Abstract
In colorectal cancer patients, prognosis is not determined by the primary tumor but by the formation of distant metastases. Molecules that have been implicated in the metastatic process are the proto-oncogene product c-Met and CD44 glycoproteins. Recently, we obtained evidence for functional collaboration between these two molecules: CD44 isoforms decorated with heparan sulfate chains (CD44-HS) can bind the c-Met ligand, the growth and motility factor hepatocyte growth factor/scatter factor (HGF/SF). This interaction strongly promotes signaling through the receptor tyrosine kinase c-Met. In the present study, we explored the expression of CD44-HS, c-Met, and HGF/SF in the normal human colon mucosa, and in colorectal adenomas and carcinomas, as well as their interaction in colorectal cancer cell lines. Compared to the normal colon, CD44v3 isoforms, which contain a site for HS attachment, and c-Met, were both overexpressed on the neoplastic epithelium of colorectal adenomas and on most carcinomas. Likewise, HGF/SF was expressed at increased levels in tumor tissue. On all tested colorectal cancer cell lines CD44v3 and c-Met were co-expressed. As was shown by immunoprecipitation and Western blotting, CD44 on these cells lines was decorated with HS. Interaction with HS moieties on colorectal carcinoma (HT29) cells promoted HGF/SF-induced activation of c-Met and of the Ras-MAP kinase pathway. Interestingly, survival analysis showed that CD44-HS expression predicts unfavorable prognosis in patients with invasive colorectal carcinomas. Taken together, our findings indicate that CD44-HS, c-Met, and HGF/SF are simultaneously overexpressed in colorectal cancer and that HS moieties promote c-Met signaling in colon carcinoma cells. These observations suggest that collaboration between CD44-HS and the c-Met signaling pathway may play an important role in colorectal tumorigenesis.
Colorectal cancer evolves through a series of morphologically recognizable stages known as the adenoma-carcinoma sequence. 1 Primarily as a result of this stepwise development, the molecular genetics of colorectal cancer are among the best studied of any solid neoplasm, 2-5 and serve as a paradigm for multistep tumorigenesis. Several important molecules implicated in the tumorigenetic process act on the cell cycle, resulting in a disturbed homeostasis between cell proliferation and apoptosis. 2 The main cause of tumor-related death in colorectal cancer however, is the formation of distant metastases, rather than the growth of the primary tumor. Although relatively little is known concerning the molecular mechanisms underlying this complex process, recent studies have identified CD44 glycoproteins 6 and the c-Met receptor tyrosine kinase 7,8 as potentially important components of the metastatic cascade.
CD44 is a family of transmembrane receptors generated from a single gene by alternative splicing and differential glycosylation. 9-13 Important biological processes involving CD44 glycoproteins include cell adhesion, 14 lymphocyte homing, 9,15,16 hematopoiesis, 9 and tumor progression and metastasis. 6,9,11,17,18 In colorectal cancer, CD44 glycoproteins, which are normally detected only in the lower crypt epithelium of the intestinal mucosa, are overexpressed. 6,19-24 This overexpression is an early event in the colorectal adenoma-carcinoma sequence 25,26 suggesting a causal relation to loss of APC tumor suppressor gene function. Indeed, recent studies in Apc and Tcf-4 mutant mice indicate that CD44 expression in normal and neoplastic intestinal epithelium is regulated by the Wnt-signaling pathway. 24
The precise mechanisms via which CD44 promotes tumorigenesis have not yet been elucidated. CD44 functions as a molecular linker between extracellular matrix molecules, specifically hyaluronate, and the cell and cytoskeleton. 9,14,27,28 Recently, CD44 isoforms decorated with heparan sulfate-side (HS) chains have been shown to bind and present growth factors. 29-31 We demonstrated that CD44-HS binds the growth and motility factor hepatocyte growth factor/scatter factor (HGF/SF). This interaction strongly promotes signaling through c-Met, the high-affinity receptor for HGF/SF. 31 The HGF/SF-c-Met pathway is essential for normal murine embryonal development 32-34 and affects a wide range of biological activities including angiogenesis, cell motility, growth, and morphogenesis. In addition, there is ample evidence for a key role of the HGF/SF-c-Met pathway in tumor growth, invasion, and metastasis. 7,8,35,36 For example, c-Met was isolated originally as the product of a human oncogene, Tpr-Met, which encodes a constitutively dimerized/activated chimeric c-Met protein possessing transforming activity. 37,38 The generation of an autocrine loop as a result of co-expression of wild-type c-Met and HGF/SF molecules in the same cell is also oncogenic. 39 The tumorigenicity of both Tpr-Met and autocrine HGF/SF-Met signaling has been verified in transgenic mouse models, which develop tumors in many different tissues including mammary glands, skeletal muscles, and melanocytes. 40,41 c-Met activation has also been shown to promote the metastatic spread of cancer, a finding that likely is because of its stimulatory effects on a variety of processes such as angiogenesis, cell motility, and protease secretion. 8,42 Recently, missense mutations in c-Met were found to be associated with human papillary renal carcinomas. 43 These mutations deregulate the enzymatic activity of the receptor, thereby unleashing its oncogenic potential. 44
To explore whether collaboration between CD44-HS and the HGF/SF-c-Met pathway is an option in colorectal cancer, the present study investigates the expression of these molecules in the normal colon mucosa as well as along the distinct steps of the colorectal adenoma-carcinoma sequence.
Materials and Methods
Antibodies
Mouse monoclonal antibodies (mAbs) used were: 3G5 (anti-CD44v3, IgG2b; R&D Systems, Abington, UK); 3G10 (anti-desaturated uronate from heparitinase-treated HS; HS stub, IgG2b) 45 ; PY-20 (anti-phosphotyrosine, IgG2b; Affiniti, Nottingham, UK); anti-human c-Met (IgG2a; R&D Systems); anti-human HGF/SF (IgG1; R&D Systems). Polyclonal antibodies used were (rabbit anti-c-Met, IgG, C12; Santa Cruz Biotechnology, Santa Cruz, CA); rabbit anti-phospho-p44/42 MAP kinase (Thr202/Tyr204; New England Biolabs, Beverly, MA); rabbit anti-ERK 1 (C-16; Santa Cruz Biotechnology); horseradish-peroxidase (HRP)-conjugated rabbit anti-mouse (DAKO, Glostrup, Denmark); HRP-conjugated goat anti-rabbit (DAKO); HRP-conjugated swine anti-rabbit (DAKO); biotin-conjugated rabbit anti-mouse (DAKO). In addition we used phycoerythrin- conjugated streptavidin (DAKO).
Cell Lines
The colon carcinoma cell lines SW480, SW620, colo 201, colo 205, colo 320, and HT-29, were purchased from the American Type Culture Collection (ATCC, Rockville, MD). HT-29 cells were cultured in modified McCoy’s 5A medium (Gibco BRL/Life Technologies, Paisley, UK), whereas SW480 and SW620 were cultured in L-15 (Leibovitz) medium (Gibco BRL/Life Technologies). The other cell lines were cultured in RPMI 1640 (Gibco BRL/Life Technologies). All media were supplemented with 10% heat-inactivated fetal calf serum, 2 mmol/L l-glutamine, 100 IU/ml penicillin, and 100 IU/ml streptomycin (all from Gibco BRL/Life Technologies).
Purification of Wild-Type and Mutant HGF/SF
The construction of pVL1393 vectors (Pharmingen, San Diego, CA) containing wild-type or mutant HGF/SF (HP1) cDNA was described elsewhere. 46 HGF/SF (wild type and HP1) was produced in a Baculovirus system as described previously. 47 In brief, sf 9 insect cells were transduced with an amplified virus stock and after 3 days media were pooled and analyzed for scattering activity in the Madin-Darby canine kidney dissociation assay. 48 Then, HGF/SF was purified with Ni-NTA-resin from the QIA expressionist system (Qiagen, Hilden, Germany). HGF/SF concentrations were measured by enzyme-linked immunosorbent assay as described previously, 49 and in addition, HGF/SF (wild type and mutant) was analyzed by Western blotting using anti-goat-HGF/SF.
Enzyme Treatments
For enzymatic cleavage of GAGs, cells were treated with either heparitinase (Flafobacterium heparinum, EC 4.2.2.8; ICN Biomedicals, Aurora, OH) or chondroitinase avidin-biotin-peroxidase complex (Proteus vulgaris, EC 4.2.2.4; Boehringer Mannheim, Almere, The Netherlands) in phosphate-buffered saline (PBS) at 37°C for the periods indicated. Enzyme treatments were followed by immunoprecipitation.
Immunoprecipitation and Western Blot Analysis
Immunoprecipitation was performed as described. 49 The only modification were that, for precipitation of CD44, cells were lysed in lysis buffer containing 50 mmol/L Tris-HCl, pH 8, 150 mmol/L NaCl, 1% Nonidet P-40, 10 μg/ml aprotinin (Sigma), 10 μg/ml leupeptin (Sigma), 1 mmol/L sodium orthovanadate (Sigma), 2 mmol/L ethylenediaminetetraacetic acid, and 5 mmol/L NaF. For precipitation of c-Met, cells were lysed in 10 mmol/L Tris-HCl (pH 8), 150 mmol/L NaCl, 10% glycerol, 1% Nonidet P-40, 10 μg/ml aprotinin (Sigma), 10 μg/ml leupeptin (Sigma), 2 mmol/L sodium orthovanadate (Sigma), 5 mmol/L ethylenediaminetetraacetic acid, and 5 mmol/L NaF.
Western blotting of immunoprecipitates and total cell lysates was essentially performed as described previously, 50 with the modification that, for analysis of phosphorylated proteins, membranes were blocked and stained in 2% bovine serum albumin, 20 mmol/L Tris-HCl, 150 mmol/L NaCl, pH 7.5, and 0.05% Tween-20 (Sigma). Films were scanned with an Eagle Eye II video system (Stratagene, La Jolla, CA) and band intensities were determined with ONE-Dscan software (Stratagene). c-Met phosphorylation was expressed as the ratio of phosphorylated c-Met to precipitated c-Met.
Activation of the MAP kinases ERK 1 and 2 was analyzed by immunoblotting of total cell lysates with the phospho-specific p44/42 MAP kinase antibody.
Tissue Samples
The study set consisted of 54 primary colorectal carcinomas, 20 removed at operation between January 1, 1983 and January 1, 1986 at the Department of Surgery, Reinier de Graaf Hospital, Delft, The Netherlands, of which snap-frozen tissue and follow-up till June 1, 1992 (6.5 to 9.5 years) was available. The mean age of the patients at diagnosis was 69.7 (range, 39 to 92) and the male-to-female ratio was 28/40. Colorectal tissue samples of six adenomas and six normal controls, removed at operation between January 1, 1992 and January 1, 1999 were obtained from the tissue bank of the Department of Pathology, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands.
Immunohistochemistry and Statistical Analysis
Frozen tissue sections were tested for the expression of CD44v3, c-Met, and HGF/SF by immunohistochemistry as described previously. 18,19 A single modification was that HRP-conjugated rabbit anti-mouse and HRP-conjugated swine anti-rabbit were used as secondary and tertiary antibodies, respectively. All slides were read by two independent observers, and discrepancies were solved by consensus. The tumor samples were scored as described previously: 20,23 0 (low/negative) = <10% of the cells positive; 1 (intermediate) = 10 to 50% of the cells positive; 2 (high) = >50% of the cells positive. Survival functions were estimated by the Kaplan-Meier method and comparison of survival functions between groups was performed by the log-rank test.
RNA Isolation and Reverse-Transcriptase-Polymerase Chain Reaction (RT-PCR)
RNA isolation and first-strand cDNA synthesis were performed as described previously. 49 PCR was performed with 1.5 U Taq DNA Polymerase (Gibco BRL/Life Technologies), 200 μmol/L dNTPs (Pharmacia Biotech, Uppsala, Sweden), and 1.5 mmol/L MgCl2 (2 mmol/L for GAPDH) in 1× PCR Buffer (both Gibco BRL/Life Technologies). Primers used were HGF-1 (5′-CGACAGTGTTTCCCTTCTCG-3′) in combination with HGF-3 (5′-GGTGGGTGCAGACACAC-3′), or GAPDH-D (5′-GGCAGAGATGATGACCCTTTTGGC-3′) in combination with GAPDH-U (5′-AAGGTGAAGGTCGGAGTCAACG-3′). PCR was started with a 5-minute denaturation step at 95°C, after which amplification was performed in 35 cycles of denaturation at 95°C for 30 seconds, annealing at 60°C (55°C for GAPDH) for 1 minute (30 seconds for GAPDH), and elongation at 72°C for 2 minutes (30 seconds for GAPDH). After a final elongation step for 10 minutes at 72°C, samples were cooled on ice and analyzed by electrophoresis in an 1.5% agarose Tris borate-ethylenediaminetetraacetic acid-gel containing ethidium bromide.
Fluorescence-Activated Cell Sorting Analysis (FACS)
For FACS analysis cells were blocked with 10% pooled human serum (CLB, Amsterdam, The Netherlands), 1% bovine serum albumin (Fraction V) (Sigma) in PBS at 4°C for 15 minutes, and washed with FACS buffer (1% bovine serum albumin in PBS), respectively. Then, the cells were incubated with the primary antibodies for 1 hour, washed, and incubated with the secondary antibody for 30 minutes. All incubations were performed in FACS buffer at 4°C. Stained cells were analyzed by flow cytometry on a FACScan (Becton Dickinson, Mountain View, CA).
HS Proteoglycan-Dependent Phosphorylation of c-Met in HT29 Cells
HT29 cells were cultured in 6-well plates until subconfluent (60 to 80%) and then starved overnight. Part of the cells were treated with heparitinase as mentioned above and activated with 100 ng/ml wild-type HGF/SF or mutant HGF/SF (HP1) in 0.5 ml serum-free, prewarmed medium for 10 minutes at 37°C. Cells were washed once with cold PBS and were immediately cooled on ice. Lysis buffer (500 μl) was added and cells were harvested. c-Met was immunoprecipitated and c-Met phosphorylation was analyzed by Western blotting.
Results
Co-Expression of CD44-HS and c-Met in Colorectal Cancer
Previous studies have shown that heparan sulfate forms of CD44 (CD44-HS) are splice variants containing exon v3. 13,31 To explore the expression of CD44-HS during colorectal tumor progression, we compared CD44v3 levels in normal colon mucosa, adenomas, and carcinomas (Table 1 ▶ and Figure 1 ▶ ). In the normal colon mucosa CD44v3 expression was low to intermediate and strictly confined to the base of the crypts. By contrast, in all adenomas and in 70% (38 of 54) of the invasive carcinomas, an intermediate to high expression of CD44v3 was observed (Table 1) ▶ .
Table 1.
Expression of CD44v3 and c-Met in Colorectal Tumorigenesis
| Tissue | Expression level* | Number of patients n (%) | |
|---|---|---|---|
| CD44v3 | c-Met | ||
| Normal | |||
| Crypt base | Negative/low | 1 (17) | 3 (60) |
| Intermediate | 4 (67) | 2 (40) | |
| High | 1 (17) | 0 | |
| Upper crypt | Negative/low | 6 (100) | 3 (60) |
| Intermediate | 0 | 2 (40) | |
| High | 0 | 0 | |
| Polyp | Negative/low | 0 | 0 |
| Intermediate | 4 (67) | 2 (33) | |
| High | 2 (33) | 4 (67) | |
| Carcinoma | Negative/low | 16 (30) | 0 |
| Intermediate | 19 (35) | 0 | |
| High | 19 (35) | 54 (100) | |
*Negative/low, intermediate, high: expression on <10%, 10 to 50%; or >50% of the tumor cells, respectively.
Figure 1.
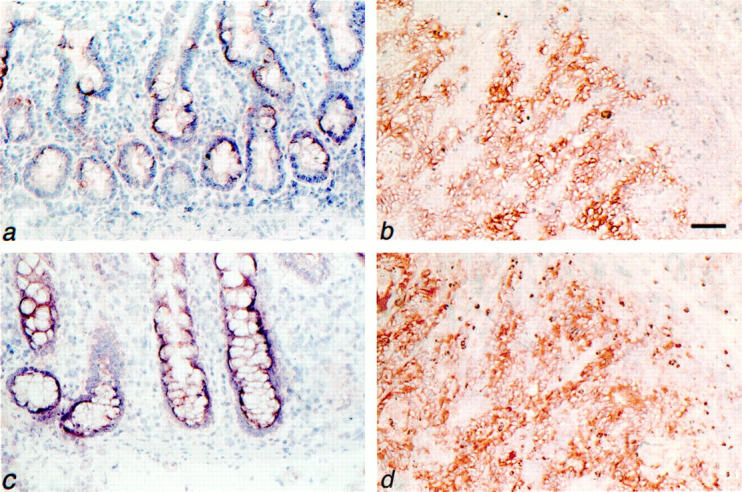
Expression of CD44v3 and c-Met by normal colon mucosa and colorectal carcinomas. Normal colon mucosa (a and c) and colorectal carcinoma (b and d) serial frozen tissue sections were stained for CD44v3 (a and b) or for c-Met (c and d) by immunohistochemistry. a: Normal colon mucosa showing weak focal expression of CD44v3 in the lower part of the crypts. b: Invasive colorectal carcinoma with strong CD44v3 expression. c: Normal colon mucosa showing weak expression of c-Met. d: Invasive colorectal carcinoma with strong c-Met expression. Tissues were counterstained with hematoxylin. Scale bars, 57 μm (a and c); 69 μm (b and d).
For c-Met, enhanced expression along the adenoma-carcinoma sequence was also observed. Whereas the epithelium of the normal colon mucosa showed a low to intermediate expression, c-Met expression in adenomas and carcinomas was intermediate to high and high, respectively (Table 1 ▶ ; Figure 1 ▶ ).
Colorectal Carcinoma Cell Lines Co-Express CD44v3 and c-Met
To strengthen the observation that colorectal carcinomas co-express CD44v3 and c-Met, the presence of these molecules was assessed by FACS on a panel of colorectal carcinoma cell lines. On all of these carcinoma cell lines, ie, colo 320, HT29, SW480, SW620, colo 201, colo 205, and colo320, both CD44v3 and c-Met were clearly expressed (Figure 2) ▶ .
Figure 2.
Expression of CD44v3 and c-Met by colon carcinoma cell lines. A: FACS analysis of the expression of CD44v3 on the colon carcinoma cell lines colo 320, HT29, SW480, SW620, colo 201, colo 205, and colo 320. Wild-type and CD44v3–10 transfected Namalwa cells are shown as negative or positive controls, respectively. Expression was analyzed with mouse anti-CD44v3 (filled histogram) or an isotype-matched control antibody (empty histogram), followed by RPE-conjugated goat anti-mouse. B: FACS analysis of the c-Met expression on the colon carcinoma cell lines shown in A. Wild-type or c-Met-transfected Namalwa cells are shown as negative and positive controls, respectively. Expression was analyzed with mouse anti-c-Met (filled histogram) or an isotype-matched control antibody (empty histogram), followed by RPE-conjugated goat anti-mouse.
Taken together, the above expression studies show that co-expression of CD44v3 and c-Met is present in most primary colorectal adenomas and carcinomas as well as in colorectal carcinoma cell lines.
CD44v3 on Colorectal Cells is Decorated with HS
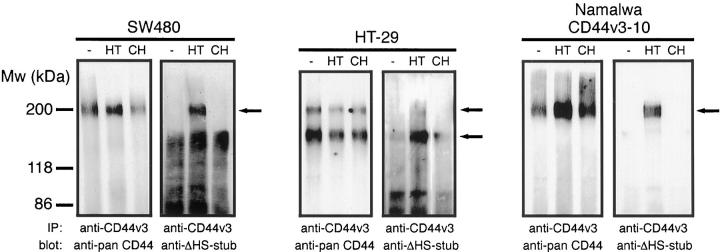
To verify whether the glycosylation machinery of colorectal cancer cells indeed decorates CD44v3 with HS chains, we studied CD44v3 immunoprecipitates from the colorectal cancer cell lines SW480 and HT-29 on Western blot with the mAb 3G10. This mAb recognizes the HS stubs that remain on HS proteoglycans after heparitinase treatment. 45 Hence, before immunoprecipitation, the tumor cells were treated with heparitinase, or as controls, were sham-treated or chondroitinase-treated. As is shown by staining with an anti-pan CD44 mAb (Figure 3) ▶ , one major CD44v3 species of ∼200 kd was precipitated from SW480 cells whereas two species of ∼150 and 200 kd were precipitated from HT-29. The size of the latter CD44 variant was identical to that from a control cell line (Namalwa) expressing a single CD44 isoform containing v3-10, 31,51 whereas the shorter species most probably contains a shorter variable domain. Staining of the blots with the anti-HS stub mAb revealed the presence of bands corresponding to those obtained after staining with the anti-pan CD44 mAb. These bands were specifically present in the precipitates of the heparitinase treated cells, but not in the precipitates of sham- or chondroitinase-treated cells (Figure 3) ▶ . Hence, CD44v3 isoforms on colorectal cancer cell lines are HS-decorated.
Figure 3.
CD44v3 isoforms on colon carcinoma cell lines are decorated with HS. CD44v3 was immunoprecipitated from the colon carcinoma cell lines SW480 and HT-29, and, as a positive control, from Namalwa cells transfected with CD44v3–10, with mouse anti-CD44v3. Before immunoprecipitation, the cells were treated with either PBS (−), 30 mU/ml heparitinase (HT), or 30 mU/ml chondroitinase ABC (CH) at 37°C for 3.5 hours. The Western blot of the precipitates was stained with the anti-pan CD44 mAb Hermes-3, stripped, and restained with the mAb 3G10 that detects ΔHS stubs after treatment of HS with heparitinase. CD44v3 isoforms decorated with HS are indicated with arrows.
Expression of HGF/SF in Colorectal Cancer Tissue Samples
To explore whether the c-Met ligand HGF/SF is also expressed within the colorectal carcinoma microenvironment, HGF/SF mRNA expression was measured by RT-PCR in paired samples of normal and neoplastic colon tissue from five patients. In addition, tissue sections of normal colon mucosa and carcinomas were stained for the presence of HGF/SF protein. As is shown in Figure 4A ▶ , HGF/SF mRNA expression was readily detectable in all colorectal carcinoma samples. Moreover, the intensity of the bands obtained from the tumor samples was clearly increased, compared to those obtained from the samples of the normal mucosa. In colorectal carcinomas HGF/SF protein expression was detected in cells present within the tumor stroma (Figure 4B) ▶ . In normal mucosa no HGF/SF-positive cells were found (data not shown).
Figure 4.
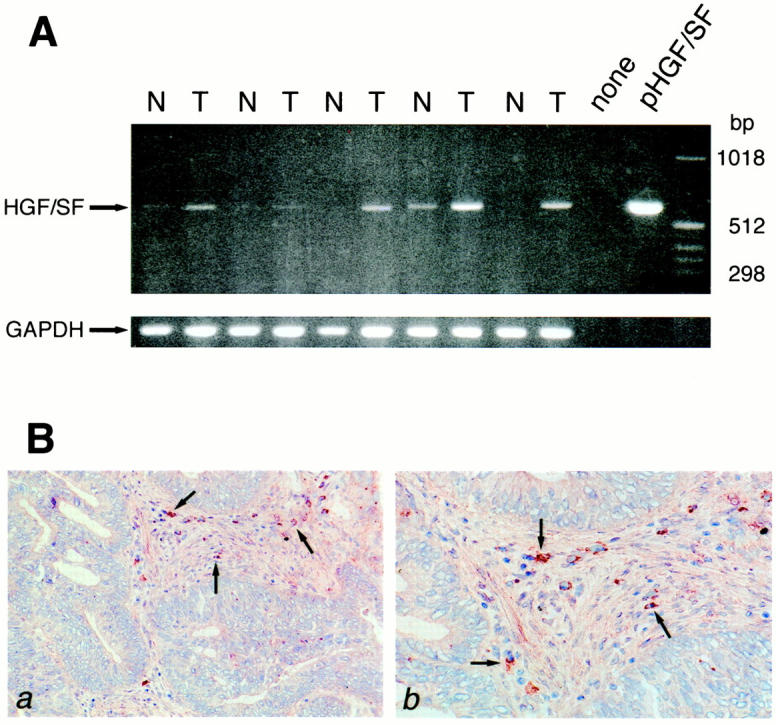
HGF/SF mRNA and protein expression in normal colon mucosa and colorectal carcinomas. A: RT-PCR was performed on total RNA isolated from five pairs of normal colon (N) and primary colorectal carcinoma (T), on water (none), and on a plasmid containing full-length human HGF/SF cDNA (pHGF/SF). Primers used were HGF/SF-specific or, as a control, glyceraldehydephosphate dehydrogenase (GAPDH)-specific. The tumors analyzed were CD44- and Met-positive. B: HGF/SF protein expression in colorectal cancer was assessed by immunohistochemistry. a and b: Frozen sections from colorectal cancer tissue were stained with anti-human HGF/SF. This identified cells (arrows) in the tumor stroma as HGF/SF-producing cells.
HS on Colorectal Cancer Cells Promotes Ligand-Induced c-Met Phosphorylation
To investigate whether HS chains on colorectal cancer cells are able to present HGF/SF to c-Met and promote signaling, the tyrosine phosphorylation of c-Met was studied in HT-29 cells that: 1) were treated with heparitinase before HGF/SF stimulation; 2) were stimulated with HP1, a non-HS-binding mutant form of HGF/SF. 46 As is shown in Figure 5A ▶ (top), heparitinase treatment of HT-29 cells led to an almost complete reduction in the HGF/SF-induced phosphorylation of c-Met. Similarly, the HGF/SF mutant HP1 was significantly less potent in inducing c-Met phosphorylation (Figure 5B) ▶ . In addition to activation of c-Met, the HGF-induced activation of the MAP kinases ERK 1 and 2 was shown to be dependent on the presence of HS moieties as well (Figure 5A ▶ , bottom). These findings indicate that interaction of HGF/SF with HS proteoglycans expressed on the surface of colorectal cancer cells facilitates c-Met signaling.
Figure 5.

The interaction of HGF with HS moieties of HS proteoglycans promotes Met signaling in HT29 cells. A: The effect of heparitinase treatment on HGF-induced Met signaling. HT29 cells were pretreated with 10 mU/ml heparitinase (HT) for 3.5 hours and subsequently stimulated with 100 ng/ml HGF for 10 minutes, as indicated. Met autophosphorylation was analyzed by immunoprecipitation (IP) of Met and immunoblotting (IB) with anti-phosphotyrosine (PY) antibody, and subsequent reprobing of the blot with anti-Met antibody (top). In addition, activation of the MAP kinases ERK1 (p44) and 2 (p42) was analyzed by immunoblotting total cell lysates with anti-phospho-ERK1/2 (P-ERK), and subsequent reprobing of the blot with anti-ERK antibody (bottom). B: Stimulation of Met autophosphorylation by wild-type HGF or a non-HS-binding HGF mutant. HT29 cells were stimulated for 10 minutes with either 100 ng/ml HGF or HP1, a non-HS-binding mutant form of HGF, as indicated, and Met autophosphorylation was analyzed by immunoprecipitation of Met and immunoblotting with anti-phosphotyrosine antibody.
CD44v3 Expression Is Related to Poor Prognosis
Previous studies from our own and other laboratories have shown that CD44 splice variants containing v6 and v8-10 are unfavorable prognosticators in colorectal cancer. Expression of these variants on the primary tumor predicts metastatic disease and tumor-related death. 20-23 In view of the ability of CD44v3 to present growth factors, which may promote metastasis, we now studied whether expression of CD44v3 also predicts prognosis. CD44v3 was assessed in a study group of 54 colorectal cancer patients with a long-term (6.5 to 9.5 years) and complete follow-up. Details on this study group have been published previously. 20,23 As is depicted in Table 2 ▶ and Figure 6 ▶ , CD44v3 expression on the primary tumor indeed predicts tumor related death. CD44v3 expression on the tumors was strongly correlated to expression of CD44v6 (data not shown).
Table 2.
Prognostic Significance of CD44v3 Expression
| Level of CD44v3 expression* | Number of patients n (%) | Median survival in days | P value† |
|---|---|---|---|
| Negative/low | 16 (30) | 1577 | |
| Intermediate | 19 (35) | 1167 | 0.03 |
| High | 19 (35) | 294 |
*Negative/low, intermediate, high: expression on <10%, 10 to 50%, or >50% of the tumor cells, respectively.
†Log-rank test from a univariate analysis.
Figure 6.
Patient survival and expression of CD44v3. Kaplan-Meier curves showing the relation between the expression of CD44v3 on primary tumors and survival in patients with colorectal carcinoma. Dotted line, low expression of CD44v3; dashed line, intermediate expression; solid line, high expression; +, censored cases.
Discussion
Deregulation of c-Met signaling can initiate and promote tumor growth and dissemination. 7,8,35-39 The present study shows that c-Met is strongly expressed on primary colorectal adenomas and carcinomas, as well as on colorectal cell lines, whereas HGF/SF is present at increased levels within tumor tissue. In addition, it demonstrates that a subset of colorectal carcinomas with unfavorable prognosis strongly expresses CD44-HS. Because CD44-HS can bind and present HGF/SF, and promotes signaling through c-Met, 31 our observations suggest a role for functional collaboration between CD44-HS and the HGF/SF-c-Met pathway in colorectal tumorigenesis.
Our observation that c-Met is strongly expressed by both colorectal adenomas and carcinomas (Table 1 ▶ and Figure 1 ▶ ) confirms previous studies, documenting overexpression of c-Met in colorectal tumors. 52-54 We extend these findings by demonstrating that, in parallel, HGF/SF is expressed in colorectal tumor tissue. HGF/SF mRNA levels in tumor tissue were consistently higher than in the normal mucosa (Figure 4A) ▶ . Moreover, cells expressing HGF/SF protein were detected in the stroma of tumors but not in the normal mucosa (Figure 4B) ▶ . These observations indicate that paracrine HGF/SF-c-Met interaction is likely to take place within the colorectal carcinoma microenvironment, promoting tumor growth and motility.
At present, the mechanism of c-Met and HGF/SF overexpression in colorectal cancer is primarily unknown. Di Renzo and colleagues 53 reported that c-Met overexpression is associated with amplification of the c-met gene in ∼10% of primary colon carcinomas and 50% of metastases. However, because high c-Met levels were present in all carcinomas tested (Table 1) ▶ other mechanisms must also be involved. As c-Met overexpression occurs from an early stage of colorectal tumor progression onwards, c-met might, like c-myc, 55 be regulated by the Wnt-signaling pathway. For HGF/SF the mechanism of overexpression and the identity of cell(s) producing the growth factor within the tumor microenvironment remains to be defined. HGF/SF expression by epithelial tumor cells, with autocrine c-Met stimulation, has been reported in human breast cancer. 56,57 Alternatively, as indicated by our immunohistochemical stainings (Figure 4B) ▶ , cells within tumor stroma present a potential paracrine source of HGF/SF. Although these cells need further identification, they presumably represent fibroblasts and/or activated macrophages, because both of these cell types have been reported to express HGF/SF. 58-60 Paracrine stimulation may also promote the outgrowth of metastases because HGF/SF is produced at the two major sites of colorectal carcinoma metastasis, ie, the liver 61 and lymphoid tissue. 49
CD44v3 isoforms were detected on colorectal adenomas, on a major subset (70%) of invasive colorectal carcinomas, and on all carcinoma cell lines studied (Table 1 ▶ and Figures 1 and 2 ▶ ▶ ). Analyses of CD44v3 immunoprecipitates showed that these isoforms were decorated with HS, and thus are HS proteoglycans (Figure 3) ▶ . Interestingly, interaction of HGF/SF with HS moieties on HT-29 cells was found to promote c-Met phosphorylation as well as activation of the MAP kinases ERK1 and 2 (Figure 5A) ▶ . Although the precise contribution of CD44-HS versus other HS proteoglycans, such as the syndecans 62 remains to be explored, our findings suggest a role of HS proteoglycans in c-Met signaling in colorectal cancer. We have recently identified CD44-HS as a functional co-receptor for HGF/SF. Binding of HGF/SF to CD44-HS promotes signaling through c-Met leading to phosphorylation of several downstream proteins and of overactivity of the Ras-MAP kinase pathway. 31 The Ras-MAP kinase pathway, which has been implicated in the processes of cell motility and invasion, 63 is also activated by Tpr-Met and by oncogenic c-Met mutants associated with human papillary renal carcinomas. 64 The enhancing effects of CD44-HS on signal transduction were critically dependent on HGF/SF interaction with HS moieties, suggesting that CD44-HS promotes the action of HGF/SF through concentration of HGF/SF on the cell surface and by presenting it to the high-affinity receptor c-Met. 31 Similar mechanisms were proposed for the role of high and low affinity receptors in fibroblast growth factor-2 functioning. 65-71 CD44, c-Met and HGF/SF are also expressed in embryonal tissues, including intestine. 24,72 Presumably, they play a role in mesenchymal-epithelial interactions regulating differentiation and morphogenesis. Interestingly, we have recently shown that CD44 is present in normal mouse embryonal intestine but absent in that of mice with a disrupted Wnt-signaling pathway. 24 Loss of CD44 in these Tcf-4 mutant mice occurred in the context of a phenotype characterized by the absence of a proliferative stem cell compartment. Binding to CD44-HS of mesenchymally derived growth factors, including HGF/SF and WNT-factors, may be required for normal intestinal stem cell differentiation. In a recent study by Sherman et al, 73 CD44-HS was shown to present fibroblast growth factor-2 in embryonal limb bud formation.
Several studies have reported a strong correlation between CD44 expression in invasive colorectal carcinomas and tumor-related death. 6,20-23,74 In these studies, antibodies recognizing different parts of the CD44 molecule, ie, CD44v6, CD44v8–10, or CD44s (the constant part of CD44) all gave similar results. We now show that CD44v3 expression also predicts prognosis. This correlation of survival with a number of CD44 domains indicates concerted overexpression of these various CD44 variant domains.
In conclusion, we demonstrate that most colorectal tumors co-express c-Met and CD44-HS, and that co-expression of these molecules in invasive carcinomas is associated with an unfavorable prognosis. Moreover, our findings suggest that during colorectal tumorigenesis, CD44-HS overexpression may enhance signaling through the HGF/SF-c-Met signaling pathway, promoting tumor growth and the development of metastatic disease.
Footnotes
Address reprint requests to Steven T. Pals, M.D., PhD, Department of Pathology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands. E-mail: s.t. pals@amc.uva.nl.
Supported by grants from the het Praeventiefonds (grant no. 28-2575) and from the Dutch Cancer Society (AMC 98-1712).
References
- 1.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AMM, Bos JL: Genetic alterations during colorectal-tumor development. N Engl J Med 1988, 319:525-532 [DOI] [PubMed] [Google Scholar]
- 2.Kinzler KW, Vogelstein B: Lessons from hereditary colorectal cancer. Cell 1996, 87:159-170 [DOI] [PubMed] [Google Scholar]
- 3.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H: Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 1997, 275:1784-1787 [DOI] [PubMed] [Google Scholar]
- 4.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW: Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 1997, 275:1787-1790 [DOI] [PubMed] [Google Scholar]
- 5.Eppert K, Scherer SW, Ozcelik H, Pirone R, Hoodless P, Kim H, Tsui LC, Bapat B, Gallinger S, Andrulis IL, Thomsen GH, Wrana L, Attisano L: MADR2 maps to 18q21 and encodes a TGF-β-regulated MAD-related protein that is mutated in colorectal carcinoma. Cell 1996, 86:543-552 [DOI] [PubMed] [Google Scholar]
- 6.Wielenga VJM, van der Neut R, Offerhaus GJA, Pals ST: CD44 glycoproteins in colorectal cancer; expression, function and prognostic value. Adv Cancer Res 1999, 77:169-187 [DOI] [PubMed] [Google Scholar]
- 7.Van der Voort R, Taher TEI, Derksen PWD, Spaargaren M, van der Neut R, Pals ST: The hepatocyte growth factor-Met pathway in development, tumorigenesis, and B cell differentiation. Adv Cancer Res 2000, 79:39-90 [DOI] [PubMed] [Google Scholar]
- 8.Jeffers M, Rong S, Vande Woude GF: Hepatocyte growth factor/scatter factor-Met signaling in tumorigenicity and invasion/metastasis. J Mol Med 1996, 74:505-513 [DOI] [PubMed] [Google Scholar]
- 9.Lesley J, Hyman R, Kincade PW: CD44 and its interactions with the extracellular matrix. Adv Immunol 1993, 4:271-335 [DOI] [PubMed] [Google Scholar]
- 10.Stamenkovic I, Amiot M, Pesando JM, Seed B: A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell 1989, 56:1057-1063 [DOI] [PubMed] [Google Scholar]
- 11.Günthert U, Hofmann M, Rudy W, Reber S, Zöller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P: A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cell lines. Cell 1991, 65:13-24 [DOI] [PubMed] [Google Scholar]
- 12.Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth K, Bell JL: Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci USA 1992, 89:12160-12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson DG, Bell JI, Dickinson R, Timans J, Shields J, Whittle N: Proteoglycan forms of the lymphocyte homing receptor CD44 are alternatively spliced variants containing the v3 exon. J Cell Biol 1995, 128:673-685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B: CD44 is the principal cell surface receptor for hyaluronate. Cell 1990, 61:1303-1313 [DOI] [PubMed] [Google Scholar]
- 15.Jalkanen S, Bargatze RF, de los Toyos J, Butcher EC: Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85–95-kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol 1987, 105:983-990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeGrendele HC, Estress P, Siegelman MH: Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science 1997, 278:672-675 [DOI] [PubMed] [Google Scholar]
- 17.Naor D, Sionov RV, Ish-Shalom D: CD44: structure, function and association with the malignant process. Adv Cancer Res 1997, 71:241-319 [DOI] [PubMed] [Google Scholar]
- 18.Koopman G, Heider KH, Horst E, Adolf GR, van den Berg F, Ponta H, Herrlich P, Pals ST: Activated human lymphocytes and aggressive non-Hodgkin’s lymphomas express a homologue of the rat metastasis-associated variant of CD44. J Exp Med 1993, 177:897-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heider K-H, Hofmann M, Horst E, Van den Berg F, Ponta H, Herrlich P, Pals ST: A human homologue of the rat metastasis-associated variant of CD44 is expressed in colorectal carcinomas and adenomatous polyps. J Cell Biol 1993, 120:227-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulder JWR, Kruyt PM, Sewnath M, Oosting J, Seldenrijk CA, Weidema WF, Offerhaus GJA, Pals ST: Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. Lancet 1994, 344:1470-1472 [DOI] [PubMed] [Google Scholar]
- 21.Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM: Expression of CD44 and variant proteins in human colorectal cancer and its relevance for prognosis. Scan J Gastroenterol 1998, 33:303-309 [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi A, Urano T, Goi T, Saito M, Hiroso K, Nakagawa G, Shiku H, Furukawa K: Expression of a CD44 variant containing exons 8 to 10 is a useful independent factor for the prediction of prognosis in colorectal cancer. J Clin Oncol 1996, 14:1122-1127 [DOI] [PubMed] [Google Scholar]
- 23.Wielenga VJM, van der Voort R, Mulder JWR, Kruyt PM, Weidema WF, Oosting J, Selderijk CA, van Krimpen C, Offerhaus GJA, Pals ST: CD44 splice variants as prognostic markers in colorectal cancer. Scan J Gastroenterol 1998, 33:82-87 [DOI] [PubMed] [Google Scholar]
- 24.Wielenga VJM, Smits R, Korinek V, Smit L, Kielman M, Fodde R, Clevers H, Pals ST: Expression of CD44 in APC and TCF mutant mice implies regulation by the Wnt-pathway. Am J Pathol 1999, 154:515-524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wielenga VJM, Heider KH, Offerhaus GJA, Adolf GR, Van den Berg F, Ponta H, Herrlich P, Pals ST: Expression of CD44 variant proteins in human colorectal cancer is related to tumor progression. Cancer Res 1993, 53:4754-4756 [PubMed] [Google Scholar]
- 26.Kim H, Yang XL, Rosada C, Hamilton SR, August JT: CD44 expression in colorectal adenomas is an early event occurring prior to K-ras and p53 gene mutation. Arch Biochem Biophys 1994, 310:504-507 [DOI] [PubMed] [Google Scholar]
- 27.Kalomiris EL, Bourguignon LY: Mouse T lymphoma cells contain a transmembrane glycoprotein (GP85) that binds ankyrin. J Cell Biol 1988, 106:319-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsukita S, Oishi K, Sagara J, Kawai A, Tsukita S: ERM family members as molecular linkers between cell surface glycoprotein CD44 and actin-based cytoskeleton. J Cell Biol 1994, 126:391-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennett KL, Jackson DG, Simon JC, Tanczos E, Peach R, Modrell B, Stamenkovic I, Plowman G, Aruffo A: CD44 isoforms containing exon v3 are responsible for the presentation of heparin-binding growth factor. J Cell Biol 1995, 128:687-698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka Y, Adams DH, Hubscher S, Shaw S: T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1b. Nature 1993, 361:79-82 [DOI] [PubMed] [Google Scholar]
- 31.van der Voort R, Taher TEI, Wielenga VJM, Spaargaren M, Prevo R, Smit C, David G, Hartmann G, Gherardi E, Pals ST: Heparan sulfate-modified CD44 promotes hepatocyte growth factor/scatter factor-induced signal transduction through the receptor tyrosine kinase c-Met. J Biol Chem 1999, 274:6499-6506 [DOI] [PubMed] [Google Scholar]
- 32.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C: Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 1995, 376:768-771 [DOI] [PubMed] [Google Scholar]
- 33.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C: Scatter factor/hepatocyte growth factor is essential for liver development. Nature 1995, 373:699-702 [DOI] [PubMed] [Google Scholar]
- 34.Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N: Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 1995, 373:702-705 [DOI] [PubMed] [Google Scholar]
- 35.Birchmeier C, Birchmeier W, Brand-Saberi B: Epithelial-mesenchymal transitions in cancer progression. Acta Anatomica 1996, 156(Suppl 3):S217-S226 [DOI] [PubMed] [Google Scholar]
- 36.Maggiora P, Gambarotta G, Olivero M, Giordano S, Di Renzo MF, Comoglio PM: Control of invasive growth by the HGF receptor family. J Cell Physiol 1997, 173:183-186 [DOI] [PubMed] [Google Scholar]
- 37.Cooper CS, Park M, Blair DG, Tainsky MA, Huebner K, Croce CM, Vande Woude GF: Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 1984, 311:29-33 [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues GA, Park M: Autophosphorylation modulates the kinase activity and oncogenic potential of the Met receptor tyrosine kinase. Oncogene 1994, 9:2019-2027 [PubMed] [Google Scholar]
- 39.Jeffers M, Rong S, Anver M, Vande Woude GF: Autocrine hepatocyte growth factor/scatter factor-Met signaling induces transformation and the invasive/metastatic phenotype in C127 cells. Oncogene 1996, 13(Suppl 4):S853-S856 [PubMed] [Google Scholar]
- 40.Liang TJ, Reid AE, Xavier R, Cardiff RD, Wang TC: Transgenic expression of tpr-met oncogene leads to development of mammary hyperplasia and tumors. J Clin Invest 1996, 97:2872-2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takayama H, LaRochelle WJ, Sharp R, Otsuka T, Kriebel P, Anver M, Aaronson SA, Merlino G: Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci USA 1997, 94:701-706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeffers M, Rong S, Vande Woude GF: Enhanced tumorigenicity and invasion-metastasis by hepatocyte growth factor/scatter factor-met signalling in human cells concomitant with induction of the urokinase proteolysis network. Mol Cell Biol 1996, 16:1115-1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt L, Duh FM, Chen F, Kishida T, Glenn G, Choyke P, Scherer SW, Zhuang Z, Lubensky I, Dean M, Allikmets R, Chidambaram A, Bergerheim U, Feltis JT, Casadevall C, Zamarron A, Bernues M, Richard S, Lips CJM, Walther MM, Tsui LC, Geil L, Orcutt ML, Stackhouse T, Lipan J, Slife L, Brauch H, Decker J, Niehans G, Hughson MD, Moch H, Storkel S, Lerman MI, Linehan WM, Zbar B: Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet 1997, 16:68-73 [DOI] [PubMed] [Google Scholar]
- 44.Jeffers M, Schmidt L, Nakaigawa N, Webb CP, Weirich G, Kishida T, Zbar B, Vande Woude GF: Activating mutations for the met tyrosine kinase receptor in human cancer. Proc Natl Acad Sci USA 1997, 94:11445-11450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.David G, Bai XM, Van Der Schueren B, Cassiman JJ, Van Den Berghe H: Developmental changes in heparan sulfate expression: in situ detection with mAbs. J Cell Biol 1992, 119:961-975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hartmann G, Prospero T, Brinkmann V, Ozcelik O, Winter G, Hepple J, Batley S, Bladt F, Sachs M, Birchmeier C: Engineered mutants of HGF/SF with reduced binding to heparan sulfate proteoglycans, decreased clearance and enhanced activity in vivo. Curr Biol 1998, 8:125-134 [DOI] [PubMed] [Google Scholar]
- 47.Hartmann G, Naldini L, Weidner KM, Sachs M, Vigna E, Comoglio PM, Birchmeier W: A functional domain in the heavy chain of scatter factor/hepatocyte growth factor binds the c-Met receptor and induces cell dissociation but not mitogenesis. Proc Natl Acad Sci USA 1992, 89:11574-11578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weidner KM, Behrens J, Vandekerckhove J, Birchmeier W: Scatter factor: molecular characteristics and effect on the invasiveness of epithelial cells. J Cell Biol 1990, 111:2097-2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Voort R, Taher TEI, Keehnen RMJ, Smit L, Groenink M, Pals ST: Paracrine regulation of germinal center B cell adhesion through the c-Met-hepatocyte growth factor/scatter factor pathway. J Exp Med 1997, 185:2121-2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taher TEI, Smit L, Griffioen W, Schilder-Tol EJM, Borst J, Pals ST: Signaling through CD44 is mediated by tyrosine kinases. Association with p56lck in T lymphocytes. J Biol Chem 1996, 271:2863-2867 [DOI] [PubMed] [Google Scholar]
- 51.van der Voort R, Manten-Horst E, Smit L, Ostermann E, Van den Berg F, Pals ST: Binding of cell-surface expressed CD44 hyaluronate is dependent on splicing and cell type. Biochem Biophys Res Comm 1995, 214:137-144 [DOI] [PubMed] [Google Scholar]
- 52.Di Renzo MF, Narsimhan RP, Olivero M, Bretti S, Giordano S, Medico E, Gaglia P, Zara P, Comoglio PM: Expression of the Met/HGF receptor in normal and neoplastic human tissues. Oncogene 1991, 6:1997-2003 [PubMed] [Google Scholar]
- 53.Di Renzo MF, Olivero M, Giacomini A, Porte H, Chastre E, Mirossay L, Nordlinger B, Bretti S, Bottardi S, Giordano S, Plebani M, Gespach C, Comoglio PM: Overexpression and amplification of the Met/HGF receptor gene during the progression of colorectal cancer. Clin Cancer Res 1995, 1:147-154 [PubMed] [Google Scholar]
- 54.Liu C, Park M, Tsao MS: Overexpression of c-met proto-oncogene but not epidermal growth factor receptor or c-erb-2 in primary human colorectal carcinomas. Oncogene 1992, 7:181-185 [PubMed] [Google Scholar]
- 55.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW: Identification of c-MYC as a target of the APC pathway. Science 1998, 281:1509-1512 [DOI] [PubMed] [Google Scholar]
- 56.Yamashita JI, Ogawa M, Yamashita SI, Nomura K, Kuramoto M, Saishoji T, Shin S: Immunoreactive hepatocyte growth factor is a strong and independent predictor of recurrence and survival in human breast cancer. Cancer Res 1994, 54:1630-1633 [PubMed] [Google Scholar]
- 57.Tuck AB, Park M, Sterns EE, Boag A, Elliot BE: Co-expression of hepatocyte growth factor and receptor (Met) in human breast carcinoma. Am J Pathol 1996, 148:225-232 [PMC free article] [PubMed] [Google Scholar]
- 58.Nakamura T, Matsumoto K, Kiritoshi A, Tano Y, Nakamura T: Induction of hepatocyte growth factor in fibroblasts by tumor-derived factors affects invasive growth of tumor cells: in vitro analysis of tumor-stromal interactions. Cancer Res 1997, 57:3305-3313 [PubMed] [Google Scholar]
- 59.Ota S, Tanaka Y, Bamba H, Kato A, Matsuzaki F: Non-steroidal antiinflammatory drugs may prevent colon cancer through suppression of hepatocyte growth factor expression. Eur J Pharmacol 1999, 367:131-138 [DOI] [PubMed] [Google Scholar]
- 60.Beilmann M, Odenthal M, Jung W, Vande Woude GF, Dienes HP, Schirmacher P: Neoexpression of the c-met/hepatocyte growth factor-scatter factor receptor gene in activated monocytes. Blood 1997, 90(Suppl 11):S4450-S4458 [PubMed] [Google Scholar]
- 61.Noji S, Tashiro K, Koyama E, Nohno T, Ohyama K, Taniguchi S, Nakamura T: Expression of hepatocyte growth factor gene in endothelial and Kupffer cells of damaged rat livers, as revealed by in situ hybridization. Biochem Biophys Res Comm 1990, 173:42-47 [DOI] [PubMed] [Google Scholar]
- 62.Steinfeld R, Van Den Berghe H, David G: Stimulation of fibroblast growth factor receptor-1 occupancy and signaling by cell surface-associated syndecans and glypican. J Cell Biol 1996, 133:405-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA: Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol 1997, 137(Suppl 2):S481-S492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeffers M, Koochekpour S, Fiscella M, Sathyanarayana BK, Vande Woude GF: Signaling requirements for oncogenic forms of the Met tyrosine kinase receptor. Oncogene 1998, 17:2691-2700 [DOI] [PubMed] [Google Scholar]
- 65.Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz D: Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 1991, 64:841-848 [DOI] [PubMed] [Google Scholar]
- 66.Maccarana M, Casu B, Lindahl U: Minimal sequence in heparin/heparan sulfate required for binding of basic fibroblast growth factor. J Biol Chem 1993, 268:23898-23905 [PubMed] [Google Scholar]
- 67.Aviezer D, Levy E, Safran M, Svahn C, Buddecke E, Schmidt A, David G, Vlodavsky I, Yayon A: Differential structural requirements of heparin and heparan sulfate proteoglycans that promote binding of basic fibroblast growth factor to its receptor. J Biol Chem 1994, 269:114-121 [PubMed] [Google Scholar]
- 68.Rapraeger AC, Krufka A, Olwin B: Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science 1992, 252:1705-1708 [DOI] [PubMed] [Google Scholar]
- 69.Schlessinger J, Lax I, Lemmon M: Regulation of growth factor activation by proteoglycans: what is the role of the low affinity receptors? Cell 1995, 83:357-360 [DOI] [PubMed] [Google Scholar]
- 70.Spivak-Kroizman T, Lemmon MA, Dikic I, Ladbury JE, Pinchasi D, Huang J, Jaye M, Crumley G, Schlessinger J, Lax I: Heparin-induced oligomerization of FGF molecules is responsible for FGF receptor dimerization, activation, and cell proliferation. Cell 1994, 79:1015-1024 [DOI] [PubMed] [Google Scholar]
- 71.Klagsbrun M, Baird A: A dual receptor system is required for basic fibroblast growth factor activity. Cell 1991, 67:229-231 [DOI] [PubMed] [Google Scholar]
- 72.Sonnenberg E, Meyer D, Weidner KM, Birchmeier C: Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol 1993, 123:223-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sherman L, Wainwright D, Ponta P, Herrlich P: A splice variant of CD44 expressed on the apical ectodermal ridge presents fibroblast growth factors to limb mesenchyme and is required for limb outgrowth. Genes Dev 1998, 12:1058-1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhatavdekar JM, Patel DD, Chikhlikar PR, Trivedi TI, Gosalia NM, Ghosh N, Shah NG, Vora HH, Suthar TP: Overexpression of CD44: a useful independent predictor of prognosis in patients with colorectal carcinomas. Ann Surg Oncol 1998, 5:495-501 [DOI] [PubMed] [Google Scholar]