectastain Standard ABC kit; Vector Laboratories) for 1 hour. Bound antibody complexes were visualized using 3,3′-diaminobenzidine.
For GCI quantification, five adjacent medium power (×200) photomicrographs were taken from the MSA cerebellar white-matter tissue sections stained with either the 3-NT pAb or Syn 202 and the total number of GCIs in all five photomicrographs was obtained. For LB quantification, the total number of LBs recognized by each antibody within entire tissue sections of cingulate cortex from DLB and LBVAD cases were counted for each antibody. The percentage of LBs or GCIs labeled with the 3-NT pAb was determined as a ratio of 3-NT pAb inclusion counts over Syn 202 inclusion counts.
Results
Immunohistochemical Localization of 3-NT in Neurodegenerative Brain Lesions
In all of the DLB and LBVAD brain sections (see Table 1 ▶ for a summary of cases studied), the 3-NT pAb robustly stained cortical and nigral LBs, as well as many LNs (Figure 1, a–c) ▶ . In the substantia nigra pars compacta, the core and peripheral halo of classical LBs were labeled by this antibody (Figure 1c) ▶ , whereas robust 3-NT immunoreactivity was observed throughout many GCIs in the cerebellar white matter in all MSA brains examined here (Figure 1, d–f) ▶ , and these 3-NT-positive GCIs were seen in satellite, interfascicular, and perivascular oligodendrocytes.
Figure 1.
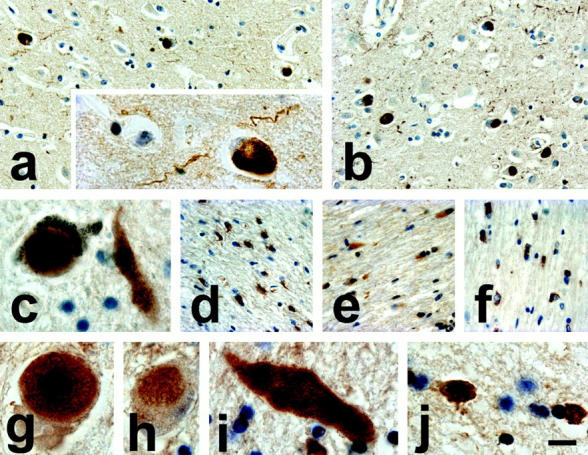
Immunostaining with the 3-NT pAb in sections of DLB, LBVAD, MSA, and NBIA1 brains. a and b: 3-NT immunostaining of α-syn lesions in the cingulate cortex of DLB and LBVAD brains, respectively. The insert in a shows a high-power magnification of a typical 3-NT-positive LB and LN from the same section. c: Intense 3-NT immunostaining throughout a classical LB in a pigmented substantia nigra pars compacta neuron of an LBVAD brain. d–f: Robust 3-NT immunostaining is seen in multiple GCIs throughout cerebellar white matter of three different MSA brains. In the NBIA1 brain, intense 3-NT immunoreactivity is seen in LB-like inclusions of the globus pallidus (g and h), as well as in neuraxonal spheroids (i), and GCIs from the insular cortex (j). Scale bars, 30 μm (a, b, d–f)and 10 μm (inset in a, c, g–j).
In the NBIA1 brain, the 3-NT pAb immunolabeled LB-like inclusions (Figure 1, g and h) ▶ and neuraxonal spheroids (Figure 1i) ▶ of the globus pallidus. As in LBs of DLB and LBVAD brains, 3-NT immunoreactivity was seen in the core and halo regions of LB-like inclusions of the NBIA1 brain (Figure 1g) ▶ , and white-matter GCIs also were extensively immunoreactive for 3-NT (Figure 1j) ▶ . The specificity of this 3-NT pAb for 3-NT residues was confirmed using control sections treated with dithionite (which reduces 3-NT to 3-aminotyrosine), and by preabsorbing the 3-NT pAb with 3-NT before immunohistochemistry because both control experiments completely abolished the labeling of all pathological inclusions by this antibody (data not shown).
Quantitation of 3-NT Immunoreactive LBs and GCIs
The percentage of α-syn-positive cortical LBs that were also 3-NT immunoreactive ranged from 61 to 100% (mean, 76.2%) in DLB brains and from 57 to 80% (mean, 67.8%) in LBVAD brains (Table 2) ▶ . Presumably because of section-to-section variation in the number of LBS, one DLB brain had slightly more 3-NT-immunolabeled LBs than those labeled by antibodies to α-syn in adjacent sections, and this case (identified by an asterisk in Table 2 ▶ ) was assigned a grade of 100%. Further, the percentage of α-syn-positive GCIs in the MSA brains that were 3-NT immunoreactive ranged from 56 to 76% (mean, 65.4%). Finally, there was no apparent relationship between the percentage of 3-NT-positive LBs or GCIs and the severity of the neuropathology, as reflected by the abundance of these α-syn inclusions.
Table 2.
Percentage of 3-NT-Positive Inclusions
| Case no. | Diagnosis | Percentage |
|---|---|---|
| 1 | MSA | 76 |
| 2 | MSA | 57 |
| 3 | MSA | 60 |
| 4 | MSA | 56 |
| 5 | MSA | 63 |
| 6 | MSA | 70 |
| 7 | MSA | 76 |
| 8 | DLB | 63 |
| 9 | DLB | 61 |
| 10 | DLB | NA |
| 11 | DLB | 100* |
| 12 | DLB | 90 |
| 13 | DLB | 67 |
| 14 | LBVAD | 79 |
| 15 | LBVAD | 60 |
| 16 | LBVAD | 57 |
| 17 | LBVAD | 80 |
| 18 | LBVAD | 63 |
Asterisk indicates a DLB brain that had slightly more 3-NT-immunolabeled LBs than those labeled by antibodies to α-syn in adjacent sections.
Western Blot Analysis of Nitrated Proteins Recognized by the 3-NT pAb
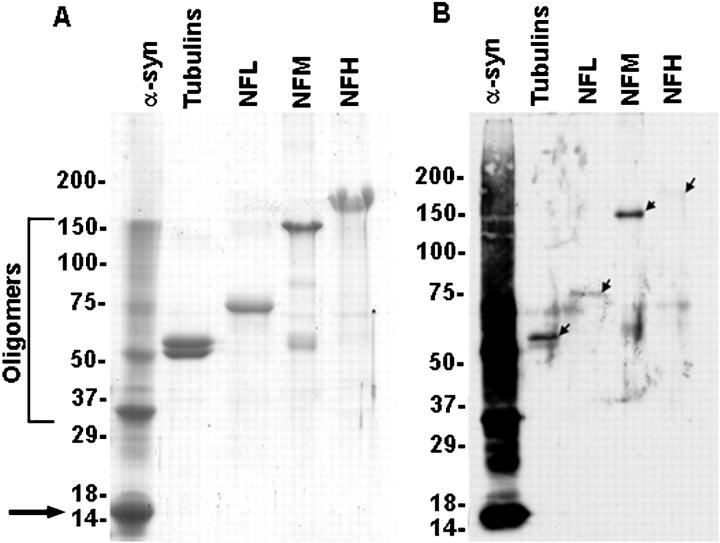
α-Syn is the major component of LBs and GCIs, however other proteins such as NF subunits 41,51,52 and tubulins 53,54 are prominent constituents of LBs and GCIs, respectively. Because it is plausible that the 3-NT pAb could recognize a variety of nitrated proteins, Western blot analysis was used to compare the relative specificity of this antibody for these proteins after in vitro nitration. Human α-syn, bovine microtubule-derived tubulins, as well as mouse NFL and bovine NFM and NFH were exposed under identical conditions to the same quantity of peroxynitrite in the presence of CO2. As shown in Figure 2 ▶ , the immunoreactivity for in vitro-nitrated α-syn was significantly more intense than that for in vitro-nitrated tubulins or NF proteins. This dramatic difference may be because of more extensive nitration of α-syn, or greater affinity of this 3-NT pAb for nitrated α-syn which is known to be nitrated in vitro at all four of its tyrosine residues, primarily because of the random coil conformation that this protein assumes in solution. 40 Indeed, other proteins may assume alternative secondary structures that confer a selective susceptibility of their tyrosine residues to nitration, as in NFL. 55 Notably, the higher molecular mass species of α-syn seen in Figure 2 ▶ probably reflect peroxynitrite-induced o-o′-dityrosine cross-link formation because α-syn has been show to be modified by this reaction more readily than other proteins. 40
Figure 2.
Western blot analysis of 3-NT pAb antibody. A: Coomassie-stained gel of in vitro nitrated α-syn, tubulin, NFL, NFM, and NFH. Five μg of each protein were resolved by electrophoresis on separate lanes of a discontinuous 6% to 10% to 12% sodium dodecyl sulfate-polyacrylamide gel. The arrow on the left denotes monomeric α-syn. B: In Western blots the 3-NT pAb variably recognizes each of the nitrated proteins but none are as intensely immunoreactive as nitrated α-syn. Note the robust recognition of monomeric α-syn as well as higher oligomeric α-syn species by this antibody. The arrows in B identify the far less intensely labeled immunobands for each of the other proteins. Fifty ng of each protein were resolved by electrophoresis on a discontinuous 6% to 10% to 12% sodium dodecyl sulfate-polyacrylamide gel. After electrophoretic transfer to nitrocellulose membrane, the blot was blocked with 5% skimmed milk, sequentially incubated with the 3-NT pAb and anti-rabbit-horseradish peroxidase-conjugated antibody and developed with enhanced luminol reagents (Dupont-New England Nuclear). The position of the molecular weight markers are indicated on the left.
Discussion
Although evidence that oxidative and nitrative modifications occur in several different neurodegenerative diseases continues to accumulate, it remains unclear if these modifications play a mechanistic role in brain degeneration, or if they deleteriously affect neurons and glia in these disorders. Similarly, although many signature lesions of a large number of distinct neurodegenerative diseases are composed of abnormal aggregates of different brain proteins, 56 it remains incompletely understood how they compromise the function and viability of neurons and glia. Because oxidative and nitrative injury may play a mechanistic role in the pathogenesis of one or more of these signature lesions, the detection of 3-NT immunoreactivity in some of these pathological protein aggregates by a limited number of published studies implies that components of these lesions have been modified by nitrative and oxidative injury. 13-15 Thus, to extend previous reports of the presence of 3-NT-modified residues in studies that focused exclusively on Alzheimer’s disease neurofibrillary tangles and LBs in Parkinson’s disease brains, 13-15 we conducted the studies described here which showed that protein nitration is not limited to the LBs of Parkinson’s disease. Indeed, we demonstrated that this also occurs in LBs of DLB and LBVAD brains, as well as in NBIA1 LB-like inclusions. The presence of 3-NT immunoreactivity also was demonstrated in all other α-syn rich neuropathological hallmarks of MSA, NBIA1, DLB, or LBVAD (ie, LNs, GCIs, neuronal cytoplasmic inclusions, and neuraxonal spheroids) and our quantitative analysis showed that the majority of these inclusions contain 3-NT modified protein.
Taken together with previous observations, our studies suggest that α-syn is nitrated in the α-syn lesions detected here by the 3-NT pAb. The abundance of α-syn in LBs and GCIs and the recent observation that α-syn is an excellent substrate for nitration 40 provide compelling support for this view. Further, because we also showed that nitrated α-syn was far more intensely labeled by the 3-NT pAb than any of the other nitrated proteins examined here by Western blot, it is highly likely that α-syn is the major 3-NT-modified protein in the lesions we studied. Nonetheless, additional studies are needed to confirm this using complementary methods and additional synucleinopathy brains.
Because the majority (57 to 100%) of the inclusions counted here, (ie, LBs and GCIs), contained 3-NT immunoreactivity, it is tempting to speculate that nitrative or oxidative damage may cause aggregation of proteins to form these inclusions. However, not all inclusions were labeled by the 3-NT pAb and this may be because of the fact that the generation of 3-NT by nitration is enzymatically reversible, and denitration of 3-NT may occur throughout an extended period of time if normal cellular reductive capacities are re-established. Thus, the generation of additional antibodies that specifically recognize the more stable o-o′-dityrosine modification induced by nitration may demonstrate the presence of nitrative injury in all of these α-syn inclusions.
The effects of α-syn nitration are undetermined, but it is possible that nitration may render α-syn more resistant to proteolysis or alter other properties of this synaptic protein thereby playing a mechanistic role in the formation of α-syn lesions as well as in the onset/progression of synucleinopathies. Nitrating species also may contribute to the pathogenesis of α-syn lesions by oxidizing tyrosine residues to form o-o′-dityrosine resulting in the covalent cross-linking of α-syn and the formation of stable α-syn polymers. 40 Indeed, o-o′-dityrosine formation may be more damaging than the 3-NT modification because this alteration may be reversed enzymatically whereas o-o′-dityrosine cross-linking is more stable and it may retard or prevent the removal of abnormal protein aggregates. 57,58
These uncertainties not withstanding, based on the data described here, together with evidence that o-o′-dityrosine cross-linking of α-syn leads to the formation of stable α-syn polymers, 40 we suggest that loss of neuronal or glial oxidative protective mechanisms may have deleterious effects on the normal functions or metabolism of α-syn and thereby contribute to the onset/progression of neurodegenerative synucleinopathies. Thus, further studies of the role of oxidative and nitrative injury in mechanisms underlying these and other neurodegenerative disorders may lead to the identification of therapeutic targets for the prevention or reversal of these diseases.
Acknowledgments
We thank Dr. Walter Mushynski for the gift of the NFL-pET-23d plasmid and the families of the patients that make this research possible.
Footnotes
Address reprint requests to John Q. Trojanowski, MD, PhD, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, 3rd Floor Maloney Building, 3600 Spruce St., Philadelphia, PA 19104. E-mail: trojanow@mail.med.upenn.edu.
Supported by grants from the National Institutes of Health and by a Pioneer Award from the Alzheimer’s Association. B. I. G. is the recipient of a fellowship from the Human Frontier Science Program Organization.
References
- 1.Markesbery WR, Carney JM: Oxidative alterations in Alzheimer’s disease. Brain Pathol 1999, 9:133-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenner P, Olanow CW: Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology 1996, 47:S161-S170 [DOI] [PubMed] [Google Scholar]
- 3.Jenner P: Oxidative mechanisms in nigral cell death in Parkinson’s disease. Mov Disord 1998, 13(Suppl 1):24-34 [PubMed] [Google Scholar]
- 4.Lyras L, Perry RH, Perry EK, Ince PG, Jenner A, Jenner P, Halliwell B: Oxidative damage to proteins, lipids, and DNA in cortical brain regions from patients with dementia with Lewy bodies. J Neurochem 1998, 71:302-312 [DOI] [PubMed] [Google Scholar]
- 5.Cookson MR, Shaw PJ: Oxidative stress and motor neurone disease. Brain Pathol 1999, 9:165-186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browne SE, Ferrante RJ, Beal MF: Oxidative stress in Huntington’s disease. Brain Pathol 1999, 9:147-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White CR, Patel RP, Darley-Usmar V: Nitric oxide donor generation from reactions of peroxynitrite. Methods Enzymol 1999, 301:288-298 [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell VB, Eiserich JP, Chumley PH, Jablonsky MJ, Krishna NR, Kirk M, Barnes S, Darley-Usmar VM, Freeman BA: Nitration of unsaturated fatty acids by nitric oxide-derived reactive nitrogen species peroxynitrite, nitrous acid, nitrogen dioxide, and nitronium ion. Chem Res Toxicol 1999, 12:83-92 [DOI] [PubMed] [Google Scholar]
- 9.Ischiropoulos H: Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys 1998, 356:1-11 [DOI] [PubMed] [Google Scholar]
- 10.Gow A, Duran D, Thom SR, Ischiropoulos H: Carbon dioxide enhancement of peroxynitrite-mediated protein tyrosine nitration. Arch Biochem Biophys 1996, 333:42-48 [DOI] [PubMed] [Google Scholar]
- 11.van der Vliet A, Eiserich JP, Halliwell B, Cross CE: Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem 1997, 272:7617-7625 [DOI] [PubMed] [Google Scholar]
- 12.Wu W, Chen Y, Hazen SL: Eosinophil peroxidase nitrates protein tyrosyl residues. Implications for oxidative damage by nitrating intermediates in eosinophilic inflammatory disorders. J Biol Chem 1999, 274:25933-25944 [DOI] [PubMed] [Google Scholar]
- 13.Good PF, Hsu A, Werner P, Perl DP, Olanow CW: Protein nitration in Parkinson’s disease. J Neuropathol Exp Neurol 1998, 57:338-342 [DOI] [PubMed] [Google Scholar]
- 14.Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G: Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J Neurosci 1997, 17:2653-2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Good PF, Werner P, Hsu A, Olanow CW, Perl DP: Evidence of neuronal oxidative damage in Alzheimer’s disease. Am J Pathol 1996, 149:21-28 [PMC free article] [PubMed] [Google Scholar]
- 16.George JM, Jin H, Woods WS, Clayton DF: Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron 1995, 15:361-372 [DOI] [PubMed] [Google Scholar]
- 17.Jakes R, Spillantini MG, Goedert M: Identification of two distinct synucleins from human brain. FEBS Lett 1994, 345:27-32 [DOI] [PubMed] [Google Scholar]
- 18.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O: Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 1998, 18:106-108 [DOI] [PubMed] [Google Scholar]
- 19.Papadimitriou A, Veletza V, Hadjigeorgiou GM, Patrikiou A, Hirano M, Anastasopoulos I: Mutated alpha-synuclein gene in two Greek kindreds with familial PD: incomplete penetrance? Neurology 1999, 52:651-654 [DOI] [PubMed] [Google Scholar]
- 20.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL: Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 1997, 276:2045-2047 [DOI] [PubMed] [Google Scholar]
- 21.Arima K, Ueda K, Sunohara N, Hirai S, Izumiyama Y, Tonozuka-Uehara H, Kawai M: Immunoelectron-microscopic demonstration of NACP/alpha-synuclein-epitopes on the filamentous component of Lewy bodies in Parkinson’s disease and in dementia with Lewy bodies. Brain Res 1998, 808:93-100 [DOI] [PubMed] [Google Scholar]
- 22.Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T: Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol 1998, 152:879-884 [PMC free article] [PubMed] [Google Scholar]
- 23.Bayer TA, Jakala P, Hartmann T, Havas L, McLean C, Culvenor JG, Li QX, Masters CL, Falkai P, Beyreuther K: Alpha-synuclein accumulates in Lewy bodies in Parkinson’s disease and dementia with Lewy bodies but not in Alzheimer’s disease beta-amyloid plaque cores. Neurosci Lett 1999, 266:213-216 [DOI] [PubMed] [Google Scholar]
- 24.Mezey E, Dehejia AM, Harta G, Tresser N, Suchy SF, Nussbaum RL, Brownstein MJ, Polymeropoulos MH: Alpha synuclein is present in Lewy bodies in sporadic Parkinson’s disease. Mol Psychiatry 1998, 3:493-499 [DOI] [PubMed] [Google Scholar]
- 25.Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M: Alpha-synuclein in Lewy bodies. Nature 1997, 388:839-840 [DOI] [PubMed] [Google Scholar]
- 26.Takeda A, Hashimoto M, Mallory M, Sundsumo M, Hansen L, Sisk A, Masliah E: Abnormal distribution of the non-Abeta component of Alzheimer’s disease amyloid precursor/alpha-synuclein in Lewy body disease as revealed by proteinase K and formic acid pretreatment. Lab Invest 1998, 78:1169-1177 [PubMed] [Google Scholar]
- 27.Wakabayashi K, Hayashi S, Kakita A, Yamada M, Toyoshima Y, Yoshimoto M, Takahashi H: Accumulation of alpha-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathol (Berl) 1998, 96:445-452 [DOI] [PubMed] [Google Scholar]
- 28.Arima K, Ueda K, Sunohara N, Arakawa K, Hirai S, Nakamura M, Tonozuka-Uehara H, Kawai M: NACP/alpha-synuclein immunoreactivity in fibrillary components of neuronal and oligodendroglial cytoplasmic inclusions in the pontine nuclei in multiple system atrophy. Acta Neuropathol (Berl) 1998, 96:439-444 [DOI] [PubMed] [Google Scholar]
- 29.Dickson DW, Liu W, Hardy J, Farrer M, Mehta N, Uitti R, Mark M, Zimmerman T, Golbe L, Sage J, Sima A, D’Amato C, Albin R, Gilman S, Yen S: Widespread alterations of alpha-synuclein in multiple system atrophy. Am J Pathol 1999, 155:1241-1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gai WP, Power JH, Blumbergs PC, Blessing WW: Multiple-system atrophy: a new alpha-synuclein disease? Lancet 1998, 352:547-548 [DOI] [PubMed] [Google Scholar]
- 31.Mezey E, Dehejia A, Harta G, Papp MI, Polymeropoulos MH, Brownstein MJ: Alpha synuclein in neurodegenerative disorders: murderer or accomplice? Nat Med 1998, 4:755-757 [DOI] [PubMed] [Google Scholar]
- 32.Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M: Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett 1998, 251:205-208 [DOI] [PubMed] [Google Scholar]
- 33.Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VM: Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol 1998, 44:415-422 [DOI] [PubMed] [Google Scholar]
- 34.Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H: Alpha-synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett 1998, 249:180-182 [DOI] [PubMed] [Google Scholar]
- 35.Arawaka S, Saito Y, Murayama S, Mori H: Lewy body in neurodegeneration with brain iron accumulation type 1 is immunoreactive for alpha-synuclein. Neurology 1998, 51:887-889 [DOI] [PubMed] [Google Scholar]
- 36.Wakabayashi K, Yoshimoto M, Fukushima T, Koide R, Horikawa Y, Morita T, Takahashi H: Widespread occurrence of alpha-synuclein/NACP-immunoreactive neuronal inclusions in juvenile and adult-onset Hallervorden-Spatz disease with Lewy bodies. Neuropathol Appl Neurobiol 1999, 25:363-368 [DOI] [PubMed] [Google Scholar]
- 37.Giasson BI, Uryu K, Trojanowski JQ, Lee VMY: Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem 1999, 274:7619-7622 [DOI] [PubMed] [Google Scholar]
- 38.Tokutake S, Hutchison SB, Pachter JS, Liem RK: A batchwise purification procedure of neurofilament proteins. Anal Biochem 1983, 135:102-105 [DOI] [PubMed] [Google Scholar]
- 39.Balin BJ, Clark EA, Trojanowski JQ, Lee VM: Neurofilament reassembly in vitro: biochemical, morphological and immuno-electron microscopic studies employing monoclonal antibodies to defined epitopes. Brain Res 1991, 556:181-195 [DOI] [PubMed] [Google Scholar]
- 40.Souza JM, Giasson BI, Chen Q, Lee VMY, Ischiropoulos H: Dityrosine cross-linking promotes formation of stable alpha-synuclein polymers: implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J Biol Chem 2000, 275:18344-18349 [DOI] [PubMed] [Google Scholar]
- 41.Schmidt ML, Murray J, Lee VMY, Hill WD, Wertkin A, Trojanowski JQ: Epitope map of neurofilament protein domains in cortical and peripheral nervous system Lewy bodies. Am J Pathol 1991, 139:53-65 [PMC free article] [PubMed] [Google Scholar]
- 42.Gelb DJ, Oliver E, Gilman S: Diagnostic criteria for Parkinson disease. Arch Neurol 1999, 56:33-39 [DOI] [PubMed] [Google Scholar]
- 43.Gilman S, Low PA, Quinn N, Albanese A, Ben Shlomo Y, Fowler CJ, Kaufmann H, Klockgether T, Lang AE, Lantos PL, Litvan I, Mathias CJ, Oliver E, Robertson D, Schatz I, Wenning GK: Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci 1999, 163:94-98 [DOI] [PubMed] [Google Scholar]
- 44.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH: Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996, 47:1113-1124 [DOI] [PubMed] [Google Scholar]
- 45.Trojanowski JQ, Schuck T, Schmidt ML, Lee VMY: Distribution of tau proteins in the normal human central and peripheral nervous system. J Histochem Cytochem 1989, 37:209-215 [DOI] [PubMed] [Google Scholar]
- 46.Beckman JS, Ye YZ, Anderson PG, Chen J, Accavitti MA, Tarpey MM, White CR: Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe-Seyler 1994, 375:81-88 [DOI] [PubMed] [Google Scholar]
- 47.Ye YZ, Strong M, Huang ZQ, Beckman JS: Antibodies that recognize nitrotyrosine. Methods Enzymol 1996, 269:201-209 [DOI] [PubMed] [Google Scholar]
- 48.Viera L, Ye YZ, Estevez AG, Beckman JS: Immunohistochemical methods to detect nitrotyrosine. Methods Enzymol 1999, 301:373-381 [DOI] [PubMed] [Google Scholar]
- 49.Giasson BI, Jakes R, Goedert M, Duda JE, Leight S, Trojanowski JQ, Lee VMY: A panel of epitope-specific antibodies detects protein domains distributed throughout human alpha-synuclein in Lewy bodies of Parkinson’s disease. J Neurosci Res 2000, 59:528-533 [DOI] [PubMed] [Google Scholar]
- 50.Lippa CF, Schmidt ML, Lee VMY, Trojanowski JQ: Antibodies to alpha-synuclein detect Lewy bodies in many Down’s syndrome brains with Alzheimer’s disease. Ann Neurol 1999, 45:353-357 [DOI] [PubMed] [Google Scholar]
- 51.Goldman JE, Yen SH, Chiu FC, Peress NS: Lewy bodies of Parkinson’s disease contain neurofilament antigens. Science 1983, 221:1082-1084 [DOI] [PubMed] [Google Scholar]
- 52.Hill WD, Lee VMY, Hurtig HI, Murray JM, Trojanowski JQ: Epitopes located in spatially separate domains of each neurofilament subunit are present in Parkinson’s disease Lewy bodies. J Comp Neurol 1991, 309:150-160 [DOI] [PubMed] [Google Scholar]
- 53.Nakazato Y, Yamazaki H, Hirato J, Ishida Y, Yamaguchi H: Oligodendroglial microtubular tangles in olivopontocerebellar atrophy. J Neuropathol Exp Neurol 1990, 49:521-530 [DOI] [PubMed] [Google Scholar]
- 54.Papp MI, Lantos PL: Accumulation of tubular structures in oligodendroglial and neuronal cells as the basic alteration in multiple system atrophy. J Neurol Sci 1992, 107:172-182 [DOI] [PubMed] [Google Scholar]
- 55.Crow JP, Ye YZ, Strong M, Kirk M, Barnes S, Beckman JS: Superoxide dismutase catalyzes nitration of tyrosines by peroxynitrite in the rod and head domains of neurofilament-L. J Neurochem 1997, 69:1945-1953 [DOI] [PubMed] [Google Scholar]
- 56.Trojanowski JQ, Goedert M, Iwatsubo T, Lee VM: Fatal attractions: abnormal protein aggregation and neuron death in Parkinson’s disease and Lewy body dementia. Cell Death Differ 1998, 5:832-837 [DOI] [PubMed] [Google Scholar]
- 57.Gow AJ, Duran D, Malcolm S, Ischiropoulos H: Effects of peroxynitrite-induced protein modifications on tyrosine phosphorylation and degradation. FEBS Lett 1996, 385:63-66 [DOI] [PubMed] [Google Scholar]
- 58.Kamisaki Y, Wada K, Bian K, Balabanli B, Davis K, Martin E, Behbod F, Lee YC, Murad F: An activity in rat tissues that modifies nitrotyrosine-containing proteins. Proc Natl Acad Sci USA 1998, 95:11584-11589 [DOI] [PMC free article] [PubMed] [Google Scholar]