Abstract
Nuclear pore complexes are large, elaborate macromolecular structures that mediate the bidirectional nucleocytoplasmic traffic. In vertebrates, nuclear pore complexes comprise 50 to 100 proteins termed nucleoporins (Nup). An 88-kd nucleoporin (Nup88) has been recently cloned and characterized, and found to be associated in a dynamic subcomplex with the oncogenic nucleoporin CAN/Nup 214. We have produced a polyclonal antiserum to Nup88, and found that it immunoreacts convincingly in conventional tissue sections of 214 samples of malignant tumors of many types. All carcinomas were stained irrespective of site or line of differentiation; the majority of cases reacted strongly and extensively. In situ carcinomas and highly dysplastic epithelia were similarly reactive. Samples of malignant mesotheliomas, gliomas, sarcomas, and lymphoreticular tumors were also stained. Substantial reactions were also found in certain fetal tissues. Focal reactions were noted in some reactive-proliferative processes. Most benign epithelial and mesenchymal tumors and hyperplasias, and normal adult tissues reacted weakly and sporadically or not at all. Immunoblot analysis of selected samples strongly corroborated those findings. If further substantiated, our findings indicate that Nup88 could be regarded as a selective yet broadly based proliferation marker of potential significance in the histological evaluation and diagnosis of malignant transformation. Its ready applicability on conventional paraffin sections and on cytological preparations may broaden its clinical and investigative significance.
In 1993, a monoclonal antibody directed against Candida krusei cytochrome c was shown to react with a cytoplasmic fraction protein of a human lung carcinoma cell line. 1 Independently, it was reported that monoclonal antibody C6, generated against Candida albicans mannoproteins, reacted specifically with a Mr 43,000 molecule from samples of human ovarian carcinoma but not with nonneoplastic counterpart cells. 2 Subsequently, we showed that this monoclonal antibody recognized in immunoblots an additional protein of Mr 88,000. 3 Using a cDNA library, we recognized the mammalian molecule as nucleoporin (Nup) 88 3 ; and, identified it as a protein located at the nuclear membrane known to be involved in nuclear-cytoplasmic transport. 4,5 In turn, Nup88 had been found to be associated with the central domain of CAN/Nup214, a nuclear pore complex component putatively implicated in nuclear protein import, nuclear mRNA export, and the regulation of the cell cycle. 6 Notably, the CAN/Nup214 proto-oncogene is involved in chromosomal rearrangements related to two variants of leukemia. 7,8
As part of the investigation that led to the recognition that the molecule bound by monoclonal antibody C6 corresponded to Nup88, a polyclonal antiserum directed to the pertinent recombinant protein was generated. By immunohistochemistry, this antiserum was shown to recognize several human tumor cell lines as well as ovarian carcinomas in tissue sections; parallel results were obtained by immunoblot analysis. 3 We now report that this antiserum immunostains richly and extensively conventional histological sections of 214 samples representing a wide spectrum of malignant tumors including carcinomas and sarcomas of diverse sites, lineages, and differentiation as well as some mesotheliomas, gliomas, melanomas, and lymphoreticular neoplasms. Certain fetal tissues were similarly stained. Notably, benign neoplasms and reparative processes showed only focal and less intense reactions whereas normal adult cells reacted only sporadically and weakly. Immunoblots of selected samples showed parallel results. Our findings indicate that this molecule most probably corresponds to Nup88. Its significant overexpression and its exceedingly wide distribution across a wide spectrum of cancers and precancerous dysplasias raise the possibility of using it as a broadly based generic histodiagnostic marker of malignant transformation.
Materials and Methods
Samples
Cases were selected on the basis of known diagnoses from the files of the Rush-Presbyterian-St. Luke’s Medical Center, Chicago, and the Hospital of the Faculty de Medicine, Bilbao, Spain. A total of 214 malignant tumors were examined; benign tumors, hyperplasias, and related conditions were also included (see Table 1 ▶ ). Most of these cases had been extensively studied and characterized in previous studies. 9-13 Surgical procedures were performed with due consent, and were based on widely accepted therapeutic and/or diagnostic protocols. Autopsy samples from adult and fetal cases were obtained from Rush-Presbyterian-St. Luke’s Medical Center; autopsies were performed based on duly obtained consent. The anonymity of the patients was protected in all cases.
Table 1.
Nup88 Immunoreactivity of Tumors and Related Conditions
| Site and diagnosis | No. of cases and reaction | Extent of reaction* | Intensity of reaction† |
|---|---|---|---|
| Epithelial tumors | |||
| Stomach | |||
| Infiltrating adenocarcinoma | 11 /11 | 3+ /5+ | m/s |
| In situ carcinoma | 2 /2 | 3+ /4+ | m/s |
| Colon | |||
| Infiltrating adenocarcinoma | 12 /12 | 3+ /5+ | m/s |
| In situ adenocarcinoma | 3 /3 | 2+ /4+ | m |
| Villous adenoma | 3 /3 | 2+ /3+ | m |
| Tubular adenoma | 5 /5 | 2+ /3+ | w/m |
| Neuroendocrine carcinoma | 2 /2 | 3+ /4+ | m/s |
| Liver | |||
| Hepatocellular carcinoma | 4 /2 | 3+ /5+ | m/s |
| Dysplastic nodules | 2 /2 | 2+ /3+ | w |
| Pancreas | |||
| Adenocarcinoma | 7 /7 | 3+ /4+ | m/s |
| Neuroendocrine carcinoma | 3 /3 | 3+ /4+ | m/s |
| Breast | |||
| Infiltrating ductal carcinoma | 14 /14 | 3+ /5 | m/s |
| Infiltrating lobular carcinoma | 12 /12 | 3+ /5+ | m/s |
| In situ ductal carcinoma | 16 /16 | 3+ /4+ | m |
| In situ lobular carcinoma | 5 /5 | 3+ | m |
| Fibroadenoma | 2 /5 | 1+ | w |
| Fibrocystic disease | 16 /28 | 1+ /3+ | w/m |
| Lung | |||
| Squamous carcinoma | 8 /8 | 3+ /5+ | m/s |
| Adenocarcinoma | 12 /12 | 3+ /5+ | m/s |
| Bronchioloalveolar carcinoma | 2 /2 | 3+ /4+ | m |
| Large-cell carcinoma | 3 /3 | 3+ /5+ | m/s |
| Neuroendocrine carcinoma | 14 /14 | 3+ /5+ | m/s |
| Carcinoid | 9 /9 | 2+ /4+ | m |
| Hyperplastic bronchi | 3 /3 | 2+ /3+ | w/m |
| Ovary | |||
| Cystadenoma | 2 /3 | 2+ | w/m |
| Benign teratoma | 1 /1 | 2+ | w/m |
| Borderline serous carcinoma | 2 /2 | 2+ /3+ | m |
| Borderline mucinous carcinoma | 2 /2 | 2+ /3+ | m |
| Serous carcinoma | 6 /6 | 3+ /5+ | m/s |
| Mucinous carcinoma | 4 /4 | 3+ /5+ | m/s |
| Endometrioid carcinoma | 1 /1 | 4+ | m/s |
| Clear-cell carcinoma | 2 /2 | 3+ /5 | m/s |
| Uterus | |||
| Endometrial carcinoma | 12 /12 | 3+ /5+ | m/s |
| Endometrial hyperplasia | 4 /10 | 0 /2+ | w |
| Prostate | |||
| Adenocarcinoma | 11 /11 | 3+ /5+ | m/s |
| PIN, high grade | 6 /6 | 2+ /3+ | m/s |
| PIN, low grade | 2 /4 | 1+ /2+ | w/m |
| Benign glandular hyperplasia | 2 /6 | (+) /2+ | w/m |
| Kidney | |||
| Clear cell carcinoma | 4 /4 | 3+ /5+ | m/s |
| Adrenal | |||
| Cortical adenoma | 2 /2 | 2+ /3+ | w |
| Mesenchymal tumors | |||
| Fibrosarcoma | 5 /5 | 3+ /4+ | m |
| Malignant fibrous histiocytoma | 7 /7 | 3+ /4+ | w/m |
| Kaposi sarcoma | 2 /2 | 3+ /4+ | m |
| Dermatofibrosarcoma protuberans | 5 /5 | 3+ /4+ | m |
| Giant-cell tumor, benign | 0 /2 | 0 | — |
| Leiomyoma | 2 /2 | 3+ /4+ | w |
| Atypical fibroxanthoma | 2 /2 | 2+ /3+ | w |
| Angiolipoma | 1 /2 | (+) | w |
| Miscellaneous tumors | |||
| Large-cell lymphoma | 3 /3 | 3+ /4+ | m |
| Lymphoblastic lymphoma | 1 /1 | 3+ /4+ | m |
| Hodgkin’s disease | 4 /4 | 2+ /3+ | m |
| Malignant mesothelioma | 5 /5 | 3+ /5+ | m/s |
| Benign mesothelioma | 0 /2 | 0 | — |
| Glioblastoma multiforme | |||
| Malignant melanoma | 4 /4 | 3+ /5+ | m/s |
| Infiltrating | 4 /4 | 3+ /5+ | m/s |
| In situ | 2 /2 | 3+ /4+ | m/s |
*(+) = <1% positive cells: 1+ = 1–5% positive cells; 2+ = 6 to 15% positive cells; 3+ = 16 to 50% positive cells; 4+ = 51 to 95% positive cells; 5+ = 95% positive cells.
†S, strong; m, moderate; w, weak.
Immunohistochemistry
Two paraffin blocks per case were selected; diagnoses were confirmed on conventional hematoxylin and eosin-stained sections by two independent observers (VEG and AO). All tissues had been fixed in formalin, conventionally processed, and embedded in paraffin. Sections for immunostaining were cut at 4 μm, set on coated slides, and placed on a warmer at 60°C for 1 hour; subsequently, they were deparaffinized in xylene and rehydrated in graded alcohols. No pretreatment with microwaves or enzymes before exposure to the primary antiserum was applied. Details on the preparation and characteristics of the antiserum have been recently published. 3 Staining was accomplished by the avidin-biotin-peroxidase method as originally outlined by Hsu et al 14 ; commercial reagents were used (DAKO, Carpinteria, CA). Best results were obtained when the antiserum was applied overnight in a humid chamber at 4°C at a concentration of 1/500; the diluent was that provided in a commercial kit (Ventana Medical Systems, Tucson, AZ). Binding sites were visualized with 3,3′-diaminobenzidine (Aldrich Chemicals, Danvers, MA); in the case of melanocytic lesions, alkaline phosphatase (DAKO) was used as chromogen. All sections were counterstained with hematoxylin to improve nuclear visualization. As negative controls, slides were similarly processed but omitting the primary antiserum. Staining intensity was rated as weak, moderate, or strong; in heterogeneous cases the rating of the predominant pattern prevailed. The extent of the reaction was defined by the percentage of reactive cells and graded as negative (0) to 1+ to 5+ as previously described. 15
Immunoblots
For immunoblot analysis, freshly obtained samples were placed in vials containing precooled isopentane, and snap-frozen in liquid nitrogen. All samples were kept in a deep freezer at −80°C until used. Tumors were homogenized in 1% sodium dodecyl sulfate-0.14 mol/L β-mercaptoethanol and then centrifuged over a Qiashredded (Qiagen, Germany) column to shear the DNA. The protein content of the samples was calculated with the DC protein assay system (Bio-Rad, Richmond, CA) and 15 μg of proteins were separated on 10% polyacrylamide gels. Proteins were electrotransferred to polyvinylidene difluoride membranes, blocked with nonfat milk in phosphate-buffered saline, and incubated with our polyclonal antiserum at a dilution of 1:500. After washing, the membranes were reacted with a horseradish peroxidase-labeled goat anti-rabbit, washed again, and the reacting bands were revealed with the Amersham enhanced chemoluminescence system (Amersham, Buckinghamshire, UK).
Results
Immunohistochemistry
All malignant neoplasms stained convincingly; most reactions were moderate to strong and 3+ to 5+ in extent. In situ carcinomas were invariably stained as were, albeit less so, dysplastic lesions that did not reach the level of carcinoma in situ. Benign tumors showed variably extensive and generally weak immunoreactivity. Salient points are summarized in Table 1 ▶ .
Gastric carcinomas were extensively and strongly stained. The contrast between the surrounding or overlying mostly negative mucosa and the convincingly reactive tumor was evident (Figures 1, a and b) ▶ . Reactions were similar in tumors showing variably differentiated glands, solid clusters, or linitis plastica single-cell pattern. One case showed intestinal metaplasia with some dilated glands as well as in situ carcinoma with the latter reacting strongly (Figure 1c) ▶ . The immunostaining was characteristically granular; the granules were rather large as compared with the delicate dots seen in synaptophysin reactions. 16,17 In a minority of cells, a perinuclear localization was noted but the distribution was predominantly cytoplasmic (see below). The cell membrane did not stain.
Figure 1.
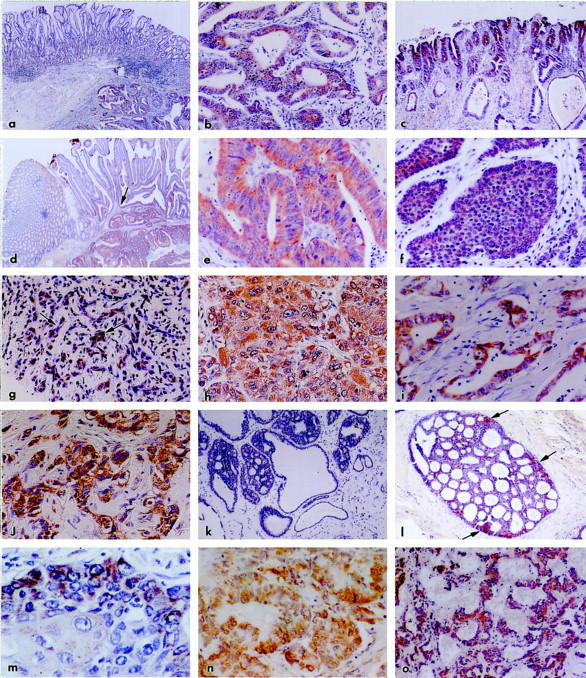
All samples were immunostained with polyclonal antiserum to Nup88; the chromogen is DAB. a: Stomach adenocarcinoma; the prominent mucosa (top) is not reactive, whereas the carcinoma invading the submucosa (bottom right) is clearly stained. Original magnification, ×60. b: Stomach adenocarcinoma; higher magnification of tumor in a shows diffuse and strong staining of the malignant glands. Original magnification, ×480. c: Stomach with extensive intestinal metaplasia; a strongly and diffusely reactive in situ carcinoma is evident. Original magnification, ×180. d: Colon adenocarcinoma; the normal mucosa on the left is negative whereas the basal aspect of the glands of a villous adenoma are moderately reactive (arrow). The overt carcinoma invading the submucosa is richly stained. Original magnification, ×120. e: Colon adenocarcinoma; higher magnification of tumor in d shows diffuse, strong, and granular reaction of malignant glands. Original magnification, ×620. f: Colon; neuroendocrine carcinoma showing portions of characteristically organoid clusters diffusely reactive for Nup88. Original magnification, ×480. g: Liver; cirrhotic septum in the vicinity of the carcinoma depicted in h. Note focal staining in proliferating bile ductules (arrows) amid nonreactive fibroconnective tissue. Original magnification, ×180. h: Liver; hepatocellular carcinoma; higher magnification shows strong, extensive, and granular reaction. Original magnification, ×620. i: Pancreas; adenocarcinoma showing strong Nup88 staining of malignant glands. Original magnification, ×420. j: Breast; infiltrating ductal carcinoma showing strongly Nup88 reactive clusters. Original magnification, ×420. k: Breast; fibrocystic changes; hyperplastic and cystic ducts are nonreactive. Original magnification, ×120. l: Breast; intraductal carcinoma, cribriform variant. The staining is diffuse but is best appreciated toward the periphery of the duct (arrows). Original magnification, ×120. m: Lung; well-differentiated squamous carcinoma; part of neoplastic cluster showing strong staining particularly of the peripheral cells; the central, more mature cells approaching pearl formation show fewer reactive granules. Original magnification, ×620. n: Lung; moderately differentiated adenocarcinoma; note strong and diffuse reaction. Original magnification, ×480. o: Bronchial carcinoid. Note moderately but diffusely reactive cell ribbons and clusters amid negative stroma. Original magnification, ×180.
Colonic adenocarcinomas were strongly and diffusely stained. Several blocks included normal mucosa, polyps with variable degrees of dysplasia, and infiltrating carcinoma. In these instances, the normal mucosa was negative, the polyps reacted paralleling the degree and extent of dysplasia, and the overt carcinomas reacted strongly (Figure 1, d and e) ▶ . Two carcinomas of mixed exocrine and neuroendocrine phenotypes and two pure neuroendocrine carcinomas were similarly stained (Figure 1f) ▶ . In polyps not associated with carcinoma, villous adenomas stained stronger and more extensively than their tubular counterparts; foci of intramucosal carcinoma stained convincingly. Hepatocellular carcinomas were strongly reactive. One of these developed in the background of advanced cirrhosis; in this case, occasional cells in proliferating bile ductules in cirrhotic septa were weakly stained (Figure 1g) ▶ whereas the adjacent carcinoma stained diffusely and strongly (Figure 1h) ▶ , and dysplastic nodules reacted moderately. Pancreatic carcinomas of variable glandular differentiation showed consistent, moderate to strong reactions (Figure 1i) ▶ ; two neuroendocrine carcinomas were similarly stained. Regardless of tumor phenotype, nonneoplastic exocrine and neuroendocrine cells in the vicinity of, or entrapped within, tumors were occasionally, focally reactive.
All infiltrating breast carcinomas of ductal (Figure 1j) ▶ or lobular types reacted convincingly, whereas ducts nearby, or entrapped within them, reacted weakly and sporadically or not at all. Mucinous (colloid) carcinomas showed strong staining of the scanty malignant cells whereas the dominant mucous pools did not react. In several carcinomas, the strongest staining was noted in the peripheral, invasive edge of the tumors. Fibrocystic changes in the vicinity of carcinomas including cysts, ductal hyperplasia, adenosis, papillomas, and apocrine metaplasia, showed weak to moderate, focal reactions, whereas similar changes not associated with carcinomas ranged from focally reactive to entirely negative (Figure 1k) ▶ . Atypical ductal hyperplasia and all variants of ductal carcinoma in situ stained convincingly (Figure 1l) ▶ , fibroadenomas reacted weakly and focally or not at all.
Pulmonary squamous, adeno-, bronchioloalveolar, large-cell, and neuroendocrine carcinomas reacted richly and extensively (Figure 1, m and n) ▶ ; in well-differentiated squamous carcinomas, the peripheral growing edge of the clusters reacted strongly whereas the central cells approaching the squamous pearls were less reactive. Hyperplastic bronchi in the vicinity or within tumors were often stained. Bronchial carcinoids stained moderately and often extensively (Figure 1o) ▶ . Ovarian carcinomas including serous, mucinous, clear-cell, and endometrioid types were convincingly stained; borderline tumors stained moderately whereas their invasive (Figure 2a) ▶ and particularly their high-grade counterparts were strongly reactive. Benign cystadenomas showed uneven, generally weak reactions. In a benign teratoma, the basal cells of the skin and adnexa, the respiratory epithelium and cartilaginous cells showed moderate staining. Endometrial carcinomas of all types reacted strongly (Figure 2b) ▶ . Several of these cases were associated with endometrial hyperplasia; and, although the carcinomas stained convincingly, the bland variants of hyperplasia did not (Figure 2c) ▶ . Also interesting was that the luminal aspect of several tumors stained less intensely than the deep, invasive aspect. In several cases, segments of fallopian tubes included in these samples showed focal, moderate reactions, as did cells comprising small follicular cysts and corpora lutea.
Figure 2.
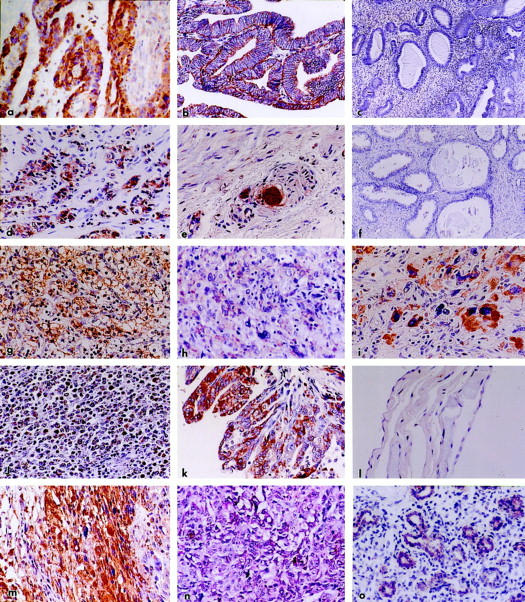
The same antiserum was used in all samples. The chromogen was DAB except for n for which alkaline phosphatase (red) was used. a: Ovary; moderately differentiated papillary serous carcinoma; note rich, diffuse reaction of neoplastic papillae. Original magnification, ×480. b: Endometrium; adenocarcinoma, well differentiated, showing strong and diffuse Nup88 reactivity. Original magnification, ×480. c: Endometrium; cystic hyperplasia; no reaction is evident. Original magnification, ×180. d: Prostate; adenocarcinoma, Gleason 4 + 5; intense reaction involving the majority of malignant cells is seen. Original magnification, ×420. e: Prostate; periphery of carcinoma shown in d. Small clusters of malignant cells are strongly stained; note also the intense reaction of neoplastic cells around and within a nerve. Original magnification, ×540. f: Prostate showing dilated, hyperplastic, and atrophic glands; no Nup88 reaction is seen. Original magnification, ×180. g: Kidney; clear cell carcinoma. A strong and extensive reaction is evident. Original magnification, ×480. h: Soft tissue; malignant fibrous histiocytoma; a weak to moderate but extensive reaction involves most neoplastic cells. Original magnification, ×480. i: Leiomyosarcoma metastatic to the lung (see Figure 3 ▶ , immunoblot B5). Strong reactivity is noted particularly in the bizarre giant cells. Original magnification, ×620. j: Lymph node; large cell lymphoma diffusely reactive for Nup88. Original magnification, ×160. k: Pleura; malignant papillary mesothelioma richly and diffusely reactive for Nup88. Original magnification, ×480. l: Abdomen; benign cystic mesothelioma; no reaction is seen. Original magnification, ×480. m: Brain; glioblastoma multiforme; a rich and extensive reaction is noted. Original magnification, ×480. n: Skin; malignant melanoma immunostained for Nup88 with alkaline phosphatase as chromogen (red). A rich and extensive reaction is evident. Original magnification, ×480. o: Fetal lung (20 weeks, 360 g). A moderate immunoreaction involves the primitive air spaces whereas the stroma appears nonreactive. Original magnification, ×320.
All prostatic carcinomas reacted strongly and diffusely irrespective of degree of differentiation (Figure 2g) ▶ whereas nonneoplastic ducts and acini entrapped within them stained weakly and sporadically or not at all. In the case of poorly differentiated and hypernephroid carcinomas, minute clusters or single reactive cells were readily detected in the stroma and, around and/or within nerves (Figure 2e) ▶ . Foci of low- and high-grade prostatic intraepithelial neoplasia were consistently stained, particularly the latter. Benign hyperplastic and atrophic glands were either negative (Figure 2f) ▶ or showed rare positive cells. All renal carcinomas examined were of clear cell type and all reacted strongly (Figure 2g) ▶ ; some reactive tubules in the vicinity stained as well but weakly. Two adrenocortical adenomas showed weak but fairly extensive reactions.
Mesenchymal Tumors
Fibrosarcomas, malignant fibrous histiocytomas (Figure 2h) ▶ , and Kaposi sarcomas stained moderately to strongly, and a single leiomyosarcoma reacted strongly in both primary and metastatic sites (Figure 2i) ▶ . Dermatofibrosarcoma protuberans, benign giant cell tumors, atypical fibroxanthomas, and angiolipomas showed focal, moderate to weak reactions. Two (uterine) leiomyomas stained rather extensively but weakly.
Miscellaneous Tumors and Fetal Tissues
Diffuse large cell lymphomas and a lymphoblastic lymphoma stained strongly and extensively (Figure 2j) ▶ . In several cases of Hodgkin’s disease, the Reed-Sternberg as well as the lacunar cells stained convincingly whereas the associated, nonneoplastic leukocytes did not. Malignant mesotheliomas stained strongly (Figure 2k) ▶ ; similar reactions were seen in epithelial, sarcomatoid, and biphasic variants. In contrast, samples from two benign cystic mesotheliomas (multiple peritoneal inclusion cysts) were consistently negative (Figure 2l) ▶ . Several small samples of glioblastoma multiforme stained strongly and extensively (Figure 2m) ▶ . In situ malignant melanomas showed convincing reactions of the dysplastic melanocytes at the base of the epidermis, and of single cells migrating upwards in the epidermis. Invasive malignant melanomas of various sites showed intense and diffuse reactions (Figure 2n) ▶ irrespective of architecture or melanin content.
Fetal samples examined ranged from 20 to 24 weeks of gestational age; tissues included lung, heart, liver, kidney, and bowel. Reactions were particularly convincing in developing bronchi and primitive air spaces (Figure 2o) ▶ as well as in the crypts of the intestinal mucosa.
Immunoblots
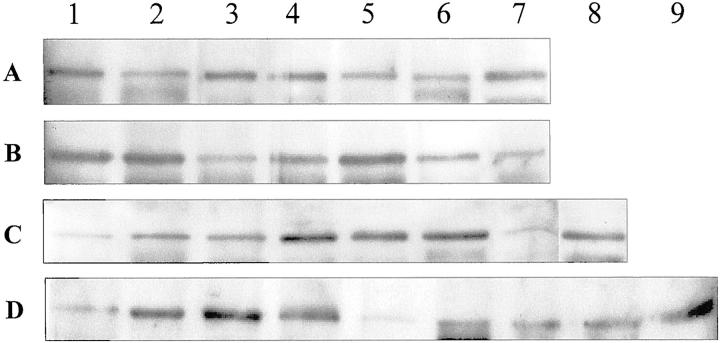
The results of these experiments are shown in Figure 3 ▶ . With a single exception, all samples of malignant tumors analyzed, showed a convincing increase in the amount of Nup88. All colon carcinomas (A1–A5) showed reactive bands that were far stronger than the controls; the comparatively weak band noted in A2 may reflect this tumor’s extensive necrosis. In the case of lung tumors, the respectable band of the bronchial carcinoid (B1) corresponds in fact to a rather intensely reactive tumor (Figure 1o) ▶ . Notice also the intense B2 and B4 bands (carcinomas) and compare with the meager B3 band that represents a carcinoma treated with radio- and chemotherapy before removal resulting in extensive tumor necrosis. The strong B5 band pertains to a leiomyosarcoma metastatic to the lung (Figure 2i) ▶ , and the relatively weak but distinct B6 band corresponds to bronchi in the vicinity of squamous carcinoma showing hyperplastic and metaplastic changes.
Figure 3.
Immunoblot analysis of Nup88 content in neoplastic, hyperplastic, and normal tissues. Total protein extracts of colon (A), lung (B), breast (C), and ovary (D) were prepared as described in Materials and Methods with the polyclonal antiserum to Nup 88. A: Colon. Lanes 1, 3, 4, and 5 represent adenocarcinomas; lane 2 represents also an adenocarcinoma but showing extensive necrosis; lane 6 is the negative control consisting of normal human lymphocytes whereas lane 7 is the positive control representing an ovarian carcinoma known to express abundant Nup88 from previous studies. B: Lung. Lane 1 represents the bronchial carcinoid depicted in Figure 1o ▶ . Lanes 2 and 4 represent an adenocarcinoma and a squamous carcinoma, respectively, whereas lane 3 is a poorly differentiated squamous carcinoma treated with chemotherapy and radiotherapy before excision. The strongly reactive lane 5 represents a metastatic leiomyosarcoma shown in Figure 2i ▶ ; lane 6 represents hyperplastic bronchi in the vicinity of but not involved by a carcinoma. Negative control in lane 7 as in A. C: Breast. Lane 1 represents typical, benign fibrocystic disease depicted in Figure 1k ▶ . Lanes 2 and 3 represent fibroadenomas; lanes 4 and 5 represent infiltrating ductal carcinomas whereas lane 6 is an infiltrating lobular carcinoma. Lane 7 is a normal control adult female breast and lane 8 is a known, positive control ovarian carcinoma. D: Ovary. Lane 1 represents a benign mucinous cystadenoma; lanes 2 and 3 represent papillary serous carcinomas, and lane 4 is a metastasis from a gastric signet-ring cell carcinoma. Lane 5 shows a surprisingly low signal given by an endometrioid carcinoma possibly reflecting sampling discrepancies. Lanes 6, 7, and 8 are negative controls and lane 9 is the positive counterpart.
The contrast between benign and malignant proliferations in the same organ is well depicted in the series of breast lesions; all carcinomas (C4–C6) show prominent bands whereas fibroadenomas (C2 and C3) and one bland variant of fibrocystic disease (C1, corresponds to Figure 1k ▶ ) show delicate bands, and the normal breast is virtually negative (C7). In the ovarian samples, the meager band noted in D1 (benign cystadenoma) is in stark contrast with the broad bands seen in the carcinomas (D2–D4). Except for problems related to limited sampling, we cannot explain the rather weak band of the endometrioid carcinoma (D5) that by immunohistochemistry was moderately stained.
Densitometric quantification of the blots showed an increased expression in carcinomas between 1.5 and 5 times greater than the normal controls. Still, it should be considered that these figures might well represent a significant underestimation for they do not take into account the considerable amount of stroma that many of these tumors had. More detailed quantitative analyses should be performed based on larger series, and subsequent to appropriate microdissection.
Discussion
Our immunohistochemical results strongly reinforced by parallel immunoblot data point to Nup88 as a molecule of exceedingly wide distribution that is consistently overexpressed in a broad spectrum of carcinomas as well as malignant mesotheliomas, and a number of sarcomas, melanomas, gliomas, and lymphoreticular tumors. Nup88 was similarly enhanced in severe dysplasias and in situ carcinomas of organs such as colon, stomach, breast, and prostate. We also showed distinct Nup88 enhancement during fetal development in sites including pulmonary primitive air spaces and intestinal crypts. Focal expression was also noted in some proliferative-reactive tissues in the vicinity of tumors, eg, hyperplastic bronchial mucosa. Conversely, Nup88 was either sporadic or not detectable in most benign tumors and hyperplasias. In normal adult tissues, Nup88 was occasionally noted in sites such as colonic crypts, bronchial mucosa, and fallopian tubes.
With regard to epithelial cancers, Nup88 enhancement was seen not only across a broad spectrum of sites but through all major differentiation lines. Thus, lung carcinomas with squamous, glandular, and neuroendocrine features were strongly positive. In the gastrointestinal tract and pancreas adeno- and neuroendocrine carcinomas were similarly reactive, whereas in the ovary, serous, mucinous, endometrioid, and clear-cell carcinomas reacted as well. Moreover, Nup88 overexpression seems to be independent of presumed histogenesis as it was found in carcinomas from diverse organs derived from all embryonal layers. In addition, as noted earlier, 3 we also found that in sites such as the ovary, high-grade carcinomas seemed to react more vigorously than their low-grade and borderline counterparts. Furthermore, in some breast and endometrial carcinomas, we noted that the invasive periphery of the tumors stained more strongly than the center. These findings suggest a spatial and temporal relationship between Nup88 overexpression and tumor expansion, and parallel previous observations wherein certain extracellular matrix proteins, eg, tenascin and cellular fibronectins are strongly enhanced in the periphery of carcinomas reflecting areas of active remodeling. 10,18
Nup88 enhancement was clearly evident in severe epithelial dysplasias and in situ carcinomas of the colon, stomach, breast, and prostate; this overexpression was noted irrespective of the presence or absence of an overt synchronous cancer. In contrast, proliferative but benign variants of conditions such as fibrocystic disease of the breast, endometrial hyperplasia, tubular adenomas of the colon, and glandular prostatic hyperplasia showed no significant Nup88 enhancement. Similar contrasts were noted in mesenchymal and other miscellaneous malignancies, eg, strong and extensive reactions in a leiomyosarcoma and in malignant mesotheliomas contrasting with weak and sporadic or absent staining in their benign counterparts. Notably, certain active reparative processes, eg, bile duct proliferation in cirrhosis, and proliferating renal tubules in the vicinity of carcinoma associated with pyelonephritis showed modest but convincing Nup88 reactions. Again, such areas are known to undergo active remodeling as reflected by the enhancement of pertinent matrix molecules. 10,18-21 These findings indicate that enhanced Nup88 reflects a selective cellular proliferation that is most often, but not exclusively, associated with the malignant or premalignant phenotype.
Some of the above findings of Nup88 overexpression in malignancy coupled with its detection in some fetal tissues suggest parallels between it and a number of oncodevelopmental marker molecules including carcinoembryonic antigen and some related substances. However, the latter molecules are for the most part cell membrane-associated glycoproteins known to have or suspected of having cell-cell adhesive functions. 22,23 In addition, they are selectively expressed in some epithelia but not in others, and are rare in nonepithelial tissues. Moreover, the punctiform, perinuclear, and cytoplasmic localization of Nup88 differs substantially from that of the above molecules. These observations added to those that Nup88 is also significantly enhanced in malignancies as diverse as carcinomas, some sarcomas and lymphomas, mesotheliomas, melanomas, and gliomas point to radical differences between Nup88 and oncodevelopmental markers currently used.
A consistent finding in the present study was the predominantly cytoplasmic localization of the overexpressed protein in the involved cells. Our previous studies on several cell lines showed that most of the protein was located at the nuclear membrane with comparatively small amounts in the cytoplasm. Noteworthy in many neoplastic and some nonneoplastic cells is the presence of aggregates of annulate lamellae 24 ; these structures are thought to derive from nuclear membranes, 25 and show features of the latter, eg, components of nuclear pore complexes including nucleoporins as described in Xenopus oocytes, 26 and in rat cells wherein they were visualized as cytoplasmic dots. 27 Therefore, we infer that the conspicuous cytoplasmic granules we found may reflect increased complements of annulate lamellae. In this context, it merits mentioning that other oncoproteins may also be aberrantly located, eg, the known nuclear-cytoplasmic mislocation of the BRCA1 gene product in breast carcinomas. 28
The polyclonal antiserum used in these experiments allowed us to recognize clearly and consistently a Mr 88,000 band in immunoblots; in addition, other weakly reactive bands were at times detected. The precise nature of these additional reactive bands remains unclear, but it should be stated that our antiserum reacts strongly with the glutathione S-transferase-Nup88 fusion proteins used for the immunization of the rabbits. 3 We cannot as yet state whether the aforementioned bands of lower molecular weight reflect degradation products of a single molecule or distinct proteins that share a similar or common epitope. The eventual identification of the epitope recognized by our antiserum should help elucidate the fragments of the proteins recognized by it. Given our current data, some questions may be said to persist as to whether the material recognized by our antiserum corresponds in fact to Nup88. Significantly, in carcinomas of various sites and in one sarcoma, strong and extensive immunostaining of tissue sections was paralleled by similarly strong Mr 88,000 reactive bands in Western blots of the same samples; conversely, in samples of hyperplasias, benign tumors, and normal tissues, weak or undetectable immunoreactions were reflected by weakly reactive bands in the corresponding immunoblots. Moreover, no differences in intensity were noted in the additional reactive bands found in tumors as well as controls thus reinforcing the notion that our antiserum is specific for Nup88. These data strongly suggest that the molecule in question corresponds indeed to Nup88.
In vertebrate cells, the nuclear pore complex is a large macromolecular aggregate 29,30 with an estimated molecular mass of 125 Mr 31 it includes 50 to 100 proteins termed nucleoporins. 32 On the other hand, in yeast, nuclear pore complexes are smaller, have a molecular mass in the range of 66 Mr, and may include 30 to 40 nucleoporins. 33,34 Among the known nucleoporins, the rather recently characterized Nup88 in its dynamic subcomplex with the oncogenic nucleoporin CAN/Nup214 seems to play several essential roles; depletion of the complex results in defective import-export processes, and eventual cell-cycle arrest; 5 and, in overexpressing cells, it seems that CAN/Nup214 and one of its interacting proteins, ie, Nup88, may function on both aspects of the nuclear pore complex. 35 Notably, preliminary experiments in our laboratory showed no increased expression of Can/Nup214 in our tumor samples thus suggesting an uncoupling of the latter from Nup88, at least in some instances. Interesting in this context is the fact that the Nup98 gene seems to be involved in therapy-related leukemias by translocation producing fusion proteins that may act as transcription factors modulating the expression of other genes. 36
We can only speculate about the possible role(s) of the overexpressed Nup88 in malignant cells. A possible explanation is that its overexpression is simply the result of increased nucleocytoplasmic transport required to meet the increased demand of proteins by transformed cells. Increased traffic is indeed known to occur in this context, and it merits mention that the diameter of the pore channel seems to be increased in transformed mammalian cells. 37 An alternative explanation is that Nup88 may play a role in the formation and maintenance of annulate lamellae as outlined above: but, although this might explain their presence it would still not clarify their function. Irrespective of these speculations, our findings suggest that Nup88 may be a potentially significant marker given its dramatic overexpression in a broad spectrum of malignant tumors of most, if not all, major types. If these results were confirmed, Nup88 might be said to approach an ideal generic marker of transformation readily demonstrable in conventional tissue sections, and possibly also in cytological samples.
Acknowledgments
The authors are grateful to Ms. Anne-Marie Fornabaro for outstanding clerical and secretarial help.
Footnotes
Address reprint requests to Dr. Victor E. Gould, Dept. of Pathology, Rush Medical College, 1653 W. Congress Pkwy, Chicago, IL 60612.
Supported by a grant from the Universidad del Pais Vasco (University of the Basque Country), Bilbao, Spain (to N. M.).
References
- 1.Yasumoto K, Setoguchi I, Kamei M, Nomoto K, Murakami H, Hasizume S: Cancer-specific binding of mouse Mab vs Candida krusei cytochrome c: an antigen recognized by a cancer-associated human Mab HB4C5. Hum Antibodies Hybridomas 1993, 4:180-186 [PubMed] [Google Scholar]
- 2.Schneider J, Moragues D, Martinez N, Romero H, Jimenez E, Ponzon J: Cross-reactivity between Candida albicans and human ovarian carcinomas revealed by monoclonal antibodies PA10F and C6. Br J Cancer 1996, 77:1015-1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez N, Alonso A, Moragues MD, Ponton J, Schneider J: The nuclear pore protein complex Nup88 is overexpressed in tumor cells. Cancer Res 1999, 59:5408-5411 [PubMed] [Google Scholar]
- 4.Fornerod M, van Deursen J, van Baal S, Reynolds A, Davis D, Murti K, Fransen J, Grosveld G: The human homologue of yeast CMR1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component Nup88. EMBO J 1997, 16:807-816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fornerod M, Boer J, van Baal S, Morreau J, Grosveld G: Interactions of cellular proteins with the leukemia-specific fusion protein DEK/CAN and SET/CAN and their normal counterpart, the nucleoporin CAN. Oncogene 1996, 13:1801-1808 [PubMed] [Google Scholar]
- 6.van Deursen J, Boer J, Kasper L, Grosveld G: G2 arrest and impaired nucleo-cytoplasmic transport in mouse embryos lacking the proto-oncogen CAN/Nup 214. EMBO J 1996, 15:5574-5583 [PMC free article] [PubMed] [Google Scholar]
- 7.von Lindern M, Fornerod M, van Baal S, Jaegle M, de Wit T, Buijs A, Grosveld G: The translocation (6;9) associated with a specific subtype of acute myeloid leukemia results in the fusion of two genes, dek and can, and the expression of a chimeric, leukemia-specific dek-can mRNA. Mol Cell Biol 1992, 12:1687-1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Lindern M, van Baal S, Wiegant J, Raap A, Hagemaijer A, Grosveld G: CAN, a putative oncogene associated with myeloid leukemogenesis can be activated by fusion of its 3′ half of different genes: characterization of the set gene. Mol Cell Biol 1992, 12:3346-3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gould VE, Shin SS, Manderino GL, Rittenhouse HG, Tomita JT, Gooch GT: Selective expression of a novel mucin-type glycoprotein in human tumors. Hum Pathol 1988, 19:623-627 [DOI] [PubMed] [Google Scholar]
- 10.Koukoulis GK, Gould VE, Bhattacharya A, Gould JE, Howeedy AA, Virtanen I: Tenascin in normal, reactive, hyperplastic and neoplastic tissues. Hum Pathol 1991, 22:636-643 [DOI] [PubMed] [Google Scholar]
- 11.Koukoulis GK, Virtanen I, Korhonen M, Laitinen L, Quaranta V, Gould VE: Immunohistochemical localization of integrins in the normal, hyperplastic and neoplastic breast. Am J Pathol 1991, 139:787-799 [PMC free article] [PubMed] [Google Scholar]
- 12.Gould VE, Doljanskaia V, Gooch GT, Bostwick DG: Immunolocalization of glycoprotein A-80 in prostatic carcinoma and prostatic intraepithelial neoplasia. Hum Pathol 1996, 27:547-552 [DOI] [PubMed] [Google Scholar]
- 13.Koukoulis GK, Shen S, Monson R, Warren WH, Virtanen I, Gould VE: Pleural mesotheliomas have an integrin profile distinct from visceral carcinomas. Hum Pathol 1998, 28:84-90 [DOI] [PubMed] [Google Scholar]
- 14.Hsu SM, Raine L, Fanger H: Use of the avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabelled antibody (PAP) procedures. J Histochem Cytochem 1981, 29:577-580 [DOI] [PubMed] [Google Scholar]
- 15.Moll R, Lee I, Gould VE, Berndt R, Roessner A, Franke WW: Immunohistochemical analysis of Ewing’s tumors: patterns of expression of intermediate filaments and desmosomal proteins indicate cell type heterogeneity and pluripotential differentiation. Am J Pathol 1987, 127:288-304 [PMC free article] [PubMed] [Google Scholar]
- 16.Wiedenmann B, Franke WW, Kuhn C, Moll R, Gould VE: Synaptophysin: a marker protein for neuroendocrine cells and neoplasms. Proc Natl Acad Sci USA 1986, 83:3500-3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gould VE, Wiedenmann B, Lee I, Dockhorn-Dworniczak B, Radosevich JA, Moll R, Franke WW: Synaptophysin expression in neuroendocrine neoplasms as determined by immunocytochemistry. Am J Pathol 1987, 126:243-257 [PMC free article] [PubMed] [Google Scholar]
- 18.Howeedy AA, Virtanen I, Laitinen L, Gould NS, Koukoulis GK, Gould VE: Differential distribution of tenascin in the normal, hyperplastic and neoplastic breast. Lab Invest 1990, 63:798-806 [PubMed] [Google Scholar]
- 19.Gould VE, Martinez-Lacabe V, Virtanen I, Sahlin KM, Schwartz MM: Differential distribution of tenascin and cellular fibronectins in acute and chronic renal allograft rejection. Lab Invest 1992, 67:71-79 [PubMed] [Google Scholar]
- 20.Assad L, Schwartz MM, Virtanen I, Gould VE: Immunolocalization of tenascin and cellular fibronectins in diverse glomerulopathies. Virchows Arch B Cell Pathol 1993, 63:307-316 [DOI] [PubMed] [Google Scholar]
- 21.Koukoulis GK, Koso-Thomas AK, Zardi L, Gabbiani G, Gould VE: Enhanced tenascin expression correlates with inflammation in primary sclerosing cholangitis. Pathol Res Pract 1999, 195:727-731 [DOI] [PubMed] [Google Scholar]
- 22.Koukoulis GK, Patriarca C, Gould VE: Adhesion molecules and tumor metastasis. Hum Pathol 1998, 29:889-892 [DOI] [PubMed] [Google Scholar]
- 23.Gould VE, Gould KA: E-cadherin as tumor differentiation marker and as architectural determinant. Hum Pathol 1999, 30:1273-1275 [DOI] [PubMed] [Google Scholar]
- 24.Ghadially FN (Ed): Ultrastructural Pathology of the Cell and Matrix, 4th ed. Boston, Butterworth-Heinemann, 1997
- 25.Kessel RG: The structure and function of annulate lamellae: porous cytoplasmic and intranuclear membranes. Int Rev Cytol 1983, 82:181-205 [DOI] [PubMed] [Google Scholar]
- 26.Cordes VC, Reidenbach S, Franke WW: High content of a nuclear pore complex protein in cytoplasmic annulate lamellae of Xenopus oocytes. Eur J Cell Biol 1995, 68:240-255 [PubMed] [Google Scholar]
- 27.Ewald A, Kossner U, Scheer U, Dabauvalle MC: A biochemical and immunological comparison of nuclear and cytoplasmic pore complexes. J Cell Sci 1996, 109:1813-1824 [DOI] [PubMed] [Google Scholar]
- 28.Chen CF, Li S, Chen Y, Chen PL, Sharp PL, Lee WH: The nuclear localization sequences of the BRCA1 protein interact with the importin-alpha subunit of the transport signal receptor. J Biol Chem 1996, 271:32863-32868 [DOI] [PubMed] [Google Scholar]
- 29.Allen TD, Cronshaw JM, Bagley JS, Kiseleva E, Goldberg MW: The nuclear pore complex: mediator of translocation between nucleus and cytoplasm. J Cell Sci 2000, 113:1651-1659 [DOI] [PubMed] [Google Scholar]
- 30.Blobel G, Wozniak RW: Proteomics for the pore. Nature 2000, 403:835-836 [DOI] [PubMed] [Google Scholar]
- 31.Reichelt R, Holzenburg A, Buhle EL, Jr, Jarnik M, Engel A, Aebi U: Correlation between structure and mass distribution of the nuclear pore complex and of distinct pore complex components. J Cell Biol 1990, 110:883-894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fontoura B, Blobel G, van Baal S, Morreau H, Grosveld G: A conserved biogenesis pathway for nucleoporins: proteolytic processing of a 186kDa precursor generates Nup98 and the novel nucleoporin Nup 96. J Cell Biol 1999, 144:1097-1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rout MP, Blobel G: Isolation of the yeast nuclear pore complex. J Cell Biol 1993, 123:771-783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rout MP, Aichison JP, Suprapto A, Hjertaas K, Zhao Y, Chait BD: The yeast nuclear pore complex: composition, architecture and transport mechanism. J Cell Biol 2000, 148:635-651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boer JM, van Deursen JMA, Croes HJ, Fransen JAM, Grosveld GC: The nucleoporin CAN/214 binds to both the cytoplasmic and the nucleoplasmic sides of the nuclear pore complex in overexpressing cells. Exp Cell Res 1997, 232:182-185 [DOI] [PubMed] [Google Scholar]
- 36.Nishiyama M, Arai Y, Tsunematsu Y, Kobayashi H, Asami K, Yabe M, Kato S, Pda M, Eguchi H, Ohki M, Kaneko Y: 11p15 translocation involving the Nup98 gene in childhood therapy-related acute myeloid leukemia/myelodysplastic syndrome. Genes Chromosom Cancer 1999, 3:215-220 [PubMed] [Google Scholar]
- 37.Feldherr CM, Aikin D: Nuclear transport as a function of cellular activity. Membr Protein Trans 1995, 2:237-259 [Google Scholar]